Recent Advances in Cardiac Resynchronization Therapy: Current Treatment and Future Direction
Abstract
:1. Introduction
2. Methods
3. Current Guidelines and Recommendations
3.1. Goals for CRT
3.2. Measured Outcomes for CRT
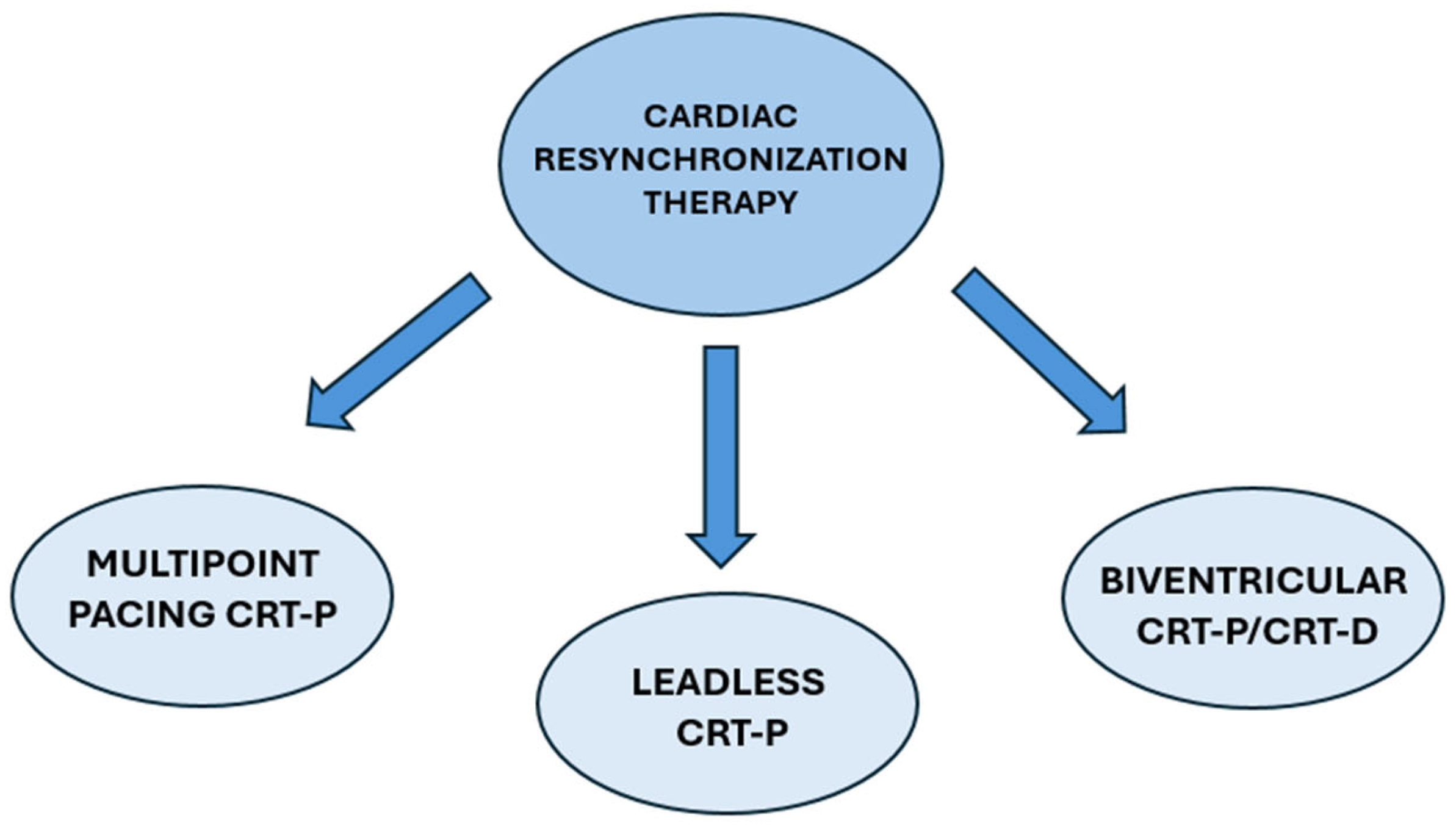
4. Types of CRT
4.1. Biventricular CRT
4.2. Multipoint Pacing CRT
4.3. CRT-Defibrillators
4.4. Image Guided CRT Placement
4.5. Remote Monitoring in CRT
4.6. Drawbacks of CRT—Non-Response and Other Complications
5. Alternatives to CRT: Conduction System Pacing
5.1. His-Bundle Pacing
5.2. Left Bundle Branch Area Pacing
6. A Route Revisited: Endovascular Lead Placement
7. Other Variations on CRT Delivery Systems
8. CRT for Special Populations
9. Emerging Therapy—Cardiac Contractility Modulation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Barra, S.; Providência, R.; Narayanan, K.; Boveda, S.; Duehmke, R.; Garcia, R.; Leyva, F.; Roger, V.; Jouven, X.; Agarwal, S.; et al. Time trends in sudden cardiac death risk in heart failure patients with cardiac resynchronization therapy: A systematic review. Eur. Heart J. 2020, 41, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Prinzen, F.W.; Auricchio, A.; Mullens, W.; Linde, C.; Huizar, J.F. Electrical management of heart failure: From pathophysiology to treatment. Eur. Heart J. 2022, 43, 1917. [Google Scholar] [CrossRef] [PubMed]
- Alpert, J.S.; Petersen, P.; Godtfredsen, J. Atrial fibrillation: Natural history, complications, and management. Annu. Rev. Med. 1988, 39, 41–52. [Google Scholar] [CrossRef]
- Namana, V.; Gupta, S.S.; Sabharwal, N.; Hollander, G. Clinical significance of atrial kick. QJM Int. J. Med. 2018, 111, 569–570. [Google Scholar] [CrossRef]
- Bessière, F.; Mondésert, B.; Chaix, M.A.; Khairy, P. Arrhythmias in adults with congenital heart disease and heart failure. Heart Rhythm O2 2021, 2 Pt B, 744–753. [Google Scholar] [CrossRef]
- Jarcho, J.A. Biventricular pacing. N. Engl. J. Med. 2006, 355, 288–294. [Google Scholar] [CrossRef]
- Nakai, T.; Ikeya, Y.; Kogawa, R.; Okumura, Y. Cardiac resynchronization therapy: Current status and near-future prospects. J. Cardiol. 2022, 79, 352–357. [Google Scholar] [CrossRef]
- Kosztin, A.; Boros, A.M.; Geller, L.; Merkely, B. Cardiac resynchronisation therapy: Current benefits and pitfalls. Pol. Heart J. Kardiologia Pol. 2018, 76, 1420–1425. [Google Scholar] [CrossRef]
- Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Card Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef]
- Miller-Davis, C.; Marden, S.; Leidy, N.K. The New York Heart Association Classes and functional status: What are we really measuring? Heart Lung J. Crit. Care. 2006, 35, 217–224. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Chia, P.L.; Foo, D. Overview of implantable cardioverter defibrillator and cardiac resynchronisation therapy in heart failure management. Singapore Med. J. 2016, 57, 354–359. [Google Scholar] [CrossRef]
- Wyman, B.T.; Hunter, W.C.; Prinzen, F.W.; McVeigh, E.R. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am. J. Physiol. 1999, 276, H881–H891. [Google Scholar] [CrossRef]
- Wyman, B.T.; Hunter, W.C.; Prinzen, F.W.; Faris, O.P.; McVeigh, E.R. Effects of single- and biventricular pacing on temporal and spatial dynamics of ventricular contraction. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H372–H379. [Google Scholar] [CrossRef]
- Kroon, W.; Lumens, J.; Potse, M.; Suerder, D.; Klersy, C.; Regoli, F.; Murzilli, R.; Moccetti, T.; Delhaas, T.; Krause, R.; et al. In vivo electromechanical assessment of heart failure patients with prolonged QRS duration. Heart Rhythm. 2015, 12, 1259–1267. [Google Scholar] [CrossRef]
- Mafi-Rad, M.; Van’t Sant, J.; Blaauw, Y.; Doevendans, P.A.; Cramer, M.J.; Crijns, H.J.; Prinzen, F.W.; Meine, M.; Vernnooy, K. Regional Left Ventricular Electrical Activation and Peak Contraction Are Closely Related in Candidates for Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2017, 3, 854–862. [Google Scholar] [CrossRef]
- Nguyên, U.C.; Verzaal, N.J.; van Nieuwenhoven, F.A.; Vernooy, K.; Prinzen, F.W. Pathobiology of cardiac dyssynchrony and resynchronization therapy. EP Eur. 2018, 20, 1898–1909. [Google Scholar] [CrossRef]
- Mashali, M.A.; Saad, N.S.; Peczkowski, K.K.; Fanning, T.; Hare, A.N.; Whitson, B.A.; Mokadam, N.A.; Janssen, P.M.L. Mechanical Dyssynchrony of Isolated Left and Right Ventricular Human Myocardium in End-stage Heart Failure. Circ. Heart Fail. 2023, 16, e009871. [Google Scholar] [CrossRef]
- Kirk, J.A.; Kass, D.A. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ. Res. 2013, 113, 765–776. [Google Scholar] [CrossRef]
- van Oosterhout, M.F.; Prinzen, F.W.; Arts, T.; Schreuder, J.J.; Vanagt, W.Y.R.; Cleutjens, J.P.M.; Reneman, R.S. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 1998, 98, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Craine, A.; Krishnamurthy, A.; Villongco, C.T.; Vincent, K.; Krummen, D.E.; Narayan, S.M.; Kerckhoffs, R.C.P.; Omens, J.H.; Contijoch, F.; McCulloch, A.D. Successful cardiac resynchronization therapy reduces negative septal work in patient-specific models of dyssynchronous heart failure. PLoS Comput. Biol. 2024, 20, e1012150. [Google Scholar] [CrossRef]
- Stevenson, W.G.; Hernandez, A.F.; Carson, P.E.; Fang, J.C.; Katz, S.D.; Spertus, J.A.; Sweitzer, N.K.; Tang, W.H.W.; Albert, N.M.; Butler, J.; et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. J. Card. Fail. 2012, 18, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Patton, K.K.; Lau, C.P.; Dal Forno, A.R.J.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS Guideline on Cardiac Physiologic Pacing for the Avoidance and Mitigation of Heart Failure. J Arrhythm. 2023, 39, 681–756. [Google Scholar] [CrossRef] [PubMed]
- Coppola, G.; Ciaramitaro, G.; Stabile, G.; D’Onofrio, A.; Palmisano, P.; Carità, P.; Mascioli, G.; Pecora, D.; De Simone, A.; Marini, M.; et al. Magnitude of QRS duration reduction after biventricular pacing identifies responders to cardiac resynchronization therapy. Int. J. Cardiol. 2016, 221, 450–455. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Zhang, Z.; Li, G.; Fragakis, N.; Liu, T. Meta-Analysis of the Usefulness of Change in QRS Width to Predict Response to Cardiac Resynchronization Therapy. Am. J. Cardiol. 2016, 118, 1368–1373. [Google Scholar] [CrossRef]
- Verma, N.; Knight, B.P. Update in Cardiac Pacing. Arrhythmia Electrophysiol. Rev. 2019, 8, 228–233. [Google Scholar] [CrossRef]
- Kaya, A.; Tatlısu, M.A.; Tekkesin, A.İ.; Alper, A.T. CRT-D or CRT-P in CRT-indicated patients? Anatol. J. Cardiol. 2017, 17, 79–80. [Google Scholar] [CrossRef]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Linde, C.; Abraham, W.T.; Gold, M.R.; St John Sutton, M.; Ghio, S.; Daubert, C. Randomized Trial of Cardiac Resynchronization in Mildly Symptomatic Heart Failure Patients and in Asymptomatic Patients With Left Ventricular Dysfunction and Previous Heart Failure Symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A., 3rd; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.F.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef]
- Romeyer-Bouchard, C.; Costa, A.D.; Abdellaoui, L.; Messier, M.; Thévenin, J.; Afif, Z.; Samuel, B.; Kihel, A.; Cerisier, A.; Convert, G.; et al. Simplified cardiac resynchronization implantation technique involving right access and a triple-guide/single introducer approach. Heart Rhythm. 2005, 2, 714–719. [Google Scholar] [CrossRef]
- Johansen, J.B.; Nielsen, J.C.; Kristensen, J.; Sandgaard, N.C. Troubleshooting the difficult left ventricular lead placement in cardiac resynchronization therapy: Current status and future perspectives. Expert. Rev. Med. Devices. 2022, 19, 341–352. [Google Scholar] [CrossRef]
- Pescariu, S.A.; Şoşdean, R.; Enache, B.; Macarie, R.I.; Tudoran, M.; Tudoran, C.; Mornoş, C.; Ionac, A.; Pescariu, S. Single-Pass VDD Pacing Lead for Cardiac Resynchronization Therapy: A Reliable Alternative. Micromachines 2021, 12, 978. [Google Scholar] [CrossRef]
- Carpio, E.F.; Gomez, J.F.; Sebastian, R.; Lopez-Perez, A.; Castellanos, E.; Almendral, J.; Ferrero, J.M.; Trenor, B. Optimization of Lead Placement in the Right Ventricle During Cardiac Resynchronization Therapy. A Simulation Study. Front. Physiol. 2019, 10, 74. [Google Scholar] [CrossRef]
- Niazi, I.; Baker, J.; Corbisiero, R.; Love, C.; Martin, D.; Sheppard, R.; Worley, S.J.; Varma, N.; Lee, K.; Tomassoni, G. Safety and Efficacy of Multipoint Pacing in Cardiac Resynchronization Therapy: The MultiPoint Pacing Trial. JACC Clin. Electrophysiol. 2017, 3, 1510–1518. [Google Scholar] [CrossRef]
- Almusaad, A.; Sweidan, R.; Alanazi, H.; Jamiel, A.; Bokhari, F.; Al Hebaishi, Y.; Al Fagih, A.; Alrawahi, N.; Al-Mandalawi, A.; Hashim, M.; et al. Long-term reverse remodeling and clinical improvement by MultiPoint Pacing in a randomized, international, Middle Eastern heart failure study. J. Interv. Card. Electrophysiol. Int. J. Arrhythm. Pacing. 2022, 63, 399–407. [Google Scholar] [CrossRef]
- Mehta, V.S.; Elliott, M.K.; Sidhu, B.S.; Gould, J.; Porter, B.; Niederer, S.; Rinaldi, C.A. Multipoint pacing for cardiac resynchronisation therapy in patients with heart failure: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2021, 32, 2577–2589. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, C.; Burri, H.; Delnoy, P.P.; Rinaldi, C.A.; Sperzel, J.; Calò, L.; Fernandez Concha, J.; Fusco, A.; Al Samadi, F.; Lee, K.; et al. Cardiac resynchronization therapy non-responder to responder conversion rate in the MORE-CRT MPP trial. EP Eur. 2023, 25, euad294. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, E.P.; Austin, A.M.; Leggett, C.G.; Gilstrap, L.G.; Friedman, D.J.; Skinner, J.S.; Al-Khatib, S.M. Complications and Mortality Following CRT-D Versus ICD Implants in Older Medicare Beneficiaries With Heart Failure. JACC Heart Fail. 2022, 10, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Tsartsalis, D.; Korela, D.; Karlsson, L.O.; Foukarakis, E.; Svensson, A.; Anastasakis, A.; Venetsanos, D.; Aggeli, C.; Tsioufis, C.; Braunschweig, F.; et al. Risk and Protective Factors for Sudden Cardiac Death: An Umbrella Review of Meta-Analyses. Front. Cardiovasc. Med. 2022, 9, 848021. [Google Scholar] [CrossRef]
- Patel, D.; Kumar, A.; Black-Maier, E.; Morgan, R.L.; Trulock, K.; Wilner, B.; Nemer, D.; Donnellan, E.; Tarakji, K.G.; Cantillon, D.J.; et al. Cardiac Resynchronization Therapy With or Without Defibrillation in Patients With Nonischemic Cardiomyopathy: A Systematic Review and Meta-Analysis. Circ. Arrhythm. Electrophysiol. 2021, 14, e008991. [Google Scholar] [CrossRef]
- Long, Y.X.; Hu, Y.; Cui, D.Y.; Hu, S.; Liu, Z.Z. The benefits of defibrillator in heart failure patients with cardiac resynchronization therapy: A meta-analysis. Pacing Clin. Electrophysiol. 2021, 44, 225–234. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.Y.; Zhu, Y.S.; Li, Z.M.; Li, K.L.; Wang, R.X. Association between CRT(D)/ICD and renal insufficiency: A systematic review and meta-analysis. Semin. Dial. 2021, 34, 17–30. [Google Scholar] [CrossRef]
- Sapp, J.L.; Sivakumaran, S.; Redpath, C.J.; Khan, H.; Parkash, R.; Exner, D.V.; Healey, J.S.; Thibault, B.; Sterns, L.D.; Lam, N.H.N.; et al. Long-Term Outcomes of Resynchronization-Defibrillation for Heart Failure. N. Engl. J. Med. 2024, 390, 212–220. [Google Scholar] [CrossRef]
- Kheiri, B.; Przybylowicz, R.; Simpson, T.F.; Merrill, M.; Osman, M.; Dalouk, K.; Rahmouni, H.; Stecker, E.; Nazer, B.; Henrikson, C.A. Imaging-guided cardiac resynchronization therapy: A meta-analysis of randomized controlled trials. Pacing Clin. Electrophysiol. 2021, 44, 1570–1576. [Google Scholar] [CrossRef]
- Hu, X.; Xu, H.; Hassea, S.R.A.; Qian, Z.; Wang, Y.; Zhang, X.; Hou, X.; Zou, J. Comparative efficacy of image-guided techniques in cardiac resynchronization therapy: A meta-analysis. BMC Cardiovasc. Disord. 2021, 21, 255. [Google Scholar] [CrossRef]
- Bazoukis, G.; Hui, J.M.H.; Lee, Y.H.A.; Chou, O.H.I.; Sfairopoulos, D.; Vlachos, K.; Saplaouras, A.; Letsas, K.P.; Efremidis, M.; Tse, G.; et al. The role of cardiac magnetic resonance in identifying appropriate candidates for cardiac resynchronization therapy—A systematic review of the literature. Heart Fail. Rev. 2022, 27, 2095–2118. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Beinart, R.; Golovchiner, G.; Bar Sheshet, A.; Swissa, M.; Bolous, M.; Rosso, R.; Medina, A.; Haim, M.; Friedman, P.; et al. Radial strain imaging-guided lead placement for improving response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy: The Raise CRT trial. EP Eur. 2022, 24, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Fyenbo, D.B.; Sommer, A.; Nørgaard, B.L.; Kronborg, M.B.; Kristensen, J.; Gerdes, C.; Jensen, H.K.; Jensen, J.M.; Nielsen, J.C. Long-term outcomes in a randomized controlled trial of multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy. EP Eur. 2022, 24, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Behar, J.M.; Rajani, R.; Pourmorteza, A.; Preston, R.; Razeghi, O.; Niederer, S.; Adhya, S.; Claridge, S.; Jackson, T.; Sieniewicz, B.; et al. Comprehensive use of cardiac computed tomography to guide left ventricular lead placement in cardiac resynchronization therapy. Heart Rhythm. 2017, 14, 1364–1372. [Google Scholar] [CrossRef]
- Gould, J.; Sidhu, B.S.; Sieniewicz, B.J.; Porter, B.; Lee, A.W.C.; Razeghi, O.; Behar, J.M.; Mehta, V.; Elliott, M.K.; Toth, D.; et al. Feasibility of intraprocedural integration of cardiac CT to guide left ventricular lead implantation for CRT upgrades. J. Cardiovasc. Electrophysiol. 2021, 32, 802–812. [Google Scholar] [CrossRef]
- Boehmer, J.P.; Hariharan, R.; Devecchi, F.G.; Smith, A.L.; Molon, G.; Capucci, A.; An, Q.; Averina, V.; Stolen, C.M.; Thakur, P.H.; et al. A Multisensor Algorithm Predicts Heart Failure Events in Patients With Implanted Devices: Results From the MultiSENSE Study. JACC Heart Fail. 2017, 5, 216–225. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: Results from the SELENE HF study. EP Eur. 2022, 24, 234–244. [Google Scholar] [CrossRef]
- Landolina, M.; Perego, G.B.; Lunati, M.; Curnis, A.; Guenzati, G.; Vicentini, A.; Parati, G.; Borghi, G.; Zanaboni, P.; Valsecchi, S.; et al. Remote monitoring reduces healthcare use and improves quality of care in heart failure patients with implantable defibrillators: The evolution of management strategies of heart failure patients with implantable defibrillators (EVOLVO) study. Circulation 2012, 125, 2985–2992. [Google Scholar] [CrossRef]
- Boriani, G.; Da Costa, A.; Ricci, R.P.; Quesada, A.; Favale, S.; Iacopino, S.; Romeo, F.; Risi, A.; Mangoni di SStefano, L.; Navarro, X.; et al. The MOnitoring Resynchronization dEvices CARdiac patiEnts (MORE-CARE) Randomized Controlled Trial: Phase 1 Results on Dynamics of Early Intervention with Remote Remote Monitoring. J. Med. Internet Res. 2013, 15, e167. [Google Scholar] [CrossRef]
- Crossley, G.H.; Boyle, A.; Vitense, H.; Chang, Y.; Mead, R.H.; CONNECT Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: The value of wireless remote monitoring with automatic clinician alerts. J. Am. Coll. Cardiol. 2011, 57, 1181–1189. [Google Scholar] [CrossRef]
- Piccini, J.P.; Mittal, S.; Snell, J.; Prillinger, J.B.; Dalal, N.; Varma, N. Impact of remote monitoring on clinical events and associated health care utilization: A nationwide assessment. Heart Rhythm. 2016, 13, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Fernandes, M.; Reis, H.; Primo, J.; Sanfins, V.; Silva, V.; Silva Cunha, P.; Silva, M.; Nicola, P.J.; PORTLink Study Investigators. Remote versus in-office monitoring for implantable cardioverter defibrillators: Results from a randomized pragmatic controlled study in Portugal. Rev. Port. De Cardiol. 2022, 41, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Ginder, C.; Li, J.; Halperin, J.L.; Akar, J.G.; Martin, D.T.; Chattopadhyay, I.; Upadhyay, G.A. Predicting Malignant Ventricular Arrhythmias Using Real-Time Remote Monitoring. J. Am. Coll. Cardiol. 2023, 81, 949–961. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Fornwalt, B.K.; Sprague, W.W.; BeDell, P.; Suever, J.D.; Gerritse, B.; Merlino, J.D.; Fyfe, D.A.; León, A.R.; Oshinski, J.N. Agreement is Poor Among Current Criteria Used to Define Response to Cardiac Resynchronization Therapy. Circulation 2010, 121, 1985–1991. [Google Scholar] [CrossRef]
- Mullens, W.; Auricchio, A.; Martens, P.; Witte, K.; Cowie, M.R.; Delgado, V.; Dickstein, K.; Linde, C.; Vernooy, K.; Leyva, F.; et al. Optimized implementation of cardiac resynchronization therapy: A call for action for referral and optimization of care. Eur. J. Heart Fail. 2020, 22, 2349–2369. [Google Scholar] [CrossRef]
- Sieniewicz, B.J.; Gould, J.; Porter, B.; Sidhu, B.S.; Teall, T.; Webb, J.; Carr-White, G.; Rinaldi, C.A. Understanding non-response to cardiac resynchronisation therapy: Common problems and potential solutions. Heart Fail. Rev. 2019, 24, 41–54. [Google Scholar] [CrossRef]
- Batta, A.; Hatwal, J. Left bundle branch pacing set to outshine biventricular pacing for cardiac resynchronization therapy? World J. Cardiol. 2024, 16, 186–190. [Google Scholar] [CrossRef]
- Varma, N.; Boehmer, J.; Bhargava, K.; Yoo, D.; Leonelli, F.; Costanzo, M.; Saxena, A.; Sun, L.; Gold, M.R.; Singh, J.; et al. Evaluation, Management, and Outcomes of Patients Poorly Responsive to Cardiac Resynchronization Device Therapy. J. Am. Coll. Cardiol. 2019, 74, 2588–2603. [Google Scholar] [CrossRef]
- Pothineni, N.V.K.; Gondi, S.; Cherian, T.; Kovelamudi, S.; Schaller, R.D.; Lakkireddy, D.; Gopinathannair, R.; Deshmukh, A. Complications of Cardiac Resynchronization Therapy: Comparison of Safety Outcomes from Real-world Studies and Clinical Trials. J. Innov. Card. Rhythm. Manag. 2022, 13, 5121–5125. [Google Scholar] [CrossRef]
- Padeletti, L.; Paoletti Perini, A.; Gronda, E. Cardiac resynchronization therapy: The issue of non-response. Heart Fail. Rev. 2012, 17, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Defaye, P.; Boveda, S.; Klug, D.; Beganton, F.; Piot, O.; Narayanan, K.; Périer, M.C.; Gras, D.; Fauchier, L.; Bordachar, P.; et al. Dual- vs. single-chamber defibrillators for primary prevention of sudden cardiac death: Long-term follow-up of the Défibrillateur Automatique Implantable-Prévention Primaire registry. EP Eur. 2017, 19, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.E.; Gleva, M.J.; Mela, T.; Chung, M.K.; Uslan, D.Z.; Borge, R.; Gottipaty, V.; Shinn, T.; Dan, D.; Feldman, L.A.; et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: Results from the REPLACE registry. Circulation 2010, 122, 1553–1561. [Google Scholar] [CrossRef]
- Ahmed, F.Z.; Fullwood, C.; Zaman, M.; Qamruddin, A.; Cunnington, C.; Mamas, M.A.; Sandoe, J.; Motwani, M.; Zaidi, A. Cardiac implantable electronic device (CIED) infections are expensive and associated with prolonged hospitalisation: UK Retrospective Observational Study. PLoS ONE 2019, 14, e0206611. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.B.; Ferguson, C.L.; Crites, K.; Crimmins-Reda, P. The additional hospital costs generated in the management of complications of pacemaker and defibrillator implantations. J. Thorac. Cardiovasc. Surg. 1996, 111, 742–751, discussion 751–752. [Google Scholar] [CrossRef]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J. Am. Coll. Cardiol. 2011, 58, 1001–1006. [Google Scholar] [CrossRef]
- Olsen, T.; Jørgensen, O.D.; Nielsen, J.C.; Thøgersen, A.M.; Philbert, B.T.; Johansen, J.B. Incidence of device-related infection in 97 750 patients: Clinical data from the complete Danish device-cohort (1982–2018). Eur. Heart J. 2019, 40, 1862–1869. [Google Scholar] [CrossRef]
- Arabia, G.; Mitacchione, G.; Cersosimo, A.; Calvi, E.; Salghetti, F.; Bontempi, L.; Giacopelli, D.; Cerini, M.; Curnis, A. Long-term outcomes following transvenous lead extraction: Data from a tertiary referral center. Int. J. Cardiol. 2023, 378, 32–38. [Google Scholar] [CrossRef]
- Herweg, B.; Ali, R.; Ilercil, A.; Madramootoo, C.; Cutro, R.; Weston, M.W.; Barold, S. Site-specific differences in latency intervals during biventricular pacing: Impact on paced QRS morphology and echo-optimized V-V interval. Pacing Clin. Electrophysiol. 2010, 33, 1382–1391. [Google Scholar] [CrossRef]
- Mafi-Rad, M.; Luermans, J.G.L.M.; Blaauw, Y.; Janssen, M.; Crijns, H.J.; Prinzen, F.W.; Vernooy, K. Feasibility and Acute Hemodynamic Effect of Left Ventricular Septal Pacing by Transvenous Approach Through the Interventricular Septum. Circ. Arrhythm. Electrophysiol. 2016, 9, e003344. [Google Scholar] [CrossRef]
- Lustgarten, D.L.; Crespo, E.M.; Arkhipova-Jenkins, I.; Lobel, R.; Winget, J.; Koehler, J.; Liberman, E.; Sheldon, T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm. 2015, 12, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Ajijola, O.A.; Upadhyay, G.A.; Macias, C.; Shivkumar, K.; Tung, R. Permanent His-bundle pacing for cardiac resynchronization therapy: Initial feasibility study in lieu of left ventricular lead. Heart Rhythm. 2017, 14, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Dandamudi, G.; Herweg, B.; Wilson, D.; Singh, R.; Naperkowski, A.; Koneru, J.N.; Ellenbogen, K.A.; Vijayaraman, P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm. 2018, 15, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, G.A.; Vijayaraman, P.; Nayak, H.M.; Verma, N.; Dandamudi, G.; Sharma, P.S.; Saleem, M.; Mandrola, J.; Genovese, D.; Tung, R.; et al. His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 157–159. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Subzposh, F.A.; Naperkowski, A.; Panikkath, R.; John, K.; Mascarenhas, V.; Bauch, T.D.; Huang, W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019, 16, 1774–1782. [Google Scholar] [CrossRef]
- da Silva Menezes Junior, A.; Melo, M.G.Z.; Barreto, L.P. Meta-analysis of clinical outcomes in cardiac resynchronisation therapy: His bundle pacing vs biventricular pacing. Expert. Rev. Med. Devices. 2023, 20, 505–515. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Pokharel, P.; Subzposh, F.A.; Oren, J.W.; Storm, R.H.; Batul, S.A.; Beer, D.A.; Hughes, G.; Leri, G.; Manganiello, M.; et al. His-Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy vs Biventricular Pacing: HOT-CRT Clinical Trial. JACC Clin. Electrophysiol. 2023, 9, 2628–2638. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Su, L.; Fu, G.; Su, Y.; Chen, K.; Zou, J.; Han, H.; Wu, S.; Sheng, X.; et al. His-bundle pacing vs biventricular pacing following atrioventricular nodal ablation in patients with atrial fibrillation and reduced ejection fraction: A multicenter, randomized, crossover study—The ALTERNATIVE-AF trial. Heart Rhythm. 2022, 19, 1948–1955. [Google Scholar] [CrossRef]
- Whinnett, Z.I.; Shun-Shin, M.J.; Tanner, M.; Foley, P.; Chandrasekaran, B.; Moore, P.; Adhya, S.; Qureshi, N.; Muthumala, A.; Lane, R.; et al. Effects of haemodynamically atrio-ventricular optimized His bundle pacing on heart failure symptoms and exercise capacity: The His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) randomized, double-blind, cross-over trial. Eur. J. Heart Fail. 2023, 25, 274–283. [Google Scholar] [CrossRef]
- Siranart, N.; Chokesuwattanaskul, R.; Prasitlumkum, N.; Huntrakul, A.; Phanthong, T.; Sowalertrat, W.; Navaravong, L.; Cheungpasitporn, W.; Jongnarangsin, K. Reverse of left ventricular remodeling in heart failure patients with left bundle branch area pacing: Systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2023, 46, 459–466. [Google Scholar] [CrossRef]
- Parlavecchio, A.; Vetta, G.; Caminiti, R.; Coluccia, G.; Magnocavallo, M.; Ajello, M.; Pistelli, L.; Dattilo, G.; Foti, R.; Carerj, S.; et al. Left bundle branch pacing versus biventricular pacing for cardiac resynchronization therapy: A systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2023, 46, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jin, Q.; Qiu, Z.; Qian, C.; Liang, Y.; Wang, J.; Qin, S.; Bai, J.; Wang, W.; Chen, H.; et al. Outcomes of Upgrading to LBBP in CRT Nonresponders: A Prospective, Multicenter, Nonrandomized, Case-Control Study. JACC Clin. Electrophysiol. 2024, 10, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, B.H.; Zhang, N.; Sun, M.; Wang, R. The electrocardiogram characteristics and pacing parameters of permanent left bundle branch pacing: A systematic review and meta-analysis. J. Interv. Card. Electrophysiol. 2022, 63, 215–224. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, W.; Shi, S.; Zhou, X.; Zhu, Z. Left bundle branch pacing for cardiac resynchronization therapy: A systematic literature review and meta-analysis. Pacing Clin. Electrophysiol. 2021, 44, 497–505. [Google Scholar] [CrossRef]
- Yu, G.I.; Kim, T.H.; Cho, Y.H.; Bae, J.S.; Ahn, J.H.; Jang, J.Y.; Park, Y.W.; Kwak, C.H. Left bundle branch area pacing in mildly reduced heart failure: A systematic literature review and meta-analysis. Clin. Cardiol. 2023, 46, 713–720. [Google Scholar] [CrossRef]
- Pujol-Lopez, M.; Jiménez-Arjona, R.; Garre, P.; Guasch, E.; Borràs, R.; Doltra, A.; Ferró, E.; García-Ribas, C.; Niebla, M.; Carro, E.; et al. Conduction System Pacing vs Biventricular Pacing in Heart Failure and Wide QRS Patients: LEVEL-AT Trial. JACC Clin. Electrophysiol. 2022, 8, 1431–1445. [Google Scholar] [CrossRef]
- Mariani, M.V.; Piro, A.; Forleo, G.B.; Della Rocca, D.G.; Natale, A.; Miraldi, F.; Vizza, C.D.; Lavalle, C. Clinical, procedural and lead outcomes associated with different pacing techniques: A network meta-analysis. Int. J. Cardiol. 2023, 377, 52–59. [Google Scholar] [CrossRef]
- Diaz, J.C.; Duque, M.; Aristizabal, J.; Marin, J.; Niño, C.; Bastidas, O.; Ruiz, L.M.; Matos, C.D.; Hoyos, C.; Hincapie, D.; et al. The Emerging Role of Left Bundle Branch Area Pacing for Cardiac Resynchronisation Therapy. Arrhythmia Electrophysiol. Rev. 2023, 12, e29. [Google Scholar] [CrossRef]
- Scott, P.A.; Yue, A.M.; Watts, E.; Zeb, M.; Roberts, P.R.; Morgan, J.M. Transseptal left ventricular endocardial pacing reduces dispersion of ventricular repolarization. Pacing Clin. Electrophysiol. 2011, 34, 1258–1266. [Google Scholar] [CrossRef]
- Mendonca Costa, C.; Neic, A.; Gillette, K.; Porter, B.; Gould, J.; Sidhu, B.; Chen, Z.; Elliott, M.; Mehta, V.; Plank, G.; et al. Left ventricular endocardial pacing is less arrhythmogenic than conventional epicardial pacing when pacing in proximity to scar. Heart Rhythm. 2020, 17, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Behar, J.M.; Jackson, T.; Hyde, E.; Claridge, S.; Gill, J.; Bostock, J.; Sohal, M.; Porter, B.; O’Neill, M.; Razavi, R.; et al. Optimized Left Ventricular Endocardial Stimulation Is Superior to Optimized Epicardial Stimulation in Ischemic Patients With Poor Response to Cardiac Resynchronization Therapy: A Combined Magnetic Resonance Imaging, Electroanatomic Contact Mapping, and Hemodynamic Study to Target Endocardial Lead Placement. JACC Clin. Electrophysiol. 2016, 2, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Biffi, M.; Gellér, L.; Leclercq, C.; Ruffa, F.; Tung, S.; Defaye, P.; Yang, Z.; Gerritse, B.; van Ginneken, M.; et al. ALternate Site Cardiac ResYNChronization (ALSYNC): A prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur. Heart J. 2016, 37, 2118–2127. [Google Scholar] [CrossRef]
- Gamble, J.H.P.; Herring, N.; Ginks, M.; Rajappan, K.; Bashir, Y.; Betts, T.R. Endocardial left ventricular pacing for cardiac resynchronization: Systematic review and meta-analysis. EP Eur. 2018, 20, 73–81. [Google Scholar] [CrossRef]
- Elencwajg, B.; López-Cabanillas, N.; Fischer, A.; Negrete, A.; Marin, J.; Delgado, L.; Glikson, M.; Molina, L.; Worley, S.; Arnez, J.; et al. Multicenter prospective observational long-term follow-up study of endocardial cardiac resynchronization therapy using the Jurdham procedure. Heart Rhythm. 2019, 16, 1453–1461. [Google Scholar] [CrossRef]
- Rademakers, L.M.; van Gelder, B.M.; Scheffer, M.G.; Bracke, F.A. Mid-term follow up of thromboembolic complications in left ventricular endocardial cardiac resynchronization therapy. Heart Rhythm. 2014, 11, 609–613. [Google Scholar] [CrossRef]
- Gellér, L.; Salló, Z.; Molnár, L.; Tahin, T.; Özcan, E.E.; Kutyifa, V.; Osztheimer, I.; Szilágyi, S.; Szegedi, N.; Ábrahám, P.; et al. Long-term single-centre large volume experience with transseptal endocardial left ventricular lead implantation. EP Eur. 2019, 21, 1237–1245. [Google Scholar] [CrossRef]
- Santos, H.; Santos, M.; Almeida, I.; Paula, S.B.; Figueiredo, M.; Portugal, G.; Valente, B.; Silva Cunha, P.; Almeida, L.; Oliveira, M. A systemic review of endocardial left ventricular pacing. Heart Lung J. Crit. Care. 2022, 51, 82–86. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Miller, M.A.; Neuzil, P.; Søgaard, P.; Butter, C.; Seifert, M.; Delnoy, P.P.; van Erven, L.; Schalji, M.; Boersma, L.V.; et al. Cardiac Resynchronization Therapy With Wireless Left Ventricular Endocardial Pacing: The SELECT-LV Study. J. Am. Coll. Cardiol. 2017, 69, 2119–2129. [Google Scholar] [CrossRef]
- Singh, J.P.; Rinaldi, C.A.; Sanders, P.; Kubo, S.H.; James, S.; Niazi, I.K.; Betts, T.; Butter, C.; Okabe, T.; Cunnane, R.; et al. Leadless Ultrasound-Based Cardiac Resynchronization System in Heart Failure. JAMA Cardiol. 2024, 9, 871–879. [Google Scholar] [CrossRef]
- White, H.D.; Norris, R.M.; Brown, M.A.; Brandt, P.W.; Whitlock, R.M.; Wild, C.J. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987, 76, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Udelson, J.E.; Anand, I.S.; Cohn, J.N. Ventricular remodeling in heart failure: A credible surrogate endpoint. J. Card. Fail. 2003, 9, 350–353. [Google Scholar] [CrossRef]
- Ypenburg, C.; van Bommel, R.J.; Borleffs, C.J.W.; Bleeker, G.B.; Boersma, E.; Schalij, M.J.; Bax, J.J. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J. Am. Coll. Cardiol. 2009, 53, 483–490. [Google Scholar] [CrossRef]
- Yu, C.M.; Bleeker, G.B.; Fung, J.W.H.; Schalij, M.J.; Zhang, Q.; van der Wall, E.E.; Chan, Y.S.; Kong, S.L.; Bax, J.J. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 2005, 112, 1580–1586. [Google Scholar] [CrossRef]
- Gould, J.; Claridge, S.; Jackson, T.; Sieniewicz, B.J.; Sidhu, B.S.; Porter, B.; Elliott, M.K.; Mehta, V.; Niederer, S.; Chadwick, H.; et al. Standard care vs. TRIVEntricular pacing in Heart Failure (STRIVE HF): A prospective multicentre randomized controlled trial of triventricular pacing vs. conventional biventricular pacing in patients with heart failure and intermediate QRS left bundle branch block. EP Eur. 2022, 24, 796–806. [Google Scholar] [CrossRef]
- Wilkoff, B.L.; Filippatos, G.; Leclercq, C.; Gold, M.R.; Hersi, A.S.; Kusano, K.; Mullens, W.; Felker, G.M.; Kantipudi, C.; El-Chami, M.F.; et al. Adaptive versus conventional cardiac resynchronisation therapy in patients with heart failure (AdaptResponse): A global, prospective, randomised controlled trial. Lancet Lond. Engl. 2023, 402, 1147–1157. [Google Scholar] [CrossRef]
- Pujol-López, M.; Tolosana, J.M.; Guasch, E.; Trucco, E.; Jiménez-Arjona, R.; Borràs, R.; Garre, P.; San Antonio, R.; Doltra, A.; Roca-Luque, I.; et al. Cardiac Resynchronization Therapy Response Is Equalized in Men and Women by Electrical Optimization: PR Matters. JACC Clin. Electrophysiol. 2021, 7, 1400–1409. [Google Scholar] [CrossRef]
- Wijesuriya, N.; Elliott, M.K.; Mehta, V.; Sidhu, B.S.; Behar, J.M.; Niederer, S.; Rinaldi, C.A. Leadless left ventricular endocardial pacing for cardiac resynchronization therapy: A systematic review and meta-analysis. Heart Rhythm. 2022, 19, 1176–1183. [Google Scholar] [CrossRef]
- Damman, K.; Valente, M.A.E.; Voors, A.A.; O’Connor, C.M.; van Veldhuisen, D.J.; Hillege, H.L. Renal impairment, worsening renal function, and outcome in patients with heart failure: An updated meta-analysis. Eur. Heart J. 2014, 35, 455–469. [Google Scholar] [CrossRef]
- Varga, C.R.; Cleland, J.G.F.; Abraham, W.T.; Lip, G.Y.H.; Leyva, F.; Hatamizadeh, P. Implantable Cardioverter Defibrillator and Resynchronization Therapy in Patients With Overt Chronic Kidney Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2024, 84, 1342–1362. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Martens, P.; L’hoyes, W.; Maass, A.H.; Damman, K.; Dupont, M.; Mullens, W. Response to Cardiac Resynchronization Therapy Across Chronic Kidney Disease Stages. J. Card. Fail. 2019, 25, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Daly, D.D.; Maran, A.; Hyer, J.M.; Funke, F.; Waring, A.; Cuoco, F.A.; Sturdivant, J.L.; Leman, R.B.; Gold, M.R. The Effect of Chronic Kidney Disease on Mortality with Cardiac Resynchronization Therapy. Pacing Clin. Electrophysiol. 2016, 39, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Katz, R.; St John Sutton, M.; Dixit, S.; Gerstenfeld, E.P.; Ghio, S.; Gold, M.R.; Linde, C.; Shlipak, M.G.; Deo, R. Chronic kidney disease and cardiac remodelling in patients with mild heart failure: Results from the REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction (REVERSE) study. Eur. J. Heart Fail. 2012, 14, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Singal, G.; Upadhyay, G.A.; Borgquist, R.; Friedman, D.J.; Chatterjee, N.A.; Kandala, J.; Park, M.Y.; Orencole, M.; Dec, G.W.; Picard, M.H.; et al. Renal Response in Patients with Chronic Kidney Disease Predicts Outcome Following Cardiac Resynchronization Therapy. Pacing Clin. Electrophysiol. 2015, 38, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Gronda, E.; Genovese, S.; Padeletti, L.; Cacciatore, F.; Vitale, D.F.; Bragato, R.; Innocenti, L.; Schiano, C.; Sommese, L.; De Pascale, M.R.; et al. Renal function impairment predicts mortality in patients with chronic heart failure treated with resynchronization therapy. Cardiol. J. 2015, 22, 459–466. [Google Scholar] [CrossRef]
- Ayoub, K.; Fry, E.; Marji, M.; Masri, A.; Hesselson, A.; Ellison, K. Implantable cardioverter-defibrillators with end stage renal disease: Nationwide inpatient sample database results. Pacing Clin. Electrophysiol. 2022, 45, 124–131. [Google Scholar] [CrossRef]
- Aggarwal, A.; Wang, Y.; Rumsfeld, J.S.; Curtis, J.P.; Heidenreich, P.A.; National Cardiovascular Data Registry. Clinical characteristics and in-hospital outcome of patients with end-stage renal disease on dialysis referred for implantable cardioverter-defibrillator implantation. Heart Rhythm. 2009, 6, 1565–1571. [Google Scholar] [CrossRef]
- Charytan, D.M.; Patrick, A.R.; Liu, J.; Setoguchi, S.; Herzog, C.A.; Brookhart, M.A.; Winkelmayer, W.C. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am. J. Kidney Dis. 2011, 58, 409–417. [Google Scholar] [CrossRef]
- Guha, A.; Maddox, W.R.; Colombo, R.; Nahman, N.S., Jr.; Kintziger, K.W.; Waller, J.L.; Diamond, M.; Murphy, M.; Kheda, M.; Litwin, S.E. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm. 2015, 12, 2395–2401. [Google Scholar] [CrossRef]
- Teruya, T.H.; Abou-Zamzam, A.M.; Limm, W.; Wong, L.; Wong, L. Symptomatic subclavian vein stenosis and occlusion in hemodialysis patients with transvenous pacemakers. Ann. Vasc. Surg. 2003, 17, 526–529. [Google Scholar] [CrossRef]
- Safdar, N.Z.; Kamalathasan, S.; Gupta, A.; Wren, J.; Bird, R.; Papp, D.; Latto, R.; Ahmed, A.; Palin, V.; Gierula, J.; et al. Outcomes following cardiac resynchronisation therapy in older people. Age Ageing. 2023, 52, afad222. [Google Scholar] [CrossRef] [PubMed]
- Behon, A.; Merkel, E.D.; Schwertner, W.R.; Kuthi, L.K.; Veres, B.; Masszi, R.; Kovács, A.; Lakatos, B.K.; Zima, E.; Gellér, L.; et al. Long-term outcome of cardiac resynchronization therapy patients in the elderly. GeroScience 2023, 45, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, E.P.; Dalgaard, F.; Abraham, W.T.; Cleland, J.G.F.; Curtis, A.B.; Friedman, D.J.; Gold, M.R.; Kutyifa, V.; Linde, C.; Tang, A.S.; et al. Benefit of cardiac resynchronization therapy among older patients: A patient-level meta-analysis. Am. Heart J. 2024, 267, 81–90. [Google Scholar] [CrossRef]
- Backman, W.D.; DiCaro, M.V.; Zuo, X.; Peralta, A.; Orkaby, A.R. Aligning goals with care: Advance directives in older adults with implantable cardioverter-defibrillators. Pacing Clin. Electrophysiol. 2024, 47, 697–701. [Google Scholar] [CrossRef]
- Martens, P.; Dupont, M.; Dauw, J.; Nijst, P.; Bertrand, P.B.; Tang, W.H.W.; Mullens, W. The effect of intravenous ferric carboxymaltose on right ventricular function—Insights from the IRON-CRT trial. Eur. J. Heart Fail. 2022, 24, 1106–1113. [Google Scholar] [CrossRef]
- Brignole, M.; Pentimalli, F.; Palmisano, P.; Landolina, M.; Quartieri, F.; Occhetta, E.; Calò, L.; Mascia, G.; Mont, L.; Vernooy, K.; et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: The APAF-CRT mortality trial. Eur. Heart J. 2021, 42, 4731–4739. [Google Scholar] [CrossRef]
- Kuschyk, J.; Kloppe, A.; Schmidt-Schweda, S.; Bonnemeier, H.; Rousso, B.; Röger, S. Cardiac Contractility Modulation: A Technical Guide for Device Implantation. Rev. Cardiovasc. Med. 2017, 18, 1–13. [Google Scholar] [CrossRef]
- Tschöpe, C.; Kherad, B.; Klein, O.; Sliwa, K.; Hamdani, N.; Lüscher, T.F.; Anker, S.D.; Pieske, B.; Van Linthout, S.; Burkhoff, D.; et al. Cardiac contractility modulation: Mechanisms of action in heart failure with reduced ejection fraction and beyond. Eur. J. Heart Fail. 2019, 21, 14–22. [Google Scholar] [CrossRef]
- Pipilas, D.C.; Hanley, A.; Singh, J.P.; Mela, T. Cardiac Contractility Modulation for Heart Failure: Current and Future Directions. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2 Pt B, 101176. [Google Scholar] [CrossRef]
- Abraham, W.T.; Kuck, K.H.; Goldsmith, R.L.; Lindenfeld, J.; Reddy, V.Y.; Carson, P.; Mann, D.L.; Gunderson, B.; Daubert, J.P.; Lawhorn, F.O.; et al. A Randomized Controlled Trial to Evaluate the Safety and Efficacy of Cardiac Contractility Modulation. JACC Heart Fail. 2018, 6, 874–883. [Google Scholar] [CrossRef]
- Borggrefe, M.M.; Lawo, T.; Butter, C.; Schmidinger, H.; Lunati, M.; Pieske, B.; Misier, A.R.; Curnis, A.; Böcker, D.; Remppis, A.; et al. Randomized, Double Blind Study of Non-Excitatory, Cardiac Contractility Modulation Electrical Impulses for Symptomatic Heart failure. Eur. Heart J. 2008, 29, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Butter, C.; Meyhöfer, J.; Seifert, M.; Neuss, M.; Minden, H.H. First use of cardiac contractility modulation (CCM) in a patient failing CRT therapy: Clinical and technical aspects of combined therapies. Eur. J. Heart Fail. 2007, 9, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Nägele, H.; Behrens, S.; Eisermann, C. Cardiac contractility modulation in non-responders to cardiac resynchronization therapy. EP Eur. 2008, 10, 1375–1380. [Google Scholar] [CrossRef]
- Kuschyk, J.; Nägele, H.; Heinz-Kuck, K.; Lawo, T.; Rousso, B.; Sakalihas, N.; Roeger, S.; Geller, J.C.; Bauer, A.; Borggrefe, M.; et al. Cardiac contractility modulation treatment in patients with symptomatic heart failure despite optimal medical therapy and cardiac resynchronization therapy (CRT). Int. J. Cardiol. 2019, 277, 173–177. [Google Scholar] [CrossRef]
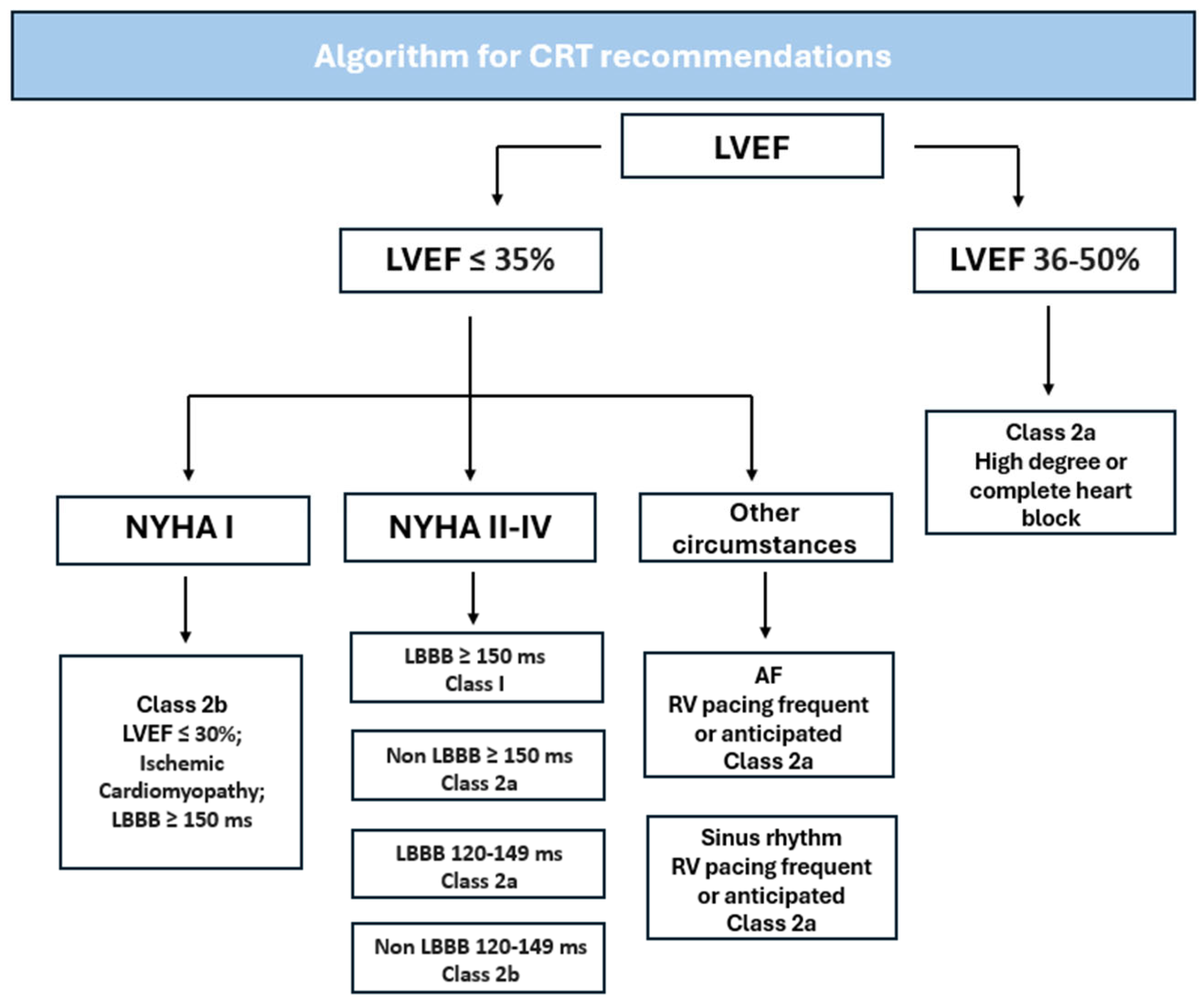

| Class 1 |
|
| Class 2a |
|
| Class 2a |
|
| Class 2a |
|
| Class 2a |
|
| Class 2a |
|
| Class 2b |
|
| Class 2b |
|
| Class 3 No benefit |
|
| Miscellaneous |
|
| Miscellaneous |
|
| Study | Study Population and Randomization | Summary of Findings |
|---|---|---|
| MIRACLE Abraham et al., 2002 [30] | 453 patients with LVEF ≤ 35%, NYHA class III or IV, 6MWT < 450 m, and QRS interval > 130 ms Randomly assigned to CRT group or to control group; followed for 6 months. | CRT group had improved 6MWT, EF, decreased hospitalization rates versus control group at 6-month follow up. |
| COMPANION, Bristow et al., 2004 [29] | 1520 patients with LVEF ≤ 35%, NYHA class III or IV and QRSd > 120 ms. Randomized in 1:2:2 ratio to receive OMT alone or in combination with CRT-P or CRT-D; followed for 15 months. | CRT with or without ICD was associated with 1-year relative risk reduction of about 20% for all-cause death or hospitalization. |
| REVERSE Linde et al., 2008 [31] | 610 patients with LVEF ≤ 40%, NYHA class I or II, QRS ≥ 120 ms. Randomly assigned to active CRT group or control group, both receiving OMT; followed for 12 months. | CRT group had reduced risk for HF hospitalization, improved ventricular structure, and NYHA I and II class. |
| MADIT-CRT Moss et al., 2009 [32] | 1820 patients with LVEF ≤ 30%, QRS ≥ 130 ms, NYHA class I or II. Randomized in 3:2 ratio to receive CRT-D or ICD alone. Mean follow-up 2.4 years | CRT-D group had decreased mortality and CHF events when compared to ICD alone group. |
| CARE-HF, Cleland et al., 2005 [33] | 813 patients with LVEF ≤ 35%, QRS ≥ 120 ms, NYHA class 3 or 4 despite OMT. Randomized to undergo BiV-CRT or medical therapy alone. Mean follow-up 29.4 months. | The BiV-CRT group had improved symptoms, QOL, less complications, and improved mortality. Broader QRS patients had overall better results. |
| RAFT, Tang et al., 2010 [34] | 1798 patients with LVEF ≤ 30%, QRS ≥ 120 ms, NYHA class II or III. Randomized to obtain CRT-D or ICD alone. Mean follow up of 40 months. | CRT-D decreased mortality when compared to ICD implantation alone (though with greater adverse effects). |
| Drawbacks of CRT | |
|---|---|
| Procedural Complications | Factors Reducing Response to CRT |
| Lead-related issues (i.e., malfunction or dislodgement) | QRS of less than 150 ms |
| Infection | Not optimized medical therapy |
| Hematoma | No myocardial viability at paced site |
| Device malfunction | Significant presence of scars |
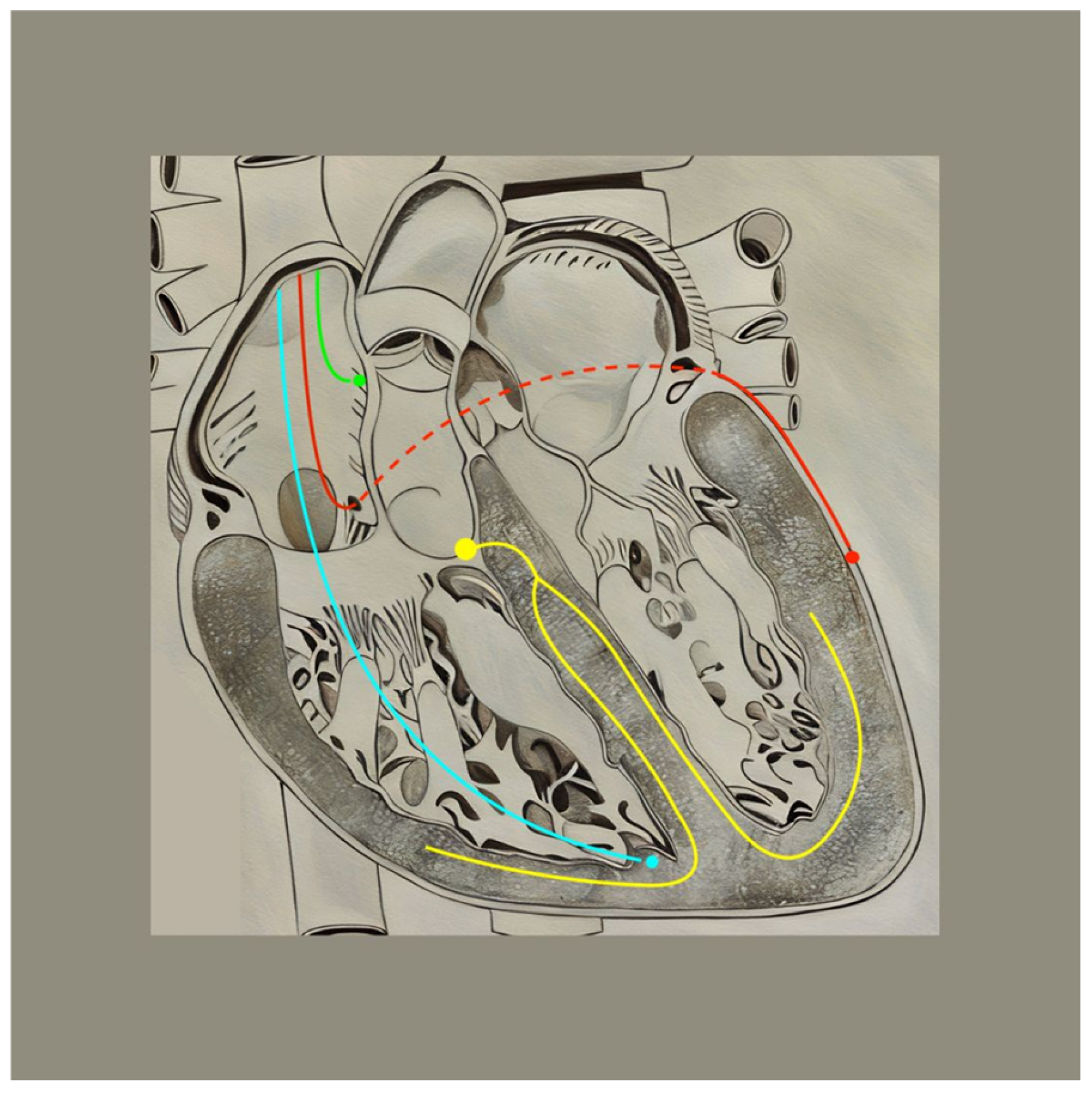
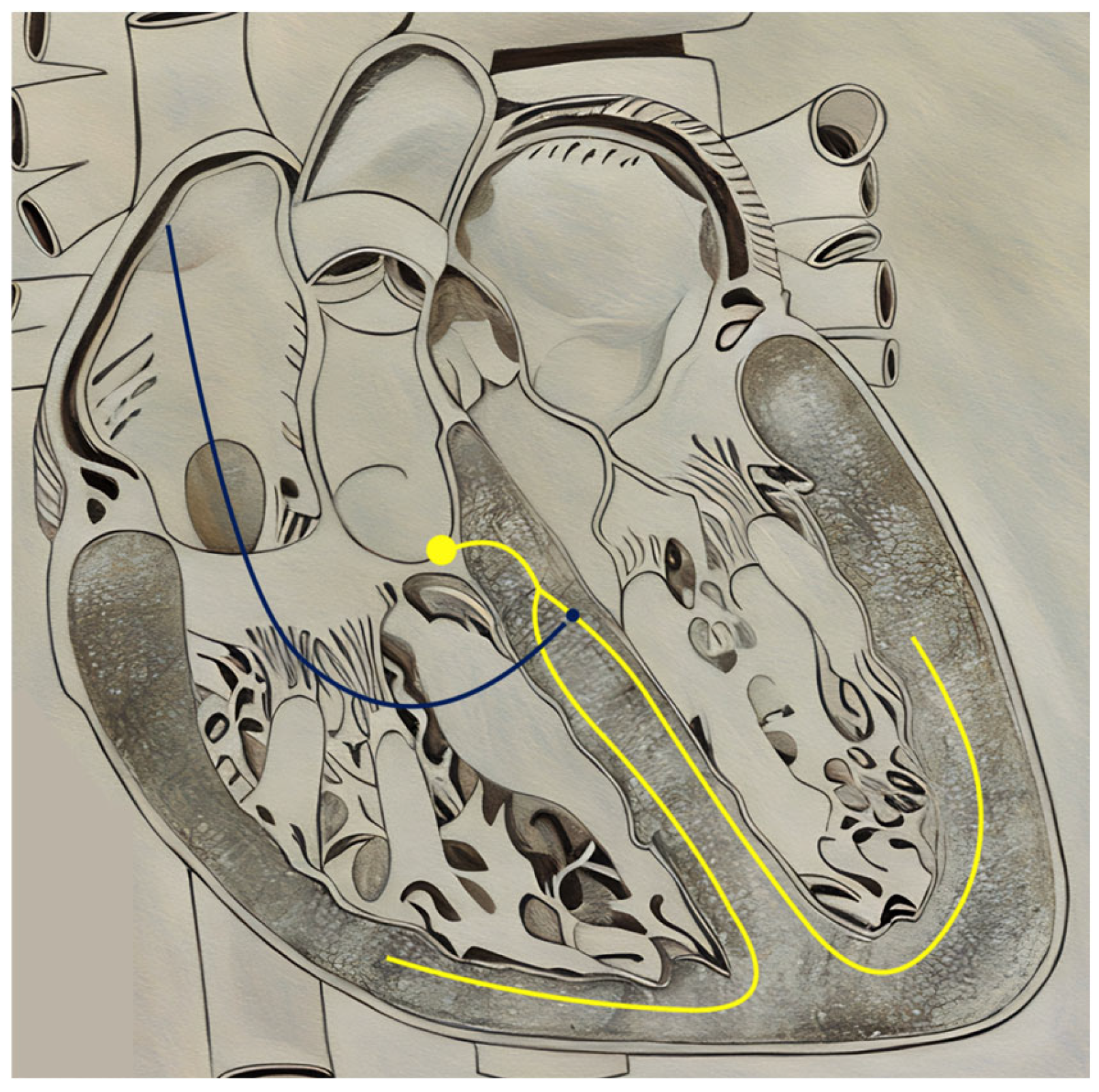
| Type of Pacing | Lead Placement | Mechanism of Action | Advantages | Disadvantages |
|---|---|---|---|---|
| Biventricular CRT (BiV-CRT) | Apex of the right ventricle and lateral left ventricular wall | An electrical impulse simultaneously stimulates both the right and left ventricle to contract | Can bypass physical barriers (e.g., scarring) that impede the electrical conduction pathway |
|
| His-bundle Pacing (HBP) | Within the membranous interventricular septum, approximately near the superior border of the tricuspid valve annulus | An electrical impulse stimulates the area approximately below the Bundle of His, eliciting a signal to the remainder of the electrical conduction pathway |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, A.; Tasouli-Drakou, V.; Ringor, M.; DiCaro, M.V.; Yee, B.; Lei, K.; Tak, T. Recent Advances in Cardiac Resynchronization Therapy: Current Treatment and Future Direction. J. Clin. Med. 2025, 14, 889. https://doi.org/10.3390/jcm14030889
Siddiqui A, Tasouli-Drakou V, Ringor M, DiCaro MV, Yee B, Lei K, Tak T. Recent Advances in Cardiac Resynchronization Therapy: Current Treatment and Future Direction. Journal of Clinical Medicine. 2025; 14(3):889. https://doi.org/10.3390/jcm14030889
Chicago/Turabian StyleSiddiqui, Arsalan, Vasiliki Tasouli-Drakou, Marc Ringor, Michael V. DiCaro, Brianna Yee, KaChon Lei, and Tahir Tak. 2025. "Recent Advances in Cardiac Resynchronization Therapy: Current Treatment and Future Direction" Journal of Clinical Medicine 14, no. 3: 889. https://doi.org/10.3390/jcm14030889
APA StyleSiddiqui, A., Tasouli-Drakou, V., Ringor, M., DiCaro, M. V., Yee, B., Lei, K., & Tak, T. (2025). Recent Advances in Cardiac Resynchronization Therapy: Current Treatment and Future Direction. Journal of Clinical Medicine, 14(3), 889. https://doi.org/10.3390/jcm14030889








