Clinical Data Mega-Collection of Obesity and Obesity-Related Trials: Primary Inclusion Criteria from All Studies and Highlights of Clinical Efficacy Analysis of GLP-1 Drugs
Abstract
1. Introduction
2. Method for Data Collection and Analysis
3. Results and Discussion
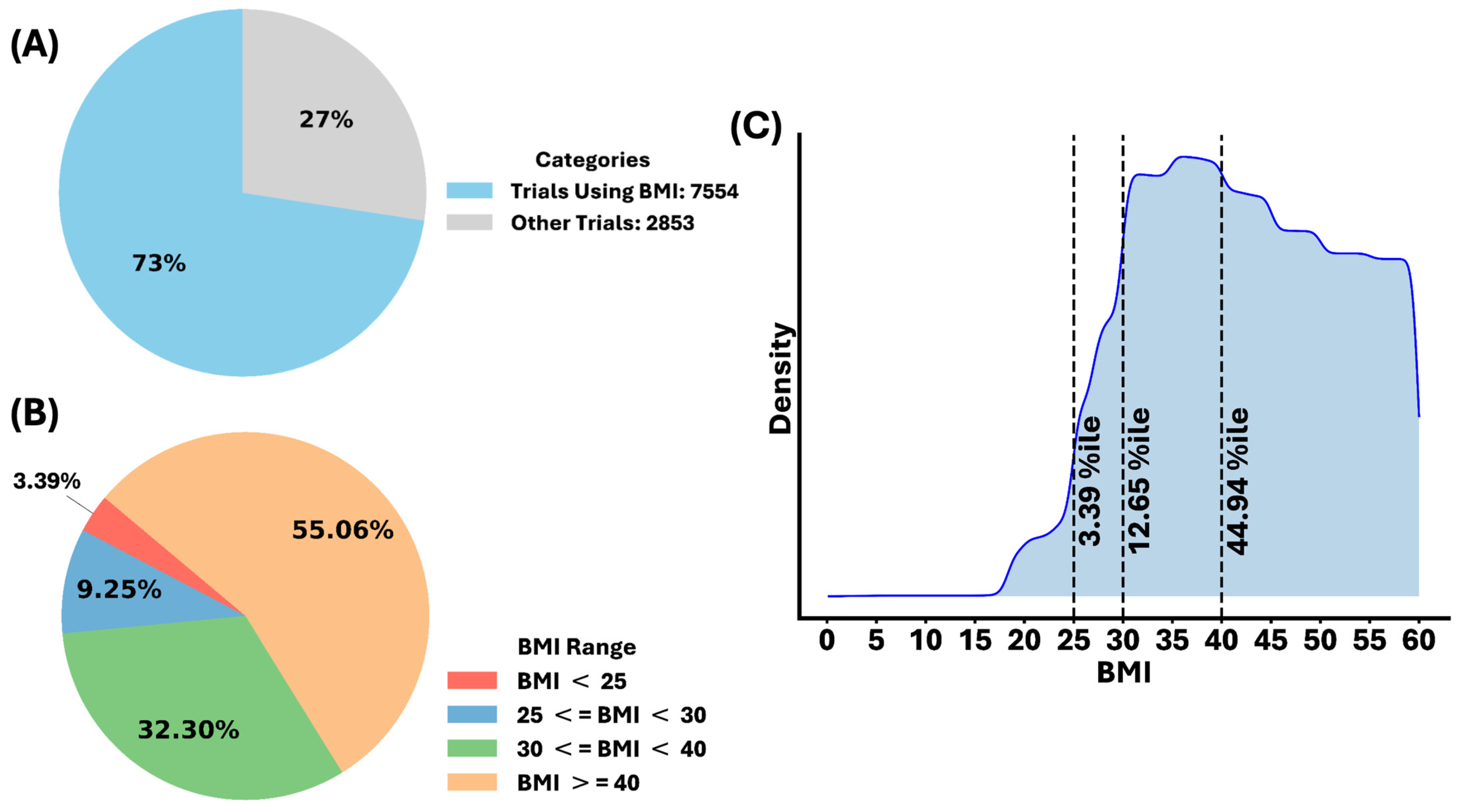
3.1. The Body Mass Index (BMI) as an Indication of Obesity
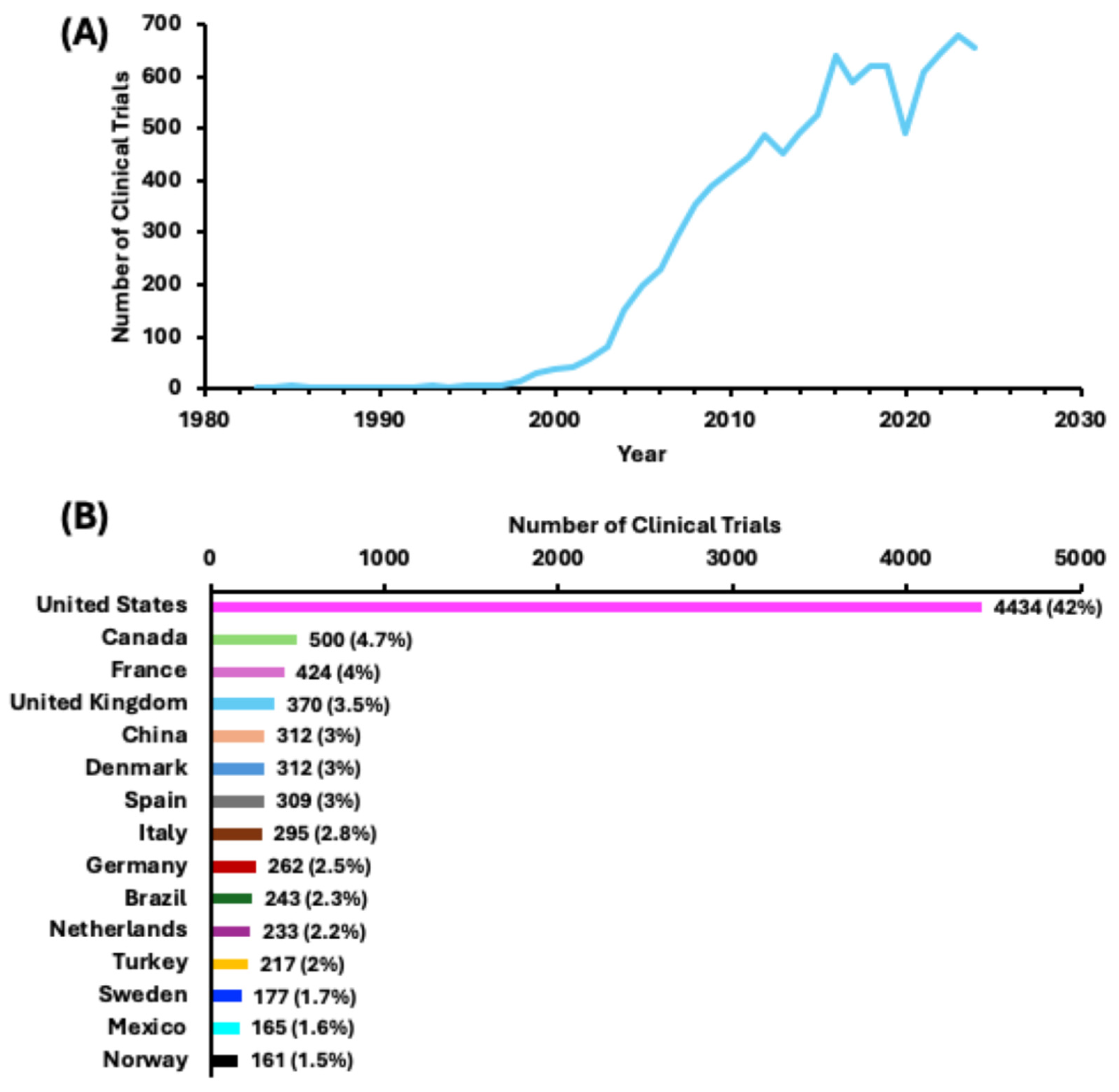
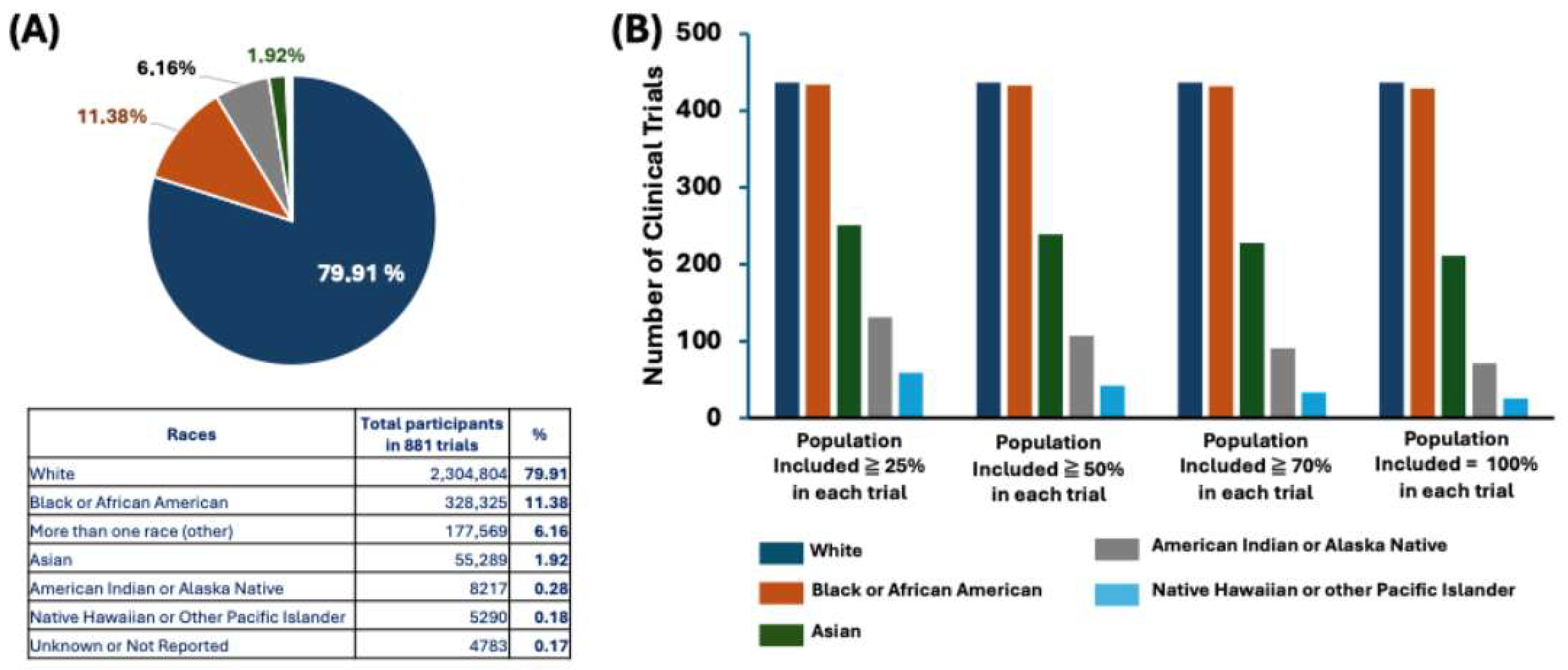
3.2. Numerous Clinical Trials for Obesity and Obesity-Related Conditions, the Disparities in Regions and Races, and the Popularity of the BMI
3.3. The Search for Medications for Weight Management
3.4. FDA-Approved Once-Weekly Injection of GLP-1 Compounds: Semaglutide and Tirzepatide
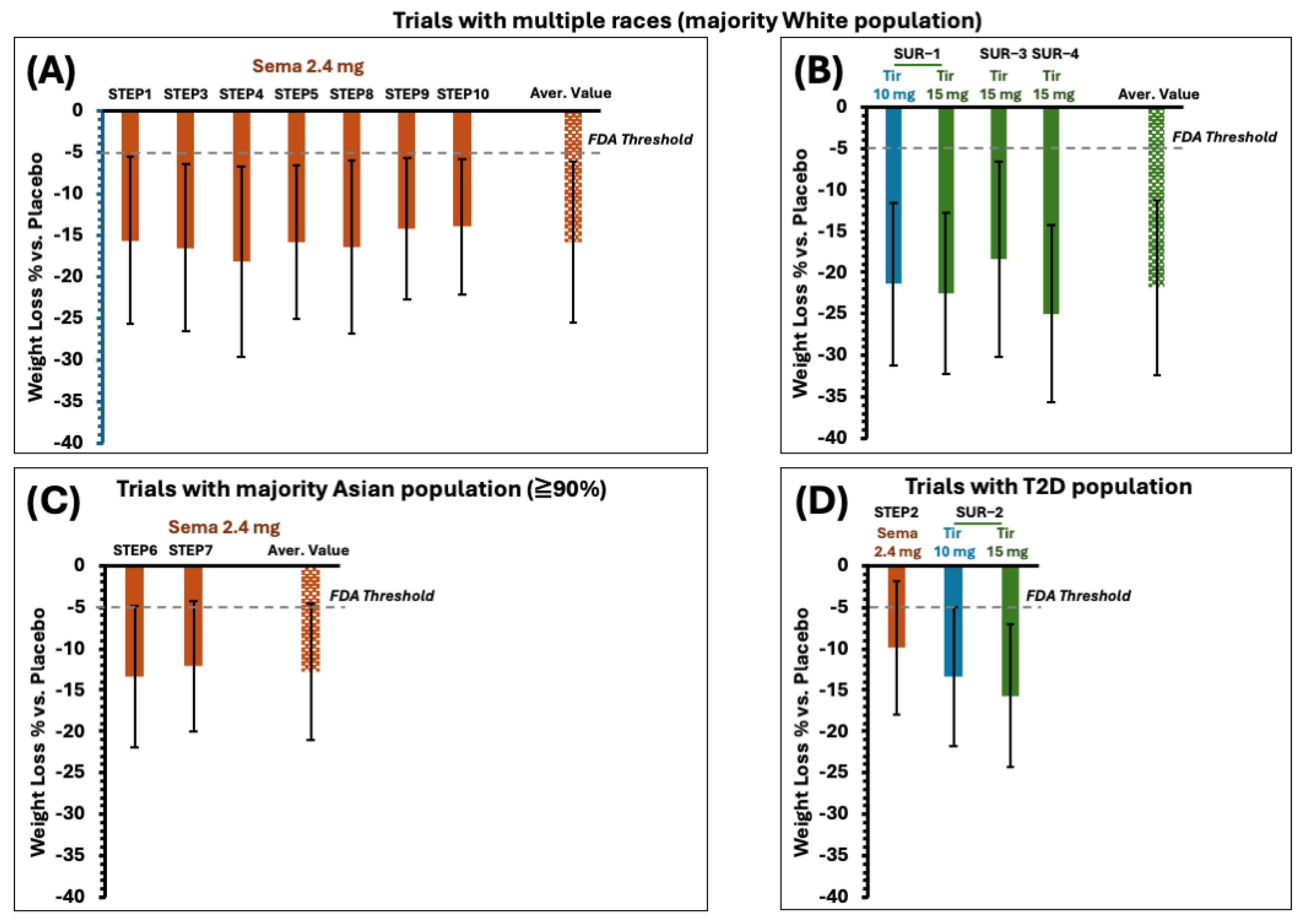
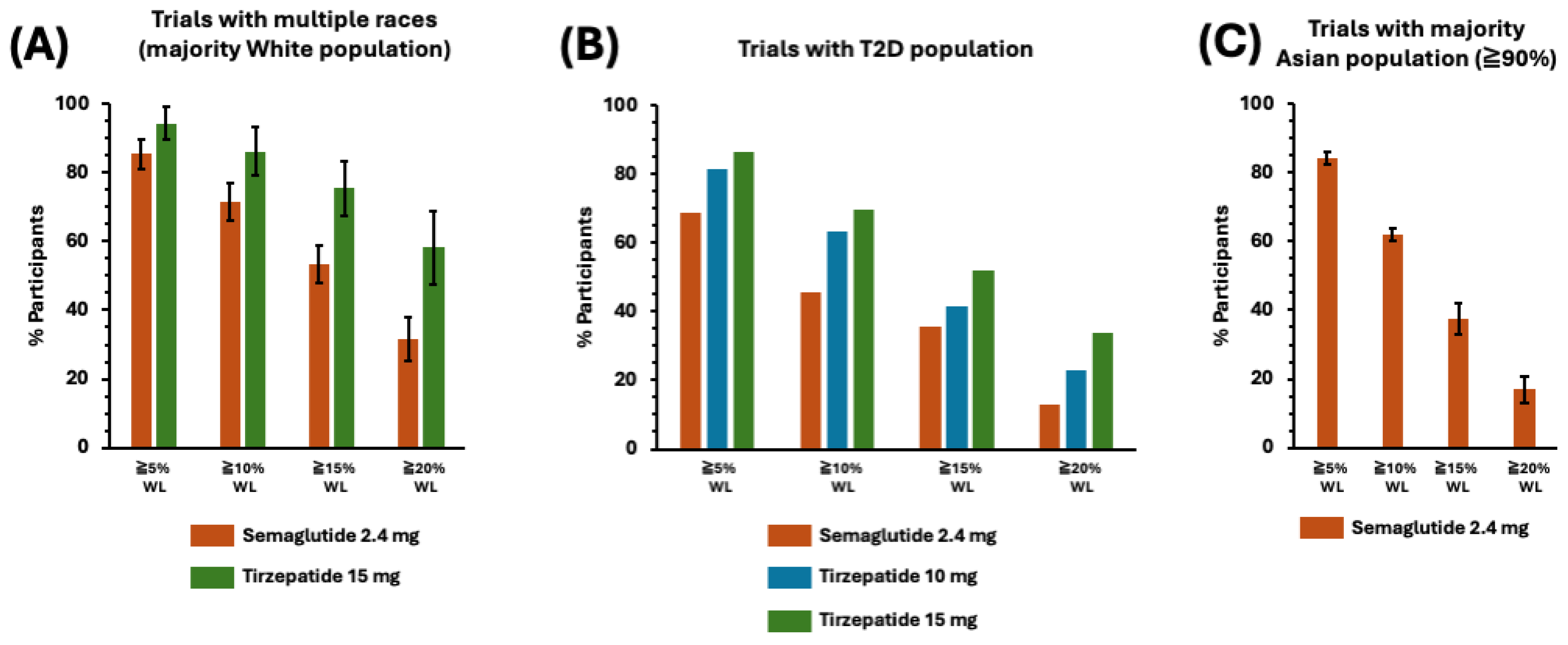
3.5. The Highlights of the Clinical Efficacy Analysis of Phase 3 Trials of Semaglutide and Tirzepatide
3.5.1. The Selection of Phase 3 Trials in This Report
3.5.2. Primary Efficacy Endpoints and Benchmarks
- There is at least 5 percent mean weight loss in the investigational therapy-treated group versus placebo-treated groups, and the difference is statistically significant.
- At least 35 percent of participants lose 5 percent or greater of their baseline body weight in the investigational therapy-treated group, which should be approximately double the proportion in the placebo-treated group, and the difference between groups is statistically significant.
3.5.3. Adverse Events
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wpt, J. WHO Recognition of the Global Obesity Epidemic. Int. J. Obes. 2008, 32, S120–S126. [Google Scholar]
- GBD 2019 Risk Factor Collaborators. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/ (accessed on 1 March 2024).
- Masood, B.; Moorthy, M. Causes of Obesity: A Review. Clin. Med. (Northfield. Il) 2023, 23, 284–291. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- Rulkiewicz, A.; Pilchowska, I.; Lisik, W.; Pruszczyk, P. Prevalence of Obesity and Severe Obesity among Professionally Active Adult Population in Poland and Its Strong Relationship with Cardiovascular Co-Morbidities-POL-O-CARIA 2016–2020 Study. J. Clin. Med. 2022, 11, 3720. [Google Scholar] [CrossRef]
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2020, 133, 155217. [Google Scholar] [CrossRef]
- GBD 2021 US besity Forcasting Collaborators. National-Level and State-Level Prevalence of Overweight and Obesity Among Children, Adolescents, and Adults in the USA, 1990–2021, and Forecasts up to 2050. Lancet 2024, 404, 2278–2298. [Google Scholar] [CrossRef]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, A.D. Years of Life Lost Due to Obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Brownell, K.D. The Heterogeneity of Obesity: Fitting Treatments To Individuals Etiologic Factors. Behav. Ther. 1991, 22, 153–177. [Google Scholar] [CrossRef]
- Hung, T.K.W.; Dong, T.S.; Chen, Z.; Elasho, D.; Sinsheimer, J.S.; Jacobs, J.P.; Lagishetty, V.; Vora, P.; Stains, J.; Mayer, E.A.; et al. Understanding the Heterogeneity of Obesity and the Relationship to the Brain-Gut Axis. Nutrients 2020, 12, 3701. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Southerland, J.; Wang, K.; Bailey, B.A.; Alamian, A.; Stevens, M.A.; Wang, Y. Ethnic Differences in Risk Factors for Obesity among Adults in California, the United States. J. Obes. 2017, 2017, 2427483. [Google Scholar] [CrossRef]
- Min, J.; Goodale, H.; Xue, H.; Brey, R.; Wang, Y. Racial-Ethnic Disparities in Obesity and Biological, Behavioral, and Sociocultural Influences in the United States: A Systematic Review. Adv. Nutr. 2021, 12, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Anekwe, C.V.; Jarrell, A.R.; Townsend, M.J.; Gabriela, I.; Hiserodt, J.M.; Stanford, F.C. Socioeconomics of Obesity. Curr. Obes. Rep. 2021, 9, 272–279. [Google Scholar] [CrossRef]
- Kostrzeba, E.; Ratajczak, J.; Horodnicka-j, A.; Raducha, D. Assessing Overweight, Obesity, and Related Risk Factors in 8–9-Year-Old Children in Szczecin, Poland. J. Clin. Med. 2024, 13, 7478. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R. Genetic Contributors to Obesity. Can. J. Cardiol. 2007, 23, 23A–27A. [Google Scholar] [CrossRef]
- Loos Ruth, J.F.; Yeo, G.S.H. The Genetics of Obesity: From Discovery to Biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Washington, T.B.; Johnson, V.R.; Kendrick, K.; Ibrahim, A.A.; Tu, L.; Sun, K.; Stanford, F.C. Disparities in Access and Quality of Obesity Care. Gastroenterol. Clin. N. Am. 2023, 52, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Mariam, A.; Miller-atkins, G.; Pantalone, K.M.; Milinovich, A.; Bauman, J.; Mocarski, M.; Ramasamy, A.; Smolarz, B.G.; Hobbs, T.M.; Zimmerman, R.S.; et al. Associations of Weight Loss with Obesity-Related Comorbidities in a Large Integrated Health System. Diabetes Obes. Metab. 2021, 23, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 8. Obesity and Weight Management for the Prevention and Treatment of Type 2 Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, 145–157. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Nakagami, T.; Oya, J.; Takahashi, K.; Isago, C.; Kurita, M.; Tanaka, Y.; Ito, A.; Kasahara, T.; Uchigata, Y. Body Weight Reduction of 5% Improved Blood Pressure and Lipid Profiles in Obese Men and Blood Glucose in Obese Women: A Four-Year Follow-up Observational Study. Metab. Syndr. Relat. Disord. 2019, 7, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.; Jebb, S.A.; Oke, J.; Nickless, A.; Ahern, A.; Boyland, E.; Caterson, I.D.; Halford, J.; Hauner, H.; Aveyard, P. Effect of Weight Loss on Cardiometabolic Risk. Br. J. Gen. Pract. 2021, 71, 312–319. [Google Scholar] [CrossRef]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the E. Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef]
- Kloock, S.; Ziegler, C.G.; Dischinger, U. Pharmacology & Therapeutics Obesity and Its Comorbidities, Current Treatment Options and Future Perspectives: Challenging Bariatric Surgery? Pharmacol. Ther. 2023, 251, 108549. [Google Scholar] [PubMed]
- Pray, R.; Riskin, S. The History and Faults of the Body Mass Index and Where to Look Next: A Literature Review. Cureus 2023, 15, e48230. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, F.Q. Body Mass Index. Nutr. Res. 2015, 50. [Google Scholar] [CrossRef] [PubMed]
- Romero-Corral, A.; Somers, V.K.; Sierra-Johnson, J.; Thomas, R.J.; Bailey, K.R.; Collazo-Clavell, M.L.; Allison, T.G.; Korinek, J.; Batsis, J.A.; Lopez-Jimenez, F. Accuracy of Body Mass Index to Diagnose Obesity In the US Adult Population. Int. J. Obes. 2010, 32, 959–966. [Google Scholar] [CrossRef]
- Global Obesity Observatory. Available online: https://data.worldobesity.org (accessed on 15 December 2024).
- Jin, X.; Qiu, T.; Li, L.; Yu, R.; Chen, X. Pathophysiology of Obesity and Its Associated Diseases. Acta Pharm. Sin. B 2023, 13, 2403–2424. [Google Scholar] [CrossRef]
- Khanna, D.; Peltzer, C.; Kahar, P.; Parmar, M.S. Body Mass Index (BMI): A Screening Tool Analysis. Cureus 2022, 14, e22119. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. Natl. Cent. Health Stat. 2020, 360, 2017–2018. [Google Scholar]
- Fitzgibbon, M.L.; Stolley, M.; Schiffer, L.; Sharp, L.; Singh, V.; Van Horn, L.; Dyer, A. Obesity Reduction Black Intervention Trial (ORBIT): Design and Baseline Characteristics. J. Women’s Health 2008, 17, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- JJih, J.; Mukherjea, A.; Vittinghoff, E.; Nguyen, T.T.; Tsoh, J.Y.; Fukuoka, Y.; Bender, M.S.; Tseng, W.; Kanaya, A.M. Using Appropriate Body Mass Index Cut Points for Overweight and Obesity among Asian Americans. Pre Med. 2014, 65, 1–6. [Google Scholar] [CrossRef]
- Baser, O.; Baser, E. Relationship between Body Mass Index and Diagnosis of Overweight or Obesity in Veterans Administration Population. Healthcare 2023, 11, 1529. [Google Scholar] [CrossRef]
- Popoviciu, M.; Lorena, P.; Yahya, G.; Metwally, K.; Cavalu, S. Emerging Role of GLP-1 Agonists in Obesity: A Comprehensive Review of Randomised Controlled Trials. Int. J. Mol. Sci. 2023, 24, 10449. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, J. FDA Green-Lights Tirzepatide, Marketed as Zepbound, for Chronic Weight Management. JAMA 2023, 330, 2143–2144. [Google Scholar] [CrossRef]
- Guidance for Industry Developing Products for Weight Management Guidance for Industry Developing Products for Weight Management; US Food and Drug Administration: Silver Spring, MD, USA, 2007.
- Hakariya, H.; Ohnishi, M.; Tanimoto, T. Japan Initiates Market Authorization of Weight-Loss Drug Semaglutide under Universal Health Coverage, but with Stringent Prescription Restrictions. Diabetes Obes. Metab. 2024, 26, 3006–3008. [Google Scholar] [CrossRef]
- Lim, Y.; Boste, J. Obesity and Comorbid Conditions; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kivimäki, M.; Strandberg, T.; Pentti, J.; Nyberg, S.T.; Frank, P.; Jokela, M.; Ervasti, J.; Suominen, S.B.; Vahtera, J. Body-Mass Index and Risk of Obesity-Related Complex Multimorbidity: An Observational Multicohort Study. Lancet 2022, 10, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Haase, C.L.; Mcewan, P. Weight Loss and Risk Reduction of Obesity-Related Outcomes in 0.5 Million People: Evidence from a UK Primary Care Database. Int. J. Obes. 2021, 45, 1249–1258. [Google Scholar] [CrossRef]
- Katsi, V.; Manta, E.; Fragoulis, C. Weight Loss Therapies and Hypertension Benefits. Biomedicines 2024, 12, 2293. [Google Scholar] [CrossRef]
- Lancet, T.; Health, R. Editorial Semaglutide and beyond: A Turning Point in Obesity Pharmacotherapy. Lancet Reg. Health Eur. 2024, 37, 100860. [Google Scholar]
- Mishra, R.; Raj, R.; Elshimy, G.; Zapata, I.; Majety, P.; Edem, D.; Correa, R. Adverse Events Related to Tirzepatide. J. Endocr. Soc. 2023, 7, bvad016. [Google Scholar] [CrossRef]
- Nunn, E.; Jaiswal, N.; Gavin, M.; Uehara, K.; Stefkovich, M.; Drareni, K.; Calhoun, R.; Lee, M.; Holman, C.D.; Baur, J.A.; et al. Antibody Blockade of Activin Type II Receptors Preserves Skeletal Muscle Mass and Enhances Fat Loss during GLP-1 Receptor Agonism. Mol. Metab. 2024, 80, 101880. [Google Scholar] [CrossRef] [PubMed]
- Hope, D.C.D.; Hinds, C.E.; Lopes, T.; Bloom, S.R.; Tan, T.M.M.; Owen, B.M.; Hope, D.C.D.; Hinds, C.E.; Lopes, T.; Vincent, M.L.; et al. Article Hypoaminoacidemia Underpins Glucagon-Mediated Energy Expenditure and Weight Loss Ll Ll Hypoaminoacidemia Underpins Glucagon-Mediated Energy Expenditure and Weight Loss. Cell Rep. Med. 2022, 3, 100810. [Google Scholar] [CrossRef]
- Martens, M.D.; Abuetabh, Y.; Schmidt, M.A.; Zolondek, M.C.P.; Silver, H.L.; Levasseur, J.L.; Ferdaoussi, M.; Dyck, J.R.B. Semaglutide Reduces Cardiomyocyte Size and Cardiac Mass in Lean and Obese Mice. JACC Basic. Transl. Sci. 2024, 9, 1429–1431. [Google Scholar] [CrossRef] [PubMed]

| General Population Classifications | |
|---|---|
| BMI (kg/m2) | Classifications |
| BMI < 16.5 | Severely underweight |
| 16.5 ≤ BMI < 18.5 | Underweight |
| 18.5 ≤ BMI < 24.9 | Normal weight |
| 25.0 ≤ BMI < 29.9 | Overweight |
| 30.0 ≤ BMI | Obesity |
| 30.0 ≤ BMI < 34.9 | Obesity class I |
| 35.0 ≤ BMI < 39.9 | Obesity class II |
| 40.0 ≤ BMI | Obesity class III |
| Asians and Asian Americans | |
| 23.0 ≤ BMI < 26.9 | Overweight |
| 27 ≤ BMI | Obesity |
| Japan and Korea ** | |
| 23.0 ≤ BMI < 24.9 | Overweight |
| 25 ≤ BMI | Obesity |
| China | |
| 24.0 ≤ BMI < 27.9 | Overweight |
| 28 ≤ BMI | Obesity |
| Drug | Number of Trials |
|---|---|
| semaglutide | 110 |
| liraglutide | 98 |
| metformin | 85 |
| tirzepatide | 44 |
| topiramate | 35 |
| orlistat | 34 |
| exenatide | 32 |
| phentermine | 30 |
| bupropion | 28 |
| sibutramine | 27 |
| Semaglutide Branded as WEGOVY® Owned by Novo Nordisk | Approved for chronic weight management in 2021 by the FDA. Indications: WEGOVY® is indicated as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in the following:
|
| Tirzepatide Branded as ZEPBOUND® Owned by Eli Lilly | Approved for chronic weight management in 2023 by the FDA. Indications: ZEPBOUND® is indicated as an adjunct to a reduced calorie diet and increased physical activity for chronic weight management in adults with an initial body mass index (BMI) of the following:
|
| Weight-Related Comorbidities | Counts in Clinical Trials |
|---|---|
| Hypertension | 41 |
| Metabolic Syndrome | 41 |
| Dyslipidemia/Dyslipidaemia (38 counts) | |
| Cardiovascular and Heart Disease | 36 |
| Cardiovascular Disease (22 counts) | |
| Cardiac Conditions (10 counts) | |
| Coronary Artery Disease (1 count) | |
| Congestive Heart Failure (1 count) | |
| Myocardial Infarction (1 count) | |
| Peripheral Arterial Disease (1 count) | |
| Sleep Apnea | 26 |
| Diabetes | 21 |
| Type 2 Diabetes Mellitus (T2D) (16 counts) | |
| Insulin Resistance (2 counts) | |
| Prediabetes (2 counts) | |
| Type 1 Diabetes (1 count) | |
| Polycystic Ovary Syndrome (PCOS) | 8 |
| Kidney Disease | 2 |
| Chronic Renal Disease (1 count) | |
| Albuminuria (1 count) | |
| Stroke | 1 |
| Non-alcoholic Fatty Liver Disease (NAFLD) | 1 |
| Trial No. | Trial Name | Title | Notes |
|---|---|---|---|
| Semaglutide: Trials | |||
| NCT03548935 | STEP 1 | Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity | Largest population: White, ~83% |
| NCT03552757 | STEP 2 | Research Study Investigating How Well Semaglutide Works in People With Type 2 Diabetes Suffering From Overweight or Obesity | Largest population: White, ~60% |
| NCT03611582 | STEP 3 | Research Study to Look at How Well Semaglutide is at Lowering Weight When Taken Together With an Intensive Lifestyle Program | Largest population: White, ~76% |
| NCT03548987 | STEP 4 | Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity | Largest population: White, ~84% |
| NCT03693430 | STEP 5 | Two-year Research Study Investigating How Well Semaglutide Works in People Suffering From Overweight or Obesity | Largest population: White, ~93% |
| NCT03811574 | STEP 6 | Research Study Investigating How Well Semaglutide Works in People Living With Overweight or Obesity | Largest population: Asian, 100% |
| NCT04251156 | STEP 7 | Research Study of How Well Semaglutide Works in People Living With Overweight or Obesity | Largest population: Asian, 90% |
| NCT04074161 | STEP 8 | Research Study to Investigate How Well Semaglutide Works Compared to Liraglutide in People Living With Overweight or Obesity | Largest population: White, 75% |
| NCT05064735 | STEP 9 | Research Study Looking at How Well Semaglutide Works in People Suffering From Obesity and Knee Osteoarthritis | Largest population: White, 62% |
| NCT05040971 | STEP 10 | Research Study Looking at How Well Semaglutide Works in People Living With Obesity and Prediabetes | Largest population: White, 90% |
| Tirzepatide: Trials | |||
| NCT04184622 | SURMONT-1 (SUR-1) | A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight | Largest population: White, ~71% |
| NCT04657003 | SURMONT-2 (SUR-2) | A Study of Tirzepatide (LY3298176) in Participants With Type 2 Diabetes Who Have Obesity or Are Overweight | Largest population: White, ~80% |
| NCT04657016 | SURMONT-3 (SUR-3) | A Study of Tirzepatide (LY3298176) In Participants After A Lifestyle Weight Loss Program | Largest population: White, ~86% |
| NCT04660643 | SURMONT-4 (SUR-4) | A Study of Tirzepatide (LY3298176) in Participants With Obesity or Overweight for the Maintenance of Weight Loss | Largest population: White, ~80% |
| Adverse Event Categories | Other Adverse Events | Serious Adverse Events | ||||||
|---|---|---|---|---|---|---|---|---|
| Semaglutide | Tirzepatide | Semaglutide | Tirzepatide | |||||
| % Reported | SD | % Reported | SD | % Reported | SD | % Reported | SD | |
| Gastrointestinal disorders | 14.70 | 12.34 | 12.90 | 9.15 | 0.19 | 0.26 | 0.09 | 0.14 |
| General disorders | 7.00 | 2.74 | 5.66 | 2.96 | 0.18 | 0.25 | 0.06 | 0.11 |
| Infections and infestations | 10.33 | 6.97 | 8.88 | 6.33 | 0.16 | 0.24 | 0.13 | 0.20 |
| Injury, poisoning, and procedural complications | 6.58 | 0.0 | - | - | 0.14 | 0.23 | 0.08 | 0.12 |
| Metabolism and nutrition disorders | 8.18 | 3.12 | 7.32 | 3.90 | 0.12 | 0.14 | 0.24 | 0.14 |
| Musculoskeletal and connective tissue disorders | 6.51 | 2.74 | 4.18 | 2.46 | 0.16 | 0.22 | 0.08 | 0.13 |
| Nervous system disorders | 8.59 | 4.57 | 5.47 | 2.02 | 0.12 | 0.17 | 0.07 | 0.13 |
| Respiratory, thoracic, and mediastinal disorders | 4.60 | 1.16 | - | - | 0.17 | 0.22 | 0.05 | 0.08 |
| Skin and subcutaneous tissue disorders | 2.38 | 0.0 | 5.27 | 1.02 | 0.08 | 0.00 | 0.00 | 0.00 |
| Vascular disorders | 5.15 | 1.70 | - | - | 0.18 | 0.24 | 0.09 | 0.13 |
| Psychiatric disorders | 3.82 | 2.04 | 3.14 | 0.0 | - | - | - | - |
| Blood and lymphatic system disorders | - | - | - | - | 0.24 | 0.35 | 0.00 | 0.00 |
| Cardiac disorders | - | - | - | - | 0.17 | 0.22 | 0.09 | 0.13 |
| Ear and labyrinth disorders | - | - | - | - | 0.14 | 0.10 | 0.05 | 0.09 |
| Endocrine disorders | - | - | - | - | 0.19 | 0.19 | 0.06 | 0.09 |
| Eye disorders | - | - | - | - | 0.13 | 0.24 | 0.06 | 0.09 |
| Hepatobiliary disorders | - | - | - | - | 0.40 | 0.44 | 0.18 | 0.20 |
| Neoplasms benign, malignant, and unspecified (incl cysts and polyps) | - | - | - | - | 0.18 | 0.26 | 0.08 | 0.15 |
| Renal and urinary disorders | - | - | - | - | 0.22 | 0.24 | 0.13 | 0.23 |
| Reproductive system and breast disorders | - | - | - | - | 0.30 | 0.33 | 0.09 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Elmaleh, D.R. Clinical Data Mega-Collection of Obesity and Obesity-Related Trials: Primary Inclusion Criteria from All Studies and Highlights of Clinical Efficacy Analysis of GLP-1 Drugs. J. Clin. Med. 2025, 14, 812. https://doi.org/10.3390/jcm14030812
Nguyen TT, Elmaleh DR. Clinical Data Mega-Collection of Obesity and Obesity-Related Trials: Primary Inclusion Criteria from All Studies and Highlights of Clinical Efficacy Analysis of GLP-1 Drugs. Journal of Clinical Medicine. 2025; 14(3):812. https://doi.org/10.3390/jcm14030812
Chicago/Turabian StyleNguyen, Trung Tin, and David R. Elmaleh. 2025. "Clinical Data Mega-Collection of Obesity and Obesity-Related Trials: Primary Inclusion Criteria from All Studies and Highlights of Clinical Efficacy Analysis of GLP-1 Drugs" Journal of Clinical Medicine 14, no. 3: 812. https://doi.org/10.3390/jcm14030812
APA StyleNguyen, T. T., & Elmaleh, D. R. (2025). Clinical Data Mega-Collection of Obesity and Obesity-Related Trials: Primary Inclusion Criteria from All Studies and Highlights of Clinical Efficacy Analysis of GLP-1 Drugs. Journal of Clinical Medicine, 14(3), 812. https://doi.org/10.3390/jcm14030812




