The Impact of Augmented Renal Clearance on Vancomycin Pharmacokinetics and Pharmacodynamics in Critically Ill Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
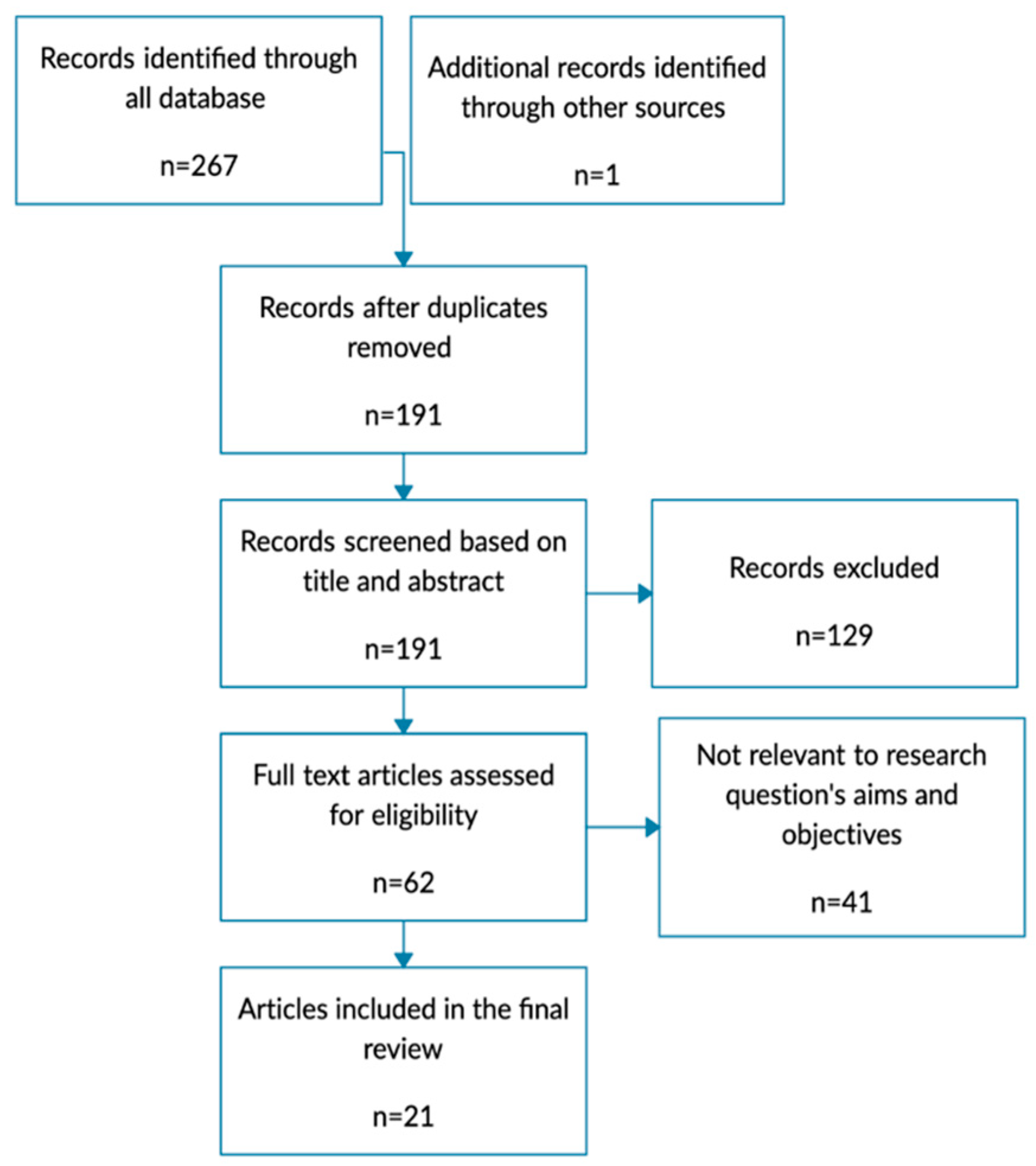
3. Results
3.1. Study Selection
3.2. ARC Definition and Its Prevalence
3.3. Impact of ARC on Vancomycin Therapy
4. Discussion
4.1. The Critical Role of CrCl in Vancomycin Therapy and Identifying ARC
4.2. Risk Factors for ARC
4.3. Vancomycin Dosage Considerations
4.4. Implication of ARC for Vancomycin PK/PD Indices
4.4.1. AUC24/MIC and Trough Concentration
4.4.2. CL, Half-Life, and Vd
4.5. Approach to Vancomycin Dosing in Patients with ARC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maki, D.G.; Crnich, C.J.; Safdar, N. Nosocomial Infection in the Intensive Care Unit. In Critical Care Medicine; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1003–1069. ISBN 978-0-323-04841-5. [Google Scholar]
- Wicha, S.G.; Märtson, A.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.; Minichmayr, I.K.; the International Society of Anti-Infective Pharmacology (ISAP), the PK/PD study group of the European Society of Clinical Microbiology, Infectious Diseases (EPASG). From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Jamal, J.-A.; Roger, C.; Roberts, J.A. Understanding the Impact of Pathophysiological Alterations during Critical Illness on Drug Pharmacokinetics. Anaesth. Crit. Care Pain Med. 2018, 37, 515–517. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic Initial β-Lactam Concentrations in Select Critically Ill Patients. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Shen, C. Augmented Renal Clearance in Critical Illness: An Important Consideration in Drug Dosing. Pharmaceutics 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Baptista, J.P.; Roberts, J.A.; Sousa, E.; Freitas, R.; Deveza, N.; Pimentel, J. Decreasing the Time to Achieve Therapeutic Vancomycin Concentrations in Critically Ill Patients: Developing and Testing of a Dosing Nomogram. Crit. Care Lond. Engl. 2014, 18, 654. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-X.; Lu, J.; Lu, H.; Li, L.; Li, J.-J.; Shi, L.; Duan, L.-F.; Zhuang, Z.-W.; Xue, S.-D.; Shen, Y.; et al. Predictive Performance of Reported Vancomycin Population Pharmacokinetic Model in Patients with Different Renal Function Status, Especially Those with Augmented Renal Clearance. Eur. J. Hosp. Pharm. Sci. Pract. 2022, 29, e6–e14. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Ishii, H.; Shimoshikiryo, T.; Shimomura, T.; Tsuji, D.; Inoue, K.; Kadoiri, T.; Itoh, K. Augmented Renal Clearance in Patients with Febrile Neutropenia Is Associated with Increased Risk for Subtherapeutic Concentrations of Vancomycin. Ther. Drug Monit. 2016, 38, 706–710. [Google Scholar] [CrossRef]
- Mikami, R.; Imai, S.; Hayakawa, M.; Sugawara, M.; Takekuma, Y. Clinical Applicability of Urinary Creatinine Clearance for Determining the Initial Dose of Vancomycin in Critically Ill Patients. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2022, 28, 199–205. [Google Scholar] [CrossRef]
- Sahraei, Z.; Saffaei, A.; Alavi Darazam, I.; Salamzadeh, J.; Shabani, M.; Shokouhi, S.; Sarvmeili, N.; Hajiesmaeili, M.; Zangi, M. Evaluation of Vancomycin Pharmacokinetics in Patients with Augmented Renal Clearances: A Randomized Clinical Trial. Front. Pharmacol. 2022, 13, 1041152. [Google Scholar] [CrossRef] [PubMed]
- Šíma, M.; Hartinger, J.; Cikánková, T.; Slanař, O. Importance of Vancomycin Loading Doses in Intermittent Infusion Regimens. J. Infect. Chemother. 2018, 24, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Mejías-Trueba, M.; Alonso-Moreno, M.; Gutiérrez-Valencia, A.; Herrera-Hidalgo, L.; Guisado-Gil, A.B.; Jiménez-Parrilla, F.; Varela-Rubio, E.; Gil-Navarro, M.V. Association between Vancomycin Pharmacokinetic Parameters and Clinical and Microbiological Efficacy in a Cohort of Neonatal Patients. Antimicrob. Agents Chemother. 2022, 66, e01109-22. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-Resistant Staphylococcus Aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef]
- Sunder, S.; Jayaraman, R.; Mahapatra, H.S.; Sathi, S.; Ramanan, V.; Kanchi, P.; Gupta, A.; Daksh, S.K.; Ram, P. Estimation of Renal Function in the Intensive Care Unit: The Covert Concepts Brought to Light. J. Intensive Care 2014, 2, 31. [Google Scholar] [CrossRef]
- John, G.; Heffner, E.; Carter, T.; Beckham, R.; Smith, N. Augmented Renal Clearance in Patients with Acute Ischemic Stroke: A Prospective Observational Study. Neurocrit. Care 2023, 38, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Luo, Y.; Jiang, M.; Zhou, B. Application of Vancomycin in Patients with Augmented Renal Clearance. Eur. J. Hosp. Pharm. Sci. Pract. 2020, 27, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Campassi, M.L.; Gonzalez, M.C.; Masevicius, F.D.; Vazquez, A.R.; Moseinco, M.; Navarro, N.C.; Previgliano, L.; Rubatto, N.P.; Benites, M.H.; Estenssoro, E.; et al. Augmented Renal Clearance in Critically Ill Patients: Incidence, Associated Factors and Effects on Vancomycin Treatment. Rev. Bras. Ter. Intens. 2014, 26, 13–20. [Google Scholar] [CrossRef]
- Ishii, H.; Hirai, K.; Sugiyama, K.; Nakatani, E.; Kimura, M.; Itoh, K. Validation of a Nomogram for Achieving Target Trough Concentration of Vancomycin: Accuracy in Patients with Augmented Renal Function. Ther. Drug Monit. 2018, 40, 693–698. [Google Scholar] [CrossRef]
- Morbitzer, K.; Jordan, D.; Sullivan, K.; Durr, E.; Olm-Shipman, C.; Rhoney, D. Enhanced Renal Clearance and Impact on Vancomycin Trough Concentration in Patients with Hemorrhagic Stroke. Pharmacotherapy 2016, 36, e218. [Google Scholar] [CrossRef]
- Baptista, J.P.; Sousa, E.; Martins, P.J.; Pimentel, J.M. Augmented Renal Clearance in Septic Patients and Implications for Vancomycin Optimisation. Int. J. Antimicrob. Agents 2012, 39, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Minkute, R.; Briedis, V.; Steponaviciute, R.; Vitkauskiene, A.; Maciulaitis, R. Augmented Renal Clearance—An Evolving Risk Factor to Consider during the Treatment with Vancomycin. J. Clin. Pharm. Ther. 2013, 38, 462–467. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.-T.; Qian, X.; Zhao, B.; Mao, E.-Q.; Chen, E.-Z.; Bian, X.-L. A Higher Dose of Vancomycin Is Needed in Critically Ill Patients with Augmented Renal Clearance. Transl. Androl. Urol. 2020, 9, 2166–2171. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Zhu, M. Effect of Augmented Renal Clearance on the Therapeutic Drug Monitoring of Vancomycin in Patients after Neurosurgery. J. Int. Med. Res. 2020, 48, 300060520949076. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, Y.; Yang, M.; Liang, X.; Wu, J.; Chen, Y.; Guo, B.; Zhang, H.; Wang, R.; Zhang, F.; et al. Association between Augmented Renal Clearance and Inadequate Vancomycin Pharmacokinetic/Pharmacodynamic Targets in Chinese Adult Patients: A Prospective Observational Study. Antibiotics 2022, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Ishigo, T.; Ibe, Y.; Fujii, S.; Kazuma, S.; Aigami, T.; Kashiwagi, Y.; Takada, R.; Takahashi, S.; Fukudo, M.; Toda, T. Effect of Renal Clearance on Vancomycin Area under the Concentration-Time Curve Deviations in Critically Ill Patients. J. Infect. Chemother. Off. J. Jpn. Soc. Chemother. 2023, 29, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Luo, Y.; Ji, S.; Jiang, M.; Zhou, B. Population Pharmacokinetics of Vancomycin in Chinese Patients with Augmented Renal Clearance. J. Infect. Public Health 2020, 13, 68–74. [Google Scholar] [CrossRef]
- Morbitzer, K.A.; Rhoney, D.H.; Dehne, K.A.; Jordan, J.D. Enhanced Renal Clearance and Impact on Vancomycin Pharmacokinetic Parameters in Patients with Hemorrhagic Stroke. J. Intensive Care 2019, 7, 51. [Google Scholar] [CrossRef]
- Zhao, S.; He, N.; Zhang, Y.; Wang, C.; Zhai, S.; Zhang, C. Population Pharmacokinetic Modeling and Dose Optimization of Vancomycin in Chinese Patients with Augmented Renal Clearance. Antibiotics 2021, 10, 1238. [Google Scholar] [CrossRef]
- Chu, Y.; Luo, Y.; Qu, L.; Zhao, C.; Jiang, M. Application of Vancomycin in Patients with Varying Renal Function, Especially Those with Augmented Renal Clearance. Pharm. Biol. 2016, 54, 2802–2806. [Google Scholar] [CrossRef]
- Nelson, N.R.; Morbitzer, K.A.; Jordan, J.D.; Rhoney, D.H. The Impact of Capping Creatinine Clearance on Achieving Therapeutic Vancomycin Concentrations in Neurocritically Ill Patients with Traumatic Brain Injury. Neurocrit. Care 2019, 30, 126–131. [Google Scholar] [CrossRef]
- Roberts, D.M. The Relevance of Drug Clearance to Antibiotic Dosing in Critically Ill Patients. Curr. Pharm. Biotechnol. 2011, 12, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Coresh, J.; Greene, T.; Levey, A.S. Assessing Kidney Function—Measured and Estimated Glomerular Filtration Rate. N. Engl. J. Med. 2006, 354, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Meseguer, I.; Rodriguez-Gascon, A.; Barrasa, H.; Isla, A.; Solinis, M.A. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef]
- Hobbs, A.L.V.; Shea, K.M.; Roberts, K.M.; Daley, M.J. Implications of Augmented Renal Clearance on Drug Dosing in Critically Ill Patients: A Focus on Antibiotics. Pharmacotherapy 2015, 35, 1063–1075. [Google Scholar] [CrossRef]
- Smit, C.; De Hoogd, S.; Brüggemann, R.J.M.; Knibbe, C.A.J. Obesity and Drug Pharmacology: A Review of the Influence of Obesity on Pharmacokinetic and Pharmacodynamic Parameters. Expert Opin. Drug Metab. Toxicol. 2018, 14, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic Monitoring of Vancomycin in Adult Patients: A Consensus Review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Mulla, H.; Pooboni, S. Population Pharmacokinetics of Vancomycin in Patients Receiving Extracorporeal Membrane Oxygenation. Br. J. Clin. Pharmacol. 2005, 60, 265–275. [Google Scholar] [CrossRef]
- Shingde, R.V.; Graham, G.G.; Reuter, S.E.; Carland, J.E.; Day, R.O.; Stocker, S.L. Comparison of the Area Under the Curve for Vancomycin Estimated Using Compartmental and Noncompartmental Methods in Adult Patients with Normal Renal Function. Ther. Drug Monit. 2019, 41, 726–731. [Google Scholar] [CrossRef]
- Reuter, S.E.; Stocker, S.L.; Alffenaar, J.-W.C.; Baldelli, S.; Cattaneo, D.; Jones, G.; Koch, B.C.P.; Kocic, D.; Mathew, S.K.; Molinaro, M.; et al. Optimal Practice for Vancomycin Therapeutic Drug Monitoring: Position Statement from the Anti-Infectives Committee of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2022, 44, 121–132. [Google Scholar] [CrossRef]
- Kim, B.; Hwang, S.; Heo, E.; Kim, H.; Jung, J.; Kim, E.S.; Kim, H.B.; Lee, K.; Park, J.S.; Song, J.; et al. Evaluation of Vancomycin TDM Strategies: Prediction and Prevention of Kidney Injuries Based on Vancomycin TDM Results. J. Korean Med. Sci. 2023, 38, e101. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Oda, K.; Shoji, K.; Hanai, Y.; Takahashi, Y.; Fujii, S.; Hamada, Y.; Kimura, T.; Mayumi, T.; Ueda, T.; et al. Clinical Practice Guidelines for Therapeutic Drug Monitoring of Vancomycin in the Framework of Model-Informed Precision Dosing: A Consensus Review by the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. Pharmaceutics 2022, 14, 489. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The Timing of Early Antibiotics and Hospital Mortality in Sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Ishigo, T.; Fujii, S.; Ibe, Y.; Aigami, T.; Nakano, K.; Fukudo, M.; Yoshida, H.; Tanaka, H.; Ebihara, F.; Maruyama, T.; et al. Flowchart for Predicting Achieving the Target Area under the Concentration-Time Curve of Vancomycin in Critically Ill Japanese Patients: A Multicenter Retrospective Study. J. Infect. Chemother. 2024, 30, 329–336. [Google Scholar] [CrossRef]
- Helset, E.; Nordoy, I.; Sporsem, H.; Bakke, V.D.; Bugge, J.F.; Gammelsrud, K.W.; Zucknick, M.; von der Lippe, E. Factors Increasing the Risk of Inappropriate Vancomycin Therapy in ICU Patients: A Prospective Observational Study. Acta Anaesthesiol. Scand. 2020, 64, 1295–1304. [Google Scholar] [CrossRef]
| Population | Age ab (Years) | CrCl ab * | Maintenance Dose ab ** | Ctrough (mg/L) ab | Ctrough < 10 mg/L, (%) | AUC24 (mg.h/L) ab | Vd (L) ab | VCM CL (L/h) ab | References |
|---|---|---|---|---|---|---|---|---|---|
| Mixed ICU | 69 (59–75) | 160.3 (144.2–199.9) | 14.7 (13.0–18.2) | NR | NR | 240 (209–300) | NR | NR | Ishigo T et al., 2023 [27] |
| Mixed ICU | 69 (50–73) | 171.6 (157.5–203.0) mL/min | 34.2 (28.3–42.1) | 9.4 (5.9–11.9) | NR | NR | NR | NR | Mikami R et al., 2022 [11] |
| Mixed ICU | BD: 44.04 ± 16.55 TDS: 42.86 ± 11.83 | BD: 166.94 ± 41.32 TDS: 171.78 ± 48.56 | 15 mg/kg | BD: 5.64 ± 1.92 TDS: 14.03 ± 2.97 | NR | BD: 397.90 ± 76.02 TDS: 611.92 ± 148.01 | BD: 44.39 ± 14.21 TDS: 41.87 ± 27.30 | BD: 5.97 ± 1.48 TDS: 5.69 ± 1.87 | Sahraei Z et al., 2022 [12] |
| ICU and non-ICU | 50.9 ± 15.1 | 141.2 ± 16.0 | 30.3 ± 6.4 mg/kg | 7.1 ± 2.9 mg/mL | 80 | JPKD: 307.4 ± 72.4 SDose: 376.6 ± 103.4 | JPKD: 72.6 ± 10.3 SDose: 44.6 ± 6.7 | NR | Yu XY et al., 2022 [8] |
| ICU and non-ICU | 50 (33–60) | 159 (144–193) | 2 g/day | 7.1 (3.9–10.6) | 71.6 | (253.8–475.0) | NR | NR | Zhao J et al., 2022 [26] |
| ICU | 33 (26–46) | 168.4 (148.5–193.2) | 1.28 ± 0.52 g | 6.45 (3.72–8.64) | 80.77 | NR | NR | NR | Chen Y et al., 2020 [25] |
| Hospitalized | 45 (33–57.25) | 180.50 (152.95–207.35) mL/min | 1000 mg every 12 h | 6.80 (3.50–13.30) | >60 | NR | NR | NR | Chu Y et al., 2020 [18] |
| Hospitalaized | 45 (33–57.25) | 175.90 (142.20–198.10) mL/min | 1000–4000 mg/d every 6, 8 and 12 h | NR | NR | NR | 155.4 | 8.52 | Chu Y et al., 2020 [28] |
| Mixed ICU | 40.0 ±11 | 180.8 ± 59.3 mL/min | 29 ± 9.4 | 6.5 ± 3.8 | 77.7 | 232.9 ± 93.6 | 69.3 ± 9.1 | 9.7 ± 3.4 | He J et al., 2020 [24] |
| ICH and aSAH | 63.3 ± 13.3 | 161.6 ± 16.7 mL/min | 15.1 ± 4.2 every 8 h (8–12) | 12 ± 3.6 | NR | NR | 71.8 ± 11.3 | NR | Morbitzer KA et al., 2019 [29] |
| Adult patients | 43.8 ± 15.9 | 187.7 ± 50.0 | 1000 mg every 8 h | NR | 62.9 | NR | NR | NR | Chu Y et al., 2016 [31] |
| Mixed ICU | 57.5 (39.0–69.3) | 157.4 (142.1–173.9) | 35.7 (30.5–40.0) | 7.4 (5.2–11.6) | NR | 447 (400–554) | 133 (112–147) | 5.3 (4.9–6.02) | Hirai K et al., 2016 [10] |
| Mixed ICU | 48±15 | 155±33 | 30 | NR | 100 | NR | NR | NR | Campassi M et al., 2014 [19] |
| ICU and non-ICU | 45.5 (21–66) | 150.5 (42); 131–324 | <15 15–30 >30 | NS | 31.8 | NR | NR | NR | Minkute R et al., 2013 [23] |
| ICU | 41 (32–56) | 158.9 (140.9–193.6) | 30 (25.0–32.3) mg/kg | D1: 14 D3: 20 | D1: (98.2) D3: (48) | NR | NR | NR | Baptista JP et al., 2012 [22] |
| CrCl (mL/min) | Dosage Regimen | PTA (%) | PD Target | Based on | References |
|---|---|---|---|---|---|
| 120–149 | 1750 mg q24 h | 62.33 | AUC24 (400–650 mg.h/L) | PopPK study (Model-based Monte Carlo Simulations) | Zhao S et al., 2021 [30] |
| 150–179 | 1000 mg q12 h | 62.56 | |||
| ≥180 | 750 mg q8 h | 61.69 | |||
| ≥130 | 46 mg/kg/day | Ctrough > 10 mg/L | PopPK study (Bayesian estimation) | He J et al., 2020 [24] | |
| 69 mg/kg/day * | Ctrough > 15 mg/L * | ||||
| ≥130 | 15 mg/kg q8h | AUC/MIC > 400 | RCT | Sahraei Z et al., 2022 [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfamariam, N.S.; Aboelezz, A.; Mahmoud, S.H. The Impact of Augmented Renal Clearance on Vancomycin Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. J. Clin. Med. 2024, 13, 2317. https://doi.org/10.3390/jcm13082317
Tesfamariam NS, Aboelezz A, Mahmoud SH. The Impact of Augmented Renal Clearance on Vancomycin Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. Journal of Clinical Medicine. 2024; 13(8):2317. https://doi.org/10.3390/jcm13082317
Chicago/Turabian StyleTesfamariam, Novel Solomon, Asma Aboelezz, and Sherif Hanafy Mahmoud. 2024. "The Impact of Augmented Renal Clearance on Vancomycin Pharmacokinetics and Pharmacodynamics in Critically Ill Patients" Journal of Clinical Medicine 13, no. 8: 2317. https://doi.org/10.3390/jcm13082317
APA StyleTesfamariam, N. S., Aboelezz, A., & Mahmoud, S. H. (2024). The Impact of Augmented Renal Clearance on Vancomycin Pharmacokinetics and Pharmacodynamics in Critically Ill Patients. Journal of Clinical Medicine, 13(8), 2317. https://doi.org/10.3390/jcm13082317





