Long-Term Clinical Outcomes of Wedge-Shaped Implants Inserted in Narrow Ridges: A 7-Year Follow-Up Multicenter Prospective Single-Arm Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
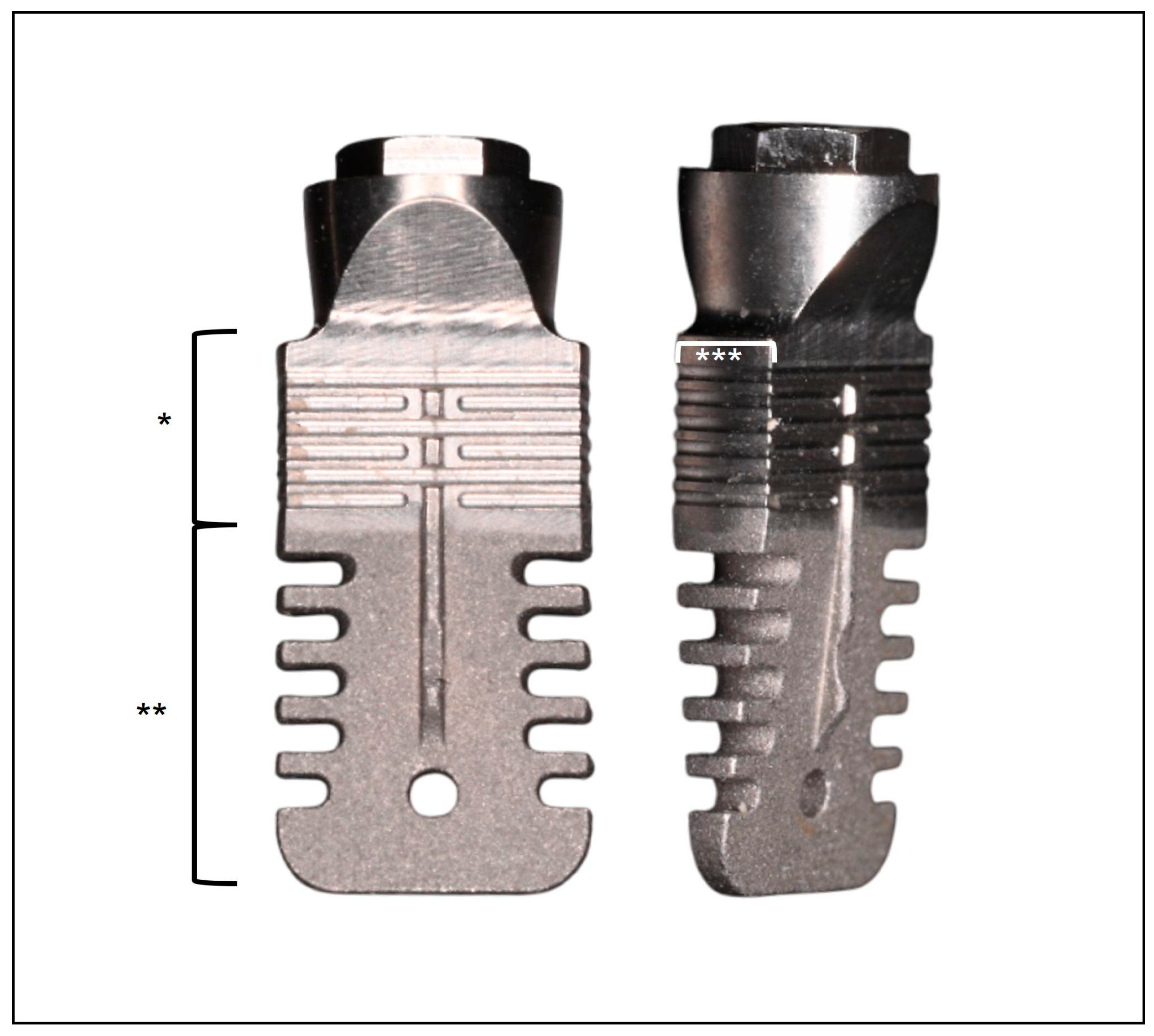
2.2. Implant Characteristics and Surgical Protocol
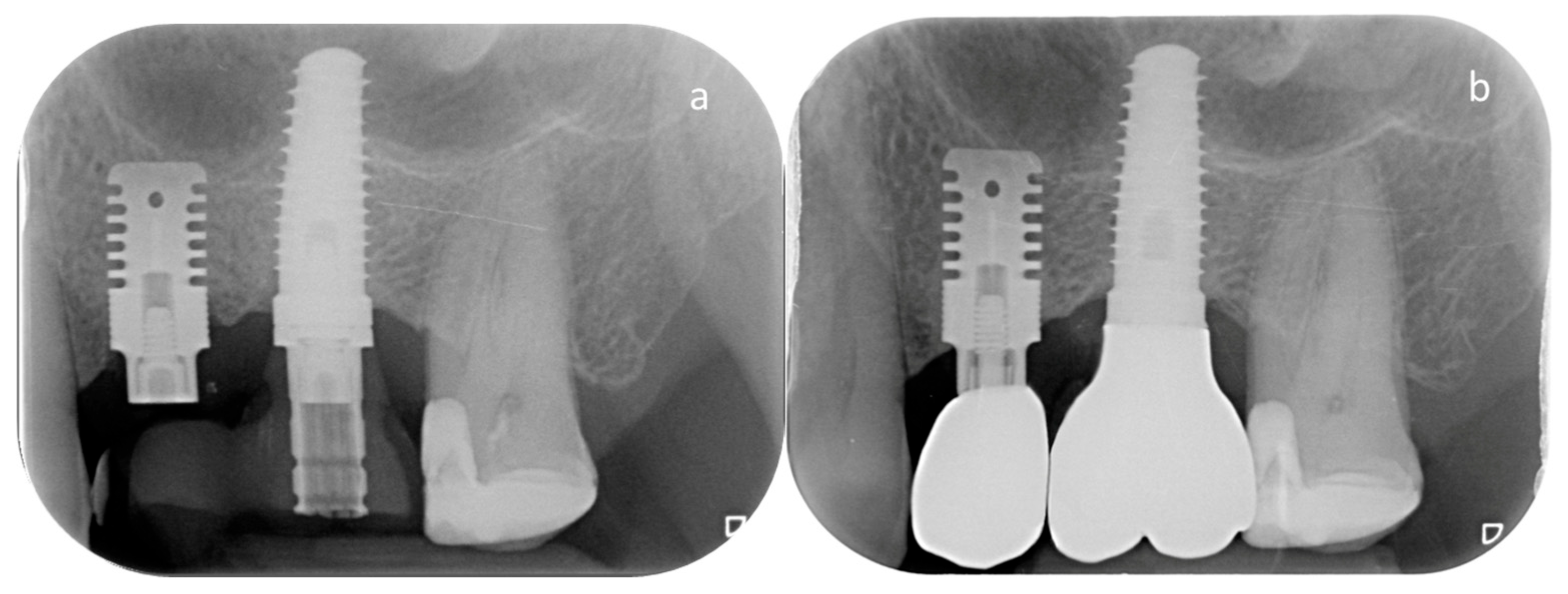
2.3. Clinical and Radiographic Evaluation
2.4. Outcome Measures
- -
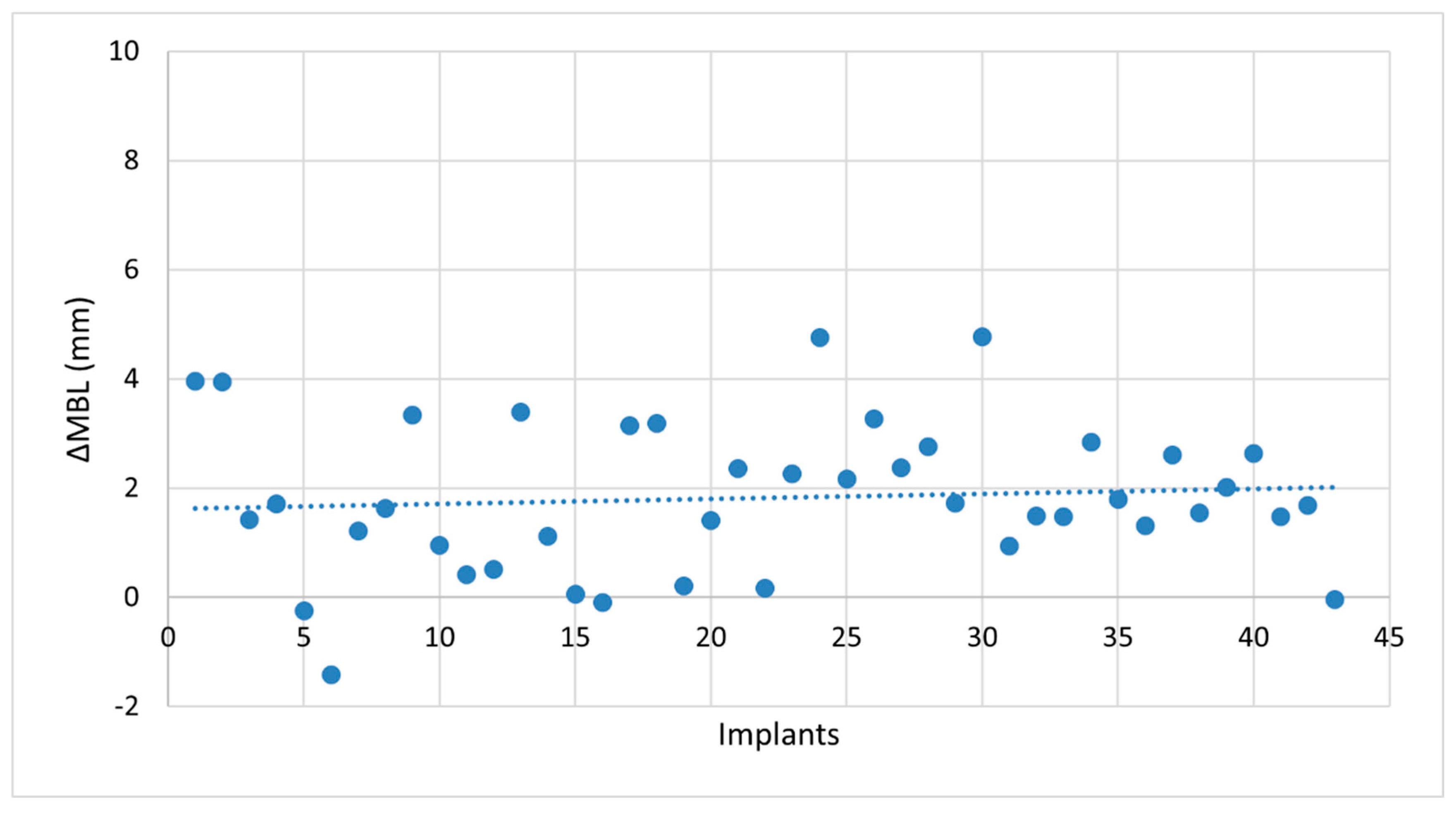
- Marginal bone loss (MBL) at T7. MBL was assessed radiographically on both mesial and distal aspects of each implant and expressed as the mean value in millimeters.
- -
- Biological complications (peri-implant mucositis or peri-implantitis) at T7. Mucositis and peri-implantitis were diagnosed according to the criteria established by Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions [23]. Peri-implant mucositis is defined by the presence of bleeding and/or suppuration on gentle probing, with signs of soft tissue inflammation and no radiographic bone loss beyond initial remodeling. The diagnostic criteria for peri-implantitis included: (1) presence of bleeding and/or suppuration on gentle probing; (2) increased probing depth relative to baseline measurements; and (3) radiographic evidence of marginal bone loss ≥0.5 mm compared to the baseline radiograph.
- -
- Mechanical complications, including screw loosening or fracture, implant fracture, prosthetic component fracture or chipping of veneering material.
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Clinical Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar] [CrossRef]
- Blackburn, T.K.; Cawood, J.I.; Stoelinga, P.J.; Lowe, D. What is the quality of the evidence base for pre-implant surgery of the atrophic jaw? Int. J. Oral Maxillofac. Surg. 2008, 37, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, V.; Rahman, L.; Buser, R.; Janner, S.F.M.; Belser, U.C.; Buser, D. Effectiveness of contour augmentation with guided bone regeneration: 10-year results. J. Dent. Res. 2018, 97, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Stacchi, C.; Visintini, E.; Di Iorio, D.; Putignano, A.; Breschi, L.; Di Lenarda, R. Clinical and histologic evaluation of fresh frozen human bone grafts for horizontal reconstruction of maxillary alveolar ridges. Int. J. Periodontics Restor. Dent. 2011, 31, 535–544. [Google Scholar]
- Starch-Jensen, T.; Becktor, J.P. Maxillary alveolar ridge expansion with split-crest technique compared with lateral ridge augmentation with autogenous bone block graft: A systematic review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef]
- Chappuis, V.; Cavusoglu, Y.; Buser, D.; von Arx, T. Lateral ridge augmentation using autogenous block grafts and guided bone regeneration: A 10-year prospective case series study. Clin. Implant Dent. Relat. Res. 2017, 19, 85–96. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Sanz-Martín, I.; Figuero, E.; Sanz, M. Effectiveness of lateral bone augmentation on the alveolar crest dimension: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 128S–142S. [Google Scholar] [CrossRef]
- Stacchi, C.; Spinato, S.; Lombardi, T.; Bernardello, F.; Bertoldi, C.; Zaffe, D.; Nevins, M. Minimally invasive management of implant-supported rehabilitation in the posterior maxilla, Part II. Surgical techniques and decision tree. Int. J. Periodontics Restor. Dent. 2020, 40, e95–e102. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Al-Nawas, B. Narrow-diameter implants: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 21–40. [Google Scholar] [CrossRef]
- Pommer, B.; Mailath-Pokorny, G.; Haas, R.; Busenlechner, D.; Millesi, W.; Fürhauser, R. Extra-short (<7 mm) and extra-narrow diameter (<3.5 mm) implants: A meta-analytic literature review. Eur. J. Oral Implantol. 2018, 11, S137–S146. [Google Scholar]
- Linkow, L.I. The blade vent—A new dimension in endosseous implantology. Dent. Concepts. 1968, 11, 3–12. [Google Scholar]
- Stacchi, C.; Bassi, F.; Troiano, G.; Rapani, A.; Lombardi, T.; Jokstad, A.; Sennerby, L.; Schierano, G. Piezoelectric bone surgery for implant site preparation compared with conventional drilling techniques: A systematic review, meta-analysis and trial sequential analysis. Int. J. Oral Implantol. 2020, 13, 141–158. [Google Scholar]
- Stacchi, C.; Troiano, G.; Montaruli, G.; Mozzati, M.; Lamazza, L.; Antonelli, A.; Giudice, A.; Lombardi, T. Changes in implant stability using different site preparation techniques: Osseodensification drills versus piezoelectric surgery. A multi-center prospective randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2023, 25, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cervino, G.; Fiorillo, L.; Iannello, G.; Santonocito, D.; Risitano, G.; Cicciù, M. Sandblasted and acid etched titanium dental implant surfaces: Systematic review and confocal microscopy evaluation. Materials 2019, 12, 1763. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Alshehri, Y.F.A.; Kruger, E.; Villata, L. Accuracy of digital versus conventional implant impressions in partially dentate patients: A systematic review and meta-analysis. J. Dent. 2025, 160, 105918. [Google Scholar] [CrossRef]
- Vercellotti, T.; Troiano, G.; Oreglia, F.; Lombardi, T.; Gregorig, G.; Morella, E.; Rapani, A.; Stacchi, C. Wedge-shaped implants for minimally invasive treatment of narrow ridges: A multicenter prospective cohort study. J. Clin. Med. 2020, 9, 3301. [Google Scholar] [CrossRef]
- Memè, L.; Mummolo, S.; Strappa, E.M.; Bambini, F.; Gallusi, G. A new wedge-shape dental implants for narrow bone ridge: Rex Piezoimplant. A case report with 12 months of follow-up. Am. J. Biomed. Sci. Res. 2022, 16, 295–300. [Google Scholar]
- Bambini, F.; Memè, L.; Rossi, R.; Grassi, A.; Grego, S.; Mummolo, S. New operative protocol for immediate post-extraction implant in lower-first-molar region with rex-blade implants: A case series with 18 months of follow-up. Appl. Sci. 2023, 13, 10226. [Google Scholar] [CrossRef]
- Giudice, A.; Attanasio, F.; Bennardo, F.; Antonelli, A.; Vercellotti, T. Usefulness of wedge-shaped implants in the full-arch rehabilitation of severe maxillary atrophy: A case report. Int. J. Periodontics Restor. Dent. 2024, 44, 347–355. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; O’Valle, F.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implants Res. 2015, 26, 28–34. [Google Scholar] [CrossRef]
- Windael, S.; Collaert, B.; De Buyser, S.; De Bruyn, H.; Vervaeke, S. Early peri-implant bone loss as a predictor for peri-implantitis: A 10-year prospective cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors influencing early marginal bone loss around dental implants positioned subcrestally: A multicenter prospective clinical study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Spinato, S.; Lombardi, T.; Stacchi, C. Bone remodeling and bone loss around subcrestal implants: Two distinct entities. Int. J. Periodontics Restor. Dent. 2023, 43, 410–411. [Google Scholar]
- Romeo, E.; Lops, D.; Margutti, E.; Ghisolfi, M.; Chiapasco, M.; Vogel, G. Long-term survival and success of oral implants in the treatment of full and partial arches: A 7-year prospective study with the ITI dental implant system. Int. J. Oral Maxillofac. Implants 2004, 19, 247–259. [Google Scholar]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Brägger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. Clin. Oral Implants Res. 2004, 15, 625–642. [Google Scholar] [CrossRef]
- Moraschini, V.; Poubel, L.A.; Ferreira, V.F.; Barboza Edos, S. Evaluation of survival and success rates of dental implants reported in longitudinal studies with a follow-up period of at least 10 years: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef]
- Ackermann, K.L.; Barth, T.; Cacaci, C.; Kistler, S.; Schlee, M.; Stiller, M. Clinical and patient-reported outcome of implant restorations with internal conical connection in daily dental practices: Prospective observational multicenter trial with up to 7-year follow-up. Int. J. Implant Dent. 2020, 6, 14. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef]
- Salvi, G.E.; Monje, A.; Tomasi, C. Long-term biological complications of dental implants placed either in pristine or in augmented sites: A systematic review and meta-analysis. Clin. Oral Implants Res. 2018, 29, 294–310. [Google Scholar] [CrossRef]
- Reis, I.N.R.D.; Huamán-Mendoza, A.A.; Ramadan, D.; Honório, H.M.; Naenni, N.; Romito, G.A.; Holzhausen, M.; Pannuti, C.M. The prevalence of peri-implant mucositis and peri-implantitis based on the world workshop criteria: A systematic review and meta-analysis. J. Dent. 2025, 160, 105914. [Google Scholar] [CrossRef] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar] [PubMed]
- Memenga-Nicksch, S.; Marschner, F.; Thomas, N.H.; Holzwart, D.; Staufenbiel, I. Systematic review and meta-analysis on marginal bone loss of dental implants placed in augmented or pristine bone sites: Findings from clinical long-term studies. J. Dent. 2025, 158, 105808. [Google Scholar] [CrossRef] [PubMed]
- Spray, J.R.; Black, C.G.; Morris, H.F.; Ochi, S. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann. Periodontol. 2000, 5, 119–128. [Google Scholar] [CrossRef]
- Chen, S.T.; Darby, I.B.; Reynolds, E.C. A prospective clinical study of non-submerged immediate implants: Clinical outcomes and esthetic results. Clin. Oral Implants Res. 2007, 18, 552–562. [Google Scholar] [CrossRef]
- Cicciù, M.; Pratella, U.; Fiorillo, L.; Bernardello, F.; Perillo, F.; Rapani, A.; Stacchi, C.; Lombardi, T. Influence of buccal and palatal bone thickness on post-surgical marginal bone changes around implants placed in posterior maxilla: A multi-centre prospective study. BMC Oral Health 2023, 23, 309. [Google Scholar] [CrossRef]
- Quarto, R.; Thomas, D.; Liang, C.T. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif. Tissue Int. 1995, 56, 123–129. [Google Scholar] [CrossRef]
- Rauner, M.; Sipos, W.; Pietschmann, P. Age-dependent Wnt gene expression in bone and during the course of osteoblast differentiation. Age 2008, 30, 273–282. [Google Scholar] [CrossRef]
- Stacchi, C.; Coyac, B.R.; Helms, J.A. Biomechanical basis for bone healing and osseointegration of implants in sinus grafts. Clin. Implant Dent. Relat. Res. 2025, 27, e13424. [Google Scholar] [CrossRef]
- Trombelli, L.; Farina, R.; Tomasi, C.; Vignoletti, F.; Paolantoni, G.; Giordano, F.; Ortensi, L.; Simonelli, A. Factors affecting radiographic marginal bone resorption at dental implants in function for at least 5 years: A multicenter retrospective study. Clin. Oral Implants Res. 2024, 35, 1406–1417. [Google Scholar] [CrossRef]
- Mertens, C.; Meyer-Bäumer, A.; Kappel, H.; Hoffmann, J.; Steveling, H.G. Use of 8-mm and 9-mm implants in atrophic alveolar ridges: 10-year results. Int. J. Oral Maxillofac. Implants 2012, 27, 1501–1508. [Google Scholar]
- Truhlar, R.S.; Orenstein, I.H.; Morris, H.F.; Ochi, S. Distribution of bone quality in patients receiving endosseous dental implants. J. Oral Maxillofac. Surg. 1997, 55, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Shapurian, T.; Damoulis, P.D.; Reiser, G.M.; Griffin, T.J.; Rand, W.M. Quantitative evaluation of bone density using the Hounsfield index. Int. J. Oral Maxillofac. Implants 2006, 21, 290–297. [Google Scholar]
- Steiner, C.; Karl, M.; Grobecker-Karl, T. Wedge shaped vs round implants: Bone strain during the insertion process. J. Oral Implantol. 2022, 48, 557–561. [Google Scholar] [CrossRef]
- Haase, F.; Siemers, C.; Rösler, J. Two novel titanium alloys for medical applications: Thermo-mechanical treatment, mechanical properties, and fracture analysis. J. Mater. Res. 2022, 37, 2589–2603. [Google Scholar] [CrossRef]
- Carrion, P.E.; Shamsaei, N.; Daniewicz, S.R.; Moser, R.D. Fatigue behavior of Ti-6Al-4V ELI including mean stress effects. Int. J. Fatigue 2017, 99, 87–100. [Google Scholar] [CrossRef]
| Variables | |
|---|---|
| Age (mean ± SD) | 59.6 ± 11.3 years |
| Gender (F; M) (total 34 patients) | 21 (61.8%); 13 (38.2%) |
| Smoking habits (smoker; no smoker) (total 34 patients) | 9 (26.5%); 25 (73.5%) |
| History of periodontitis (yes; no) (total 34 patients) | 14 (41.2%); 20 (58.8%) |
| Bone crest width at T0 (mean ± SD) | 3.73 ± 0.36 mm |
| Location (mandible; maxilla) (total 45 implants) | 33; 12 |
| Variables | Coefficient (β) | Std. Error (Cluster Patient) | 95% CI Lower | 95% Ci Upper | p-Value |
|---|---|---|---|---|---|
| Intercept | 3.568 | 2.333 | −1.203 | 8.34 | 0.137 |
| Age | 0.040 | 0.015 | 0.009 | 0.071 | 0.012 * |
| Gender (F vs. M) | −0.517 | 0.470 | −1.477 | 0.443 | 0.280 |
| Smoking | 0.049 | 0.751 | −1.488 | 1.586 | 0.948 |
| History of periodontitis | 0.326 | 0.638 | −0.979 | 1.631 | 0.612 |
| Bone crest width at T0 | −0.031 | 0.566 | −1.188 | 1.126 | 0.957 |
| Location (mandible) | 1.390 | 0.473 | 0.419 | 2.353 | 0.007 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapani, A.; Vercellotti, T.; Stacchi, C.; Gregorig, G.; Oreglia, F.; Morella, E.; Lombardi, T. Long-Term Clinical Outcomes of Wedge-Shaped Implants Inserted in Narrow Ridges: A 7-Year Follow-Up Multicenter Prospective Single-Arm Cohort Study. J. Clin. Med. 2025, 14, 6299. https://doi.org/10.3390/jcm14176299
Rapani A, Vercellotti T, Stacchi C, Gregorig G, Oreglia F, Morella E, Lombardi T. Long-Term Clinical Outcomes of Wedge-Shaped Implants Inserted in Narrow Ridges: A 7-Year Follow-Up Multicenter Prospective Single-Arm Cohort Study. Journal of Clinical Medicine. 2025; 14(17):6299. https://doi.org/10.3390/jcm14176299
Chicago/Turabian StyleRapani, Antonio, Tomaso Vercellotti, Claudio Stacchi, Gianluca Gregorig, Francesco Oreglia, Emanuele Morella, and Teresa Lombardi. 2025. "Long-Term Clinical Outcomes of Wedge-Shaped Implants Inserted in Narrow Ridges: A 7-Year Follow-Up Multicenter Prospective Single-Arm Cohort Study" Journal of Clinical Medicine 14, no. 17: 6299. https://doi.org/10.3390/jcm14176299
APA StyleRapani, A., Vercellotti, T., Stacchi, C., Gregorig, G., Oreglia, F., Morella, E., & Lombardi, T. (2025). Long-Term Clinical Outcomes of Wedge-Shaped Implants Inserted in Narrow Ridges: A 7-Year Follow-Up Multicenter Prospective Single-Arm Cohort Study. Journal of Clinical Medicine, 14(17), 6299. https://doi.org/10.3390/jcm14176299









