Thicker Inner Nuclear Layer as a Predictor of Glaucoma Progression and the Impact of Intraocular Pressure Fluctuation
Abstract
:1. Introduction
2. Materials and Methods
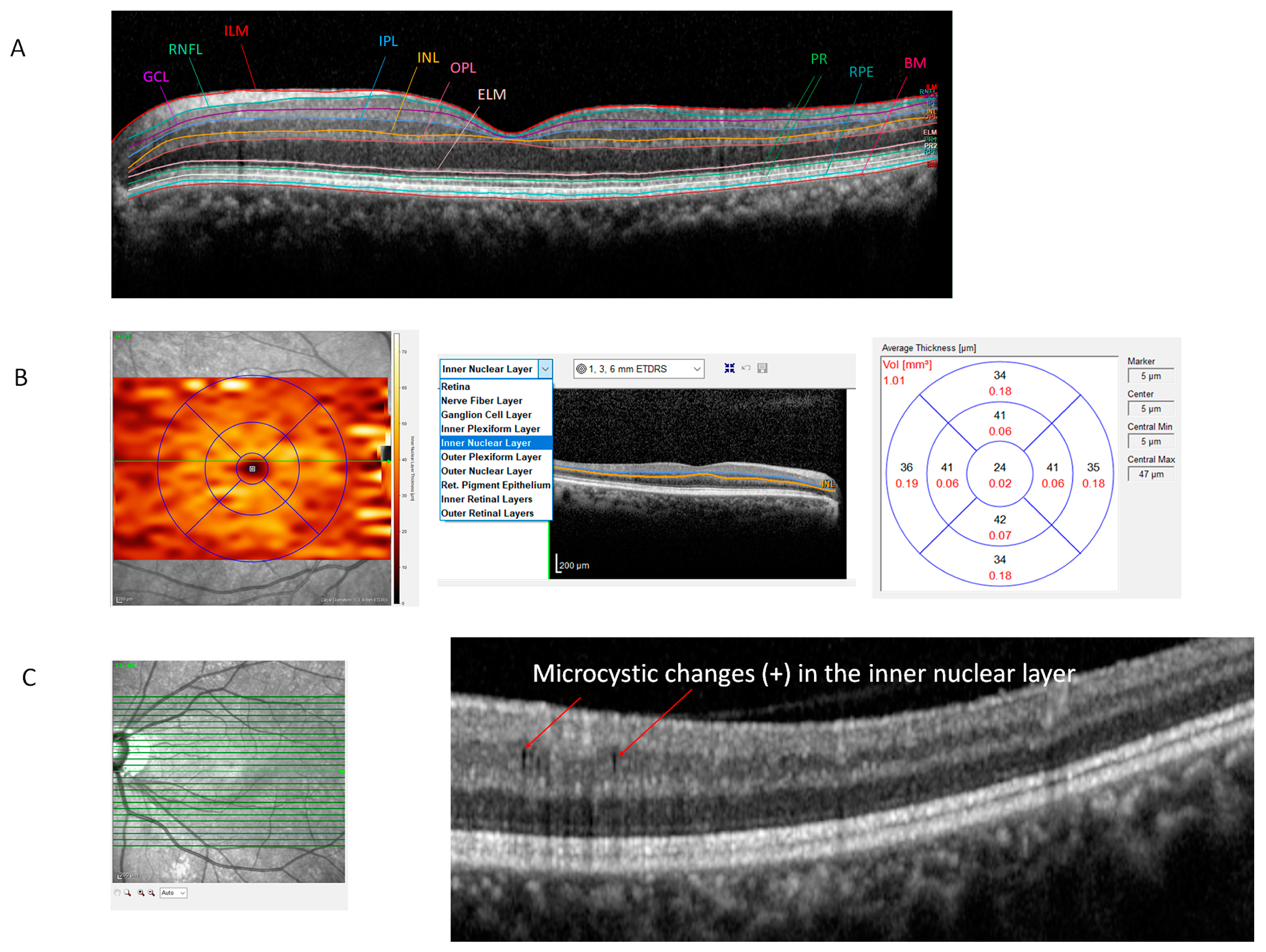
2.1. OCT and Automated Segmentation of the Retina
2.2. VF Testing
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nork, T.M.; Ver Hoeve, J.N.; Poulsen, G.L.; Nickells, R.W.; Davis, M.D.; Weber, A.J.; Vaegan; Sarks, S.H.; Lemley, H.L.; Millecchia, L.L. Swelling and loss of photoreceptors in chronic human and experimental glaucomas. Arch. Ophthalmol. 2000, 118, 235–245. [Google Scholar] [CrossRef]
- Velten, I.M.; Korth, M.; Horn, F.K. The a-wave of the dark adapted electroretinogram in glaucomas: Are photoreceptors affected? Br. J. Ophthalmol. 2001, 85, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Kessel, L.; Hamann, S.; Wegener, M.; Tong, J.; Fraser, C.L. Microcystic macular oedema in optic neuropathy: Case series and literature review. Clin. Exp. Ophthalmol. 2018, 46, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Abegg, M.; Dysli, M.; Wolf, S.; Kowal, J.; Dufour, P.; Zinkernagel, M. Microcystic macular edema: Retrograde maculopathy caused by optic neuropathy. Ophthalmology 2014, 121, 142–149. [Google Scholar] [CrossRef]
- Saidha, S.; Sotirchos, E.S.; Ibrahim, M.A.; Crainiceanu, C.M.; Gelfand, J.M.; Sepah, Y.J.; Ratchford, J.N.; Oh, J.; Seigo, M.A.; Newsome, S.D.; et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: A retrospective study. Lancet Neurol. 2012, 11, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Brazerol, J.; Iliev, M.E.; Hohn, R.; Frankl, S.; Grabe, H.; Abegg, M. Retrograde Maculopathy in Patients with Glaucoma. J. Glaucoma 2017, 26, 423–429. [Google Scholar] [CrossRef]
- Mahmoudinezhad, G.; Salazar, D.; Morales, E.; Tran, P.; Lee, J.; Hubschman, J.P.; Nouri-Mahdavi, K.; Caprioli, J. Risk factors for microcystic macular oedema in glaucoma. Br. J. Ophthalmol. 2023, 107, 505–510. [Google Scholar] [CrossRef]
- Kim, E.K.; Park, H.L.; Park, C.K. Relationship between Retinal Inner Nuclear Layer Thickness and Severity of Visual Field Loss in Glaucoma. Sci. Rep. 2017, 7, 5543. [Google Scholar] [CrossRef]
- Hasegawa, T.; Akagi, T.; Yoshikawa, M.; Suda, K.; Yamada, H.; Kimura, Y.; Nakanishi, H.; Miyake, M.; Unoki, N.; Ikeda, H.O.; et al. Microcystic Inner Nuclear Layer Changes and Retinal Nerve Fiber Layer Defects in Eyes with Glaucoma. PLoS ONE 2015, 10, e0130175. [Google Scholar] [CrossRef]
- Burggraaff, M.C.; Trieu, J.; de Vries-Knoppert, W.A.; Balk, L.; Petzold, A. The clinical spectrum of microcystic macular edema. Investig. Ophthalmol. Vis. Sci. 2014, 55, 952–961. [Google Scholar] [CrossRef]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Measuring visual field progression in the Early Manifest Glaucoma Trial. Acta Ophthalmol. Scand. 2003, 81, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Fleiss, J.L. Reliability of Measurements. In The Design and Analysis of Clinical Experiments; Wiley: New York, NY, USA, 1986; pp. 1–32. [Google Scholar]
- Wolff, B.; Basdekidou, C.; Vasseur, V.; Mauget-Faysse, M.; Sahel, J.A.; Vignal, C. Retinal inner nuclear layer microcystic changes in optic nerve atrophy: A novel spectral-domain OCT finding. Retina 2013, 33, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Agnifili, L.; Mastropasqua, R.; Frezzotti, P.; Fasanella, V.; Motolese, I.; Pedrotti, E.; Di Iorio, A.; Mattei, P.A.; Motolese, E.; Mastropasqua, L. Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta Ophthalmol. 2015, 93, e14–e21. [Google Scholar] [CrossRef]
- Tojo, N.; Abe, S.; Ishida, M.; Yagou, T.; Hayashi, A. The Fluctuation of Intraocular Pressure Measured by a Contact Lens Sensor in Normal-Tension Glaucoma Patients and Nonglaucoma Subjects. J. Glaucoma 2017, 26, 195–200. [Google Scholar] [CrossRef]
- Moon, Y.; Lee, J.Y.; Jeong, D.W.; Kim, S.; Han, S.; Kook, M.S. Relationship Between Nocturnal Intraocular Pressure Elevation and Diurnal Intraocular Pressure Level in Normal-Tension Glaucoma Patients. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, J.S.; Lee, S.Y.; Ha, A.; Lee, J.; Park, Y.J.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Twenty-four-Hour Intraocular Pressure-Related Patterns from Contact Lens Sensors in Normal-Tension Glaucoma and Healthy Eyes: The Exploring Nyctohemeral Intraocular pressure related pattern for Glaucoma Management (ENIGMA) Study. Ophthalmology 2020, 127, 1487–1497. [Google Scholar] [CrossRef]
- Sakata, R.; Yoshitomi, T.; Iwase, A.; Matsumoto, C.; Higashide, T.; Shirakashi, M.; Aihara, M.; Sugiyama, K.; Araie, M.; Lower Normal Pressure Glaucoma Study Members in Japan Glaucoma Society. Factors Associated with Progression of Japanese Open-Angle Glaucoma with Lower Normal Intraocular Pressure. Ophthalmology 2019, 126, 1107–1116. [Google Scholar] [CrossRef]
- Nouri-Mahdavi, K.; Hoffman, D.; Coleman, A.L.; Liu, G.; Li, G.; Gaasterland, D.; Caprioli, J.; Advanced Glaucoma Intervention Study. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004, 111, 1627–1635. [Google Scholar] [CrossRef]
- Baek, S.U.; Ha, A.; Kim, D.W.; Jeoung, J.W.; Park, K.H.; Kim, Y.K. Risk factors for disease progression in low-teens normal-tension glaucoma. Br. J. Ophthalmol. 2020, 104, 81–86. [Google Scholar] [CrossRef]
- Shin, D.Y.; Park, H.L.; Shin, H.; Oh, S.E.; Kim, S.A.; Jung, Y.; Lee, M.Y.; Park, C.K. Fluctuation of Intraocular Pressure and Vascular Factors Are Associated with the Development of Epiretinal Membrane in Glaucoma. Am. J. Ophthalmol. 2023, 254, 69–79. [Google Scholar] [CrossRef]
- Joos, K.M.; Li, C.; Sappington, R.M. Morphometric changes in the rat optic nerve following short-term intermittent elevations in intraocular pressure. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6431–6440. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Kim, J.H.; Park, C.K. alpha2-Adrenergic modulation of the glutamate receptor and transporter function in a chronic ocular hypertension model. Eur. J. Pharmacol. 2015, 765, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Govetto, A.; Su, D.; Farajzadeh, M.; Megerdichian, A.; Platner, E.; Ducournau, Y.; Virgili, G.; Hubschman, J.P. Microcystoid Macular Changes in Association with Idiopathic Epiretinal Membranes in Eyes with and without Glaucoma: Clinical Insights. Am. J. Ophthalmol. 2017, 181, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Lee, M.W.; Baek, S.K.; Lee, Y.H. Comparison of Retinal Layer Thicknesses of Highly Myopic Eyes and Normal Eyes. Korean J. Ophthalmol. 2020, 34, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, M.; Yuan, Y.; Huang, S.; Zhu, D.; Ma, Q.; Ye, X.; Lu, F. Macular Thickness Profiles of Intraretinal Layers in Myopia Evaluated by Ultrahigh-Resolution Optical Coherence Tomography. Am. J. Ophthalmol. 2015, 160, 53–61.e52. [Google Scholar] [CrossRef]
- Jung, K.I.; Woo, J.E.; Park, C.K. Intraocular pressure fluctuation and neurodegeneration in the diabetic rat retina. Br. J. Pharmacol. 2020, 177, 3046–3059. [Google Scholar] [CrossRef]



| Eyes with Glaucoma (n = 162) | |
|---|---|
| Age (years) | 54.2 ± 13.6 |
| Male/Female | 78/84 |
| Diabetes (%) | 25 (15.4%) |
| Systemic hypertension (%) | 44 (27.2%) |
| Migraine (%) | 30 (18.5%) |
| Cold hand (%) | 28 (17.3%) |
| Cardiovascular disease (%) | 22 (13.6%) |
| Thyroid disease | 18 (11.1%) |
| Systolic blood pressure (mmHg) | 126.4 ± 15.0 |
| Diastolic blood pressure (mmHg) | 79.1 ± 8.4 |
| Body mass index (kg/m2) | 23.3 ± 3.0 |
| Central corneal thickness (µm) | 536.4 ± 35.7 |
| Axial length (mm) | 24.8 ± 1.5 |
| Spherical equivalent (diopter) | −2.5 ± 3.5 |
| Mean IOP (mmHg) | 14.7 ± 2.4 |
| SD of IOP (mmHg) | 2.3 ± 0.7 |
| Presence of disc hemorrhage (%) | 21 (13.3%) |
| Presence of microcystic macular changes | 12 (7.4%) |
| Baseline MD of VF 24-2 (dB) | −4.0 ± 4.4 |
| Baseline PSD of VF 24-2 (dB) | 4.7 ± 4.0 |
| OCT Parameters | ICC | 95% CI | p Value | |
|---|---|---|---|---|
| Retina | Average (µm) | 0.906 | 0.821–0.951 | <0.001 |
| Volume (mm3) | 0.960 | 0.923–0.980 | <0.001 | |
| RNFL | Average (µm) | 0.778 | 0.604–0.882 | <0.001 |
| Volume (mm3) | 0.907 | 0.823–0.952 | <0.001 | |
| GCL | Average (µm) | 0.928 | 0.863–0.963 | <0.001 |
| Volume (mm3) | 0.951 | 0.905–0.975 | <0.001 | |
| IPL | Average (µm) | 0.950 | 0.903–0.974 | <0.001 |
| Volume (mm3) | 0.960 | 0.922–0.980 | <0.001 | |
| INL | Average (µm) | 0.884 | 0.782–0.940 | <0.001 |
| Volume (mm3) | 0.917 | 0.840–0.957 | <0.001 | |
| OCT Parameters | r | p Value | |
|---|---|---|---|
| Retinal thickness | Average (µm) | 0.287 | <0.001 |
| Volume (mm3) | 0.323 | <0.001 | |
| RNFL thickness | Average (µm) | 0.295 | <0.001 |
| Volume (mm3) | 0.384 | <0.001 | |
| GCL thickness | Average (µm) | 0.406 | <0.001 |
| Volume (mm3) | 0.412 | <0.001 | |
| IPL thickness | Average (µm) | 0.377 | <0.001 |
| Volume (mm3) | 0.350 | <0.001 | |
| INL thickness | Average (µm) | −0.166 | 0.035 |
| Volume (mm3) | −0.144 | 0.068 | |
| Microcystic Macular Changes (−) (n = 150) | Microcystic Macular Changes (+) (n = 12) | p Value | |
|---|---|---|---|
| Age (years) | 54.1 ± 13.3 | 56.1 ± 16.8 | 0.628 |
| Male/Female | 72/78 | 6/6 | 1.000 |
| Diabetes (%) | 19 (12.7%) | 6 (50.0%) | 0.004 |
| Systemic hypertension (%) | 40 (26.7%) | 4 (33.3%) | 0.736 |
| Migraine (%) | 30 (20%) | 0 (0%) | 0.125 |
| Cold hand (%) | 25 (16.7%) | 3 (25.0%) | 0.437 |
| Cardiovascular disease (%) | 19 (12.7%) | 3 (25.0%) | 0.211 |
| Thyroid disease | 18 (12.0%) | 0 (0.0%) | 0.364 |
| Systolic blood pressure (mmHg) | 125.9 ± 15.1 | 134.6 ± 7.8 | 0.207 |
| Diastolic blood pressure (mmHg) | 79.0 ± 8.6 | 82.2 ± 3.5 | 0.416 |
| Body mass index (kg/m2) | 23.4 ± 3.1 | 22.8 ± 1.1 | 0.615 |
| Central corneal thickness (µm) | 535.8 ± 36.3 | 543.9 ± 32.6 | 0.497 |
| Axial length (mm) | 24.9 ± 1.5 | 24.9 ± 1.5 | 0.919 |
| BCVA (log MAR) | 0.03 ± 0.05 | 0.03 ± 0.05 | 0.589 |
| Spherical equivalent (diopter) | −2.5 ± 3.5 | −3.8 ± 4.4 | 0.246 |
| Mean IOP (mmHg) | 14.7 ± 2.4 | 14.7 ± 2.0 | 0.928 |
| SD of IOP (mmHg) | 2.2 ± 0.7 | 2.7 ± 0.8 | 0.032 |
| IOP fluctuation (Higher/lower) | 70 (46.7%)/80 (53.3%) | 11 (91.7%)/1 (8.3%) | 0.005 |
| Presence of disc hemorrhage (%) | 19 (13.4%) | 1 (8.3%) | 1.000 |
| Presence of ERM | 2 (1.3%) | 3 (25.0%) | 0.003 |
| Baseline MD of VF 24-2 (dB) | −3.7 ± 4.2 | −7.6 ± 5.0 | 0.003 |
| Baseline PSD of VF 24-2 (dB) | 4.5 ± 3.9 | 7.8 ± 4.6 | 0.008 |
| OCT Parameters | Microcystic Macular Change (−) (n = 150) | Microcystic Macular Change (+) (n = 12) | p Value | |
|---|---|---|---|---|
| Retina | Average (µm) | 302.06 ± 15.03 | 307.24 ± 25.99 | 0.283 |
| Volume (mm3) | 8.30 ± 0.43 | 8.39 ± 0.66 | 0.509 | |
| RNFL | Average (µm) | 23.88 ± 4.17 | 25.13 ± 7.23 | 0.351 |
| Volume (mm3) | 0.80 ± 0.16 | 0.79 ± 0.19 | 0.566 | |
| GCL | Average (µm) | 34.96 ± 5.84 | 33.50 ± 8.41 | 0.426 |
| Volume (mm3) | 0.95 ± 0.15 | 0.92 ± 0.22 | 0.516 | |
| IPL | Average (µm) | 30.56 ± 3.75 | 30.63 ± 5.62 | 0.950 |
| Volume (mm3) | 0.82 ± 0.10 | 0.83 ± 0.15 | 0.748 | |
| INL | Average (µm) | 34.43 ± 2.38 | 38.29 ± 4.28 | 0.010 |
| Volume (mm3) | 0.96 ± 0.06 | 1.06 ± 0.10 | <0.001 | |
| Nonprogressors (n = 129) | Progressors (n = 33) | p Value | |
|---|---|---|---|
| Age (years) | 54.6 ± 13.0 | 53.0 ± 15.8 | 0.546 |
| Male/Female | 65/64 | 13/20 | 0.330 |
| Diabetes (%) | 21 (16.3%) | 4 (12.1%) | 0.787 |
| Systemic hypertension (%) | 36 (27.9%) | 8 (24.2%) | 0.827 |
| Migraine (%) | 23 (17.8%) | 7 (21.2%) | 0.624 |
| Cold hand (%) | 18 (14.0%) | 10 (30.3%) | 0.029 |
| Cardiovascular disease (%) | 20 (15.5%) | 2 (6.1%) | 0.253 |
| Thyroid disease | 15 (11.6%) | 3 (9.1%) | 1.000 |
| Systolic blood pressure (mmHg) | 127.2 ± 15.3 | 122.4 ± 12.1 | 0.279 |
| Diastolic blood pressure (mmHg) | 79.4 ± 8.6 | 78.1 ± 7.3 | 0.580 |
| Body mass index (kg/m2) | 23.4 ± 2.9 | 23.2 ± 3.7 | 0.787 |
| Central corneal thickness (µm) | 534.7 ± 36.3 | 540.3 ± 33.0 | 0.487 |
| Axial length (mm) | 24.9 ± 1.5 | 24.8 ± 1.4 | 0.867 |
| Spherical equivalent (diopter) | −2.6 ± 3.6 | −2.6 ± 3.2 | 0.950 |
| Mean IOP (mmHg) | 14.6 ± 2.5 | 14.7 ± 2.1 | 0.833 |
| SD of IOP (mmHg) | 2.2 ± 0.7 | 2.4 ± 0.7 | 0.396 |
| Presence of disc hemorrhage (%) | 16 (12.4%) | 5 (15.2%) | 0.771 |
| Baseline MD of VF 24-2 (dB) | −3.9 ± 4.3 | −4.5 ± 4.6 | 0.488 |
| Baseline PSD of VF 24-2 (dB) | 4.5 ± 3.9 | 5.8 ± 4.5 | 0.096 |
| Follow-up duration (months) | 97.1 ± 25.2 | 104.2 ± 24.1 | 0.148 |
| Findings Observed on OCT | Nonprogressors (n = 129) | Progressors (n = 33) | p Value | ||
|---|---|---|---|---|---|
| Microcystic macular changes | 5 (3.9%) | 7 (21.2%) | 0.003 | ||
| Epiretinal membrane | 1 (0.8%) | 4 (12.1%) | 0.006 | ||
| Each retinal layer thickness | Retina | Average (µm) | 302.08 ± 15.32 | 303.88 ± 18.75 | 0.567 |
| Volume (mm3) | 8.31 ± 0.43 | 8.33 ± 0.51 | 0.829 | ||
| RNFL | Average (µm) | 24.00 ± 4.48 | 23.87 ± 4.35 | 0.879 | |
| Volume (mm3) | 0.81 ± 0.16 | 0.78 ± 0.14 | 0.478 | ||
| GCL | Average (µm) | 34.91 ± 6.01 | 34.64 ± 6.29 | 0.827 | |
| Volume (mm3) | 0.95 ± 0.15 | 0.94 ± 0.16 | 0.783 | ||
| IPL | Average (µm) | 30.57 ± 3.78 | 30.52 ± 4.39 | 0.943 | |
| Volume (mm3) | 0.82 ± 0.10 | 0.81 ± 0.11 | 0.839 | ||
| INL | Average (µm) | 34.47 ± 2.55 | 35.72 ± 3.24 | 0.019 | |
| Volume (mm3) | 0.96 ± 0.06 | 0.99 ± 0.08 | 0.015 | ||
| Parameters | Trend-Based Analysis (MD Change/Year) | Event-Based Analysis (EMGT Criteria) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | |
| Presence of cold hand | −0.180 (−0.377~0.017) | 0.073 | 2.681 (1.097~6.555) | 0.031 | 2.283 (0.864~6.035) | 0.096 | ||
| Presence of microcystic macular changes | −0.349 (−0.631~−0.066) | 0.016 | −0.164 (−0.473~0.145) | 0.297 | 6.677 (1.965~22.685) | 0.002 | 4.037 (0.992~16.430) | 0.051 |
| Presence of ERM | −0.554 (−0.981~−0.127) | 0.011 | −0.286 (−0.755~0.183) | 0.230 | 6.378 (1.902~163.874) | 0.012 | 4.918 (0.419~57.681) | 0.205 |
| Average INL thickness | −0.043 (−0.070~−0.016) | 0.002 | −0.030 (−0.060~−0.001) | 0.045 | 1.176 (1.018~1.357) | 0.027 | 1.062 (0.893~1.262) | 0.497 |
| Parameters | Total Patients | Patients without Microcystic Macular Changes | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | |
| Age | 0.029 (−0.002~0.061) | 0.064 | 0.019 (−0.009~0.048) | 0.186 | ||||
| Sex | −0.460 (−1.312~0.392) | 0.288 | −0.621 (−1.387~0.145) | 0.111 | ||||
| Diabetes | 0.841 (−0.334~2.016) | 0.160 | 0.748 (−0.406~1.901) | 0.202 | ||||
| Systemic hypertension | −0.053 (−1.013~0.908) | 0.914 | 0.070 (−0.802~0.942) | 0.874 | ||||
| Systolic BP (mmHg) | 0.012 (−0.022~0.046) | 0.489 | 0.009 (−0.024~0.043) | 0.576 | ||||
| Diastolic BP (mmHg) | −0.005 (−0.065~0.055) | 0.869 | −0.008 (−0.067~0.051) | 0.787 | ||||
| CCT (µm) | 0.007 (−0.007~0.020) | 0.336 | −0.002(−0.014~0.010) | 0.750 | ||||
| Axial length (mm) | 0.067 (−0.216~0.351) | 0.639 | −0.372 (−0.656~−0.088) | 0.011 | −0.310 (−0.583~0.037) | 0.026 | ||
| Mean IOP (mmHg) | 0.072 (−0.105~0.249) | 0.424 | 0.067 (−0.091~0.225) | 0.401 | ||||
| SD of IOP (mmHg) | 1.081 (0.471~1.692) | 0.001 | 0.861 (0.288~1.434) | 0.003 | 0.815 (0.244~1.386) | 0.005 | 1.107 (0.477~1.737) | 0.001 |
| Presence of optic disc hemorrhage | −0.735 (−2.029~0.559) | 0.264 | 0.261 (−0.873~1.395) | 0.650 | ||||
| Baseline VF MD | −0.104 (−0.200~0.008) | 0.035 | 0.049 (0.130~0.227) | 0.593 | −0.056 (−0.148~0.035) | 0.225 | ||
| Baseline VF PSD | 0.107 (0.002~0.211) | 0.046 | 0.102 (0.090~0.294) | 0.295 | 0.061 (−0.038~0.159) | 0.226 | ||
| Presence of ERM | 5.884 (3.591~8.176) | <0.001 | 4.331 (2.004~6.658) | <0.001 | 3.332 (0.012~6.652) | 0.049 | 2.329 (−1.971~6.629) | 0.285 |
| Presence of microcystic macular changes | 3.852 (2.336~5.368) | <0.001 | 2.315 (0.734~3.896) | 0.004 | ||||
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| β Coefficient (95% CI) | p Value | β Coefficient (95% CI) | p Value | |
| Presence of diabetes | 6.895 (2.016~23.580) | 0.002 | 5.838 (1.301~26.201) | 0.021 |
| Presence of ERM | 24.667 (3.647~1666.834) | 0.001 | 14.590 (0.364~156.115) | 0.027 |
| Baseline MD | 0.860 (0.773~0.957) | 0.006 | 0.906 (0.796~1.041) | 0.169 |
| Higher IOP fluctuation | 12.571 (1.583~99.835) | 0.017 | 11.089 (1.288~95.430) | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.I.; Ryu, H.K.; Oh, S.E.; Shin, H.J.; Park, C.K. Thicker Inner Nuclear Layer as a Predictor of Glaucoma Progression and the Impact of Intraocular Pressure Fluctuation. J. Clin. Med. 2024, 13, 2312. https://doi.org/10.3390/jcm13082312
Jung KI, Ryu HK, Oh SE, Shin HJ, Park CK. Thicker Inner Nuclear Layer as a Predictor of Glaucoma Progression and the Impact of Intraocular Pressure Fluctuation. Journal of Clinical Medicine. 2024; 13(8):2312. https://doi.org/10.3390/jcm13082312
Chicago/Turabian StyleJung, Kyoung In, Hee Kyung Ryu, Si Eun Oh, Hee Jong Shin, and Chan Kee Park. 2024. "Thicker Inner Nuclear Layer as a Predictor of Glaucoma Progression and the Impact of Intraocular Pressure Fluctuation" Journal of Clinical Medicine 13, no. 8: 2312. https://doi.org/10.3390/jcm13082312
APA StyleJung, K. I., Ryu, H. K., Oh, S. E., Shin, H. J., & Park, C. K. (2024). Thicker Inner Nuclear Layer as a Predictor of Glaucoma Progression and the Impact of Intraocular Pressure Fluctuation. Journal of Clinical Medicine, 13(8), 2312. https://doi.org/10.3390/jcm13082312





