The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Malignant and Benign Subpleural Lung Lesions
Abstract
1. Introduction
2. Material and Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
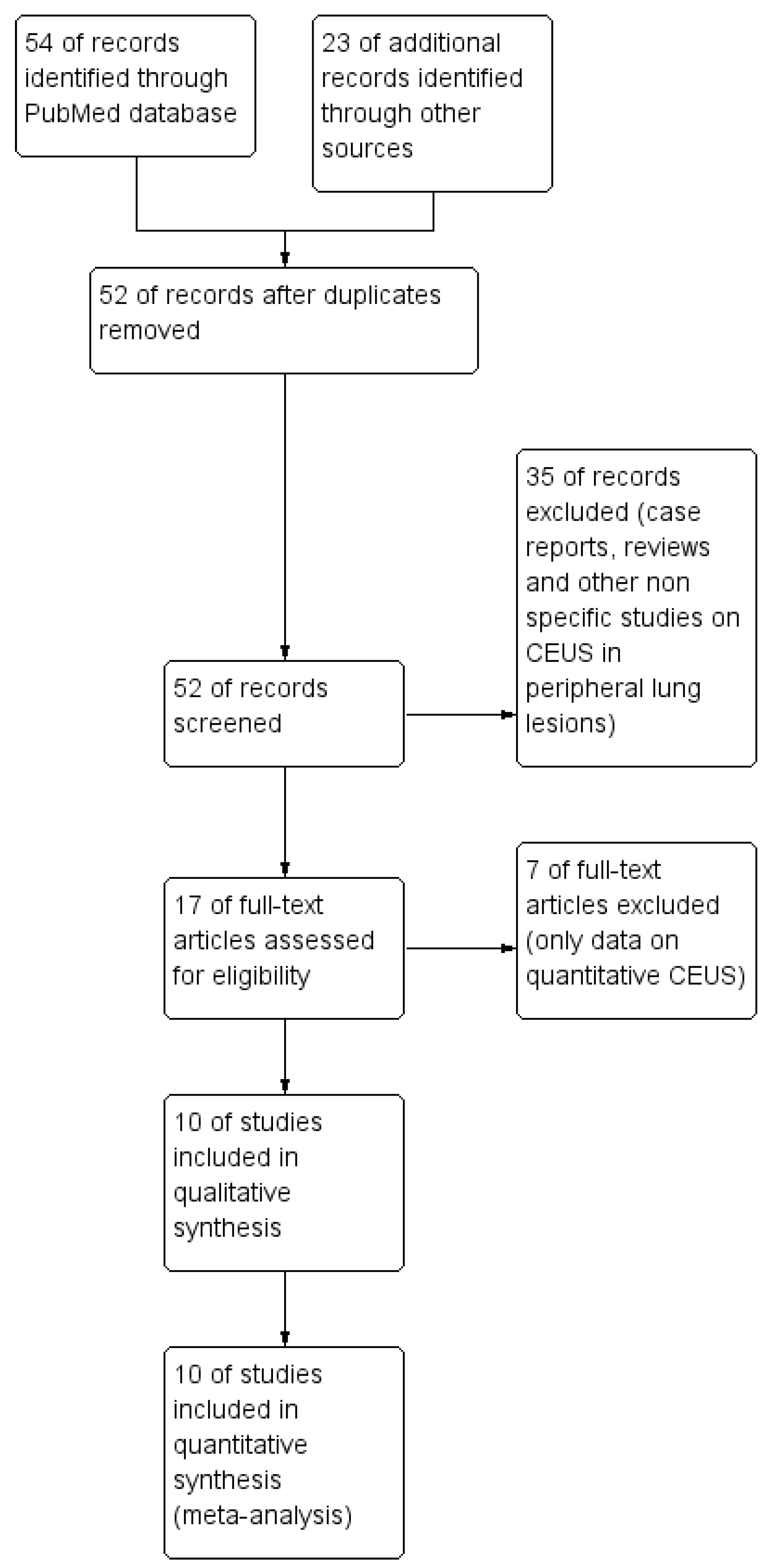
3.1. Study Identification
3.2. Study Characteristics
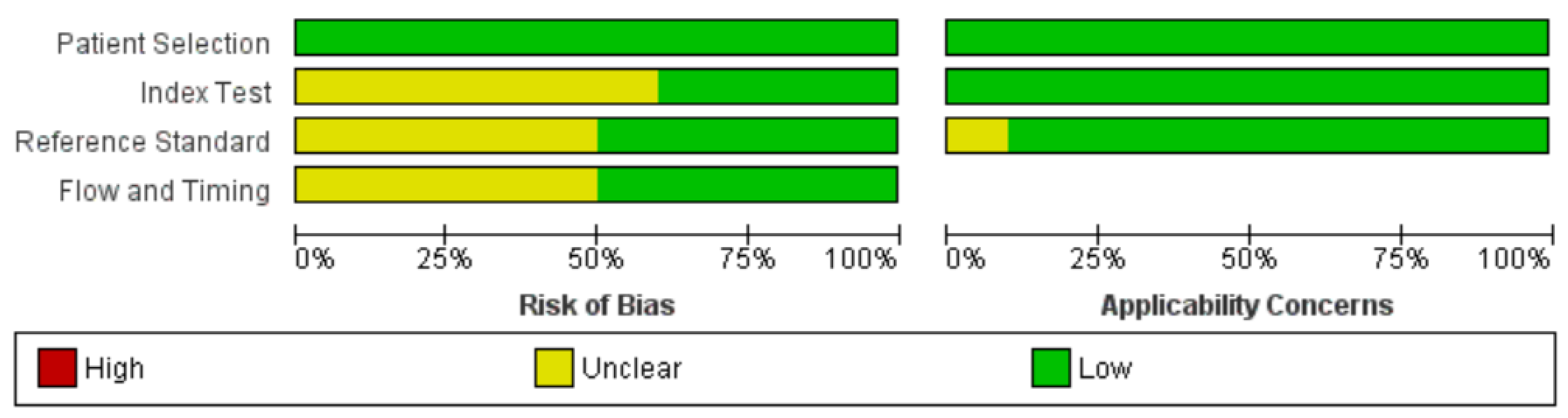
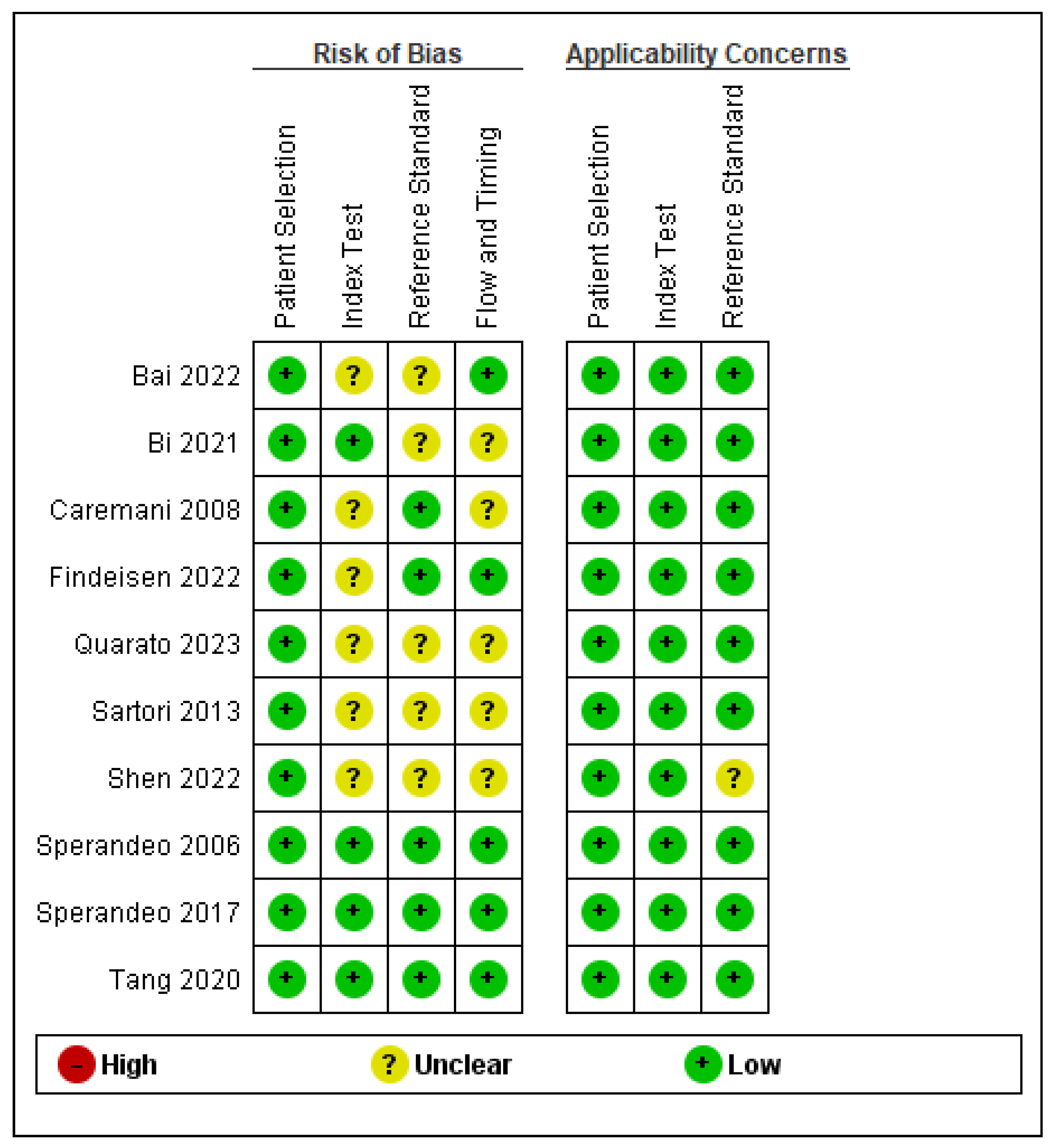
3.3. Quality Assessment
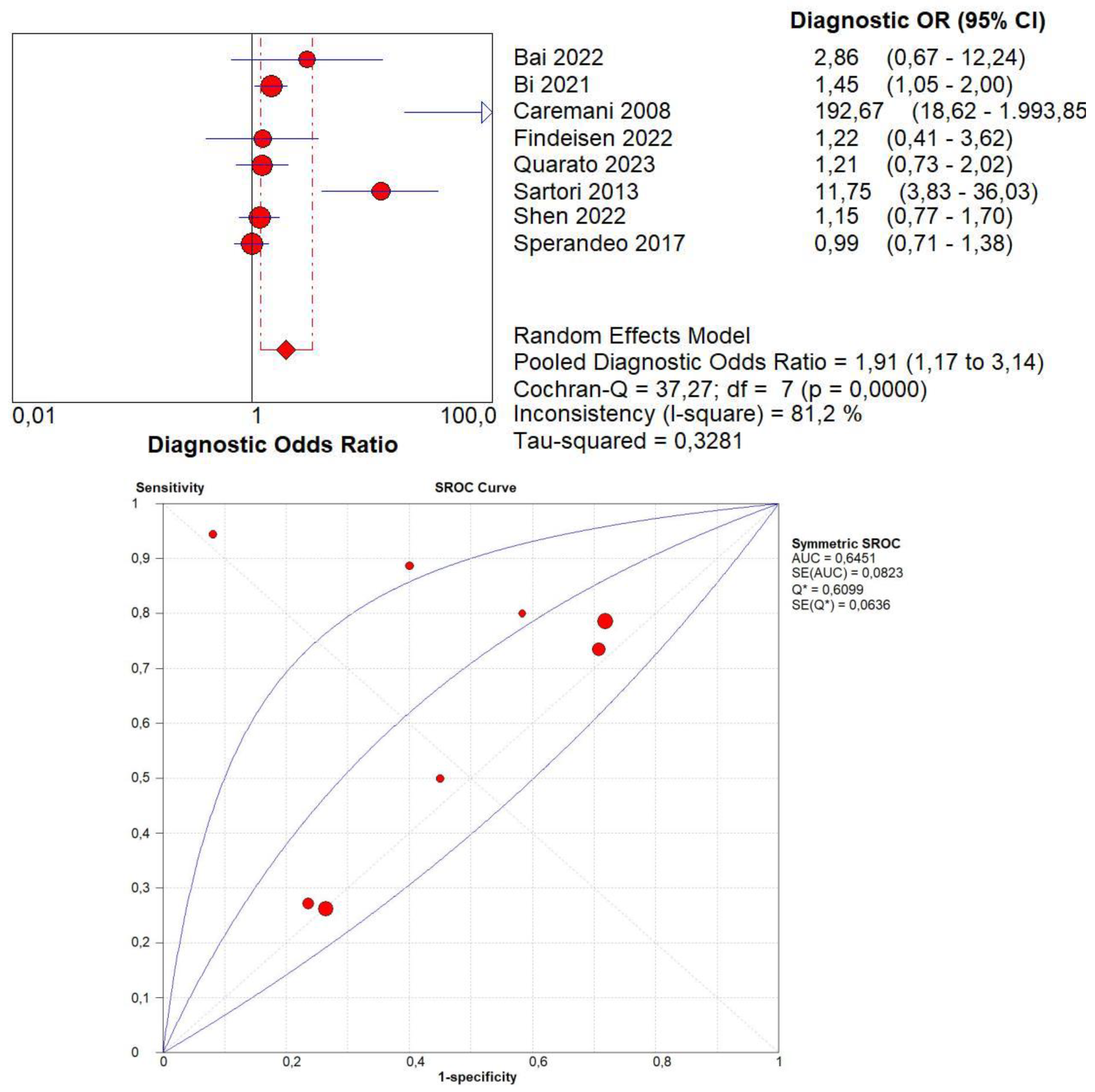
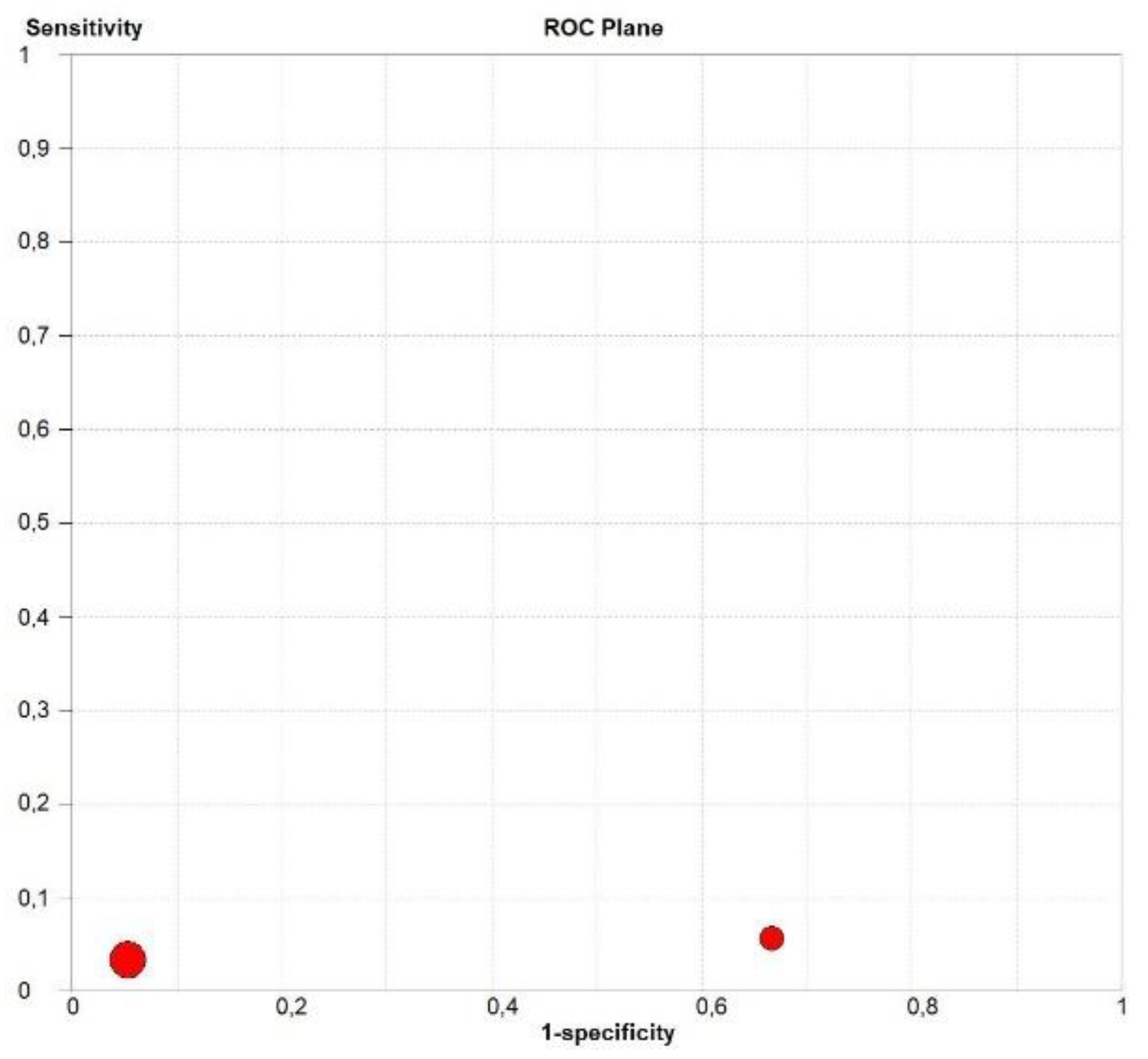
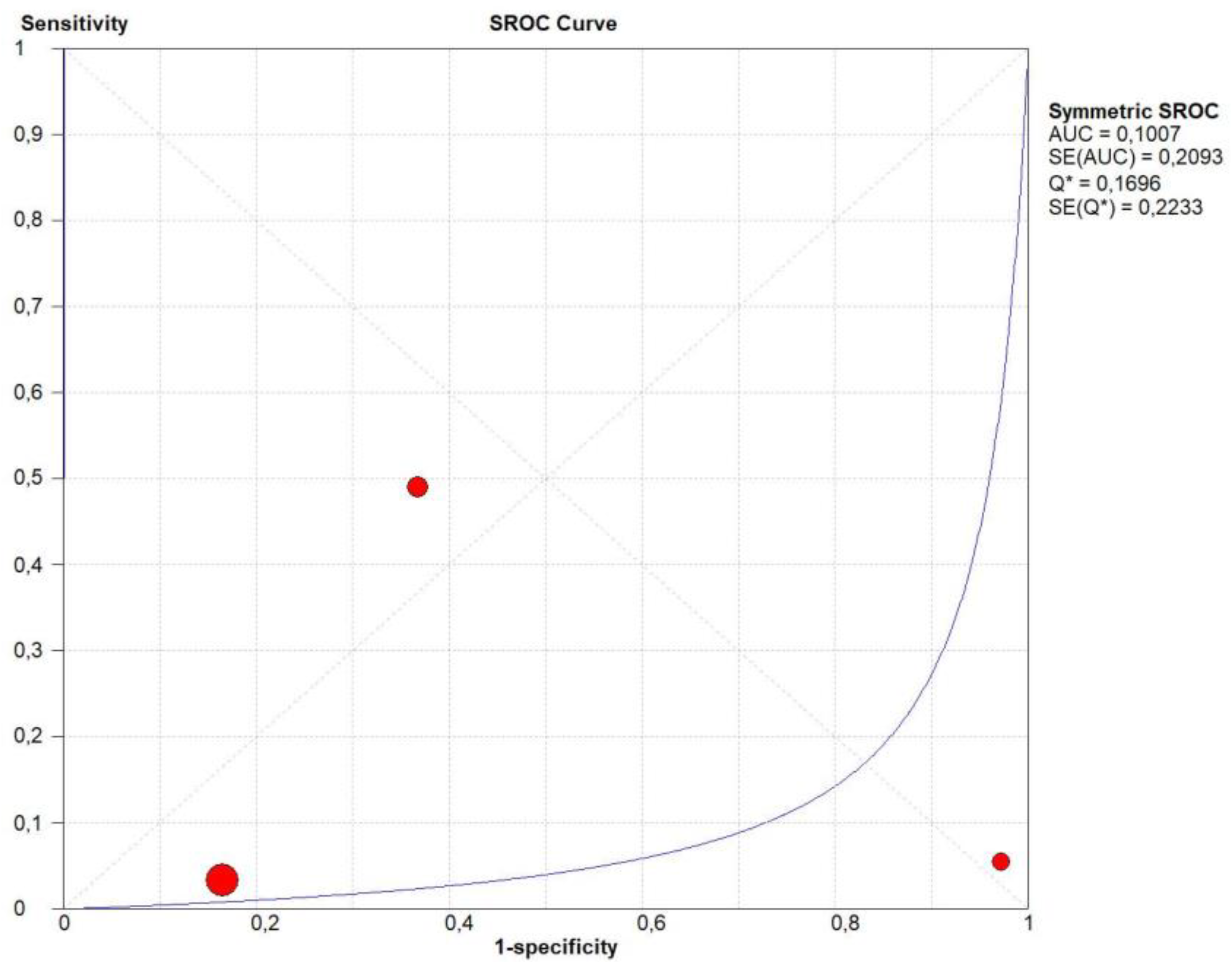
3.4. Overall Diagnostic Accuracy of CEUS
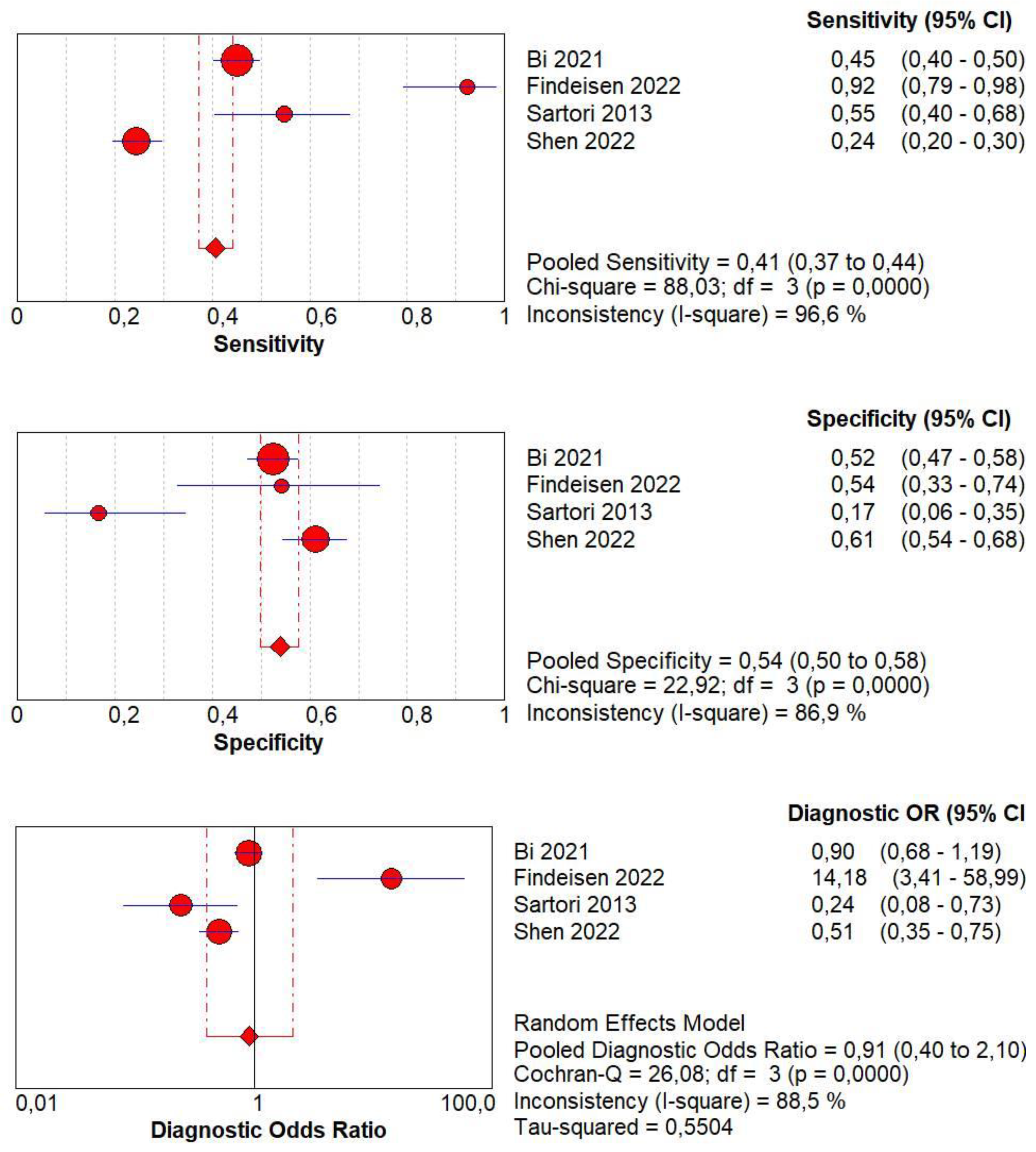
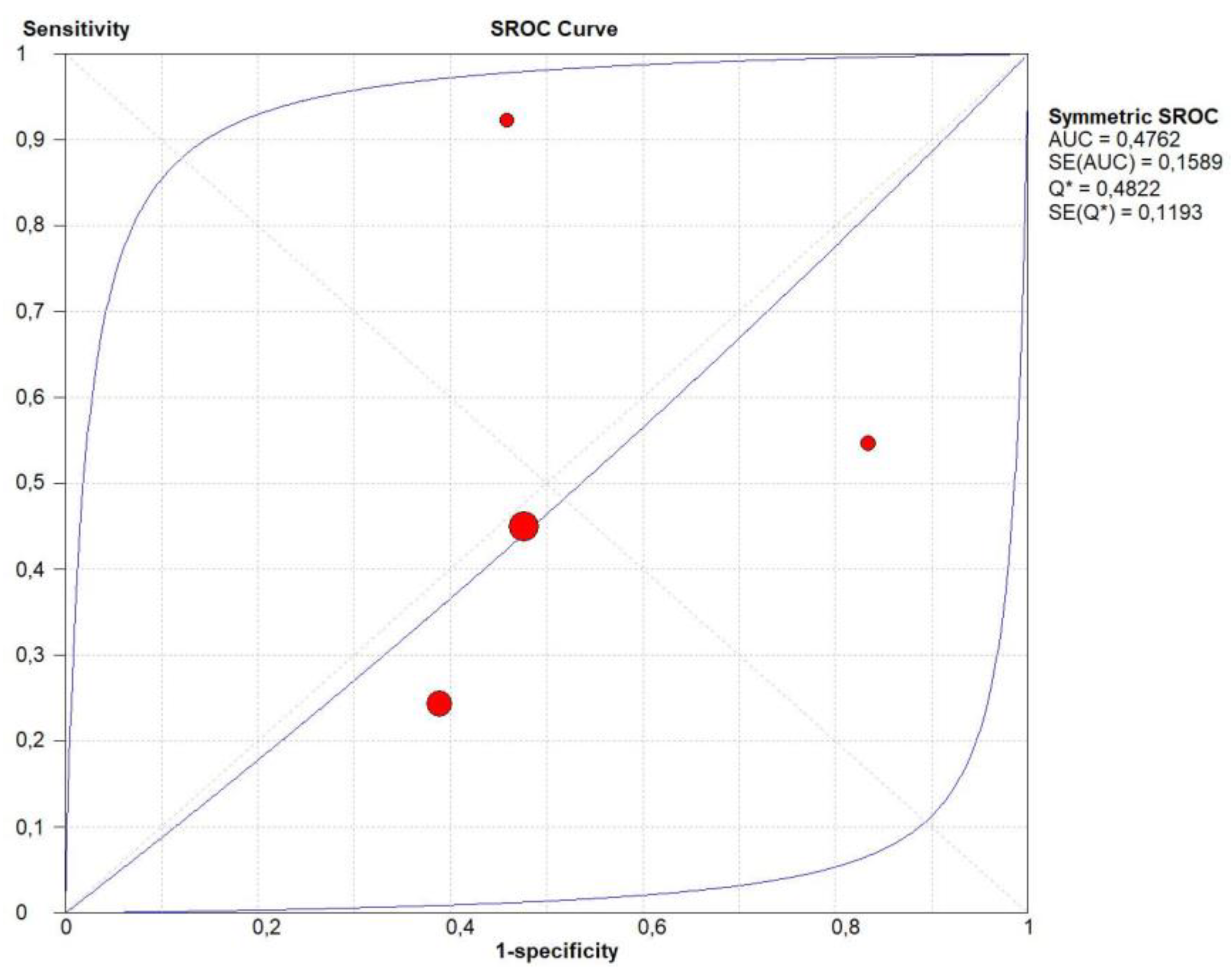
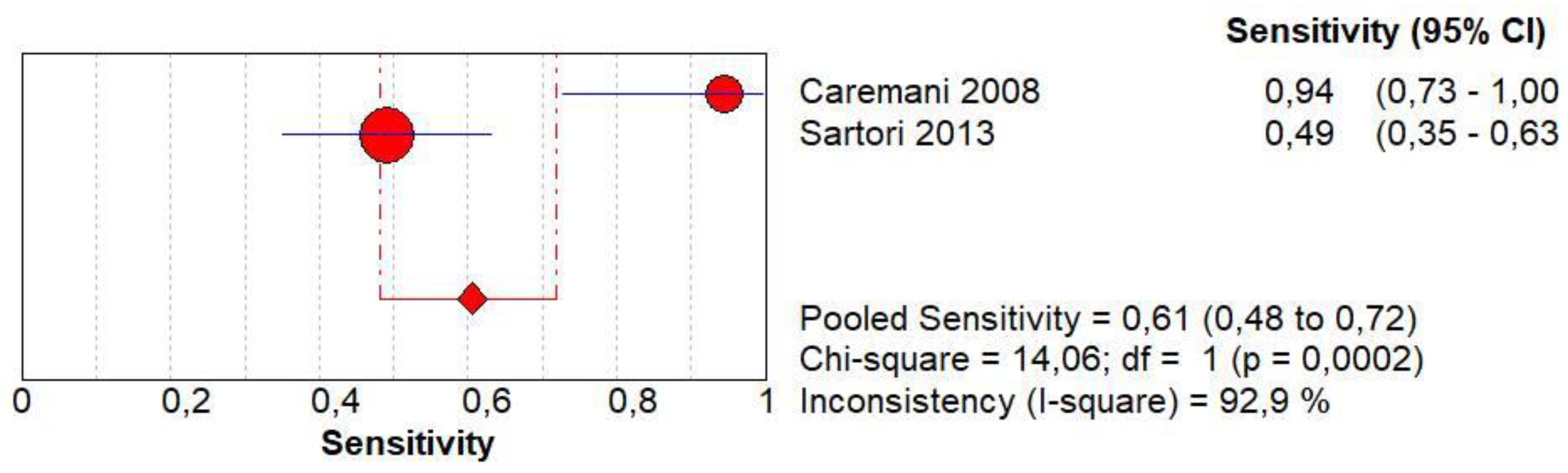
3.5. Diagnostic Accuracy of Homogeneous CE
3.6. Diagnostic Accuracy of Non-Homogeneous CE
3.7. Diagnostic Accuracy of Lack of CE
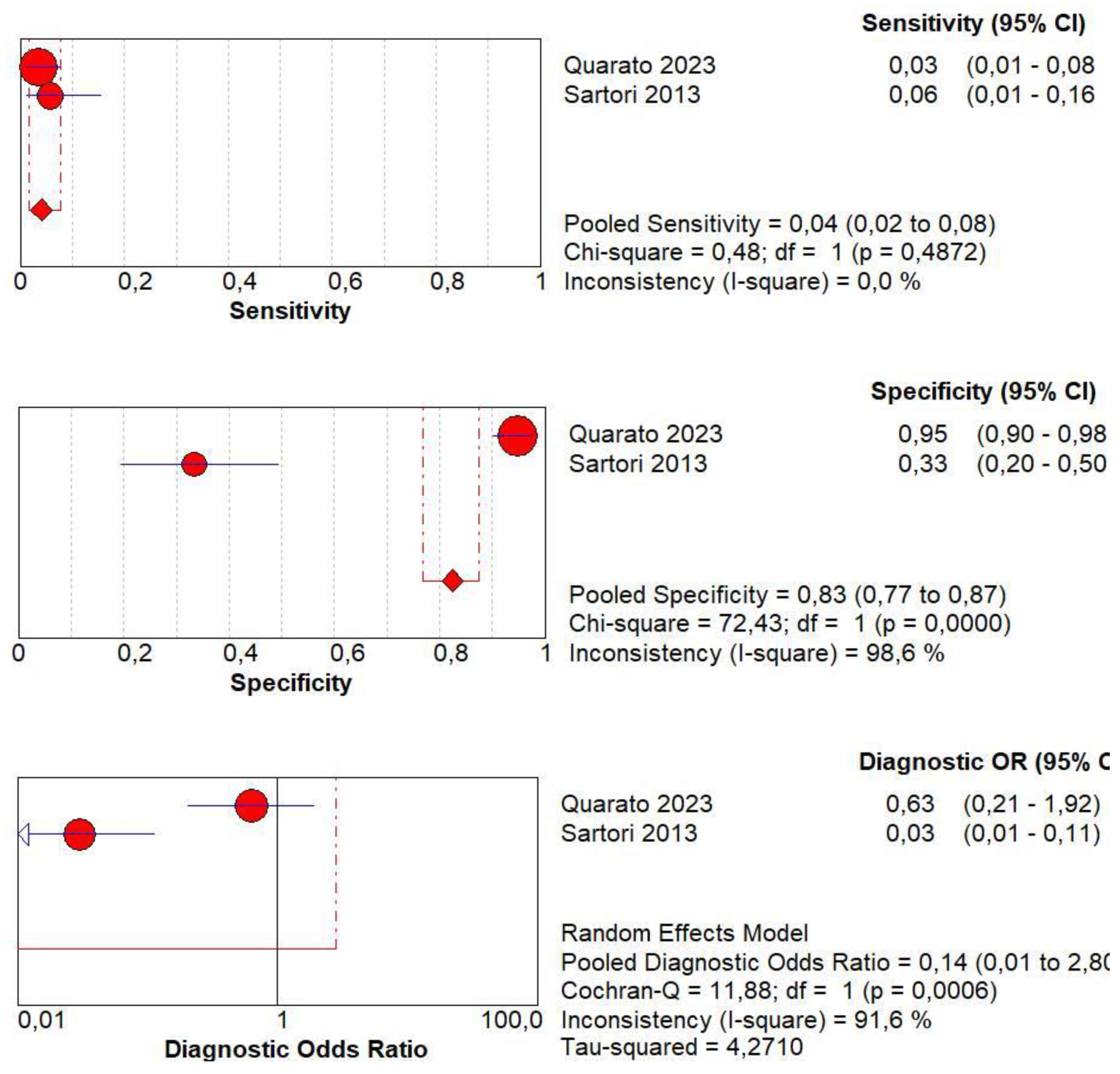
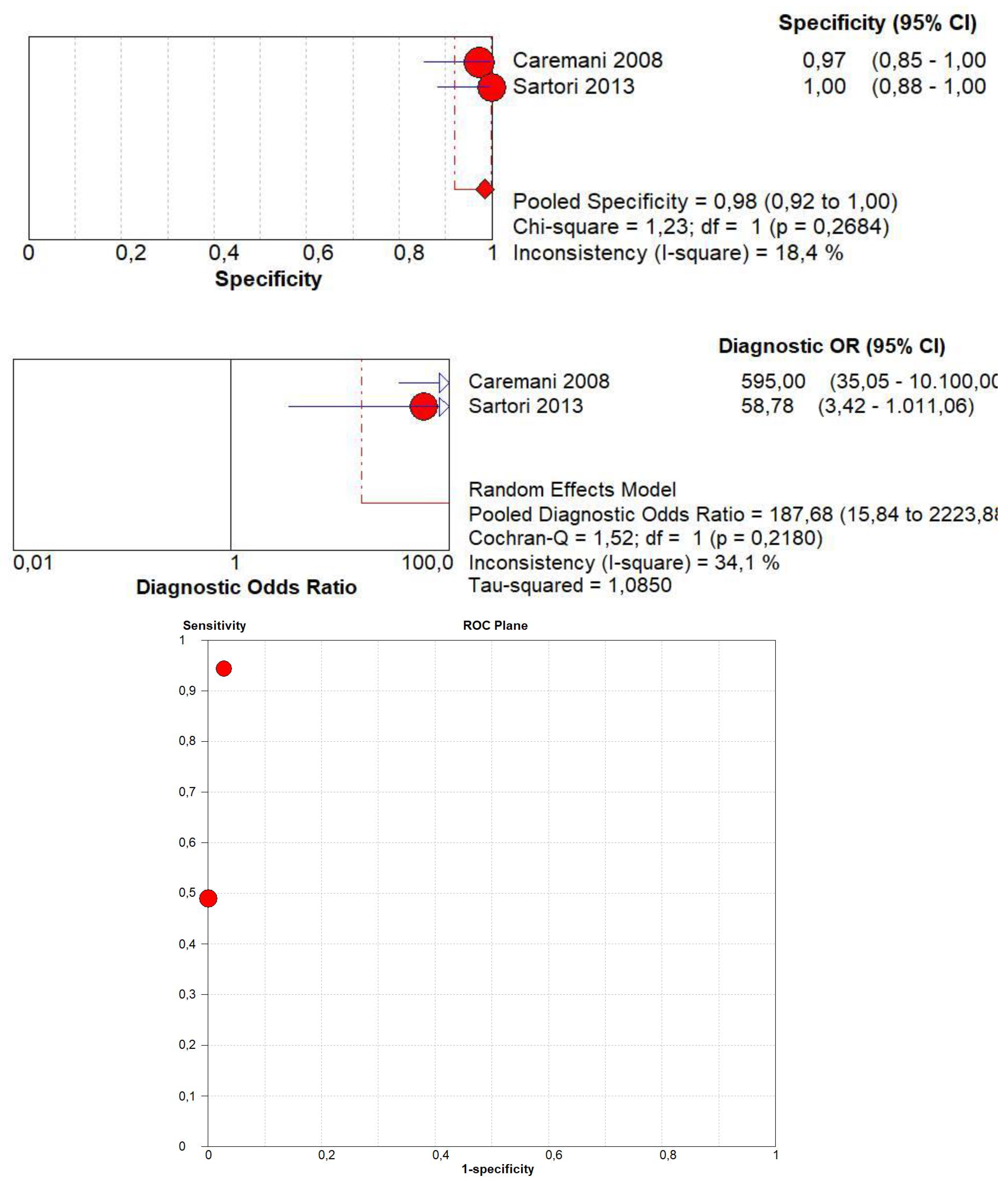
3.8. Diagnostic Accuracy of Marked CE
3.9. Diagnostic Accuracy of Non-Marked CE
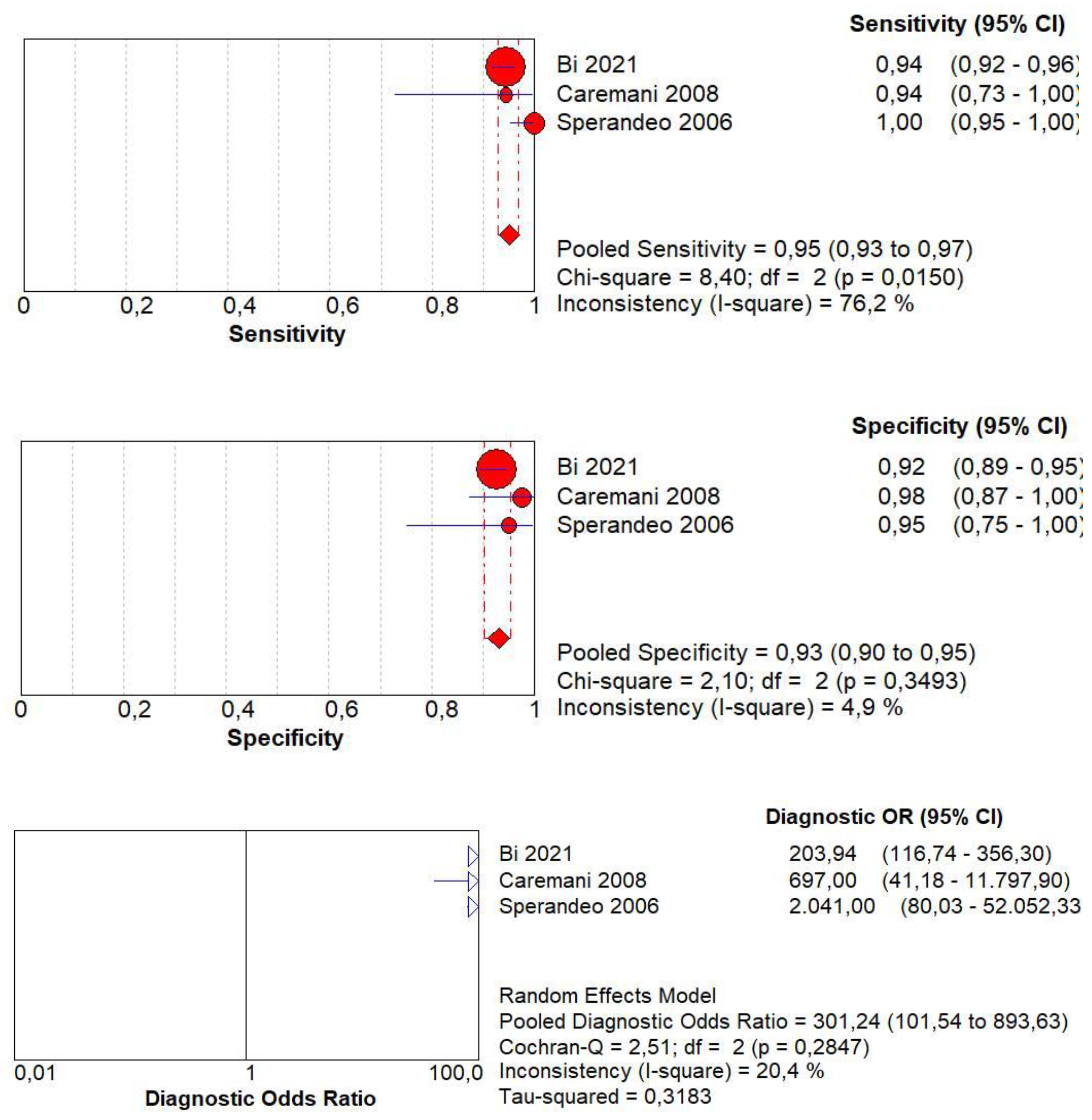
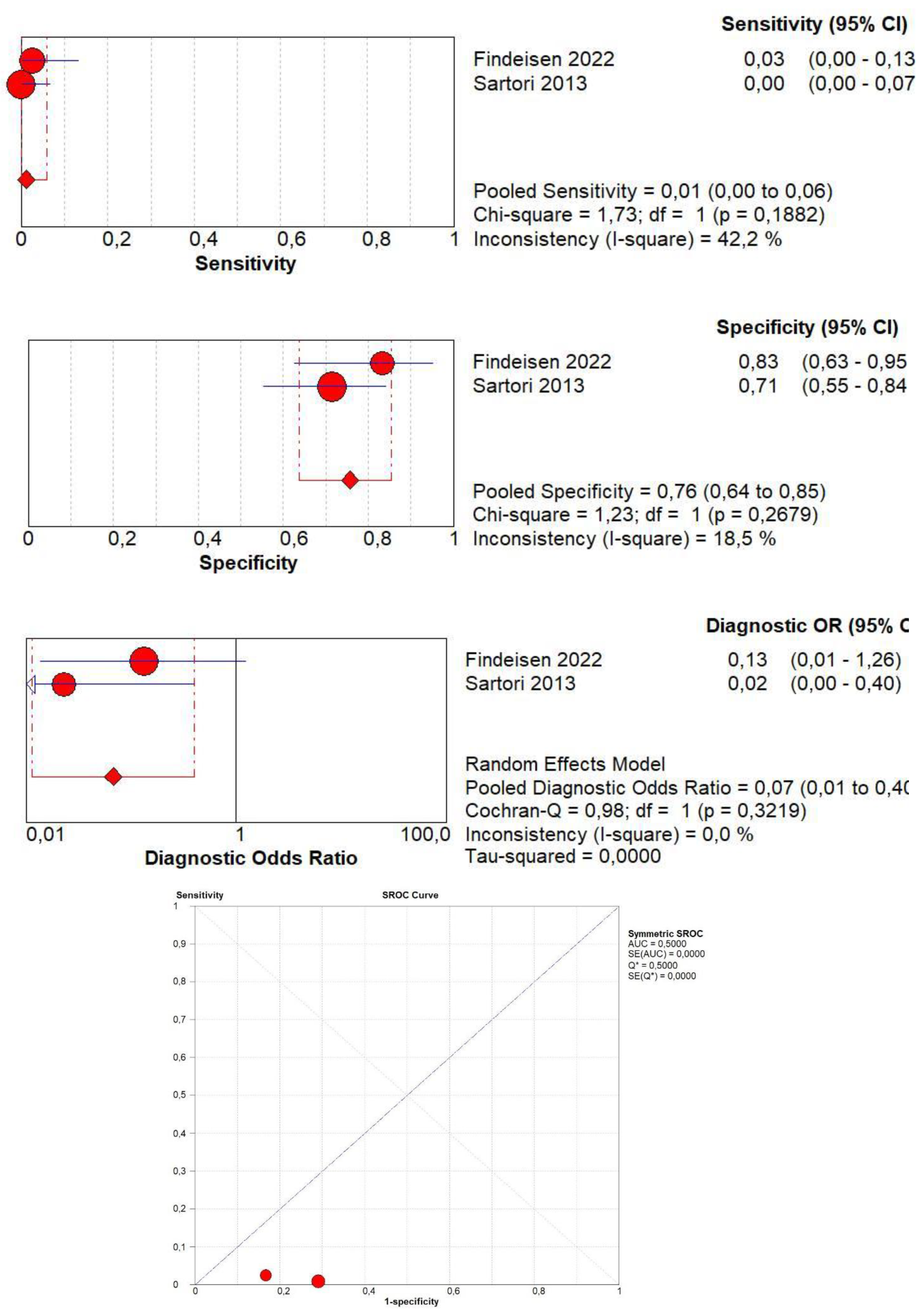
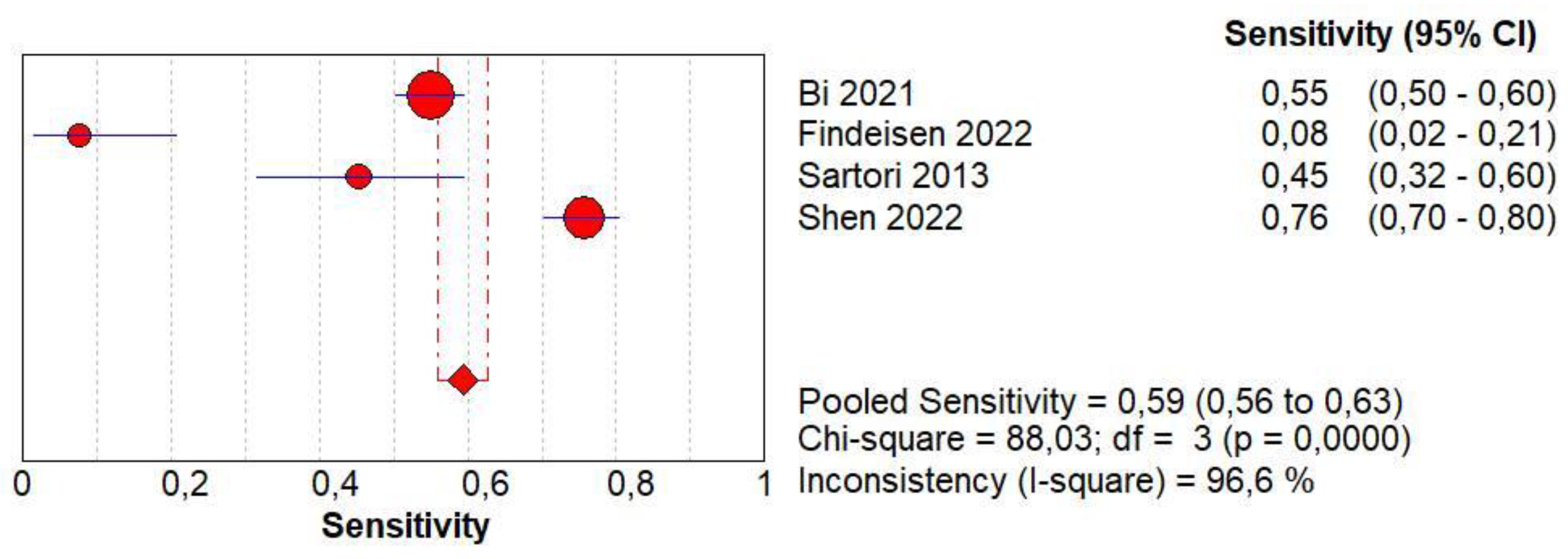
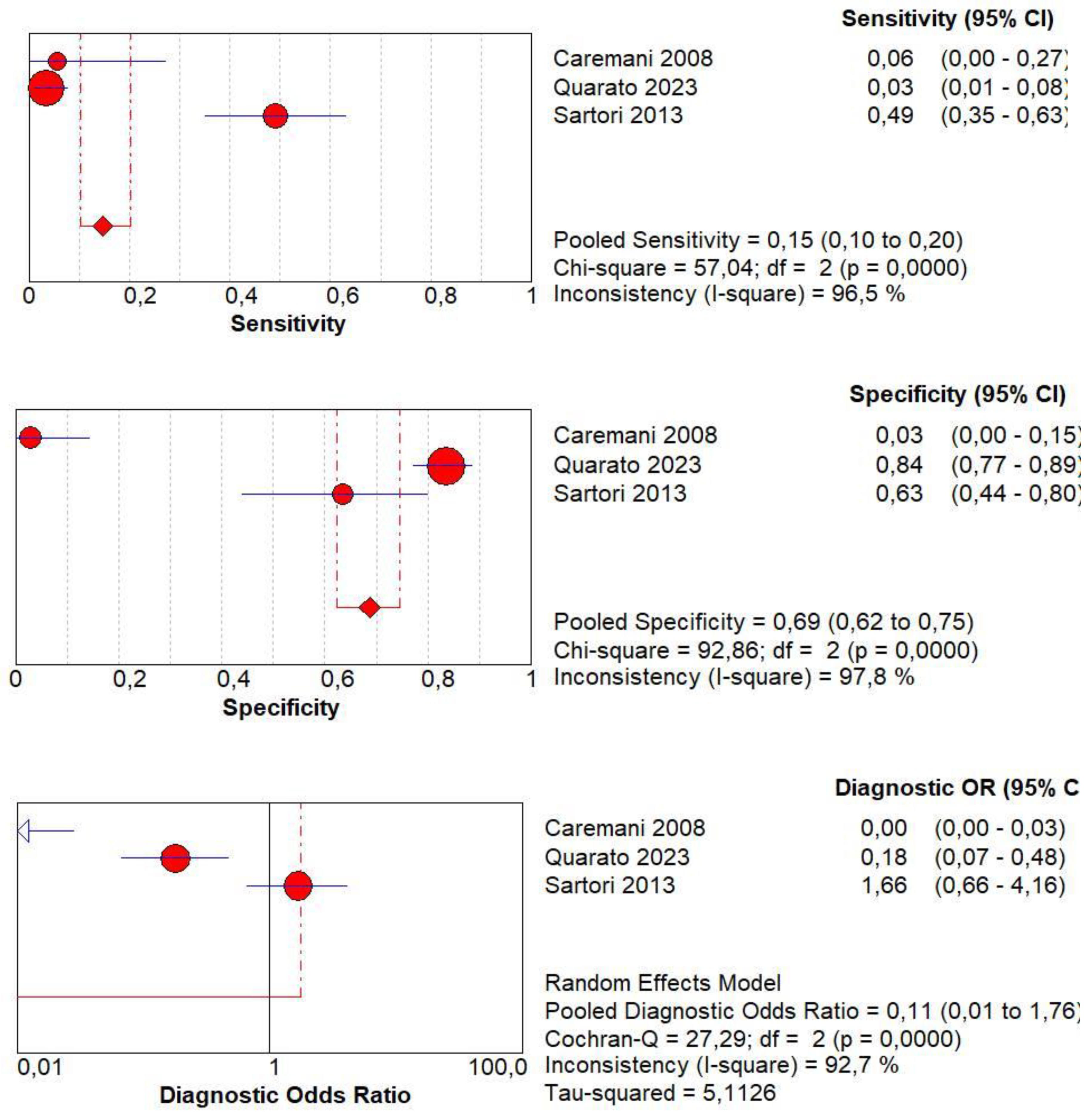
3.10. Diagnostic Accuracy of Early AT
3.11. Diagnostic Accuracy of Early Wash Out
3.12. Diagnostic Accuracy of Delayed Wash Out
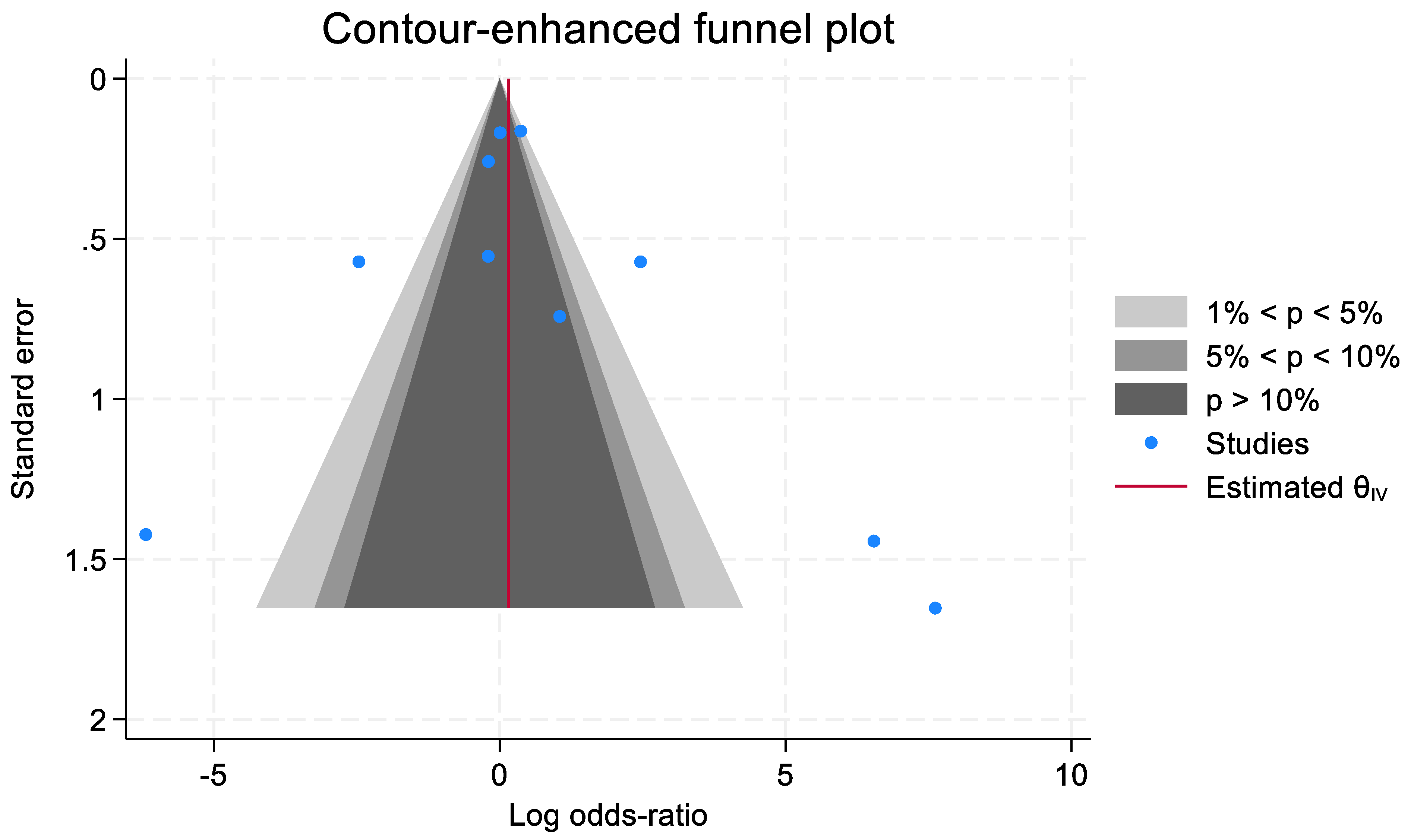
3.13. Publication Bias
4. Discussion
5. Strengths and Limitations of CEUS Characterization of Subpleural Lung Lesions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Safai Zadeh, E.; Alhyari, A.; Kroenig, J.; Görg, C.; Trenker, C.; Dietrich, C.F.; Findeisen, H. B-mode ultrasound and contrast-enhanced ultrasound for evaluation of pneumonia: A pictorial essay. Australas. J. Ultrasound Med. 2023, 26, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Cocco, G.; D’Ardes, D.; Delli Pizzi, A.; Vidili, G.; De Molo, C.; Vicari, S.; Serra, C.; Cipollone, F.; Schiavone, C.; et al. Infectious Pneumonia and Lung Ultrasound: A Review. J. Clin. Med. 2023, 12, 1402. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Andreetto, L.; Vicari, S.; Campello, E.; Simioni, P.; Ageno, W. The Diagnostic Role of Lung Ultrasound and Contrast-Enhanced Ultrasound in Pulmonary Embolism. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: Leipzig, Germany, 2023. [Google Scholar] [CrossRef]
- Buz Yaşar, A.; Tarhan, M.; Atalay, B.; Kabaalioğlu, A.; Girit, S. Investigation of Childhood Pneumonia with Thoracic Ultrasound: A Comparison Between X-ray and Ultrasound. Ultrasound Q. 2023, 39, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Calamai, I.; Greco, M.; Finazzi, S.; Savi, M.; Vitiello, G.; Garbero, E.; Spina, R.; Montisci, A.; Mongodi, S.; Bertolini, G. Thoracic UltrasONOgraphy Reporting: The TUONO Study. J. Clin. Med. 2022, 11, 7126. [Google Scholar] [CrossRef]
- Shah, A.; Oliva, C.; Stem, C.; Cummings, E.Q. Application of dynamic air bronchograms on lung ultrasound to diagnose pneumonia in undifferentiated respiratory distress. Respir. Med. Case Rep. 2022, 39, 101706. [Google Scholar] [CrossRef] [PubMed]
- Görg, C.; Bert, T. Transcutaneous colour Doppler sonography of lung consolidations: Review and pictorial essay. Part 2: Colour Doppler sonographic patterns of pulmonary consolidations. Ultraschall Der Med.-Eur. J. Ultrasound 2004, 25, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Görg, C.; Bert, T. Transcutaneous colour Doppler sonography of lung consolidations: Review and pictorial essay. Part 1: Pathophysiologic and colour Doppler sonographic basics of pulmonary vascularity. Ultraschall Der Med.-Eur. J. Ultrasound 2004, 25, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Cocco, G.; D’Ardes, D.; Schiavone, C. GB-RADS score, a possible role for CEUS? Abdom. Radiol. 2023, 48, 1199–1201. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cekuolis, A.; Schreiber-Dietrich, D.; Augustiniene, R.; Schwarz, S.; Möller, K.; Nourkami-Tutdibi, N.; Chen, S.; Cao, J.Y.; Huang, Y.L.; et al. Review on Pediatric Malignant Focal Liver Lesions with Imaging Evaluation: Part I. Diagnostics 2023, 13, 3568. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast-Enhanced Ultrasound (CEUS) in the Liver-Update 2020 WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med. Biol. 2020, 46, 2579–2604. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Lei, Y.; Cui, L.; Du, T.; Mei, F. Can Ultrasound and Contrast-Enhanced Ultrasound Help Differentiate between Subpleural Focal Organizing Pneumonia and Primary Lung Malignancy? Diagnostics 2022, 12, 2074. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, V.; Andronikou, S.; Mentzel, H.J.; Piskunowicz, M.; Squires, J.H.; Barnewolt, C.E. Contrast-enhanced ultrasound of pediatric lungs. Pediatr. Radiol. 2021, 51, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, H.; Trenker, C.; Figiel, J.; Greene, B.H.; Görg, K.; Görg, C. Vascularization of Primary, Peripheral Lung Carcinoma in CEUS—A Retrospective Study (n = 89 Patients). Ultraschall Med. 2019, 40, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Liu, T.; Liu, W.; Li, Z.; Zheng, H.; Li, X. Application value of contrast-enhanced ultrasound in the diagnosis of peripheral pulmonary focal lesions. Medicine 2022, 101, e29605. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Xia, D.M.; Fan, L.; Ye, X.F.; Zhang, Y.; Shen, M.J.; Chen, H.W.; Cong, Y.; Zhu, H.M.; Tang, C.H.; et al. Development and Prospective Validation of an Ultrasound Prediction Model for the Differential Diagnosis of Benign and Malignant Subpleural Pulmonary Lesions: A Large Ambispective Cohort Study. Front. Oncol. 2021, 11, 656060. [Google Scholar] [CrossRef] [PubMed]
- Caremani, M.; Benci, A.; Lapini, L.; Tacconi, D.; Caremani, A.; Ciccotosto, C.; Magnolfi, A.L. Contrast enhanced ultrasonography (CEUS) in peripheral lung lesions: A study of 60 cases. J. Ultrasound 2008, 11, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Findeisen, H.; Görg, C.; Hartbrich, R.; Dietrich, C.F.; Görg, K.; Trenker, C.; Safai Zadeh, E. Contrast-enhanced ultrasound is helpful for differentiating benign from malignant parietal pleural lesions. J. Clin. Ultrasound 2022, 50, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Quarato, C.M.I.; Feragalli, B.; Lacedonia, D.; Rea, G.; Scioscia, G.; Maiello, E.; Di Micco, C.; Borelli, C.; Mirijello, A.; Graziano, P.; et al. Contrast-Enhanced Ultrasound in Distinguishing between Malignant and Benign Peripheral Pulmonary Consolidations: The Debated Utility of the Contrast Enhancement Arrival Time. Diagnostics 2023, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Postorivo, S.; Vece, F.D.; Ermili, F.; Tassinari, D.; Tombesi, P. Contrast-enhanced ultrasonography in peripheral lung consolidations: What’s its actual role? World J. Radiol. 2013, 5, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Bi, K.; Cong, Y.; Chen, H.; Zhang, Y.; Zhu, H.; Wang, Y. Application of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Benign and Malignant Subpleural Pulmonary Lesions. J. Ultrasound Med. 2022, 41, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Sperandeo, G.; Varriale, A.; Filabozzi, P.; Decuzzi, M.; Dimitri, L.; Vendemiale, G. Contrast-enhanced ultrasound (CEUS) for the study of peripheral lung lesions: A preliminary study. Ultrasound Med. Biol. 2006, 32, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, M.; Rea, G.; Grimaldi, M.A.; Trovato, F.; Dimitri, L.M.; Carnevale, V. Contrast-enhanced ultrasound does not discriminate between community acquired pneumonia and lung cancer. Thorax 2017, 72, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Xie, Q.; Wang, J.; Zhai, X.; Lin, H.; Zheng, X.; Wei, G.; Tang, Y.; Zeng, F.; Chu, Y.; et al. Time Difference of Arrival on Contrast-Enhanced Ultrasound in Distinguishing Benign Inflammation from Malignant Peripheral Pulmonary Lesions. Front. Oncol. 2020, 10, 578884. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Lencioni, R.; Sagrini, E.; Pina, C.D.; Cioni, D.; Vidili, G.; Bolondi, L. Characterization of focal liver lesions with contrast-enhanced ultrasound. Ultrasound Med. Biol. 2010, 36, 531–550. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, M.; Romanini, L.; Serra, C.; Magnolfi, F.; Bertolotto, M.; Quaia, E.; Puntel, G.; Colleoni, A.; Fiorini, E.; Cenci, C.; et al. Contrast enhancement ultrasound application in focal liver lesions characterization: A retrospective study about guidelines application (SOCEUS-CEUS survey). J. Ultrasound 2016, 19, 99–106. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Shen, Y.J.; Aragaki, M.; Motooka, Y.; Koga, T.; Gregor, A.; Bernards, N.; Cherin, E.; Demore, C.E.M.; Yasufuku, K.; et al. Imaging Microbubbles with Contrast-Enhanced Endobronchial Ultrasound. Ultrasound Med. Biol. 2024, 50, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Petrone, M.C.; Arcidiacono, P.G. A narrative review of the role of endoscopic ultrasound (EUS) in lung cancer staging. Mediastinum 2021, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Pillay, J.; Rahman, S.; Klarenbach, S.; Reynolds, D.L.; Tessier, L.A.; Thériault, G.; Persaud, N.; Finley, C.; Leighl, N.; McInnes, M.D.F.; et al. Screening for lung cancer with computed tomography: Protocol for systematic reviews for the Canadian Task Force on Preventive Health Care. Syst. Rev. 2024, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Henry, T.S.; Berry, M.F.; Johnson, G.B.; Kelly, A.M.; Ko, J.P.; Kuzniewski, C.T.; Lee, E.; Maldonado, F.; Morris, M.F.; et al. ACR Appropriateness Criteria® Incidentally Detected Indeterminate Pulmonary Nodule. J. Am. Coll. Radiol. 2023, 20, S455–S470. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zheng, S.; Zhang, W.; Fan, S.; Wang, J.; Song, F.; Liu, X.; Zhu, W.; Ye, Z. Prediction of histologic types in solid lung lesions using preoperative contrast-enhanced CT. Eur. Radiol. 2023, 33, 4734–4745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, Y.; Chen, W.; Jiang, J.; Chen, Y.; Zhou, X.; Tang, Q. Enhanced ultrasound-guided versus non-enhanced ultrasound-guided percutaneous needle biopsy in tissue cellularity of lung malignancies: A propensity score matched study. Quant. Imaging Med. Surg. 2022, 12, 5056–5067. [Google Scholar] [CrossRef] [PubMed]
- Quarato, C.M.I.; Cipriani, C.; Dimitri, L.; Lacedonia, D.; Graziano, P.; Copetti, M.; De Cosmo, S.; Simeone, A.; Scioscia, G.; Foschino Barbaro, M.; et al. Assessing value of contrast-enhanced ultrasound vs. conventional transthoracic ultrasound in improving diagnostic yield of percutaneous needle biopsy of peripheral lung lesions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Z.; Huang, H.; Zhou, X.; Xian, M. Application of quantitative contrast-enhanced ultrasound for evaluation and guiding biopsy of peripheral pulmonary lesions: A preliminary study. Clin. Radiol. 2020, 75, 79.e19–79.e24. [Google Scholar] [CrossRef] [PubMed]
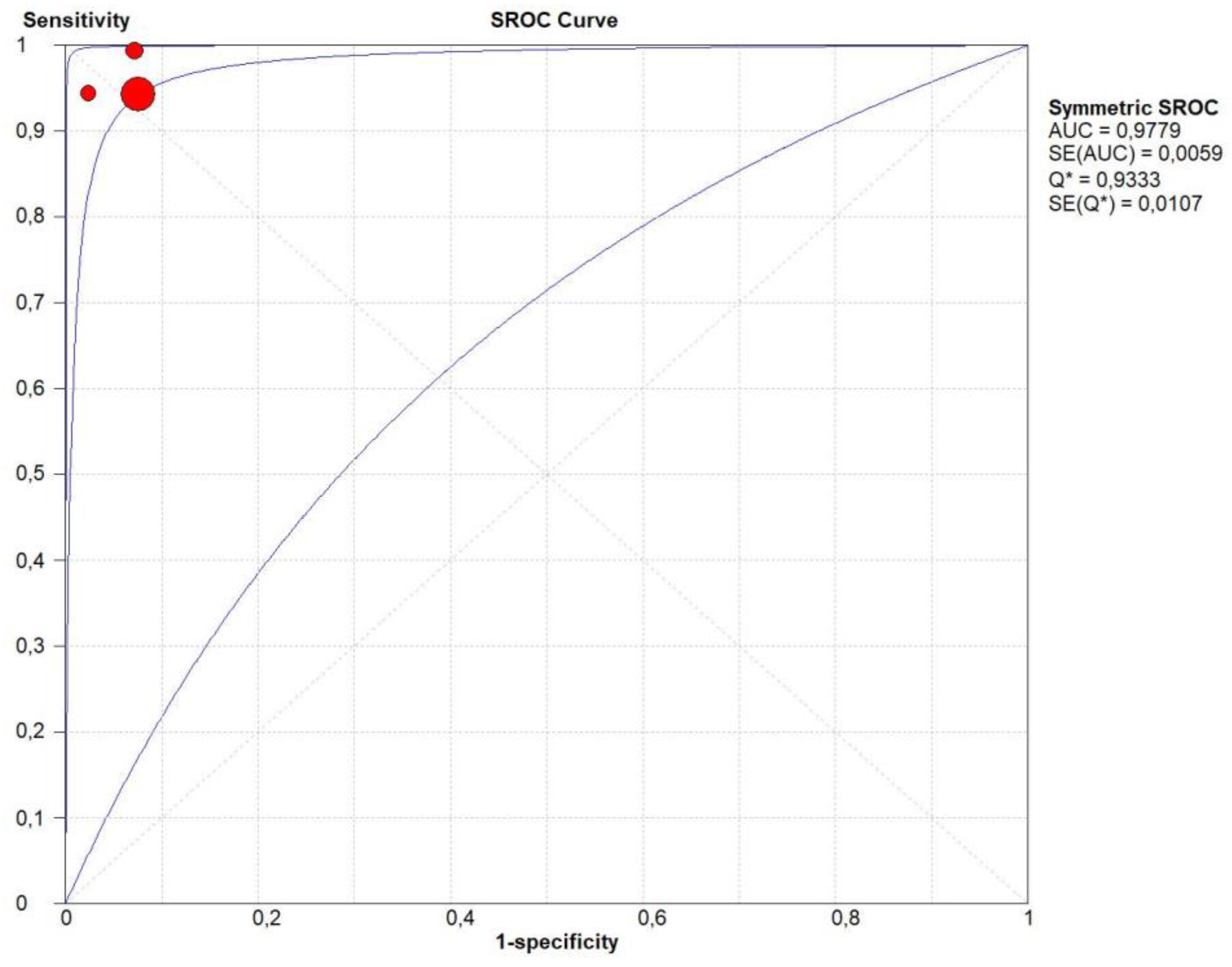
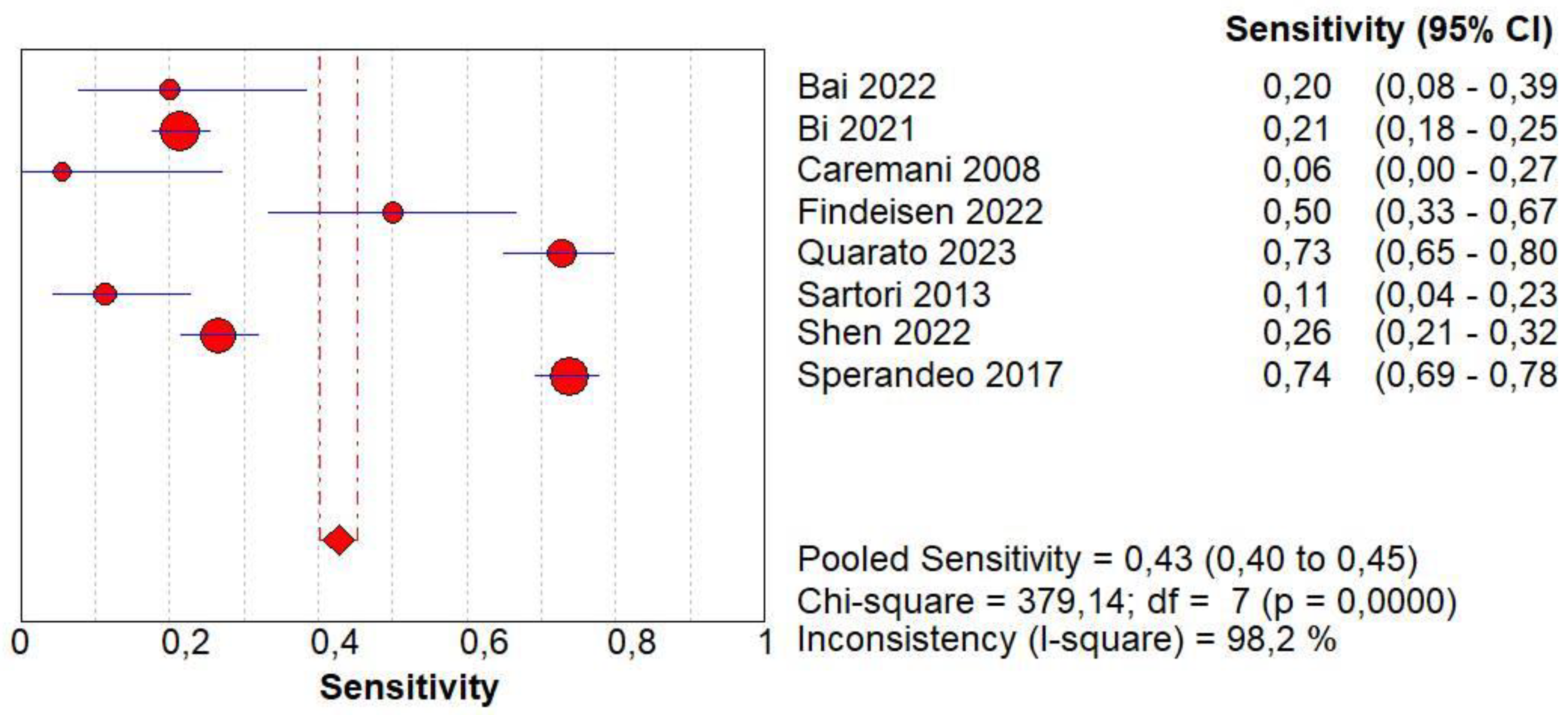
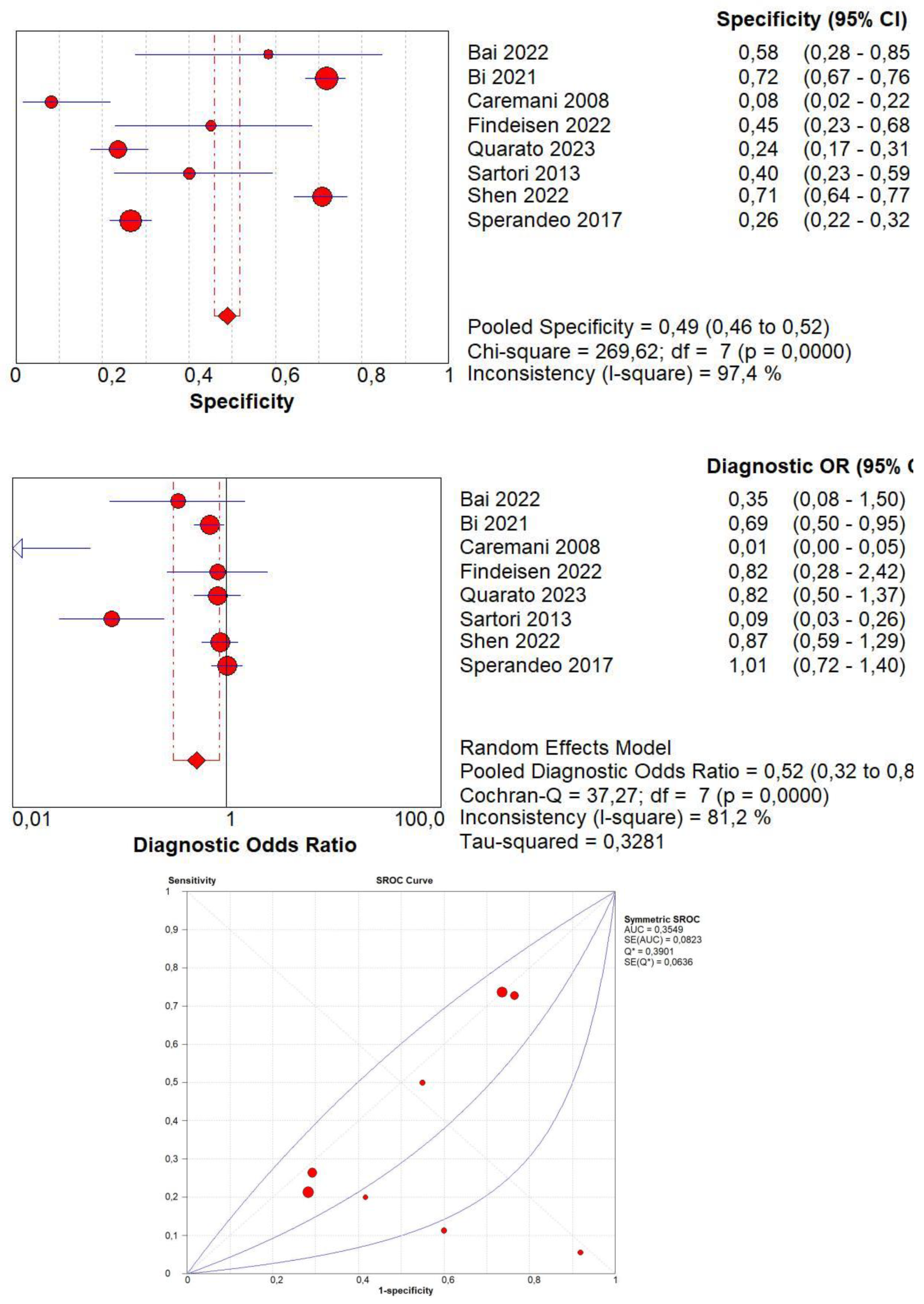
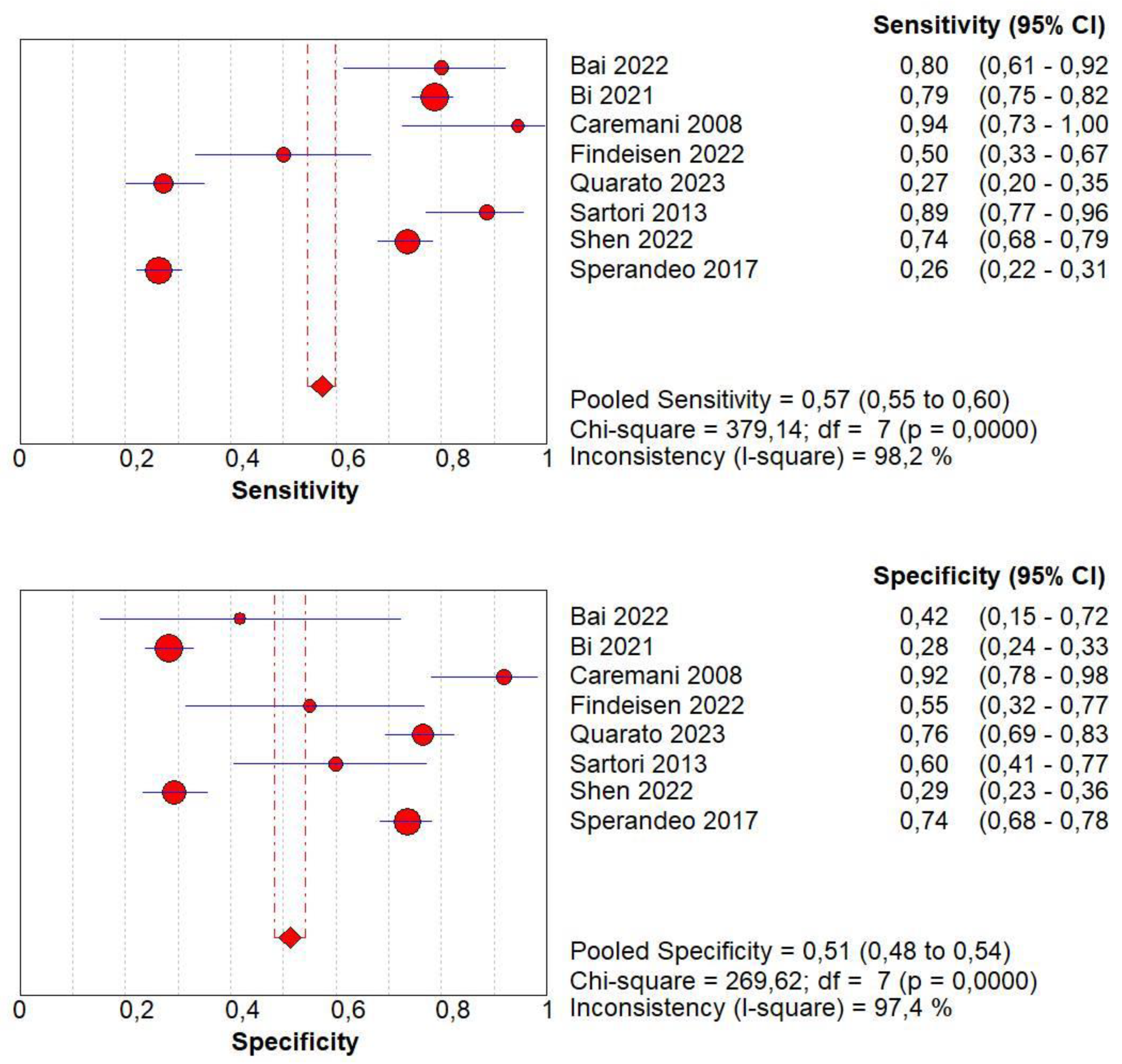


| Author | Year | Study Design | Patients | Age | Male/Female | Benign | Malignant | Size of Lesions | Standard Reference | Operators | CEUS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bai et al. [15] | 2022 | Retrospective observational | 42 | 55.6 | 26/16 | 12 | 30 | 5.57 ± 1.73 cm | Biopsy | 1 operator | Convex probe; Sulfur hexafluoride powder (Italian Bracco Company) was dissolved in 5 mL of 0.9% sodium chloride and immediately and fully shaken to form a suspension. For the CEUS process, 2.0 mL of the suspension was obtained and quickly pushed into the median cubital vein, followed by an injection of 5 mL of 0.9% sodium chloride. |
| Bi et al. [16] | 2021 | Retrospective and prospective | 812 | 59.0 | 587/225 | 372 | 440 | M: 4.65 cm (3.49–6.83) B: 4.26 cm (3.13–5.32) p = 0.010 | Histopathology is the priority; when histopathology could not make a definite diagnosis, microbial evidence, imaging findings, clinical symptoms, and treatment effects were used. All cases were followed up with for at least 12 months. | Two operators performed this process together (with 4 and 5 years of experience in lung US). For discordant assessments, a third senior operator (with 18 years of experience of lung US) was consulted on the cases and would make the final decision | 1–6 MHz convex probe; the mechanical index was set at 0.1, and the gain was adjusted to show the surface of air-filled lungs only (20 dB). Then, 1.5 mL of UCA was injected into the median cubital vein within 2s via a 20 gauge needle followed by an immediate flush with 5 mL of normal saline. |
| Caremani et al. [17] | 2008 | Prospective | 60 | 56.0 | 34/26 | 42 | 18 | Not specified | CT and biopsy | Not specified | 3.5 MHz convex probe with a low mechanical index; SonoVue 2.4 mL bolus injection followed by a flush of saline solution. |
| Findeisen et al. [18] | 2022 | Prospective | 63 | 64.0 | 46/17 | 24 | 39 | M: 31.2 ± 18.9 mm B: 18.3 ± 15.1 mm p = 0.003 | Biopsy | The evaluation of the CEUS parameters was carried out retrospectively by two independent, experienced operators based on the images. In the event of disagreement, a third investigator adjudicated. | 3–6 MHz convex ultrasound probe; CEUS was performed in 1.5 MHz cadence pulse sequence mode (a contrast-specific, continuous mode software and low mechanical index). |
| Quarato et al. [19] | 2023 | Prospective | 317 | 52.1 | 215/102 | 170 | 147 | B: 3.2 ± 0.9 cm (1.5–8.0) M: 2.7 ± 0.5 cm (1.25–5.75) p = 0.0004 | Biopsy or clinical and radiological follow-up | Ultrasound examination was independently performed by 2 operators with over 20 years of experience in lung ultrasonography The clips were blindly reviewed by another operator with 35 years of experience. Cohen’s k values of the diagnostic results ranged from 0.81 to 1.00, indicating almost perfect agreement between operators. | Multifrequency convex probe (3.5–5 MHz); the pre-setting for thoracic ultrasound in B-mode (i.e., gain compensation), 40–50%; dynamic range, 60–70 dB; depth, 70–140 mm; electronic imaging focus on the pleural line; tissue harmonics on) and US contrast setting (low mechanical index ≤ 0.1). Intravenous injection of 4.8 mL of SonoVue (Bracco, Milan, Italy) followed by 10 mL of regular saline. |
| Sartori et al. [20] | 2013 | Prospective | 95 | 61.0 | - | 42 | 53 | 3.5 cm (range 1–12 cm). | Biopsy or clinical follow up | One of two physicians with at least 5 years of experience in CEUS examination of abdominal organs and well experienced with US of the lung. There was no concordance between the readers in TE and EW evaluation in 5/100 cases and 4/100 cases, respectively (r = 0.899, and r = 0.9, respectively). In all these cases, final consensus was reached after collegial review and discussion of the CEUS clips. | 3.5 to 5.0 MHz convex transducer and a 5.0 to 7.5 MHz linear transducer; CEUS was performed with a low mechanical index contrast-specific non-linear technique and an 8 microliters/mL solution of SonoVue; acoustic power was set at 40 kilo pascal for both high-frequency linear transducer and low-frequency convex transducer. |
| Shen et al. [21] | 2022 | Retrospective observational | 506 | 59.0 | 351/155 | 219 | 287 | B: 4.2 ± 2.1 cm M: 5.9 ± 4.7 cm | All malignant cases were diagnosed by pathology, while benign cases were diagnosed by two respiratory physicians after comprehensive analysis of pathology, etiology, imaging, and clinical symptoms. | The imaging data were blindly reviewed by 2 experienced operators in pulmonary CEUS examinations. If the two radiologists agreed on a diagnosis, the final result was the same; if they disagreed, a third senior radiologist analyzed it, and they all discussed it together. | 2.8–5.0 MHz convex probe; The mechanical index was adjusted to 0.10, the total gain was 20, and the dynamic range was 69 DB; SonoVue solution (1.5 mL) was administered by bolus injection via the antecubital vein, followed by 5 mL of saline. |
| Sperandeo et al. [22] | 2006 | Prospective | 98 | 60.0 | 65/33 | 20 | 78 | - | Biopsy | Single physician experienced with ultrasonography of the lung | Multifrequency convex array transducers (3.5 MHz); SonoVue 4.8-mL bolus was administered via a 20-gauge IV cannula in an antecubital vein. The injection was immediately followed by a 10-mL bolus of 0.9% sodium chloride. The CEUS scan was performed in the harmonic mode with a mechanical index of 0.04 or less. |
| Sperandeo et al. [23] | 2017 | Prospective | 728 | 65.0 | - | 329 | 399 | - | Clinical course, imaging, and laboratory and/or histology test | A single physician with 25 years of experience in lung ultrasonography performed and digitally recorded all CEUS scans. The clips were blindly reviewed by another operator with 20 years of experience. Inter-reader agreement was excellent (Spearman’s coefficient ≥ 0.90 for all parameters) | Multifrequency (3.5–5 MHz and 3–8 MHz) convex probe; SonoVue bolus of 4.8 mL of Sonovue followed by 10 mL regular saline was injected Intravenously; a CEUS scan was performed with a mechanical index of ≤0.04. |
| Tang et al. [24] | 2020 | Retrospective | 96 | 61.7 | 71/25 | 45 | 51 | B: 5.54 cm (0.26) M: 7.71 cm (0.40) p < 0.001 | Biopsy | Two operators with 5 years’ experience in ultrasound diagnosis who were blinded to the pathological diagnosis of the patient, observed the CEUS analysis together and reached an agreement | 3.5–5 MHz probe SonoVue 2.4 mL were rapidly injected via the elbow vein, followed by a rapid bolus injection of 5 mL normal saline. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccatonda, A.; Guagnano, M.T.; D’Ardes, D.; Cipollone, F.; Vetrugno, L.; Schiavone, C.; Piscaglia, F.; Serra, C. The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Malignant and Benign Subpleural Lung Lesions. J. Clin. Med. 2024, 13, 2302. https://doi.org/10.3390/jcm13082302
Boccatonda A, Guagnano MT, D’Ardes D, Cipollone F, Vetrugno L, Schiavone C, Piscaglia F, Serra C. The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Malignant and Benign Subpleural Lung Lesions. Journal of Clinical Medicine. 2024; 13(8):2302. https://doi.org/10.3390/jcm13082302
Chicago/Turabian StyleBoccatonda, Andrea, Maria Teresa Guagnano, Damiano D’Ardes, Francesco Cipollone, Luigi Vetrugno, Cosima Schiavone, Fabio Piscaglia, and Carla Serra. 2024. "The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Malignant and Benign Subpleural Lung Lesions" Journal of Clinical Medicine 13, no. 8: 2302. https://doi.org/10.3390/jcm13082302
APA StyleBoccatonda, A., Guagnano, M. T., D’Ardes, D., Cipollone, F., Vetrugno, L., Schiavone, C., Piscaglia, F., & Serra, C. (2024). The Role of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Malignant and Benign Subpleural Lung Lesions. Journal of Clinical Medicine, 13(8), 2302. https://doi.org/10.3390/jcm13082302










