Adipocentric Strategy for the Treatment of Type 2 Diabetes Mellitus
Abstract
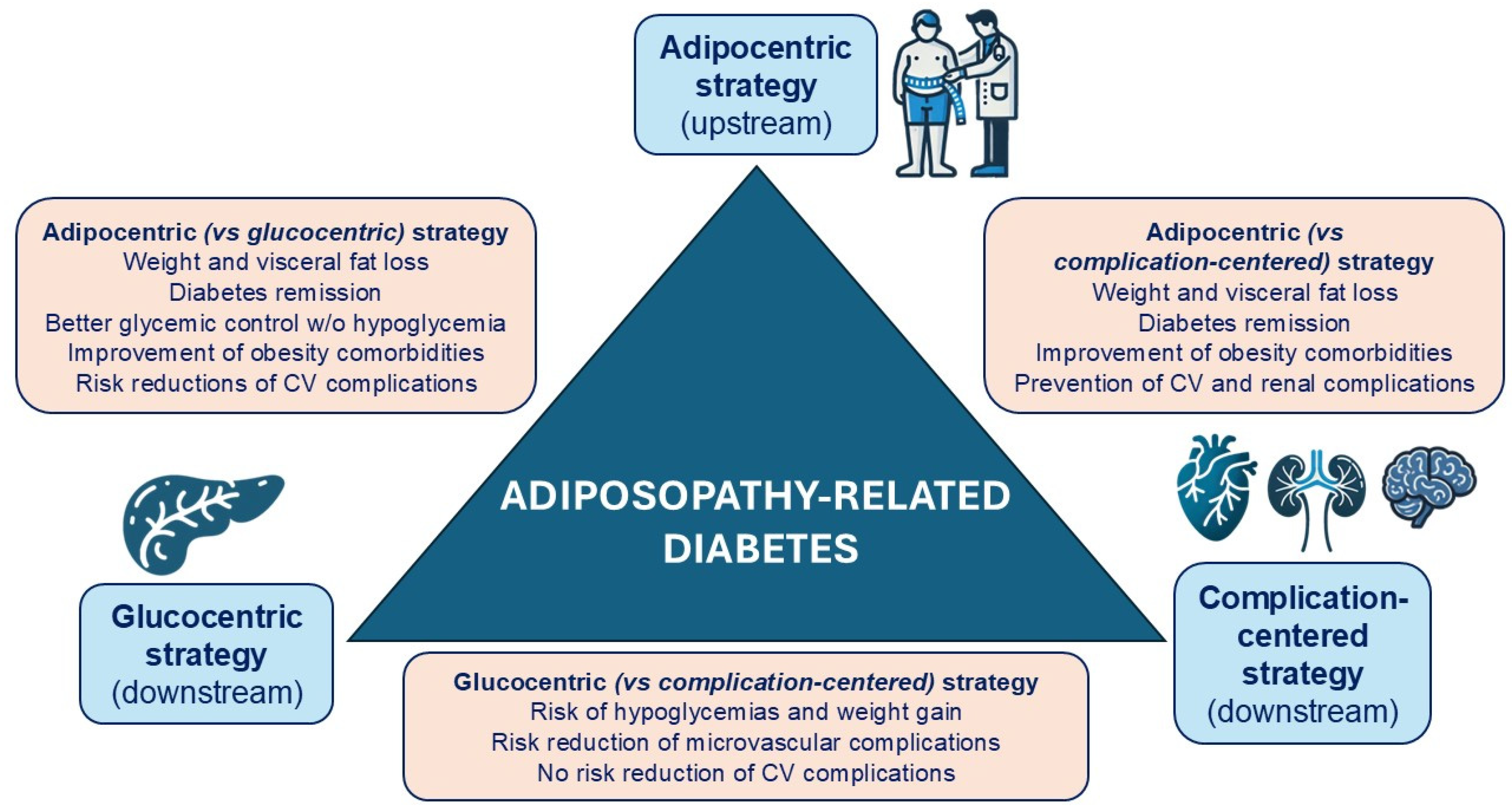
:1. Introduction: The Crisis of the Glucocentric Model
2. Adiposopathy as the Most Frequent Cause of T2D
2.1. Central Features of Adiposopathy
- (a)
- Increased ectopic and visceral fat: Abnormal accumulation of white adipose tissue in non-physiological locations (e.g., liver, pancreas, heart, and skeletal muscle) and within the visceral compartment (intra-abdominal and retroperitoneal fat).
- (b)
- Adipokine imbalance: A shift towards a pro-inflammatory profile of cytokines produced by adipose tissue.
- (c)
- Insulin resistance.
2.2. Adipose Tissue as an Active Endocrine Organ
2.3. Inflammation
2.4. Visceral Obesity, Lipotoxicity, and Beta-Cell Dysfunction
3. Heterogeneity vs. Homogeneity in the Pathophysiology of T2D
4. Diagnosis of ARD
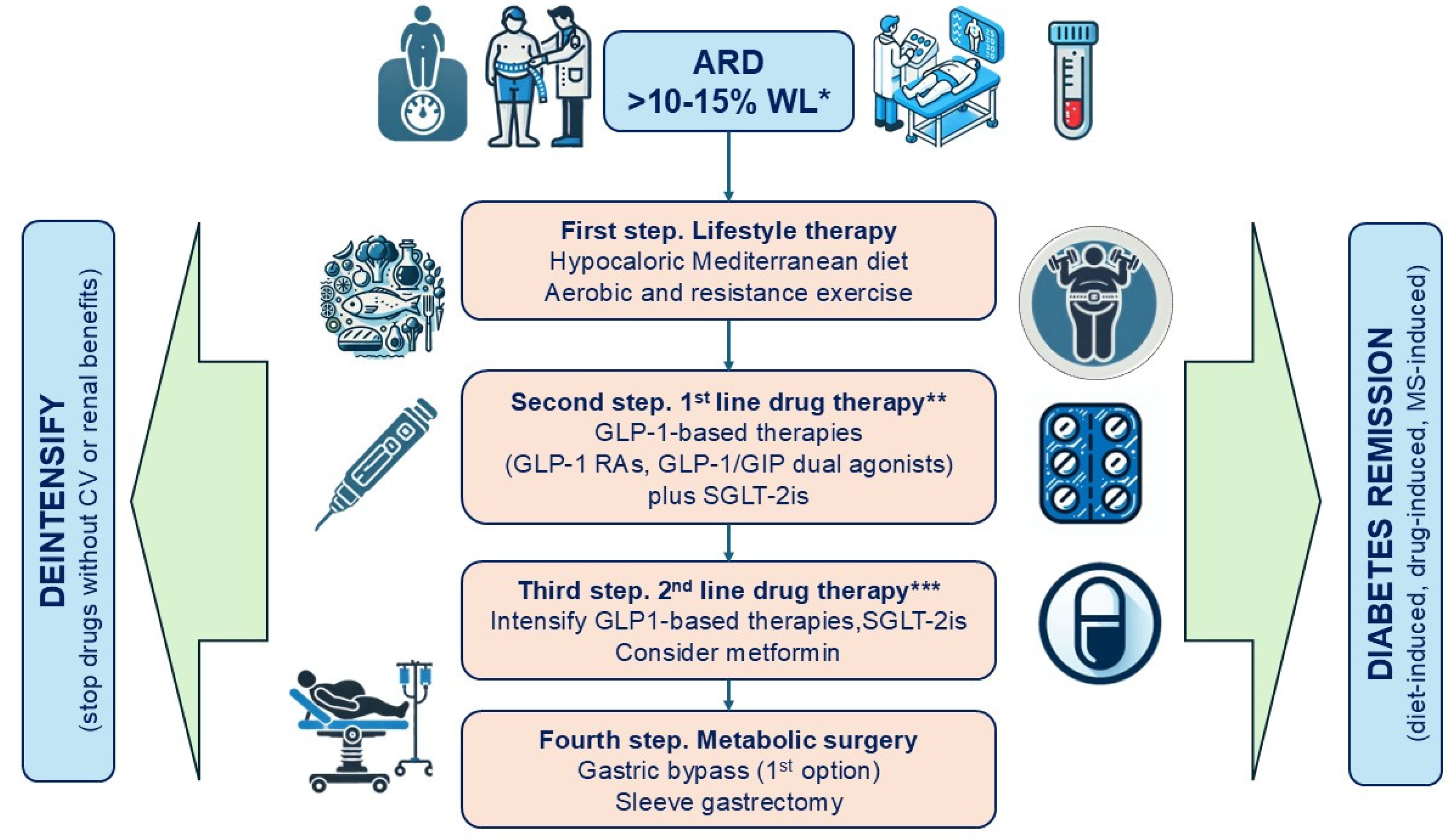
5. Importance of Weight Loss in the Remission of ARD
6. Importance of Weight Loss in Metabolic Control and Cardiorenal Protection in Patients with ARD
6.1. Lifestyle Modification
6.2. Antihyperglycemic Drugs with Weight and Cardiorenal Benefits
- Metabolic effects: Reduction in glucotoxicity, visceral fat, and uric acid levels;
- Hemodynamic effects: Lowered preload (volemia) and afterload (BP);
- Direct myocardial effects: Activation of nutrient deprivation signals, inhibition of the myocardial sodium-hydrogen exchanger type 1 (NHE-1), and reduction of myocardial fibrosis and pro-inflammatory adipokines derived from epicardial and perivascular fat.
- Metabolic mechanisms: Reductions in glucotoxicity, peri-/intrarenal fat, and activation of nutrient deprivation pathways;
- Systemic hemodynamic effects: Decreased systolic BP transmitted to the kidney;
- Intrarenal hemodynamic effects: Restoration of tubule-glomerular feedback;
- Tubulointerstitial mechanisms: Reduced glucotoxicity, proteinuria, and activation of anti-inflammatory and antifibrotic pathways.
- Direct mechanisms: Improved coronary vascularization, vasodilation, anti-inflammatory and antioxidant effects, enhanced endothelial function, inhibition of smooth muscle proliferation, plaque stabilization, and increased ischemia tolerance;
- Indirect mechanisms: Reduced glucotoxicity and lipotoxicity, improved myocardial glucose utilization, lowered BP, and decreased inflamed epicardial fat.
- Systemic effects: Reduced glucotoxicity, body weight, and systolic BP;
- Hemodynamic effects: Inhibition of NHE-3 in the proximal tubule, inducing natriuresis and restoring tubule-glomerular feedback;
- Anti-inflammatory effects: Reduced proteinuria and activation of antifibrotic pathways.
6.3. Metabolic Surgery
7. Proposed Therapeutic Strategy for Patients with ARD
8. Conclusions
Funding
Conflicts of Interest
References
- GBD 2021 Risk Factors Collaborators. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Palmer, M.K.; Toth, P.P. Trends in Lipids, Obesity, Metabolic Syndrome, and Diabetes Mellitus in the United States: An NHANES Analysis (2003–2004 to 2013–2014). Obesity 2019, 27, 309–314. [Google Scholar] [CrossRef]
- Caspard, H.; Jabbour, S.; Hammar, N.; Fenici, P.; Sheehan, J.J.; Kosiborod, M. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: An analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes. Metab. 2018, 20, 667–671. [Google Scholar] [CrossRef]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef]
- National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 19 December 2024).
- Mata-Cases, M.; Franch-Nadal, J.; Real, J.; Cedenilla, M.; Mauricio, D. Prevalence and coprevalence of chronic comorbid conditions in patients with type 2 diabetes in Catalonia: A population-based cross-sectional study. BMJ Open 2019, 9, e031281. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Gil, M.J.; Valentí, V.; Rotellar, F.; Ramírez, B.; Salvador, J.; et al. Body adiposity and type 2 diabetes: Increased risk with a high body fat percentage even having a normal BMI. Obesity 2011, 19, 1439–1444. [Google Scholar] [CrossRef]
- Taylor, R.; Holman, R.R. Normal weight individuals who develop type 2 diabetes: The personal fat threshold. Clin. Sci. 2015, 128, 405–410. [Google Scholar] [CrossRef]
- Stokes, A.; Preston, S.H. The contribution of rising adiposity to the increasing prevalence of diabetes in the United States. Prev. Med. 2017, 101, 91–95. [Google Scholar] [CrossRef]
- Tobias, D.K.; Merino, J.; Ahmad, A.; Aiken, C.; Benham, J.L.; Bodhini, D.; Clark, A.L.; Colclough, K.; Corcoy, R.; Cromer, S.J.; et al. Second international consensus report on gaps and opportunities for the clinical translation of precision diabetes medicine. Nat. Med. 2023, 29, 2438–2457. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S27–S49. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Understanding the cause of type 2 diabetes. Lancet Diabetes Endocrinol. 2024, 12, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Gorgojo Martínez, J.J. Glucocentrismo o adipocentrismo: Una visión crítica de los consensos y guías clínicas para el tratamiento de la diabetes mellitus tipo 2 [Glucocentricity or adipocentricity: A critical view of consensus and clinical guidelines for the treatment of type 2 diabetes mellitus]. Endocrinol. Nutr. 2011, 58, 541–549. [Google Scholar]
- Kahn, S.E.; Haffner, S.M.; Heise, M.A.; Herman, W.H.; Holman, R.R.; Jones, N.P.; Kravitz, B.G.; Lachin, J.M.; O’Neill, M.C.; Zinman, B.; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006, 355, 2427–2443. [Google Scholar] [CrossRef]
- Nissen, S.E.; Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007, 356, 2457–2471. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C., Jr.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar]
- Ray, K.K.; Seshasai, S.R.; Wijesuriya, S.; Sivakumaran, R.; Nethercott, S.; Preiss, D.; Erqou, S.; Sattar, N. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials. Lancet 2009, 373, 1765–1772. [Google Scholar] [CrossRef]
- Samson, S.L.; Vellanki, P.; Blonde, L.; Christofides, E.A.; Galindo, R.J.; Hirsch, I.B.; Isaacs, S.D.; Izuora, K.E.; Low Wang, C.C.; Twining, C.L.; et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm—2023 Update. Endocr. Pract. 2023, 29, 305–340. [Google Scholar] [CrossRef]
- UKPDS Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of metformin on cardiovascular disease: A meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S167–S180. [Google Scholar] [CrossRef] [PubMed]
- Castro Conde, A.; Marzal Martín, D.; Campuzano Ruiz, R.; Fernández Olmo, M.R.; Morillas Ariño, C.; Gómez Doblas, J.J.; Gorriz Teruel, J.L.; Mazón Ramos, P.; García-Moll Marimon, X.; Soler Romeo, M.J.; et al. Comprehensive Cardiovascular and Renal Protection in Patients with Type 2 Diabetes. J. Clin. Med. 2023, 12, 3925. [Google Scholar] [CrossRef] [PubMed]
- Gorgojo Martínez, J.J. Relevance of weight in the management of patients with type 2 diabetes mellitus: Towards an adipocentric approach to diabetes. Med. Clin. 2016, 147, 8–16. [Google Scholar] [CrossRef]
- Muzurović, E.; Dragnić, S.; Medenica, S.; Smolović, B.; Bulajić, P.; Mikhailidis, D.P. Weight-centric pharmacological management of type 2 diabetes mellitus—An essential component of cardiovascular disease prevention. J. Diabetes Complicat. 2020, 34, 107619. [Google Scholar] [CrossRef]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Koufakis, T.; Liberopoulos, E.N.; Kokkinos, A.; Zebekakis, P.; Kotsa, K. Weight Loss Versus Glycemic Control as the Primary Treatment Target in Newly Diagnosed Type 2 Diabetes: Why Choose When You Can Have Both? Drugs 2023, 83, 469–477. [Google Scholar] [CrossRef]
- Arsenault, B.J.; Carpentier, A.C.; Poirier, P.; Després, J.P. Adiposity, type 2 diabetes and atherosclerotic cardiovascular disease risk: Use and abuse of the body mass index. Atherosclerosis 2024, 394, 117546. [Google Scholar] [CrossRef]
- Artasensi, A.; Mazzolari, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Obesity and Type 2 Diabetes: Adiposopathy as a Triggering Factor and Therapeutic Options. Molecules 2023, 28, 3094. [Google Scholar] [CrossRef]
- Michaelidou, M.; Pappachan, J.M.; Jeeyavudeen, M.S. Management of diabesity: Current concepts. World J. Diabetes 2023, 14, 396–411. [Google Scholar] [CrossRef]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Castro Conde, A.; Gorgojo Martínez, J.J.; Górriz Teruel, J.L.; Manito Lorite, N.; Cobo Marcos, M.; Freixa-Pamias, R.; Obaya Rebollar, J.C.; Álvarez Hermida, A.B.; Campuzano Ruiz, R.; Fernández Olmo, R.; et al. Obesidad y enfermedad cardiovascular y renal. Posicionamiento de las Asociaciones de Cardiología Preventiva, Cardiología Clínica e Insuficiencia Cardiaca de la SEC. REC CardioClinics 2024, 59, 212–224. [Google Scholar] [CrossRef]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Yaghootkar, H.; Scott, R.A.; White, C.C.; Zhang, W.; Speliotes, E.; Munroe, P.B.; Ehret, G.B.; Bis, J.C.; Fox, C.S.; Walker, M.; et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes 2014, 63, 4369–4377. [Google Scholar] [CrossRef]
- Gadde, K.M.; Martin, C.K.; Berthoud, H.R.; Heymsfield, S.B. Obesity: Pathophysiology and Management. J. Am. Coll. Cardiol. 2018, 71, 69–84. [Google Scholar] [CrossRef]
- Gustafson, B.; Smith, U. Regulation of white adipogenesis and its relation to ectopic fat accumulation and cardiovascular risk. Atherosclerosis 2015, 241, 27–35. [Google Scholar] [CrossRef]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Lotta, L.A.; Gulati, P.; Day, F.R.; Payne, F.; Ongen, H.; van de Bunt, M.; Gaulton, K.J.; Eicher, J.D.; Sharp, S.J.; Luan, J.; et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat. Genet. 2017, 49, 17–26. [Google Scholar] [CrossRef]
- Preda, A.; Carbone, F.; Tirandi, A.; Montecucco, F.; Liberale, L. Obesity phenotypes and cardiovascular risk: From pathophysiology to clinical management. Rev. Endocr. Metab. Disord. 2023, 24, 901–919. [Google Scholar] [CrossRef]
- Yamazaki, H.; Tauchi, S.; Machann, J.; Haueise, T.; Yamamoto, Y.; Dohke, M.; Hanawa, N.; Kodama, Y.; Katanuma, A.; Stefan, N.; et al. Fat Distribution Patterns and Future Type 2 Diabetes. Diabetes 2022, 71, 1937–1945. [Google Scholar] [CrossRef]
- Linge, J.; Whitcher, B.; Borga, M.; Dahlqvist Leinhard, O. Sub-phenotyping Metabolic Disorders Using Body Composition: An Individualized, Nonparametric Approach Utilizing Large Data Sets. Obesity 2019, 27, 1190–1199. [Google Scholar] [CrossRef] [PubMed]
- Caleyachetty, R.; Thomas, G.N.; Toulis, K.A.; Mohammed, N.; Gokhale, K.M.; Balachandran, K.; Nirantharakumar, K. Metabolically Healthy Obese and Incident Cardiovascular Disease Events Among 3.5 Million Men and Women. J. Am. Coll. Cardiol. 2017, 70, 1429–1437. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Wadden, T.A. Mechanisms, Pathophysiology, and Management of Obesity. N. Engl. J. Med. 2017, 376, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.S.; Epstein, S.; Corkey, B.E.; Grant, S.F.; Gavin JR 3rd Aguilar, R.B. The Time Is Right for a New Classification System for Diabetes: Rationale and Implications of the β-Cell-Centric Classification Schema. Diabetes Care 2016, 39, 179–186. [Google Scholar] [CrossRef]
- Ahlqvist, E.; Prasad, R.B.; Groop, L. Subtypes of Type 2 Diabetes Determined From Clinical Parameters. Diabetes 2020, 69, 2086–2093. [Google Scholar] [CrossRef]
- Herder, C.; Roden, M. A novel diabetes typology: Towards precision diabetology from pathogenesis to treatment. Diabetologia 2022, 65, 1770–1781. [Google Scholar] [CrossRef]
- Smith, R.J.; Nathan, D.M.; Arslanian, S.A.; Groop, L.; Rizza, R.A.; Rotter, J.I. Individualizing therapies in type 2 diabetes mellitus based on patient characteristics: What we know and what we need to know. J. Clin. Endocrinol. Metab. 2010, 95, 1566–1574. [Google Scholar] [CrossRef]
- Hattersley, A.T.; Patel, K.A. Precision diabetes: Learning from monogenic diabetes. Diabetologia 2017, 60, 769–777. [Google Scholar] [CrossRef]
- Udler, M.S.; Kim, J.; von Grotthuss, M.; Bonàs-Guarch, S.; Cole, J.B.; Chiou, J.; Christopher, D.; Anderson on behalf of METASTROKE and the ISGC; Boehnke, M.; Laakso, M.; et al. Type 2 diabetes genetic loci informed by multi-trait associations point to disease mechanisms and subtypes: A soft clustering analysis. PLoS Med. 2018, 15, e1002654. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A.J.; Ahlqvist, E.; Udler, M.S. Phenotypic and genetic classification of diabetes. Diabetologia 2022, 65, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R. Type 2 diabetes and remission: Practical management guided by pathophysiology. J. Intern. Med. 2021, 289, 754–770. [Google Scholar] [CrossRef] [PubMed]
- García Almeida, J.M.; García García, C.; Vegas Aguilar, I.M.; Bellido Castañeda, V.; Bellido Guerrero, D. Morphofunctional assessment of patient’s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar]
- Bellido, D.; García-García, C.; Talluri, A.; Lukaski, H.C.; García-Almeida, J.M. Future lines of research on phase angle: Strengths and limitations. Rev. Endocr. Metab. Disord. 2023, 24, 563–583. [Google Scholar] [CrossRef]
- Salmón-Gómez, L.; Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Relevance of body composition in phenotyping the obesities. Rev. Endocr. Metab. Disord. 2023, 24, 809–823. [Google Scholar] [CrossRef]
- Jochem, C.; Leitzmann, M.; Volaklis, K.; Aune, D.; Strasser, B. Association Between Muscular Strength and Mortality in Clinical Populations: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2019, 20, 1213–1223. [Google Scholar] [CrossRef]
- Nakhleh, A.; Halfin, E.; Shehadeh, N. Remission of type 2 diabetes mellitus. World J. Diabetes 2024, 15, 1384–1389. [Google Scholar] [CrossRef]
- Riddle, M.C.; Cefalu, W.T.; Evans, P.H.; Gerstein, H.C.; Nauck, M.A.; Oh, W.K.; Rothberg, A.E.; le Roux, C.W.; Rubino, F.; Schauer, P.; et al. Consensus Report: Definition and Interpretation of Remission in Type 2 Diabetes. Diabetes Care 2021, 44, 2438–2444. [Google Scholar] [CrossRef]
- Kalra, S.; Singal, A.; Lathia, T. What’s in a Name? Redefining Type 2 Diabetes Remission. Diabetes Ther. 2021, 12, 647–654. [Google Scholar] [CrossRef]
- Gregg, E.W.; Chen, H.; Bancks, M.P.; Manalac, R.; Maruthur, N.; Munshi, M.; Wing, R.; Look AHEAD Research Group. Impact of remission from type 2 diabetes on long-term health outcomes: Findings from the Look AHEAD study. Diabetologia 2024, 67, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019, 7, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Al-Mrabeh, A.; Zhyzhneuskaya, S.V.; Peters, C.; Barnes, A.C.; Melhem, S.; Jesuthasan, A.; Aribisala, B.; Hollingsworth, K.G.; Lietz, G.; Mathers, J.C.; et al. Hepatic Lipoprotein Export and Remission of Human Type 2 Diabetes after Weight Loss. Cell Metab. 2020, 31, 233–249.e4. [Google Scholar] [CrossRef]
- Taheri, S.; Zaghloul, H.; Chagoury, O.; Elhadad, S.; Ahmed, S.H.; El Khatib, N.; Amona, R.A.; El Nahas, K.; Suleiman, N.; Alnaama, A.; et al. Effect of intensive lifestyle intervention on bodyweight and glycaemia in early type 2 diabetes (DIADEM-I): An open-label, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 477–489. [Google Scholar] [CrossRef]
- Sattar, N.; Welsh, P.; Leslie, W.S.; Thom, G.; McCombie, L.; Brosnahan, N.; Richardson, J.; Gill, J.M.R.; Crawford, L.; Lean, M.E.J. Dietary weight-management for type 2 diabetes remissions in South Asians: The South Asian diabetes remission randomised trial for proof-of-concept and feasibility (STANDby). Lancet Reg. Health Southeast. Asia 2023, 9, 100111. [Google Scholar] [CrossRef]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Kelly, T.; Irvine, K.; Peters, C.; Zhyzhneuskaya, S.; et al. 5-year follow-up of the randomised Diabetes Remission Clinical Trial (DiRECT) of continued support for weight loss maintenance in the UK: An extension study. Lancet Diabetes Endocrinol. 2024, 12, 233–246. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lingvay, I.; Catarig, A.M.; Frias, J.P.; Kumar, H.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Viljoen, A.; McCrimmon, R.J. Efficacy and safety of once-weekly semaglutide versus daily canagliflozin as add-on to metformin in patients with type 2 diabetes (SUSTAIN 8): A double-blind, phase 3b, randomised controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 834–844. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Catarig, A.M.; Frias, J.P.; Lausvig, N.L.; le Roux, C.W.; Thielke, D.; Lingvay, I. Effects of once-weekly semaglutide vs once-daily canagliflozin on body composition in type 2 diabetes: A substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia 2020, 63, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Cusi, K.; Fernández Landó, L.; Bray, R.; Brouwers, B.; Rodríguez, Á. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): A substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 2022, 10, 393–406. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Vázquez, L.; Del Prato, S.; Franco, D.R.; Weerakkody, G.; Dai, B.; Landó, L.F.; Bergman, B.K.; Rodríguez, A. Achieving Normoglycemia With Tirzepatide: Analysis of SURPASS 1-4 Trials. Diabetes Care 2023, 46, 1986–1992. [Google Scholar] [CrossRef]
- Rubino, F.; Nathan, D.M.; Eckel, R.H.; Schauer, P.R.; Alberti, K.G.; Zimmet, P.Z.; Del Prato, S.; Ji, L.; Sadikot, S.M.; Herman, W.H.; et al. Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations. Diabetes Care 2016, 39, 861–877. [Google Scholar] [CrossRef]
- Hanipah, Z.N.; Rubino, F.; Schauer, P.R. Remission with an Intervention: Is Metabolic Surgery the Ultimate Solution? Endocrinol. Metab. Clin. N. Am. 2023, 52, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Chumakova-Orin, M.; Vanetta, C.; Moris, D.P.; Guerron, A.D. Diabetes remission after bariatric surgery. World J. Diabetes. 2021, 12, 1093–1101. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, H.S. Not Control but Conquest: Strategies for the Remission of Type 2 Diabetes Mellitus. Diabetes Metab. J. 2022, 46, 165–180. [Google Scholar] [CrossRef]
- De Luca, M.; Zese, M.; Bandini, G.; Chiappetta, S.; Iossa, A.; Merola, G.; Piatto, G.; Tolone, S.; Vitiello, A.; Silverii, G.A.; et al. Metabolic bariatric surgery as a therapeutic option for patients with type 2 diabetes: A meta-analysis and network meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2023, 25, 2362–2373. [Google Scholar] [CrossRef]
- Yang, Y.; Miao, C.; Wang, Y.; He, J. The long-term effect of bariatric/metabolic surgery versus pharmacologic therapy in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2024, 40, e3830. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Lei, X.; Chen, G.; Wang, Z.; Song, H.; Feng, X.; Wu, Y.; Jia, V.; Hu, J.; Tian, Y. Update on comparison of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass: A systematic review and meta-analysis of weight loss, comorbidities, and quality of life at 5 years. BMC Surg. 2024, 24, 219. [Google Scholar] [CrossRef] [PubMed]
- Elsaigh, M.; Awan, B.; Shabana, A.; Sohail, A.; Asqalan, A.; Saleh, O.; Szul, J.; Khalil, R.; Elgohary, H.; Marzouk, M.; et al. Comparing Safety and Efficacy Outcomes of Gastric Bypass and Sleeve Gastrectomy in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e52796. [Google Scholar] [CrossRef]
- Mingrone, G.; Panunzi, S.; De Gaetano, A.; Guidone, C.; Iaconelli, A.; Capristo, E.; Chamseddine, G.; Bornstein, S.R.; Rubino, F. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2021, 397, 293–304. [Google Scholar] [CrossRef]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cummings, D.E. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care 2016, 39, 893–901. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Axelrod, C.; Belmont, K.P.; Kirwan, J.P. Physiology Reconfigured: How Does Bariatric Surgery Lead to Diabetes Remission? Endocrinol. Metab. Clin. N. Am. 2023, 52, 49–64. [Google Scholar] [CrossRef]
- Look AHEAD Research Group; Gregg, E.W.; Jakicic, J.M.; Blackburn, G.; Bloomquist, P.; Bray, G.A.; Clark, J.M.; Coday, M.; Curtis, J.M.; Egan, C.; et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S86–S127. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, C.; Liu, Q.; Luo, Y.; Yang, Z.; Lin, Y.; Sun, L.; Fan, G. A network meta-analysis of the comparative efficacy of different dietary approaches on glycaemic control and weight loss in patients with type 2 diabetes mellitus and overweight or obesity. Food Funct. 2024, 15, 11961–11974. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Corella, D.; Fitó, M.; Ros, E.; PREDIMED INVESTIGATORS. Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a Lifestyle Intervention Program With Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: A systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Salah, H.M.; Al’Aref, S.J.; Khan, M.S.; Al-Hawwas, M.; Vallurupalli, S.; Mehta, J.L.; Mounsey, J.P.; Greene, S.J.; McGuire, D.K.; Lopes, R.D.; et al. Effects of sodium-glucose cotransporter 1 and 2 inhibitors on cardiovascular and kidney outcomes in type 2 diabetes: A meta-analysis update. Am. Heart J. 2021, 233, 86–91. [Google Scholar] [CrossRef]
- Giugliano, D.; Longo, M.; Caruso, P.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Sodium-glucose co-transporter-2 inhibitors for the prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis. Diabetes Obes. Metab. 2021, 23, 1672–1676. [Google Scholar] [CrossRef]
- Badve, S.V.; Bilal, A.; Lee, M.M.Y.; Sattar, N.; Gerstein, H.C.; Ruff, C.T.; McMurray, J.J.V.; Rossing, P.; Bakris, G.; Mahaffey, K.W.; et al. Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2024, 13, 15–28. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Zile, M.R.; Kramer, C.M.; Baum, S.J.; Litwin, S.E.; Menon, V.; Ge, J.; Weerakkody, G.J.; Ou, Y.; Bunck, M.C.; et al. Tirzepatide for Heart Failure with Preserved Ejection Fraction and Obesity. N. Engl. J. Med. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Novo-Nordisk. Oral Semaglutide Demonstrates a 14% Reduction in Risk of Major Adverse Cardiovascular Events in Adults with Type 2 Diabetes in the SOUL Trial. Company Announcement No 76/2024. Available online: https://www.novonordisk.com/news-and-media/news-and-ir-materials/news-details.html?id=171480 (accessed on 23 December 2024).
- Gorgojo-Martínez, J.J. Nuevos fármacos para la reducción del riesgo cardiovascular en pacientes con diabetes mellitus tipo 2 [New glucose-lowering drugs for reducing cardiovascular risk in patients with type2 diabetes mellitus]. Hipertens. Riesgo Vasc. 2019, 36, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Cherney, D.Z.I.; Udell, J.A.; Drucker, D.J. Cardiorenal mechanisms of action of glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors. Med 2021, 2, 1203–1230. [Google Scholar] [CrossRef]
- Tuttle, K.R.; Brosius FC 3rd Cavender, M.A.; Fioretto, P.; Fowler, K.J.; Heerspink, H.J.L.; Manley, T.; McGuire, D.K.; Molitch, M.E.; Mottl, A.K.; Perreault, L.; et al. SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Diabetes 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Drucker, D.J. Efficacy and Safety of GLP-1 Medicines for Type 2 Diabetes and Obesity. Diabetes Care 2024, 47, 1873–1888. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Glucagon-like peptide 1 receptor agonists: Cardiovascular benefits and mechanisms of action. Nat. Rev. Cardiol. 2023, 20, 463–474. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Gorgojo-Martinez, J.J.; Ferreira-Ocampo, P.J.; Galdón Sanz-Pastor, A.; Cárdenas-Salas, J.; Antón-Bravo, T.; Brito-Sanfiel, M.; Almodóvar-Ruiz, F. Effectiveness and Tolerability of the Intensification of Canagliflozin Dose from 100 mg to 300 mg Daily in Patients with Type 2 Diabetes in Real Life: The INTENSIFY Study. J. Clin. Med. 2023, 12, 4248. [Google Scholar] [CrossRef]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Samms, R.J.; Coghlan, M.P.; Sloop, K.W. How May GIP Enhance the Therapeutic Efficacy of GLP-1? Trends Endocrinol. Metab. 2020, 31, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.K. Mechanisms of action and therapeutic applications of GLP-1 and dual GIP/GLP-1 receptor agonists. Front. Endocrinol. 2024, 15, 1431292. [Google Scholar] [CrossRef]
- Gasbjerg, L.S.; Rosenkilde, M.M.; Meier, J.J.; Holst, J.J.; Knop, F.K. The importance of glucose-dependent insulinotropic polypeptide receptor activation for the effects of tirzepatide. Diabetes Obes. Metab. 2023, 25, 3079–3092. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- Frías, J.P.; Davies, M.J.; Rosenstock, J.; Pérez Manghi, F.C.; Fernández Landó, L.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K.; SURPASS-2 Investigators. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Mirna, A.E.A.; Quast, D.R. Meta-analysis of head-to-head clinical trials comparing incretin-based glucose-lowering medications and basal insulin: An update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide. Diabetes Obes. Metab. 2023, 25, 1361–1371. [Google Scholar]
- American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S321–S334. [Google Scholar] [CrossRef]
- Miras, A.D.; Pérez-Pevida, B.; Aldhwayan, M.; Kamocka, A.; McGlone, E.R.; Al-Najim, W.; Chahal, H.; Batterham, R.L.; McGowan, B.; Khan, O.; et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 549–559. [Google Scholar] [CrossRef]
- Mok, J.; Adeleke, M.O.; Brown, A.; Magee, C.G.; Firman, C.; Makahamadze, C.; Jassil, F.C.; Marvasti, P.; Carnemolla, A.; Devalia, K.; et al. Safety and Efficacy of Liraglutide, 3.0 mg, Once Daily vs. Placebo in Patients With Poor Weight Loss Following Metabolic Surgery: The BARI-OPTIMISE Randomized Clinical Trial. JAMA Surg. 2023, 158, 1003–1011. [Google Scholar] [CrossRef]
- Svanevik, M.; Lorentzen, J.; Borgeraas, H.; Sandbu, R.; Seip, B.; Medhus, A.W.; Hertel, J.K.; Kolotkin, R.L.; Småstuen, M.C.; Hofsø, D.; et al. Patient-reported outcomes, weight loss, and remission of type 2 diabetes 3 years after gastric bypass and sleeve gastrectomy (Oseberg); a single-centre, randomised controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 555–566. [Google Scholar] [CrossRef]
- Murphy, R.; Plank, L.D.; Clarke, M.G.; Evennett, N.J.; Tan, J.; Kim, D.D.W.; Cutfield, R.; Booth, M.W.C. Effect of Banded Roux-en-Y Gastric Bypass Versus Sleeve Gastrectomy on Diabetes Remission at 5 Years Among Patients With Obesity and Type 2 Diabetes: A Blinded Randomized Clinical Trial. Diabetes Care 2022, 45, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Pullman, J.S.; Plank, L.D.; Nisbet, S.; Murphy, R.; Booth, M.W.C. Seven-Year Results of a Randomized Trial Comparing Banded Roux-en-Y Gastric Bypass to Sleeve Gastrectomy for Type 2 Diabetes and Weight Loss. Obes. Surg. 2023, 33, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Wilding, J.P.H.; Gu, X.S. Combining GLP-1 receptor agonists and SGLT-2 inhibitors for cardiovascular disease prevention in type 2 diabetes: A systematic review with multiple network meta-regressions. World J. Diabetes. 2024, 15, 2135–2146. [Google Scholar] [CrossRef]
- Neuen, B.L.; Heerspink, H.J.L.; Vart, P.; Claggett, B.L.; Fletcher, R.A.; Arnott, C.; de Oliveira Costa, J.; Falster, M.O.; Pearson, S.A.; Mahaffey, K.W.; et al. Estimated Lifetime Cardiovascular, Kidney, and Mortality Benefits of Combination Treatment With SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Nonsteroidal MRA Compared With Conventional Care in Patients With Type 2 Diabetes and Albuminuria. Circulation 2024, 149, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Fletcher, R.A.; Heath, L.; Perkovic, A.; Vaduganathan, M.; Badve, S.V.; Tuttle, K.R.; Pratley, R.; Gerstein, H.C.; Perkovic, V.; et al. Cardiovascular, Kidney, and Safety Outcomes With GLP-1 Receptor Agonists Alone and in Combination With SGLT2 Inhibitors in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Circulation 2024, 150, 1781–1790. [Google Scholar] [CrossRef]
- Gorgojo-Martínez, J.J.; Gargallo-Fernández, M.A.; Galdón Sanz-Pastor, A.; Antón-Bravo, T.; Brito-Sanfiel, M.; Wong-Cruz, J. Real-World Clinical Outcomes Associated with Canagliflozin in Patients with Type 2 Diabetes Mellitus in Spain: The Real-Wecan Study. J. Clin. Med. 2020, 9, 2275. [Google Scholar] [CrossRef]
- Rosenstock, J.; Frías, J.P.; Rodbard, H.W.; Tofé, S.; Sears, E.; Huh, R.; Fernández Landó, L.; Patel, H. Tirzepatide vs. Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS-6 Randomized Clinical Trial. JAMA 2023, 330, 1631–1640. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2020, 44, 258–279. [Google Scholar] [CrossRef]
- Frias, J.P.; Hsia, S.; Eyde, S.; Liu, R.; Ma, X.; Konig, M.; Kazda, C.; Mather, K.J.; Haupt, A.; Pratt, E.; et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: A multicentre, randomised, dose-response, phase 2 study. Lancet 2023, 402, 472–483. [Google Scholar] [CrossRef]
- Aroda, V.R.; Aberle, J.; Bardtrum, L.; Christiansen, E.; Knop, F.K.; Gabery, S.; Pedersen, S.D.; Buse, J.B. Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (PIONEER PLUS): A multicentre, randomised, phase 3b trial. Lancet 2023, 402, 693–704. [Google Scholar] [CrossRef]
- Blüher, M.; Rosenstock, J.; Hoefler, J.; Manuel, R.; Hennige, A.M. Dose-response effects on HbA1c and bodyweight reduction of survodutide, a dual glucagon/GLP-1 receptor agonist, compared with placebo and open-label semaglutide in people with type 2 diabetes: A randomised clinical trial. Diabetologia 2024, 67, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Frias, J.; Jastreboff, A.M.; Du, Y.; Lou, J.; Gurbuz, S.; Thomas, M.K.; Hartman, M.L.; Haupt, A.; Milicevic, Z.; et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: A randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet 2023, 402, 529–544. [Google Scholar] [CrossRef]
- Frias, J.P.; Deenadayalan, S.; Erichsen, L.; Knop, F.K.; Lingvay, I.; Macura, S.; Mathieu, C.; Pedersen, S.D.; Davies, M. Efficacy and safety of co-administered once-weekly cagrilintide 2·4 mg with once-weekly semaglutide 2·4 mg in type 2 diabetes: A multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 2023, 402, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Hejjaji, V.; Gorgojo-Martinez, J.J.; Tang, F.; Garnelo, J.B.; Cooper, A.; Medina, J.; Mutiozabal, M.S.; Khunti, K.; Nicolucci, A.; Shestakova, M.V.; et al. Factors associated with weight loss in people with overweight or obesity living with type 2 diabetes mellitus: Insights from the global DISCOVER study. Diabetes Obes. Metab. 2022, 24, 1734–1740. [Google Scholar] [CrossRef] [PubMed]
| MUNW | MHO | MUO | SO | |
|---|---|---|---|---|
| BMI (kg/m2) | Normal | High | High/ Very high | High/ normal |
| Waist circumference | Normal/ High | Normal | High | High |
| Metabolic syndrome | Present | Absent | Present | Present |
| Visceral fat | High | Low | High | High |
| Lean mass | Normal | High | Normal/ High | Low |
| Physical performance | Low | High | Low/ Very low | Very low |
|
| Anthropometry |
| a. BMI |
| b. Waist circumference * |
| c. Waist-to-hip ratio |
| d. Waist-to-height ratio |
| Body composition |
| a. Bioelectrical impedance: vector analysis, phase angle |
| b. Nutritional ultrasound |
| c. Liver ultrasound |
| d. Liver elastography |
| e. Others: plethysmography, DEXA, CT, MRI |
| Muscle functionality |
| a. Dynamometry |
| b. Sit-to-stand test |
| c. 6-min walk test |
| Laboratory tests |
| a. Metabolic syndrome |
| -HbA1c |
| -HOMA-IR |
| -Lipid profile |
| -C-reactive protein |
| -FIB-4 score |
| -Adipokines |
| -Urinary albumin/creatinine ratio |
| b. Beta-cell function |
| -Fasting plasma glucose, HbA1c, continuous glucose monitoring |
| -Basal and/or stimulated C-peptide |
| -HOMA-B |
| -Pancreatic autoimmunity |
| -HLA genotypes at risk for type 1 diabetes |
| Differential Diagnosis with Other Types of Diabetes |
| a. Tests for monogenic diabetes |
| -Online probability calculators |
| -Genetic testing |
| b. Tests for diabetes associated with pancreatic disease |
| -Fecal elastase |
| -Tumor markers |
| -Pancreatic imaging studies |
| c. Hormonal tests for endocrinopathies (e.g., Cushing’s disease, acromegaly) |
| d. Tests for hereditary hemochromatosis |
| -Transferrin saturation index |
| -Genetic testing |
| Drugs with CV Benefit in Specific Study Populations | Clinical Trial | Primary and Secondary Endpoints with a Significant Risk Reduction | |
|---|---|---|---|
| Major CV Events and HF Hospitalization | Renal Outcome | ||
| Established CVD or multiple CV risk factors | |||
| GLP-1 receptor agonists | |||
| Liraglutide | LEADER | MACE3, mortality | Lower progression of CKD |
| Semaglutide (sc) | SUSTAIN-6 | MACE3 | Lower progression of CKD |
| Dulaglutide | REWIND | MACE3 | Lower progression of CKD |
| Semaglutide (oral) | SOUL | MACE3 | |
| SGLT2 inhibitors | |||
| Empagliflozin | EMPA-REG OUTCOME | MACE3,mortality, HF hospitalization | Lower progression of CKD |
| Canagliflozin | CANVAS | MACE3, HF hospitalization | Lower progression of CKD |
| Dapagliflozin | DECLARE-TIMI | HF hospitalization | Lower progression of CKD |
| Heart failure with reduced ejection fraction | |||
| SGLT2 inhibitors | |||
| Dapagliflozin | DAPA-HF * | HF hospitalization, mortality | |
| Empagliflozin | EMPEROR-Reduced * | HF hospitalization | Lower progression of CKD |
| Heart failure with preserved ejection fraction | |||
| SGLT2 inhibitors | |||
| Empagliflozin | EMPEROR-Preserved * | HF hospitalization | |
| Dapagliflozin | DELIVER * | HF hospitalization | |
| GLP-1 receptor agonists | |||
| Semaglutide 2.4 (sc) | STEP-HFpEF DM | HF clinical improvement | |
| Dual GLP-1/GIP receptor agonists | |||
| Tirzepatide 15 mg | SUMMIT | HF hospitalization, HF clinical improvement | |
| Chronic kidney disease with albuminuria | |||
| SGLT2 inhibitors | |||
| Canagliflozin | CREDENCE | MACE3, HF hospitalization | Lower progression of CKD |
| Dapagliflozin | DAPA-CKD * | HF hospitalization, mortality | Lower progression of CKD |
| GLP-1 receptor agonists | |||
| Semaglutide (sc) | FLOW | MACE3, mortality | Lower progression of CKD |
| Chronic kidney disease with or without albuminuria | |||
| SGLT2 inhibitors | |||
| Empagliflozin | EMPA-KIDNEY * | Lower progression of CKD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorgojo-Martínez, J.J. Adipocentric Strategy for the Treatment of Type 2 Diabetes Mellitus. J. Clin. Med. 2025, 14, 678. https://doi.org/10.3390/jcm14030678
Gorgojo-Martínez JJ. Adipocentric Strategy for the Treatment of Type 2 Diabetes Mellitus. Journal of Clinical Medicine. 2025; 14(3):678. https://doi.org/10.3390/jcm14030678
Chicago/Turabian StyleGorgojo-Martínez, Juan J. 2025. "Adipocentric Strategy for the Treatment of Type 2 Diabetes Mellitus" Journal of Clinical Medicine 14, no. 3: 678. https://doi.org/10.3390/jcm14030678
APA StyleGorgojo-Martínez, J. J. (2025). Adipocentric Strategy for the Treatment of Type 2 Diabetes Mellitus. Journal of Clinical Medicine, 14(3), 678. https://doi.org/10.3390/jcm14030678






