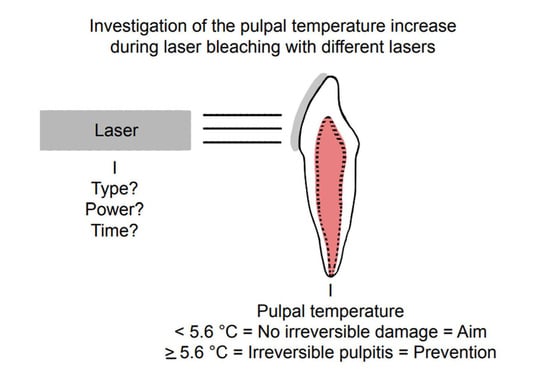
Thermal Effects on Dental Pulp during Laser-Assisted Bleaching Procedures with Diode Lasers in a Clinical Study
Abstract
:1. Introduction
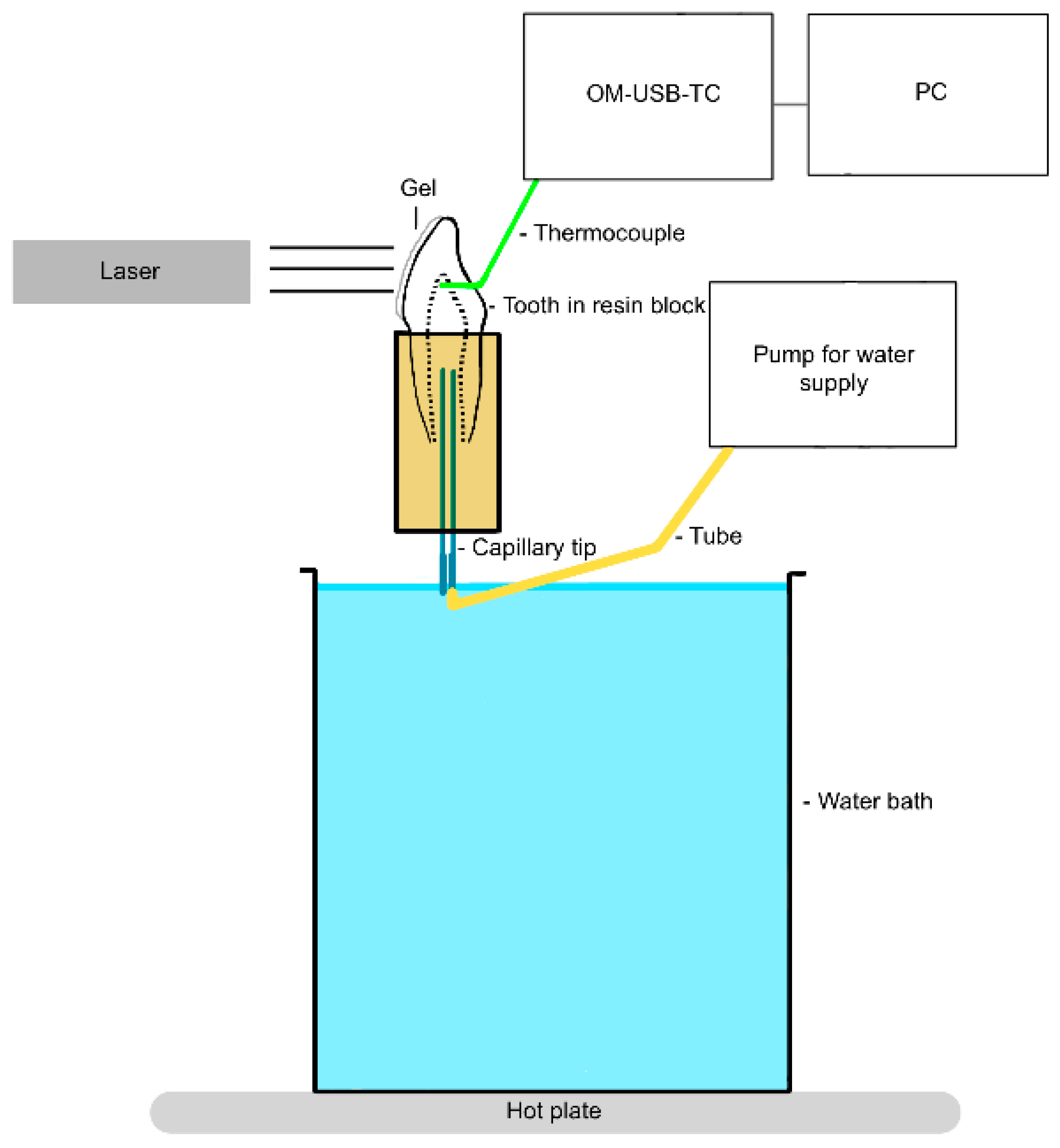
2. Materials and Methods
2.1. Investigation of the Thermal Effect
- Group 1 (control): no laser activation, bleaching gel (Perfect Bleach Office+, VOCO GmbH, Cuxhaven, Germany), and resting time 15 min.
- Group 2 (test): diode laser EzLase 940 nm 7 W (BIOLASE, Inc., Foothill Ranch, CA, USA), bleaching handpiece (EzLase* Whitening Handpiece; BIOLASE, Inc., Foothill Ranch, CA, USA), bleaching gel (Laserwhite*20 Tooth Whitening Gel Kit; BIOLASE, Inc. Aachen, Germany), irradiation time 30 s, 1.5 min rest phase, repeat irradiation, resting time 15 min, distance between tooth and laser approx. 1 mm, and 105 J/cm2 of applied energy (two cycles).
- Group 3 (test): diode laser EzLase 940 nm 2 W, single fiber 300 µm (EzTip E3–9 mm, BIOLASE, Inc.), bleaching gel (Laserwhite*20), irradiation time 30 s, repeat irradiation, resting time 15 min, distance between tooth and laser approx. 1.5–2.0 cm, spot size on tooth: 154–255 mm², and 47–78 J/cm2 per tooth (two cycles).
- Group 4 (test): diode laser EzLase 940 nm 1.5 W, single fiber 300 µm, bleaching gel (Laserwhite*20), irradiation time 30 s, repeat irradiation, resting time 15 min, distance between tooth and laser approx. 1.5–2.0 cm, spot size on tooth: 154–255 mm², and 35–58 J/cm2 per tooth (two cycles).
- Group 5 (test): diode laser SiroLaser Blue 445 nm 0.5 W (Dentsply Sirona, Charlotte, NC, USA), single fiber 320 µm (Dentsply Sirona), bleaching gel (Perfect Bleach Office+, VOCO GmbH, Cuxhaven, Germany), irradiation time 30 s, resting time 15 min, distance between tooth and laser approx. 1.5–2.0 cm, spot size on tooth: 20–28 mm², and 53–77 J/cm2 per tooth (one cycle).
- Group 6 (test): diode laser SiroLaser Blue 445 nm 0.3 W, single fiber 320 µm, bleaching gel (Perfect Bleach Office+), irradiation time 30 s, resting time 15 min, distance between tooth and laser approx. 1.5–2 cm, spot size on tooth: 20–28 mm², and 32–46 J/cm2 per tooth (one cycle).
- Group 7 (test): diode laser SiroLaser Blue 970 nm 1 W (Dentsply Sirona), single fiber 320 µm, bleaching gel (Perfect Bleach Office+), irradiation time 30 s, resting time 15 min, distance between tooth and laser approx. 1.5–2.0 cm, spot size on tooth: 20–28 mm², and 106–153 J/cm2 per tooth (one cycle).
- Group 8 (test): diode laser SiroLaser Blue 970 nm 0.5 W, single fiber 320 µm, bleaching gel (Perfect Bleach Office+), irradiation time 30 s, resting time 15 min, distance between tooth and laser approx. 1.5–2.0 cm, spot size on tooth: 20–28 mm², and 53–77 J/cm2 per tooth (one cycle).
2.2. Method Comparison
- Group 9/10: no laser activation and bleaching gel (Perfect Bleach Office+).
- Group 11/12: EzLase 940 nm 1.5 W, single fiber 300 µm, bleaching gel (Laserwhite*20), and irradiation time 30 s.
- Group 13/14: SiroLaser Blue 445 nm 0.3 W, single fiber 320 µm, bleaching gel (Perfect Bleach Office+), and irradiation time 30 s.
- Group 15/16: SiroLaser Blue 970 nm 0.5 W, single fiber 320 µm, bleaching gel (Perfect Bleach Office+), and irradiation time 30 s.
2.3. Evaluation and Statistics
3. Results
3.1. Investigation of the Thermal Effect
3.2. Method Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, R.; Omrani, L.R.; Abbasi, M.; Chiniforush, N.; Kargar, M. Effect of Three Wavelengths of Diode Laser on the Efficacy of Bleaching of Stained Teeth. Front. Dent. 2019, 16, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, R.; Pantaleo, G.; Lizio, A.; Romeo, U.; Castiello, G.; Spagnuolo, G.; Lo Giudice, G. Clinical and Spectrophotometric Evaluation of LED and Laser Activated Teeth Bleaching. Open Dent. J. 2016, 10, 242–250. [Google Scholar] [CrossRef]
- Saberi, S.; Rouzsaz, M.; Shafie, F.; Einizadeh, S.; Kharazifard, M.J.; Shahabi, S. The effect of laser-activated bleaching with 445 nm and 915 nm diode lasers on enamel micro-hardness; an in vitro study. Photodiagnosis Photodyn. Ther. 2020, 31, 101952. [Google Scholar] [CrossRef]
- Coceska, E.; Gjorgievska, E.; Coleman, N.J.; Gabric, D.; Slipper, I.J.; Stevanovic, M.; Nicholson, J.W. Enamel alteration following tooth bleaching and remineralization. J. Microsc. 2016, 262, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Dionysopoulos, D.; Strakas, D.; Koliniotou-Koumpia, E.; Koumpia, E. Effect of Er,Cr:YSGG laser irradiation on bovine enamel surface during in-office tooth bleaching ex vivo. Odontology 2017, 105, 320–328. [Google Scholar] [CrossRef]
- Ergin, E.; Ruya Yazici, A.; Kalender, B.; Usumez, A.; Ertan, A.; Gorucu, J.; Sari, T. In vitro comparison of an Er:YAG laser-activated bleaching system with different light-activated bleaching systems for color change, surface roughness, and enamel bond strength. Lasers Med. Sci. 2018, 33, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.L.d.S.G.; Besegato, J.F.; Kuga, M.C. Bleaching and microstructural effects of low concentration hydrogen peroxide photoactivated with LED/laser system on bovine enamel. Photodiagnosis Photodyn. Ther. 2021, 35, 102352. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Basso, F.G.; Scheffel, D.S.; Hebling, J.; de Souza Costa, C.A. Responses of human dental pulp cells after application of a low-concentration bleaching gel to enamel. Arch. Oral Biol. 2015, 60, 1428–1436. [Google Scholar] [CrossRef]
- Soares, D.G.; Sacono, N.T.; Ribeiro, A.P.D.; Leite, M.L.; Duque, C.C.d.O.; Gallinari, M.d.O.; Pacheco, L.E.; Hebling, J.; de Souza Costa, C.A. Pro-inflammatory mediators expression by pulp cells following tooth whitening on restored enamel surface. Braz. Dent. J. 2022, 33, 83–90. [Google Scholar] [CrossRef]
- Silva-Costa, R.S.G.d.; Ribeiro, A.E.d.L.; Assunção, I.V.d.; Araújo, R.M., Jr.; Araújo, A.A.d.; Guerra, G.C.B.; Borges, B.C.D. In-office tooth bleaching with 38% hydrogen peroxide promotes moderate/severe pulp inflammation and production of ll-1β, TNF-β, GPX, FGF-2 and osteocalcin in rats. J. Appl. Oral Sci. 2018, 26, e20170367. [Google Scholar] [CrossRef]
- Silva, I.J.P.; Cintra, L.T.A.; Ervolino, E.; Chaves, H.G.D.S.; Sivieri-Araújo, G.; Briso, A.L.F.; Cosme-Silva, L.; Benetti, F. Photobiomodulation reduces inflammation but does not influence the hypoxia-inducible factor-1α in pulp tissue of rats after bleaching. J. Appl. Oral Sci. 2022, 30, e20210559. [Google Scholar] [CrossRef] [PubMed]
- Terayama, A.M.; Benetti, F.; de Araújo Lopes, J.M.; Barbosa, J.G.; Silva, I.J.P.; Sivieri-AraúJo, G.; Briso, A.L.F.; Cintra, L.T.A. Influence of low-level laser therapy on inflammation, collagen fiber maturation, and tertiary dentin deposition in the pulp of bleached teeth. Clin. Oral Investig. 2020, 24, 3911–3921. [Google Scholar] [CrossRef]
- Raszewski, Z.; Brząkalski, D.; Derpeński, Ł.; Jałbrzykowski, M.; Przekop, R.E. Aspects and Principles of Material Connections in Restorative Dentistry-A Comprehensive Review. Materials 2022, 15, 7131. [Google Scholar] [CrossRef]
- Dahl, J.E.; Becher, R. Acute toxicity of carbamide peroxide and a commercially available tooth-bleaching agent in rats. J. Dent. Res. 1995, 74, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Sari, T.; Celik, G.; Usumez, A. Temperature rise in pulp and gel during laser-activated bleaching: In vitro. Lasers Med. Sci. 2015, 30, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, H.; Brännström, M. Pulp reaction to heat. J. Prosthet. Dent. 1968, 19, 605–612. [Google Scholar] [CrossRef]
- Zach, L.; Cohen, G. Pulp response to externally applied heat. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 515–530. [Google Scholar] [CrossRef]
- Al-Karadaghi, T.S.; Al-Saedi, A.A.; Al-Maliky, M.A.; Mahmood, A.S. The effect of bleaching gel and (940 nm and 980 nm) diode lasers photoactivation on intrapulpal temperature and teeth whitening efficiency. Aust. Endod. J. 2016, 42, 112–118. [Google Scholar] [CrossRef]
- Braun, A.; Kecsmar, S.; Krause, F.; Berthold, M.; Frentzen, M.; Frankenberger, R.; Schelle, F. Effect of simulated pulpal fluid circulation on intrapulpal temperature following irradiation with an Nd:YVO4 laser. Lasers Med. Sci. 2015, 30, 1197–1202. [Google Scholar] [CrossRef]
- Gutknecht, N.; Franzen, R.; Meister, J.; Lukac, M.; Pirnat, S.; Zabkar, J. A Novel Er:YAG Laser-Assisted Tooth Whitening Method. J. Laser Health Acad. 2011, 1, 1–10. [Google Scholar]
- Gutknecht, N.; Franzen, R.; Schippers, M.; Lampert, F. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J. Clin. Laser Med. Surg. 2004, 22, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, J.; Mishra, R.; Bhadauria, V. Antibacterial Efficacy of Sodium Hypochlorite, Ozonated Water, and 980 nm Diode Laser Used for Disinfection of Root Canal against Enterococcus faecalis: A Microbiological Study. Int. J. Clin. Pediatr. Dent. 2020, 13, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Saydjari, Y.; Kuypers, T.; Gutknecht, N. Laser Application in Dentistry: Irradiation Effects of Nd:YAG 1064 nm and Diode 810 nm and 980 nm in Infected Root Canals-A Literature Overview. Biomed Res. Int. 2016, 2016, 8421656. [Google Scholar] [CrossRef]
- Rosa, M.I.; Schambeck, V.S.; Dondossola, E.R.; Alexandre, M.C.; Tuon, L.; Grande, A.J.; Hugo, F. Laser fluorescence of caries detection in permanent teeth in vitro: A systematic review and meta-analysis. J. Evid. Based Med. 2016, 9, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Fornaini, C.; Rocca, J.-P.; Merigo, E. 450 nm diode laser: A new help in oral surgery. World J. Clin. Cases 2016, 4, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Azma, E.; Razaghi, M. Laser Treatment of Oral and Maxillofacial Hemangioma. J. Lasers Med. Sci. 2018, 9, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Alagl, A.S.; Madi, M.; Bedi, S.; Al Onaizan, F.; Al-Aql, Z.S. The Effect of Er,Cr:YSGG and Diode Laser Applications on Dental Implant Surfaces Contaminated with Acinetobacter baumannii and Pseudomonas aeruginosa. Materials 2019, 12, 2073. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, Y.T.; Marques, M.M.; Franzen, R.; Diniz, I.M.A.; Gutknecht, N. Immediate clinical evaluation of a 940-nm diode laser-assisted in-office bleaching technique. Laser Dent. Sci. 2018, 2, 239–245. [Google Scholar] [CrossRef]
- Strakas, D.; Tolidis, K.; Koliniotou-Koumpia, E.; Vanweersch, L.; Franzen, R.; Gutknecht, N. Intra-pulpal temperature rise of different tooth types during dental bleaching supported by an Er,Cr:YSGG laser. A pilot study. Lasers Med. Sci. 2016, 31, 77–82. [Google Scholar] [CrossRef]
- Raab, W.H. Temperature related changes in pulpal microcirculation. Proc. Finn. Dent. Soc. 1992, 88, 469–479. [Google Scholar]
- Papadopoulou, A.; Dionysopoulos, D.; Strakas, D.; Kouros, P.; Kolokitha, O.-E.; Tolidis, K. Temperature changes in the pulp chamber and bleaching gel during tooth bleaching assisted by diode laser (445 nm) using different power settings. Lasers Med. Sci. 2023, 38, 209. [Google Scholar] [CrossRef] [PubMed]
- Saberi, S.; Shahabi, S.; Tohidkhah, S.; Jafarnia, S.; Pedram, S. The effect of laser-activated bleaching with 445 nm and 970 nm diode lasers on pulp chamber temperature rise: An in vitro study. Laser Phys. 2021, 31, 55601. [Google Scholar] [CrossRef]
- Boulnois, J.L. Photophysical process in recent medical laser developments: A review. Lasers Med. Sci. 1986, 1, 47–66. [Google Scholar] [CrossRef]
- Reichelt, J.; Winter, J.; Meister, J.; Frentzen, M.; Kraus, D. A novel blue light laser system for surgical applications in dentistry: Evaluation of specific laser-tissue interactions in monolayer cultures. Clin. Oral Investig. 2017, 21, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Matthews, B.; Andrew, D. Microvascular architecture and exchange in teeth. Microcirculation 1995, 2, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Méndez, J.D.; Zarzoza, E. Rapid determination of dry weight in human dental pulp by a colorimetric reaction. J. Endod. 1999, 25, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Falkenstein, F.; Gutknecht, N.; Franzen, R. Analysis of laser transmission and thermal effects on the inner root surface during periodontal treatment with a 940-nm diode laser in an in vitro pocket model. J. Biomed. Opt. 2014, 19, 128002. [Google Scholar] [CrossRef]
- Morsi, A.; Haidary, D.; Franzen, R.; Gutknecht, N. Intra-pulpal temperature evaluation during diode laser (445 nm) irradiation for treatment of dentine hypersensitivity: In vitro a pilot study. Laser Dent Sci 2020, 4, 139–144. [Google Scholar] [CrossRef]
- Luk, K.; Tam, L.; Hubert, M. Effect of light energy on peroxide tooth bleaching. J. Am. Dent. Assoc. 2004, 135, 194–201, quiz 228-9. [Google Scholar] [CrossRef]
- Al-Qudah, A.A.; Mitchell, C.A.; Biagioni, P.A.; Hussey, D.L. Thermographic investigation of contemporary resin-containing dental materials. J. Dent. 2005, 33, 593–602. [Google Scholar] [CrossRef]
- Kodonas, K.; Gogos, C.; Tziafas, D. Effect of simulated pulpal microcirculation on intrapulpal temperature changes following application of heat on tooth surfaces. Int. Endod. J. 2009, 42, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.L.d.S.G.; Besegato, J.F.; Zaniboni, J.F.; Kuga, M.C. LED/laser photoactivation enhances the whitening efficacy of low concentration hydrogen peroxide without microstructural enamel changes. Photodiagnosis Photodyn. Ther. 2021, 36, 102511. [Google Scholar] [CrossRef] [PubMed]
- Loiola, A.B.A.; Souza-Gabriel, A.E.; Scatolin, R.S.; Corona, S.A.M. Impact of hydrogen peroxide activated by lighting-emitting diode/laser system on enamel color and microhardness: An in situ design. Contemp. Clin. Dent. 2016, 7, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Strakas, D.; Tolidis, K.; Koliniotou-Koumpia, E.; Vanweersch, L.; Franzen, R.; Gutknecht, N. Comparative study of intrapulpal temperature stress during Er,Cr:YSGG-supported dental bleaching versus conventional dental power bleaching. An in vitro study. Laser Dent. Sci. 2017, 1, 57–64. [Google Scholar] [CrossRef]
- Sehgal, M.; Sharma, P.; Juneja, A.; Kumar, P.; Verma, A.; Chauhan, V. Effect of different stripping techniques on pulpal temperature: In vitro study. Dent. Press J. Orthod. 2019, 24, 39–43. [Google Scholar] [CrossRef]



| Group | Minimum of Maximum Pulpal Temperature Increase in Degrees Celsius (°C) | Maximum of Maximum Pulpal Temperature Increase in Degrees Celsius (°C) | Maximum Duration of the Pulpal Temperature Increase ≥5.6 °C in Seconds (s) |
|---|---|---|---|
| 1 | 0 | 4.3 | |
| 2 | 1.0 (first bleaching procedure) 0.8 (second bleaching procedure) | 4.7 (first bleaching procedure) 6.4 (second bleaching procedure) | 12 |
| 3 | 3.4 (first bleaching procedure) 2.5 (second bleaching procedure) | 8.2 (first bleaching procedure) 11.3 (second bleaching procedure) | 132 |
| 4 | 0 (first bleaching procedure) –2.1 (second bleaching procedure) | 5.4 (first bleaching procedure) 5.5 (second bleaching procedure) | |
| 5 | 0.4 | 5.6 | 19 |
| 6 | 0 | 4.1 | |
| 7 | 0 | 9.2 | 58 |
| 8 | 0 | 4.6 |
| Group | Minimum of Temperature Differences in Degrees Celsius (°C) per Second | Maximum of Temperature Differences in Degrees Celsius (°C) per Second | Average of Temperature Differences in Degrees Celsius (°C) per Second |
|---|---|---|---|
| 9/10 | 0.3 | 3.2 | 2.2 |
| 11/12 | 0.3 | 3.7 | 2.2 |
| 13/14 | 0.9 | 4.9 | 2.5 |
| 15/16 | 1.1 | 3.6 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, M.; Braun, A.; Franzen, R. Thermal Effects on Dental Pulp during Laser-Assisted Bleaching Procedures with Diode Lasers in a Clinical Study. J. Clin. Med. 2024, 13, 2301. https://doi.org/10.3390/jcm13082301
Petersen M, Braun A, Franzen R. Thermal Effects on Dental Pulp during Laser-Assisted Bleaching Procedures with Diode Lasers in a Clinical Study. Journal of Clinical Medicine. 2024; 13(8):2301. https://doi.org/10.3390/jcm13082301
Chicago/Turabian StylePetersen, Marlene, Andreas Braun, and Rene Franzen. 2024. "Thermal Effects on Dental Pulp during Laser-Assisted Bleaching Procedures with Diode Lasers in a Clinical Study" Journal of Clinical Medicine 13, no. 8: 2301. https://doi.org/10.3390/jcm13082301