A Narrative Review on the Sperm Selection Methods in Assisted Reproductive Technology: Out with the New, the Old Is Better?
Abstract
:1. Introduction
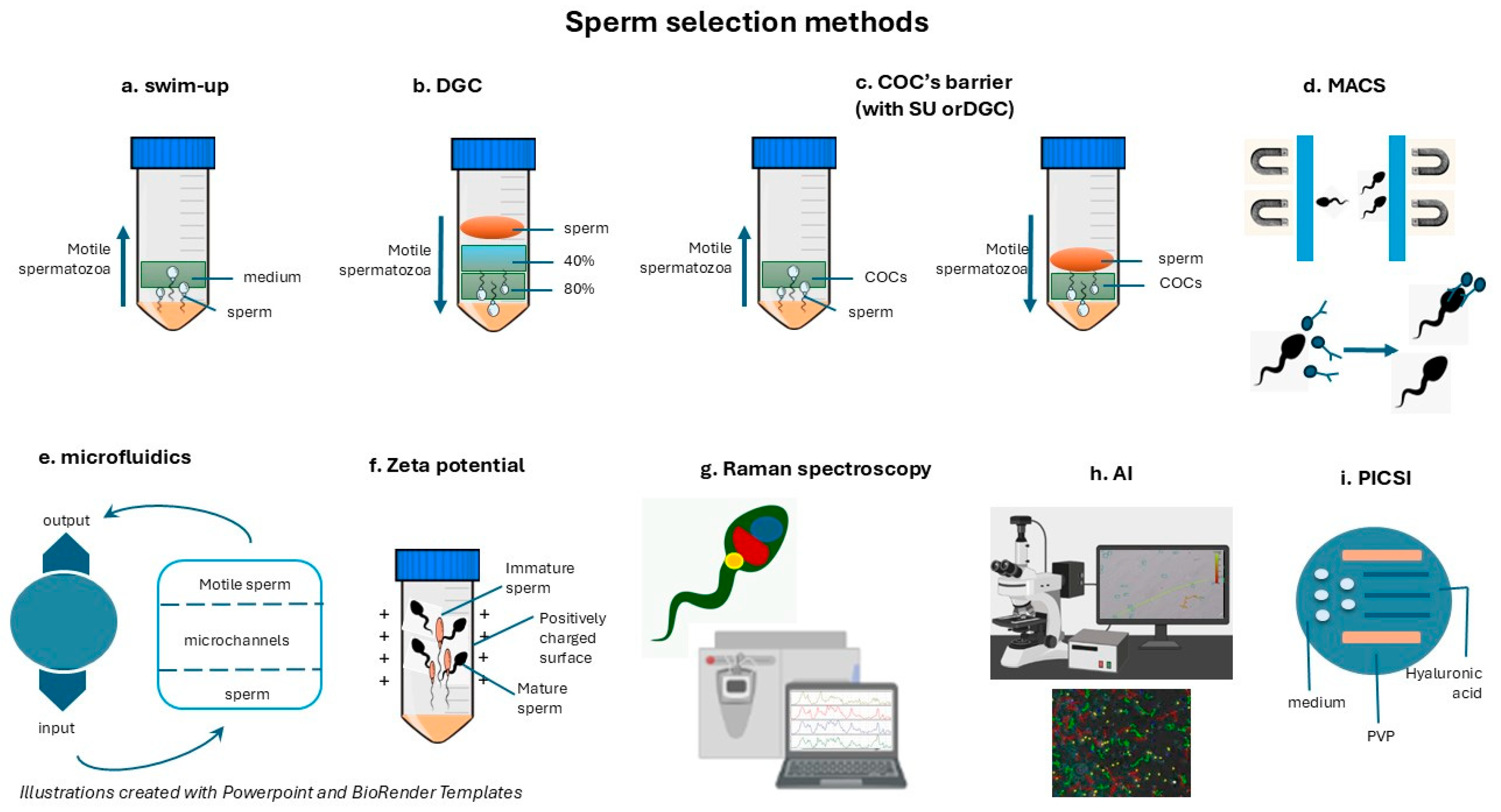
2. Sperm Selection Techniques
2.1. Conventional Sperm Selection Methods
2.1.1. The Swim-Up Technique
2.1.2. Density Gradient Centrifugation (DGC)
2.1.3. Comparison of Swim-Up and DGC
2.1.4. “Physiological” ICSI (COC Barriers)
2.1.5. Brief Overview of Other Conventional Methods
2.2. Advanced Sperm Selection Techniques
2.2.1. Magnetic-Activated Cell Sorting (MACS)
2.2.2. Microfluidic Sperm Sorting
2.2.3. Zeta Potential Selection
2.2.4. Hyaluronic Acid (HA)-Binding Assay (PICSI)
2.2.5. Raman Spectroscopy and Artificial Intelligence (AI)
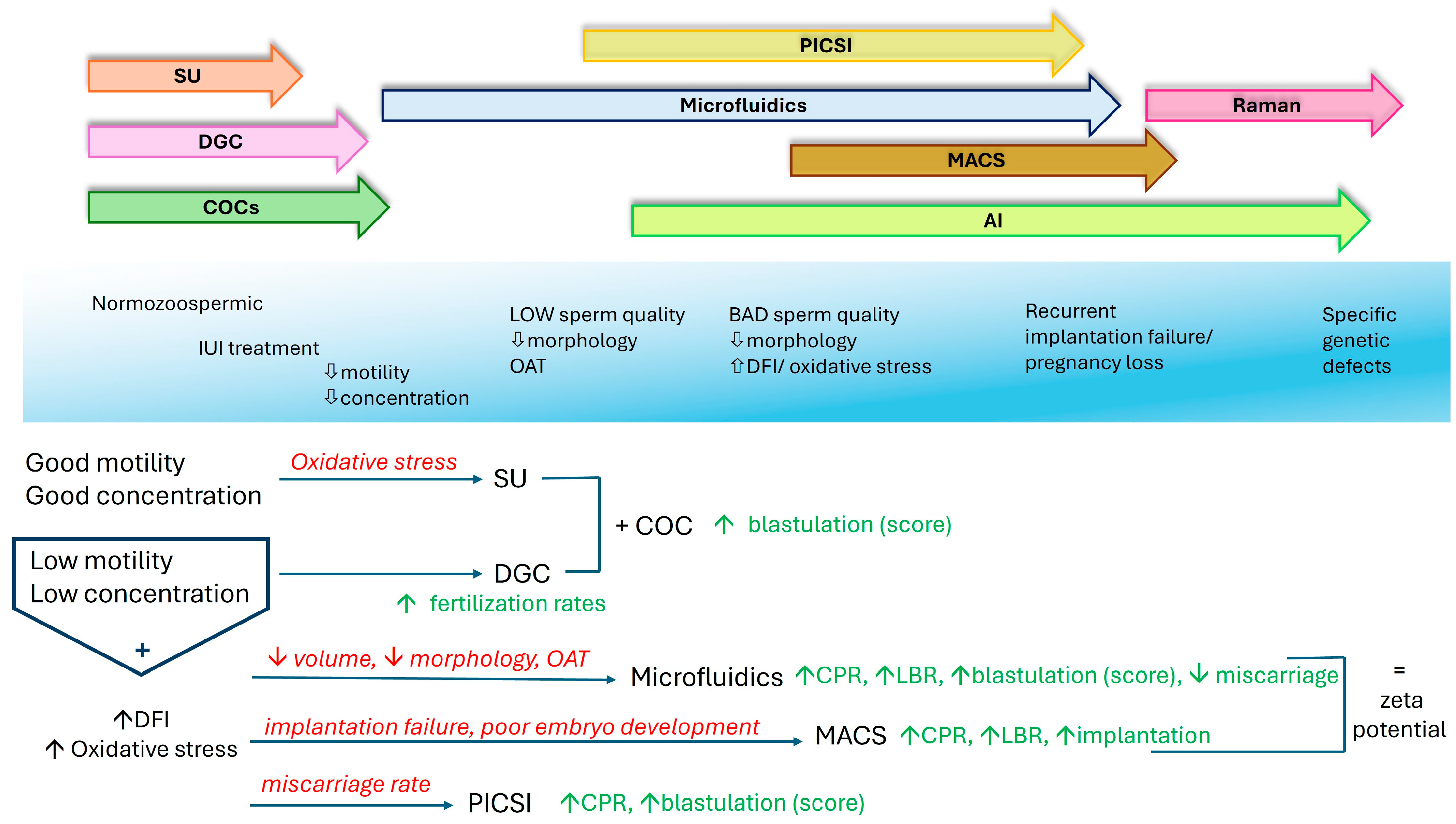
2.3. Comparative Analysis of the Techniques
| Method | Principle | Operator | Reagents-Equipment | Cost-Effectivenss | Invasiveness-Time | Advantages-Limitations | References |
|---|---|---|---|---|---|---|---|
| Swim-up | Separates motile sperm in culture medium. Spermatozoa swim upwords in the supernatant | Easy to perform but requires some essential expertise | Widely available | Cost-effective (~30 euro/cycle) | Non invasive, time efficient | Selects motile sperm with fair DNA integrity—effective for IUI and normal/ fair sperm parameters | [123,142,143,144,145] |
| Sperm with DNA fragmentation remains—ineffective in low motility/ concentration cases | |||||||
| Density Gradient Centrifugation | Seperates sperm through a density gradient by centrifugation | Easy to perform but requires some essential expertise | Widely available | Cost-effective (~50 euro/cycle) | Non invasive, time efficient | Motile sperm with good morphology, removes debris. Improves fertilization rates in oligo/ astheno-zoospermia | [3,32,79,143,146,147,148] |
| Sperm with high DFI may not benefit due to centrifugation, ineffective in severe infertility/ immotile spermatozoa | |||||||
| COC’s barrier | Uses cumulus oophorus complex to mimic natural sperm selection | Easy to perform but requires preparation | In fresh cycles available COCs | Cost-effective (~70 euro/cycle) | Non invasive, time efficient | High motility and morphology sperm, capacitated. Mainly used in combination with SU or DGC to improve blastulation | [86,87,88,89] |
| Limited COC availability in cycles with cryopreserved oocytes | Ineffective in low concentration cases | ||||||
| MACS | Sperm selection based on the exclusion of apoptotic cells with magnetic microbeads coated with antibodies | Requires special training but can be automated | Not universally available, specialized reagents and equipment. Technical considerations for clinical use (calibration - quality guarantee) | High cost (~180 euro/cycle), plus high equipment and functional cost | Non invasive, time efficient | Sperm with intact membranes and low DNA fragmentation. Fertilization and pregnancy rates imrpovent in OS/ repeated implantation failure | [106,109,149,150,151,152,153] |
| Basically used to severe asthenospermia with high DFI and absolute immotility | |||||||
| Microfluidics | Sperm selection through microchannels. Mimics natural selection | Easy to perform but requires some essential expertise | Widely available | Moderate cost (~100 euro/cycle) | Non invasive, time efficient | Sperm with high motility and good morphology. Requires low semen volume. Good for high DFI and OAT to improve CPR, LBR | [121,154,155,156,157,158,159,160] |
| Not suitable for severe oligo or/ and asthenozoospermia and absolute immotility | |||||||
| Zeta Potential Selection | Viable sperm isolation with electrical charge | Easy to perform but requires training | Need for special equipment | High cost (~160 euro/cycle), plus equipment cost | Non invasive, moderate duration | Viable sperm isolation. Reduced sperm loss. Improvement of CPR, LBR | [123,156,161,162,163,164] |
| Not suitable for severe oligo or/ and asthenozoospermia and absolute immotility. Early clinical adoption | |||||||
| Raman Spectroscopy | Molecular fingerprinting of sperm | Requires expert training | Need for special equipme | High cost (~300 euro/cycle), plus high equipment cost | Non invasive, time efficient | Detailed molecular analysis of sperm - genetic profile | [130,131,135,165,166,167,168] |
| Limited clinical validation - ethical considerations. Mostly experimental technique | |||||||
| AI (Artificial Inteligence) | Analyze sperm parameters with algorithms | Automated. Requires extensive data for training | Need for special equipment. Requires IT infrastructure | High cost (~500 euro/data setup), plus high equipment cost | Non invasive, time efficient | High motility and morphology sperm, good DNA integrity. Reduces human error | [128,139,169,170,171] |
| User evaluation is advised. Used mostly for analysis, rather sorting in clinical use | |||||||
| PICSI | Sperm selection with hyaluronan binding. Mimics natural fertilization | Easy to perform but requires ICSI training | Widely available, but needs ICSI equipment | Moderate cost (~100 euro/cycle), ICSI station considered installed | Non invasive, time efficient (combining ICSI duration) | Ideal for OAT, high DFI, high misscarriage rate. Improves blastulation, CPR | [32,125,172,173,174,175,176,177] |
| Not applicable to all infertility types |
3. Discussion
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| MACS | Magnetic-Assisted Cell Sorting |
| DGC | Density Gravity Centrifugation |
| SU | Swim-Up |
| ART | Assisted Reproduction Technology |
| AI | Artificial Intelligence |
| HPV | Human Papilloma Virus |
| HBV | Hepatitis B Virus |
| ICSI | Intracytoplasmic Sperm Injection |
| IVF | In Vitro Fertilization |
| HOS test/HOST | Hypo-Osmotic Swelling Test |
| WHO | World Health Organization |
| CASA | Computer-Assisted Sperm Analysis |
| ROS | Reactive Oxygen Species |
| NO | Nitric Oxide |
| DFI | DNA Fragmentation Index |
| SCD | Sperm Chromatin Dispersion |
| SCSA | Sperm Chromatin Structure Assay |
| OS | Oxidative Stress |
| TUNEL | Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling |
| COC | Cumulus Oophorus Complex |
| PICSI | Physiological Intracytoplasmic Sperm Injection |
| IUI | Intrauterine Insemination |
| CPR | Clinical Pregnancy Rate |
| LBR | Live Birth Rate |
| IMSI | Intracytoplasmic Morphologically Selected Sperm Injection |
| OAT | Oligoasthenoteratozoospermia |
| TESA | Testicular Sperm Aspiration |
| HA | Hyaluronic Acid |
| SDF | Sperm DNA Fragmentation |
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Sharma, R.; Panner Selvam, M.K.; Henkel, R. Male infertility. Lancet 2022, 400, 190–202. [Google Scholar]
- Krausz, C.; Casamonti, E. Genetics of spermatogenic failure. Nat. Rev. Urol. 2017, 14, 73–88. [Google Scholar]
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male infertility due to testicular disorders. J. Clin. Endocrinol. Metab. 2020, 106, e442–e459. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C.; Riera-Escamilla, A. Genetics of male infertility. Nat. Rev. Urol. 2018, 15, 369–384. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Blanco, J.; Vidal, F. Genes in human fertility and infertility. Int. J. Mol. Sci. 2019, 20, 5213. [Google Scholar]
- Skoracka, K.; Eder, P.; Łykowska-Szuber, L.; Dobrowolska, A.; Krela-Kaźmierczak, I. Diet and nutritional factors in male (in)fertility-underestimated factors. J. Clin. Med. 2020, 9, 1400. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Jenkins, T.G.; Carrell, D.T. Diet and sperm quality: Nutrients, foods and dietary patterns. Reprod. Biol. 2019, 19, 219–224. [Google Scholar] [CrossRef]
- Caponecchia, L.; Cimino, G.; Sacchetto, R.; Fiori, C.; Sebastianelli, A.; Salacone, P.; Marcucci, I.; Tomassini, S.; Rago, R. Do malignant diseases affect semen quality? Sperm parameters of men with cancers. Andrologia 2016, 48, 333–340. [Google Scholar] [CrossRef]
- Nissi, J.; Kalam, L.; Catalini, L.; Fedder, J. Effects of chemotherapy on aneuploidy rates in sperm from male patients with testicular cancer or Hodgkins lymphoma—A systematic review. J. Clin. Med. 2024, 13, 3650. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, Y.; Zheng, R.; Yan, J.; Li, W.; Xu, Y.; Yan, X.; Ke, Y.; Li, Y.; Xiang, L. Correlation between viral infections in male semen and infertility: A literature review. Virol. J. 2024, 21, 167. [Google Scholar] [CrossRef]
- Wen, L.; Tian, H.; Huang, X.; Song, T.; Tang, L.; Wei, W.; Tian, S.; Huang, Y.; Zhang, X. Effect of SARS-CoV-2 on semen parameters: A meta-analysis of 39 articles from 15 countries. J. Glob. Health 2024, 14, 05021. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.; Barone, B.; Crocetto, F.; Capece, M.; La Rocca, R. The COVID-19 Pandemic: Is it a wolf consuming fertility? Int. J. Fertil. Steril. 2020, 14, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Graziani, A.; Grande, G.; Ortolani, C.; Ferlin, A. HPV-related diseases in male patients: An underestimated conundrum. J. Endocrinol. Investig. 2024, 47, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Olivera, C.; Paira, D.A.; Olmedo, A.; Olmedo, J.J.; Tissera, A.D.; Molina, R.I.; Motrich, R.D.; Cuffini, C.G.; Rivero, V.E. Impact of high-risk and low-risk human papillomavirus infections on the male genital tract: Effects on semen inflammation and sperm quality. Front. Cell. Infect. Microbiol. 2024, 14, 1420307. [Google Scholar] [CrossRef] [PubMed]
- Notari, T.; Buttà, M.; Serra, N.; Sucato, A.; Rizzo, G.; Capra, G.; Bosco, L. Human papillomavirus and male infertility correlation analysis following World Health Organization 2021 guidelines. Sci. Rep. 2024, 14, 27422. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, G.; Feng, Y.; Zhang, J.; Liu, Y.; Yang, X.; Liu, P.; Feng, Y.; Xia, X. The association between male viral infections and infertility: A systematic review and meta-analysis. Rev. Med. Virol. 2024, 34, e70002. [Google Scholar] [CrossRef]
- Paira, D.A.; Olivera, C.; Tissera, A.D.; Molina, R.I.; Olmedo, J.J.; Rivero, V.E.; Saka, H.A.; Motrich, R.D. Ureaplasma urealyticum and Mycoplasma hominis urogenital infections associate with semen inflammation and decreased sperm quality. J. Leukoc. Biol. 2023, 113, 18–26. [Google Scholar] [CrossRef]
- Xianchun Esteves, F.; Jun, F.; Zhijun, D.; Mingyun, H. Effects of Ureaplasma urealyticum infection on semen quality and sperm morphology. Front. Endocrinol. 2023, 14, 1113130. [Google Scholar] [CrossRef]
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. Clinical and surgical evaluation of infertile males. Int. Braz. J. Urol. 2020, 46, 139–157. [Google Scholar]
- Akhatova, A.; Jones, C.; Coward, K.; Yeste, M. How do lifestyle and environmental factors influence the sperm epigenome? Effects on sperm fertilising ability, embryo development, and offspring health. Clin. Epigenetics 2025, 17, 7. [Google Scholar] [CrossRef]
- Glazer, C.H.; Eisenberg, M.L.; Tøttenborg, S.S.; Sandin, S.; Sørensen, H.T.; Hargreave, M. Male infertility and mortality risk: A Danish study. Fertil. Steril. 2017, 107, 1044–1051. [Google Scholar]
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Martini, F.O.; McLachlan, R.; Tournaye, H. The diagnosis of male infertility: Evidence for WHO guidance. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.D.; Neri, Q.V.; Takeuchi, T.; Sosa, N.; Rosenwaks, Z. IVF and ICSI: Advantages and disadvantages. J. Assist. Reprod. Genet. 2015, 32, 1253–1260. [Google Scholar]
- Schuster, T.G.; Cho, B.; Keller, L.M.; Takayama, S.; Smith, G.D. Microfluidics for sperm sorting. Fertil. Steril. 2021, 116, 284–291. [Google Scholar]
- Cho, C.L.; Esteves, S.C.; Agarwal, A. Novel concepts in male infertility. Int. Braz. J. Urol. 2017, 43, 375–384. [Google Scholar]
- Barazani, Y.; Katz, B.F.; Nagler, H.M.; Stember, D.S. Lifestyle, environment, and male reproductive health. Urology 2014, 84, 784–792. [Google Scholar] [CrossRef]
- Tomlinson, M.J.; Moffatt, O.; Taylor, A. Semen quality and sperm DNA integrity. Fertil. Steril. 2016, 106, 1154–1160. [Google Scholar]
- Agarwal, A.; Majzoub, A. Sperm DNA fragmentation: A critical assessment of clinical practice guidelines. Transl. Androl. Urol. 2017, 6, 30. [Google Scholar] [CrossRef]
- Evenson, D.P.; Wixon, R. Data analysis of two sperm DNA fragmentation assays. Reprod. Biomed. Online 2006, 12, 466–472. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Matsuyama, H. Free radical scavenger levels in seminal plasma. World J. Mens Health 2018, 36, 103–113. [Google Scholar]
- Sharma, R.; Agarwal, A. ROS in male infertility. Biol. Reprod. 2018, 99, 717–724. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef]
- Aitken, R.J.; Curry, B.J. Redox regulation of human sperm function: From capacitation to infertility and DNA damage. Antioxid. Redox Signal. 2012, 16, 964–976. [Google Scholar]
- Saleh, R.; Sallam, H.; Elsuity, M.A.; Dutta, S.; Sengupta, P.; Nasr, A. Antioxidant therapy for infertile couples: A comprehensive review of the current status and consideration of future prospects. Front. Endocrinol. 2025, 15, 1503905. [Google Scholar] [CrossRef]
- Koppers, A.J.; Mitchell, L.A.; Wang, P.; Lin, M.; Aitken, R.J. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem. J. 2011, 436, 687–698. [Google Scholar] [CrossRef]
- Makker Doshi, K.; Agarwal, A.; Sharma, R. Oxidative stress and male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Doshi, S.B.; Khullar, K.; Sharma, R.K.; Agarwal, A. Role of reactive nitrogen species in male infertility. Reprod. Biol. Endocrinol. 2012, 10, 109. [Google Scholar] [CrossRef]
- Esteves, S.C.; Sanchez-Martin, F.; Sanchez-Martin, P.; Schneider, D.T.; Gosálvez, J. Sperm DNA fragmentation in male infertility. J. Androl. 2015, 33, 331–340. [Google Scholar]
- Nasr-Esfahani, M.H.; Razavi, S.; Vahdati, A.A.; Fathi, F.; Tavalaee, M. Evaluation of sperm selection procedure based on hyaluronic acid binding ability on ICSI outcome. J. Assist. Reprod. Genet. 2008, 25, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Miyaoka, R.; Agarwal, A. Update on clinical assessment of infertile males. Clinics 2015, 66, 2073–2080. [Google Scholar] [CrossRef]
- Dirican, E.K.; Ozgün, O.D.; Akarsu, S.; Akin, K.O.; Ercan, O.; Uğurlu, M.; Camsari, C.; Kanyilmaz, O.; Kaya, A.; Unsal, A. Clinical outcome of magnetic activated cell sorting of non-apoptotic spermatozoa before density gradient centrifugation for assisted reproduction. J. Assist. Reprod. Genet. 2008, 25, 375–381. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Q.; Wang, Y.; Li, Y. Effects of high DNA fragmentation in ART. J. Assist. Reprod. Genet. 2018, 35, 943–950. [Google Scholar]
- Zini, A.; Agarwal, A. Sperm DNA fragmentation testing in clinical practice. Fertil. Steril. 2018, 110, 450–454. [Google Scholar]
- Evenson, D.P. The sperm chromatin structure assay (SCSA®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 2016, 169, 56–75. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms and impact on outcomes. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Muriel, L.; Garrido, N.; Fernández, J.L.; Remohí, J.; Pellicer, A.; Meseguer, M. Sperm chromatin dispersion test and pregnancy outcome. Hum. Reprod. 2006, 21, 738–744. [Google Scholar] [CrossRef]
- Yazdanpanah Ghadikolaei, P.; Ghaleno, L.R.; Vesali, S.; Janzamin, E.; Gilani, M.A.S.; Sajadi, H.; Dizaj, A.V.T.; Shahverdi, A.; Drevet, J.R.; Moghadam Masouleh, A.A. Epidemiology of sperm DNA fragmentation in a retrospective cohort of 1191 men. Andrology 2023, 11, 1663–1672. [Google Scholar] [CrossRef]
- Yang, T.; Yu, L.; Xu, J.; Ying, L.; Jia, Y.; Zheng, Y.; Zhou, B.; Li, F. Correlation between standard sperm parameters and sperm DNA fragmentation from 11,339 samples. Syst. Biol. Reprod. Med. 2024, 70, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Váncsa, S.; Hegyi, P.; Váradi, A.; Forintos, A.; Filipov, T.; Ács, J.; Ács, N.; Szarvas, T.; Nyirády, P.; et al. Lifestyle, environmental, and additional health factors associated with an increased sperm DNA fragmentation: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Besada, S.; Lamb, D.J. Proteomic analysis of human sperm. Asian J. Androl. 2015, 17, 545–553. [Google Scholar]
- Liu, K.; Chen, Y. The Mechanism and Clinical Significance of Sperm DNA Damage in Assisted Reproductive. Front. Biosci. 2024, 29, 416. [Google Scholar] [CrossRef]
- Erenpreiss, J.; Spano, M.; Erenpreisa, J.; Bungum, M.; Giwercman, A. Sperm chromatin structure and male fertility: Biological and clinical aspects. Asian J. Androl. 2006, 8, 11–29. [Google Scholar] [CrossRef]
- Dada, R.; Kumar, M.; Jesudasan, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2018, 15, 144–146. [Google Scholar]
- Ferreira Aderaldo, J.; da Silva Maranhão, K.; Ferreira Lanza, D.C. Does microfluidic sperm selection improve clinical pregnancy and miscarriage outcomes in assisted reproductive treatments? A systematic review and meta-analysis. PLoS ONE 2023, 18, e0292891. [Google Scholar] [CrossRef]
- Raheem, K.A.; Pant, N.; Chaturvedi, P.K. Sperm DNA fragmentation in male infertility work-up. Int. J. Androl. 2013, 36, 116–119. [Google Scholar]
- Jeyendran, R.S.; Caroppo, E.; Rouen, A.; Anderson, A.; Puscheck, E. Selecting the most competent sperm for assisted reproductive technologies. Fertil. Steril. 2019, 11, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Shirota, K.; Yotsumoto, F.; Itoh, H.; Obama, H.; Hidaka, N.; Nakajima, K.; Miyamoto, S. Separation efficiency of a microfluidic sperm sorter to minimize sperm DNA damage. Fertil. Steril. 2016, 105, 315–321.e1. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Markou, E.; Zachariou, A.; Stavropoulos, M.; Kratiras, Z.; Symeonidis, E.N.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M. Proteomics and Metabolomics in Varicocele-Associated Male Infertility: Advancing Precision Diagnostics and Therapy. J Clin Med. 2024, 13, 7390. [Google Scholar] [CrossRef]
- Intasqui, P.; Camargo, M.; Bertolla, R.P. Proteomics of male infertility. J. Proteom. 2018, 181, 9–20. [Google Scholar]
- Zhao, X.; Zhou, Y.; Sun, L.; Li, Z.; Yang, H. Sperm bioenergetics and reproductive health. Biol. Reprod. 2018, 99, 77–92. [Google Scholar]
- Castillo, J.; Amaral, A.; Azpiazu, R.; Vavouri, T.; Estanyol, J.M.; Ballescà, J.L.; Oliva, R. Genomic and proteomic dissection and characterization of the human sperm chromatin. Hum. Reprod. Update. 2014, 20, 40–62. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Afeiche, M.C.; Gaskins, A.J.; Williams, P.L.; Mendiola, J.; Jørgensen, N.; Chavarro, J.E. Fruit and vegetable intake, pesticide residues, and semen quality. Hum. Reprod. 2016, 31, 1342–1351. [Google Scholar]
- Dohle, G.R.; Colpi, G.M.; Hargreave, T.B.; Papp, G.K.; Jungwirth, A.; Weidner, W. EAU guidelines on male infertility. EAU Working Group on Male Infertility. Eur. Urol. 2005, 48, 703–711. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, J.Y.; Sun, Z.G. Exploring the impact of environmental factors on male reproductive health through epigenetics. Reprod Toxicol. 2025, 132, 108832. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; James, E.R.; Carrell, D.T.; Kramer, J. Epigenetics and human reproductive failure. Epigenomics 2017, 9, 1583–1599. [Google Scholar]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T. Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for idiopathic male infertility. World J. Mens Health 2022, 40, 197–206. [Google Scholar] [CrossRef]
- Simon, L.; Brunborg, G.; Stevenson, M.; Lutton, D.; McManus, J.; Lewis, S.E. Sperm DNA damage in assisted reproduction outcomes. Hum. Reprod. 2017, 32, 2340–2349. [Google Scholar]
- Prasad, S.; Tiwari, M.; Tripathi, A. Sperm DNA damage and male infertility. Int. J. Health Allied Sci. 2016, 5, 210–218. [Google Scholar]
- Bashiri, A.; Wang, T.; Adams, N.; Wu, S.P.; Young, S.L.; Spencer, T.E.; DeMayo, F. Evaluation and treatment of male infertility: A consensus review. J. Assist. Reprod. Genet. 2014, 31, 603–611. [Google Scholar]
- Henkel, R. Sperm preparation: State-of-the-art—Physiological aspects and application of advanced sperm preparation methods. Asian J. Androl. 2011, 14, 260–269. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, Z.; Shan, G.; Chu, L.T.; Huang, Z.; Moskovtsev, S.; Librach, C.; Jarvi, K.; Sun, Y. Advances in sperm analysis: Techniques, discoveries and applications. Nat. Rev. Urol. 2021, 18, 447–467. [Google Scholar] [CrossRef]
- Fasano, C.; DAndolfi, G.; Di Matteo, L.; Forte, C.; Dale, B.; Tosti, E. Comparison of sperm preparation methods to improve the recovery of mature spermatozoa in sub-fertile males. Zygote 2022, 30, 664–673. [Google Scholar] [CrossRef]
- Charles, D.K.; Lange, M.J.; Ortiz, N.M.; Purcell, S.; Smith, R.P. A narrative review of sperm selection technology for assisted reproduction techniques. Transl. Androl. Urol. 2024, 13, 2119–2133. [Google Scholar] [CrossRef]
- Esteves, S.C.; Agarwal, A.; Majzoub, A. Advanced sperm selection techniques. Reprod. Biol. 2019, 19, 211–222. [Google Scholar]
- De Vos, A.; Van de Velde, H.; Bocken, G.; Eylenbosch, G.; Franceus, N.; Meersdom, G.; Tistaert, S.; Vankelecom, A.; Tournaye, H.; Verheyen, G. Does intracytoplasmic morphologically selected sperm injection improve embryo development? A randomized sibling-oocyte study. Hum. Reprod. 2013, 28, 617–626. [Google Scholar] [CrossRef]
- Muratori, M.; Tarozzi, N.; Carpentiero, F.; Danti, S.; Perrone, F.M.; Cambi, M.; Casini, A.; Azzari, C.; Boni, L.; Maggi, M.; et al. Sperm selection with density gradient centrifugation and swim up: Effect on DNA fragmentation in viable spermatozoa. Sci. Rep. 2019, 9, 7492. [Google Scholar] [CrossRef]
- Palini, S.; Stefani, S.; Primiterra, M.; Benedetti, S.; Barone, S.; Carli, L.; Vaccari, E.; Murat, U.; Feichtinger, W. Comparison of in vitro fertilization outcomes in ICSI cycles after human sperm preparation by density gradient centrifugation and direct micro swim-up without centrifugation. JBRA Assist. Reprod. 2017, 21, 89–93. [Google Scholar] [CrossRef]
- Ricci, G.; Perticarari, S.; Boscolo, R.; Montico, M.; Guaschino, S.; Presani, G. Semen preparation methods and sperm apoptosis: Swim-up versus gradient-density centrifugation technique. Fertil. Steril. 2009, 91, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Carrell, D.T.; Middleton, R.G.; Peterson, C.M.; Jones, K.P.; Urry, R.L. Role of the cumulus in the selection of morphologically normal sperm and induction of the acrosome reaction during human in vitro fertilization. Arch. Androl. 1993, 31, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Franken, D.R.; Bastiaan, H.S. Can a cumulus cell complex be used to select spermatozoa for assisted reproduction? Andrologia 2009, 41, 369–376. [Google Scholar] [CrossRef]
- Rijsdijk, M.C.; Franken, D.R. Use of the capillary-cumulus oophorus model for evaluating the selection of spermatozoa. Fertil. Steril. 2007, 88, 1595–1602. [Google Scholar] [CrossRef]
- Hong, S.J.; Chiu, P.C.; Lee, K.F.; Tse, J.M.; Ho, P.C.; Yeung, W.S. Establishment of a capillary-cumulus model to study the selection of sperm for fertilization by the cumulus oophorus. Hum. Reprod. 2004, 19, 1562–1569. [Google Scholar] [CrossRef]
- Naknam, W.; Salang, L.; Sothornwit, J.; Amnatbuddee, S.; Seejorn, K.; Pongsritasana, T.; Sukkasame, S. Effect of sperm selection method by cumulus oophorus complexes and conventional sperm preparation method on sperm quality and DNA fragmentation for assisted reproduction technology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 46–50. [Google Scholar] [CrossRef]
- Wang, C.; Feng, G.; Shu, J.; Zhou, H.; Zhang, B.; Chen, H.; Lin, R.; Gan, X.; Wu, Z.; Wei, T. Cumulus oophorus complexes favor physiologic selection of spermatozoa for intracytoplasmic sperm injection. Fertil. Steril. 2018, 109, 823–831. [Google Scholar] [CrossRef]
- Sabet, S.; Najafi, M.H.; Tavalaee, M.; Sadeghi, N.; Nasr-Esfahani, M.H. Single-blind clinical trial: Sperm selection based on capacity to pass through cumulus oophorus column improves ICSI outcomes. Andrology 2021, 9, 1560–1570. [Google Scholar] [CrossRef]
- Luongo, F.P.; Perez Casasus, S.; Haxhiu, A.; Barbarulo, F.; Scarcella, M.; Governini, L.; Piomboni, P.; Scarica, C.; Luddi, A. Exposure to cumulus cell secretome improves sperm function: New perspectives for sperm selection in vitro. Cells 2023, 12, 2349. [Google Scholar] [CrossRef]
- Bloch, A.; Rogers, E.J.; Nicolas, C.; Martin-Denavit, T.; Monteiro, M.; Thomas, D.; Morel, H.; Lévy, R.; Siffroi, J.P.; Dupont, C.; et al. Detailed cell-level analysis of sperm nuclear quality among the different hypo-osmotic swelling test (HOST) classes. J. Assist. Reprod. Genet. 2021, 38, 2491–2499. [Google Scholar] [CrossRef]
- Casper, R.F.; Meriano, J.S.; Jarvi, K.A.; Cowan, L.; Lucato, M.L. The hypo-osmotic swelling test for selection of viable sperm for intracytoplasmic injection in men with complete asthenozoospermia. Fertil. Steril. 1996, 65, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Baldini, D.; Ferri, D.; Baldini, G.M.; Lot, D.; Catino, A.; Vizziello, D.; Vizziello, G. Sperm selection for ICSI: Do we have a winner? Cells 2021, 10, 3566. [Google Scholar] [CrossRef] [PubMed]
- Romany, L.; Garrido, N.; Motato, Y.; Aparicio, B.; Remohí, J.; Meseguer, M. Removal of annexin V-positive sperm cells for intracytoplasmic sperm injection in ovum donation cycles does not improve reproductive outcome: A controlled and randomized trial in unselected males. Fertil. Steril. 2014, 102, 1567–1575.e1. [Google Scholar] [CrossRef]
- Kovacic, B.; Vlaisavljevic, V.; Reljic, M. Clinical use of pentoxifylline for activation of immotile testicular sperm before ICSI in patients with azoospermia. J. Androl. 2006, 27, 45–52. [Google Scholar] [CrossRef]
- Gala, B.; Badge, A.; Bawaskar, P.; Gajbe, U.; Singh, B.R.; Kohale, M. The potential of theophylline and pentoxifylline in sperm optimization and its intracytoplasmic sperm injection outcomes. Cureus 2023, 15, e48192. [Google Scholar] [CrossRef]
- Tarlatzis, B.C.; Kolibianakis, E.M.; Bontis, J.; Tousiou, M.; Lagos, S.; Mantalenakis, S. Effect of pentoxifylline on human sperm motility and fertilizing capacity. Arch. Androl. 1995, 34, 33–42. [Google Scholar] [CrossRef]
- Simopoulou Leandri, M.; Gkoles, L.; Bakas, P.; Giannelou, P.; Kalampokas, T.; Pantos, K.; Koutsilieris, M. Improving ICSI: A review from the spermatozoon perspective. Syst. Biol. Reprod. Med. 2016, 62, 359–371. [Google Scholar] [CrossRef]
- Leandri, R.D.; Gachet, A.; Pfeffer, J.; Celebi, C.; Rives, N.; Carre-Pigeon, F.; Kulski, O.; Mitchell, V.; Parinaud, J. Is intracytoplasmic morphologically selected sperm injection (IMSI) beneficial in the first ART cycle? A multicentric randomized controlled trial. Andrology 2013, 1, 692–697. [Google Scholar] [CrossRef]
- Teixeira, D.M.; Hadyme Miyague, A.; Barbosa, M.A.; Navarro, P.A.; Raine-Fenning, N.; Nastri, C.O. Regular (ICSI) versus ultra-high magnification (IMSI) sperm selection for assisted reproduction. Cochrane Database Syst. Rev. 2020, 2, CD010167. [Google Scholar] [CrossRef]
- Baldini, D.; Baldini, A.; Silvestris, E.; Vizziello, G.; Ferri, D.; Vizziello, D. A fast and safe technique for sperm preparation in ICSI treatments within a randomized controlled trial (RCT). Reprod. Biol. Endocrinol. 2020, 18, 88. [Google Scholar] [CrossRef]
- Ghaemi, M.; Seighali, N.; Shafiee, A.; Beiky, M.; Gargari, O.K.; Azarboo, A.; Shafti, V.; Jafarabady, K.; Eshraghi, N.; Haddadi, M.; et al. The effect of Myo-inositol on improving sperm quality and IVF outcomes: A systematic review and meta-analysis. Food Sci. Nutr. 2024, 12, 8515–8524. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Unfer, V.; Facchinetti, F.; Soulage, C.O.; Greene, N.D.; Bizzarri, M.; Laganà, A.S.; Chan, S.Y.; Bevilacqua, A.; Pkhaladze, L.; et al. Inositols: From Established Knowledge to Novel Approaches. Int. J. Mol. Sci. 2021, 22, 10575. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Sobhani, A.; Khalili, M.A.; Agha-Rahimi, A.; Nabi, A.; Findikli, N.J. Comparison of the Efficiency of Magnetic-Activated Cell Sorting (MACS) and Physiological Intracytoplasmic Sperm Injection (PICSI) for Sperm Selection in Cases with Unexplained Infertility. Reprod. Infertil. 2022, 23, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chao, S.; Ye, N.; Ouyang, D. Emerging trends in sperm selection: Enhancing success rates in assisted reproduction. Reprod. Biol. Endocrinol. 2024, 22, 67. [Google Scholar] [CrossRef]
- Ziarati, N.; Tavalaee, M.; Bahadorani, M.; Esfahani, M.H.N. Clinical outcomes of magnetic activated sperm sorting in infertile men candidate for ICSI. Hum. Fertil. 2019, 22, 118–125. [Google Scholar] [CrossRef]
- Mantravadi, K.C.; Rao, D. In cases with raised sperm DNA fragmentation, can sperm selection by magnetic-activated cell sorting or testicular sperm aspiration help improve reproductive outcomes? J. Assist. Reprod. Genet. 2024, 41, 1507–1515. [Google Scholar] [CrossRef]
- Salehi Novin, M.; Mehdizadeh, A.; Artimani, T.; Bakhtiari, M.; Mehdizadeh, M.; Aflatoonian, R.; Zandieh, Z. MACS-DGC sperm preparation method resulted in high-quality sperm, top-quality embryo, and higher blastocyst rate in male factor infertile couples with high DNA fragmented sperm. Hum. Fertil. 2023, 26, 1408–1416. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Barranco, I.; Sorolla-Segura, M.; Llavanera, M.; Delgado-Bermúdez, A.; Yeste, M. Advanced sperm selection strategies as a treatment for infertile couples: A systematic review. Int. J. Mol. Sci. 2022, 23, 13859. [Google Scholar] [CrossRef]
- Pacheco, A.; Blanco, A.; Bronet, F.; Cruz, M.; García-Fernández, J.; García-Velasco, J.A. Magnetic-Activated Cell Sorting (MACS): A useful sperm-selection technique in cases of high levels of sperm DNA fragmentation. J. Clin. Med. 2020, 9, 3976. [Google Scholar] [CrossRef]
- Cakar, Z.; Cetinkaya, B.; Aras, D.; Koca, B.; Ozkavukcu, S.; Kaplanoglu, İ.; Can, A.; Cinar, O. Does combining magnetic-activated cell sorting with density gradient or swim-up improve sperm selection? J. Assist. Reprod. Genet. 2016, 33, 1059–1065. [Google Scholar] [CrossRef]
- Nouri, M.; Sadeghi, M.R.; Nazari, S. The use of magnetic-activated cell sorting (MACS) for sperm selection in assisted reproduction: A review. Reprod. Biol. Endocrinol. 2017, 15, 65. [Google Scholar]
- Bouloorchi Tabalvandani, M.; Saeidpour, Z.; Habibi, Z.; Javadizadeh, S.; Firoozabadi, S.A.; Badieirostami, M. Microfluidics as an emerging paradigm for assisted reproductive technology: A sperm separation perspective. Biotechnol. Bioeng. 2024, 26, 23. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, F.; Giménez Rodríguez, C.; Rivera Egea, R.; Carrión Sisternas, L.; Remohí, J.A.; Meseguer, M. Can Microfluidics Improve Sperm Quality? A Prospective Functional Study. Biomedicines 2024, 12, 1131. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, Y.; Yan, J.; Zhang, J.; Wang, S. Microfluidic chip as a promising evaluation method in assisted reproduction: A systematic review. Bioeng. Transl. Med. 2023, 9, e10625. [Google Scholar] [CrossRef] [PubMed]
- Banti, M.; Van Zyl, E.; Kafetzis, D. Sperm preparation with microfluidic sperm sorting chip may improve intra-cytoplasmic sperm injection outcomes compared to density gradient centrifugation. Reprod. Sci. 2024, 31, 1695–1704. [Google Scholar] [CrossRef]
- Rosenbluth, M.J.; Zhong, X.; Yang, H. Microfluidics for sperm selection: Moving toward clinical applications. Bioeng. Transl. Med. 2014, 1, 3–14. [Google Scholar]
- Giuliani, V.; Pandolfi, C.; Santucci, R.; Pelliccione, F.; Macerola, B.; Focarelli, R.; Rosati, F.; Della Giovampaola, C.; Francavilla, F.; Francavilla, S. Expression of gp20, a human sperm antigen of epididymal origin, is reduced in spermatozoa from subfertile men. Mol. Reprod. Dev. 2004, 69, 235–240. [Google Scholar] [CrossRef]
- Vahidi, S.; Narimani, N.; Dehghan Marvast, L.; Mangoli, E.; Nabi, A.; Sadeghi, M. Comparison of zeta potential and physiological intracytoplasmic sperm injection in obtaining sperms with a lower DNA fragmentation index: A cross-sectional study. Int. J. Reprod. Biomed. 2022, 20, 357–364. [Google Scholar] [CrossRef]
- Chan, P.J.; Jacobson, J.D.; Corselli, J.U.; Patton, W.C. A simple zeta method for sperm selection based on membrane charge. Fertil. Steril. 2006, 85, 481–486. [Google Scholar] [CrossRef]
- Ghorbani-Sini, R.; Izadi, T.; Tavalaee, M.; Azadi, L.; Hajian, M.; Rahimi Zamani, M.; Nasr-Esfahani, M.H. Comparison of sperm telomere length between two sperm selection procedures: Density gradient centrifugation and zeta potential. Int. J. Fertil. Steril. 2020, 14, 51–56. [Google Scholar] [CrossRef]
- Zahedi, A.; Tavalaee, M.; Deemeh, M.R.; Azadi, L.; Fazilati, M.; Nasr-Esfahani, M.H. Zeta potential vs apoptotic marker: Which is more suitable for ICSI sperm selection? J. Assist. Reprod. Genet. 2013, 30, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Ionov, M.; Gontarek, W.; Bryszewska, M. Zeta potential technique for analyzing semen quality. MethodsX 2020, 7, 100895. [Google Scholar] [CrossRef] [PubMed]
- Vahidi, N.; Eyni, H.; Sabz, F.T.K.; Narimani, N.; Zandieh, Z.; Amjadi, F. Microfluidic in comparison with Zeta potential, MACS and swim up methods, resulted in improved chromatin integrity and high-quality sperms. JBRA Assist. Reprod. 2024, 20, 357–364. [Google Scholar] [CrossRef]
- Bui, M.; Lim, J.; Lee, C. Zeta potential analysis for sperm selection: Potential and challenges. Reprod. Sci. 2020, 27, 473–482. [Google Scholar]
- Lepine, S.; McDowell, S.; Searle, L.M.; Kroon, B.; Glujovsky, D.; Yazdani, A. Advanced sperm selection techniques for assisted reproduction. Cochrane Database Syst. Rev. 2019, 2019, CD010461. [Google Scholar] [CrossRef]
- Glatthorn, H.N.; Decherney, A. The efficacy of add-ons: Selected IVF “add-on” procedures and future directions. J. Assist. Reprod. Genet. 2022, 39, 581–589. [Google Scholar] [CrossRef]
- Hussein, A.M.; El-Shafei, A.; Abdelrahman, M. The role of PICSI in sperm selection for ICSI: A systematic review. Reprod. BioMedicine Online 2020, 41, 180–188. [Google Scholar]
- Zheng, C.; Zhang, L.; Huang, H.; Wang, X.; Van Schepdael, A.; Ye, J. Raman spectroscopy: A promising analytical tool used in human reproductive medicine. J. Pharm. Biomed. Anal. 2024, 249, 116366. [Google Scholar] [CrossRef]
- Amaral, S.; Da Costa, R.; Wübbeling, F.; Redmann, K.; Schlatt, S. Raman micro-spectroscopy analysis of different sperm regions: A species comparison. Mol. Hum. Reprod. 2018, 24, 185–202. [Google Scholar] [CrossRef]
- Mallidis, C.; Sanchez, V.; Wistuba, J.; Wübbeling, F.; Burger, M.; Fallnich, C.; Schlatt, S. Raman microspectroscopy: Shining a new light on reproductive medicine. Hum. Reprod. Update 2014, 20, 403–414. [Google Scholar] [CrossRef]
- Li, M.; Ji, Y.; Wang, D.; Zhang, Y.; Zhang, H.; Tang, Y.; Lin, G.; Hu, L. Evaluation of laser confocal Raman spectroscopy as a non-invasive method for detecting sperm DNA contents. Front. Physiol. 2022, 13, 827941. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, V.; Redmann, K.; Wistuba, J.; Wübbeling, F.; Burger, M.; Oldenhof, H.; Wolkers, W.F.; Kliesch, S.; Schlatt, S. Oxidative DNA damage in human sperm can be detected by Raman microspectroscopy. Fertil. Steril. 2012, 98, 1124–1129.e1-3. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Dechant, M.; Sanchez, V.; Wistuba, J.; Boiani, M.; Pilatz, A.; Stammler, A.; Middendorff, R.; Schuler, G.; Bhushan, S.; et al. Structural and functional integrity of spermatozoa is compromised as a consequence of acute uropathogenic E. coli-associated epididymitis. Biol. Reprod. 2013, 89, 59. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, A.; Trzciński, M.; Kościński, J. Raman spectroscopy for sperm quality assessment: Applications in assisted reproduction. J. Microsc. 2019, 276, 112–120. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique look at the impact of sperm DNA damage on assisted reproductive outcomes. J. Urol. 2017, 197, 1460–1467. [Google Scholar]
- Kandel, M.E.; Rubessa, M.; He, Y.R.; Schreiber, S.; Meyers, S.; Matter Naves, L.; Sermersheim, M.K.; Sell, G.S.; Szewczyk, M.J.; Sobh, N.; et al. Reproductive outcomes predicted by phase imaging with computational specificity of spermatozoon ultrastructure. Proc. Natl. Acad. Sci. USA 2020, 117, 28647–28656. [Google Scholar] [CrossRef]
- Ory, J.; Tradewell, M.B.; Blankstein, U.; Lima, T.F.; Nackeeran, S.; Gonzalez, D.C.; Nwefo, E.; Moryousef, J.; Madhusoodanan, V.; Lau, S.; et al. Artificial intelligence based machine learning models predict sperm parameter upgrading after varicocele repair: A multi-institutional analysis. World J. Men’s Health 2024, 40, 618. [Google Scholar] [CrossRef]
- Bromfield, E.; An, J.; Tu, X. Artificial intelligence for sperm analysis: A comprehensive review. Reprod. BioMedicine Online 2020, 41, 205–216. [Google Scholar]
- Kharazmi, E.; Vahidi, R.; Shamsi, M.B. Artificial intelligence in sperm selection: Current applications and future perspectives. Hum. Reprod. Update 2022, 28, 1021–1037. [Google Scholar]
- Vaughan, D.A.; Sakkas, D. Sperm selection methods in the 21st century. Biol. Reprod. 2019, 101, 1076–1082. [Google Scholar] [CrossRef]
- Marzano, G.; Chiriacò, M.S.; Primiceri, E.; Dell’aquila, M.E.; Ramalho-Santos, J.; Zara, V.; Ferramosca, A.; Maruccio, G. Sperm selection in assisted reproduction: A review of established methods and cutting-edge possibilities. Biotechnol. Adv. 2019, 40, 107498. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; Orsi, M.G.; Bagnulo, F.; Del Mondo, D.; Vigilante, L.; De Rosa, M.; Sciorio, R.; Conforti, A.; Fleming, S.; Alviggi, C. Advanced Sperm Selection Techniques for Assisted Reproduction. J. Pers Med. 2024, 14, 726. [Google Scholar] [CrossRef] [PubMed]
- Esteves, S.C.; Roque, M.; Bedoschi, G.; Haahr, T.; Humaidan, P.; Elter, K. ICSI for male infertility and offspring outcomes. Nat. Rev. Urol. 2020, 17, 233–252. [Google Scholar]
- Tsou, T.C.; Ray, S.; Maruf, M.; Kohn, T.P.; Zaman, M.H.; Ayenew, M.F.; George, A.K.; Herati, A.S. Methods and Efficacy of Processing Testicular Sperm Samples in Obstructive and Non-Obstructive Azoospermia: A Systematic Review. J. Mens Health. 2024, 20, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Feyzioglu, B.S.; Avul, Z. Effects of sperm separation methods before intrauterine insemination on pregnancy outcomes and live birth rates: Differences between the swim-up and microfluidic chip techniques. Medicine 2023, 102, e36042. [Google Scholar] [CrossRef]
- Madaan, M.; Saha, S.; Saravanan, S.; Naha, M.; Parikh, H. Sperm preparation techniques in ART. Fertil. Steril. 2022, 118, 345–355. [Google Scholar]
- Amano, K.; Oigawa, S.; Ichizawa, K.; Tokuda, Y.; Unagami, M.; Sekiguchi, M.; Furui, M.; Nakaoka, K.; Ito, A.; Hayashi, R.; et al. Swim-up method is superior to density gradient centrifugation for preserving sperm DNA integrity during sperm processing. Reprod. Med. Biol. 2024, 23, e12562. [Google Scholar] [CrossRef]
- Guler, C.; Melil, S.; Ozekici, U.; Donmez Cakil, Y.; Selam, B.; Cincik, M. Sperm Selection and Embryo Development: A Comparison of the Density Gradient Centrifugation and Microfluidic Chip Sperm Preparation Methods in Patients with Astheno-Teratozoospermia. Life 2021, 11, 933. [Google Scholar] [CrossRef]
- Mei, J.; Chen, L.J.; Zhu, X.X.; Yu, W.; Gao, Q.Q.; Sun, H.X.; Ding, L.J.; Wang, J.X. Magnetic-activated cell sorting of nonapoptotic spermatozoa with a high DNA fragmentation index improves the live birth rate and decreases transfer cycles of IVF/ICSI. Asian J. Androl. 2022, 24, 367–372. [Google Scholar] [CrossRef]
- Baldi, E.; Colpi, G.M.; Huang, Z.W.; Balagobi, B.; Boitrelle, F.; Shah, R.; Agarwal, A. High sperm DNA fragmentation—finding a needle in the haystack: Tips on selecting the best sperm for ICSI and ART. Asian J. Androl. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Gil Juliá, M.; Hervás, I.; Navarro-Gómez Lechón, A.; Quintana, F.; Amorós, D.; Pacheco, A.; González-Ravina, C.; Rivera-Egea, R.; Garrido, N. Sperm Selection by Magnetic-Activated Cell Sorting before Microinjection of Autologous Oocytes Increases Cumulative Live Birth Rates with Limited Clinical Impact: A Retrospective Study in Unselected Males. Biology 2021, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Deemeh, M.R.; Mesbah-Namin, S.A.; Movahedin, M. Selecting motile, non-apoptotic and induced spermatozoa for capacitation without centrifuging by MACS-Up method. Andrologia 2022, 54, e14405. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K. Magnetic sorting of sperm: Advances and applications in clinical practice. J. Assist. Reprod. Genet. 2021, 24, 367–372. [Google Scholar]
- Bhat, G.R.; Lone, F.A.; Dalal, J. Microfluidics-A novel technique for high-quality sperm selection for greater ART outcomes. FASEB Bioadvances 2024, 6, 406–423. [Google Scholar] [CrossRef]
- Jahangiri, J.; Xie, Q.; Zhang, Y.; Xiao, Q.; Liu, X.; Qiao, C.; Tian, Y. Advances in microfluidic technology for sperm screening and in vitro fertilization. Anal. Bioanal. Chem. 2024, 416, 3717–3735. [Google Scholar]
- Sadeghi, M.; Bakhtiari, M.; Ghasemi, M. Advanced sperm selection techniques: An overview and impact on ART outcomes. J. Assist. Reprod. Genet. 2021, 38, 617–626. [Google Scholar]
- Hsu, C.T.; Lee, C.I.; Lin, F.S.; Wang, F.Z.; Chang, H.C.; Wang, T.E.; Huang, C.C.; Tsao, H.M.; Lee, M.S.; Agarwal, A. Live motile sperm sorting device for enhanced sperm-fertilization competency: Comparative analysis with density-gradient centrifugation and microfluidic sperm sorting. J. Assist. Reprod. Genet. 2023, 40, 1855–1864. [Google Scholar] [CrossRef]
- Martínez-González, D.; Olmedo, J.; Rios, E. Novel approaches in sperm sorting: Progress and clinical applications. Clin. Reprod. Res. 2020, 29, 301–315. [Google Scholar]
- Cabello, Y.; Belchín, P.; González-Martínez, M.; López-Fernández, C.; Johnston, S.; Gosálvez, J. The efficacy of novel centrifugation-free sperm selection (Io-Lix) on sperm parameters and ICSI reproductive outcomes. Reprod. Biomed. Online 2023, 46, 267–273. [Google Scholar] [CrossRef]
- Aydın, Ş.; Kılıçdağ, E.B.; Aytaç, P.Ç.; Çok, T.; Şimşek, E.; Haydardedeoğlu, B. Prospective randomized controlled study of a microfluidic chip technology for sperm selection in male infertility patients. Andrologia 2022, 54, e14415. [Google Scholar] [CrossRef]
- Singh, S.; Nair, N.; Pareek, C.; More, A.; Kalbande, A. Enhancing intracytoplasmic sperm injection outcomes with zeta sperm selection: A case report. Cureus 2024, 16, e64809. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Li, Y.; Wei, D. Current insights on sperm selection technologies and their clinical impact. Hum. Reprod. Update 2023, 29, 245–261. [Google Scholar]
- Wei, W.; Xu, B.; Shi, Y. Enhancing sperm quality in assisted reproduction: A review of advanced techniques. Andrology 2021, 9, 345–356. [Google Scholar]
- Moghaddam, S.M.; Azizi, Y.; Taran, P. Techniques in sperm selection for ART and their influence on pregnancy rates. J. Reprod. Med. 2020, 25, 145–152. [Google Scholar]
- Pedrosa, M.L.; Furtado, M.H.; Ferreira, M.C.F.; Carneiro, M.M. Sperm selection in IVF: The long and winding road from bench to bedside. JBRA Assist. Reprod. 2020, 24, 332–339. [Google Scholar] [CrossRef]
- Ramos, L.; Giraud, M.N.; Heijnen, E. Current trends in sperm selection: Implications for ART outcomes. Fertil. Steril. 2022, 117, 213–222. [Google Scholar]
- Tsutsui, T.; Ishii, K.; Sakai, T. Microfluidic sperm selection: A promising tool for improving ART outcomes. Reprod. Biol. Endocrinol. 2021, 19, 56–64. [Google Scholar]
- Zhao, C.; Lee, K.; Chen, J. Sperm selection technologies in ART: A comprehensive review. Front. Endocrinol. 2020, 11, 278–288. [Google Scholar] [CrossRef]
- Cherouveim, P.; Velmahos, C.; Bormann, C.L. Artificial intelligence for sperm selection—A systematic review. Fertil. Steril. 2023, 120, 24–31. [Google Scholar] [CrossRef]
- Hammadeh, M.E.; Anke, M.; Renneberg, E. Novel methods in sperm selection for assisted reproduction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 44–52. [Google Scholar]
- Wu, F.; Wang, X.; Lu, Z. Sperm selection for ART: A new horizon. J. Reprod. Health 2020, 12, 312–320. [Google Scholar]
- Liperis, G.; Sharma, K.; Ammar, O.F.; Fraire-Zamora, J.J.; da Silva, S.M.; Thomson, A.; Pini, T.; Mincheva, M. #ESHREjc report: Are sperm selection techniques a panacea? Indications for the use of physiological intracytoplasmic sperm injection (PICSI) in medically assisted reproduction. Hum. Reprod. 2022, 37, 2492–2496. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Zhang, Y.; Liu, X. Sperm selection and ART outcomes: Recent advances and future directions. Asian J. Androl. 2021, 23, 565–574. [Google Scholar]
- Saeed, S.; Jamal, F.; Nawaz, R. Approaches in sperm sorting for improving fertility outcomes in ART. J. Assist. Reprod. Genet. 2022, 39, 879–889. [Google Scholar]
- Mosa, K.; Hossen, F.; Rahman, T. New sperm selection techniques for ART: A clinical review. Middle East Fertil. Soc. J. 2020, 25, 159–166. [Google Scholar]
- Coticchio, G.; Guglielmo, M.C.; De Santis, L. Trends in sperm selection: Evaluation of current methods and new approaches. Hum. Reprod. 2021, 36, 78–88. [Google Scholar]
- Hozyen, M.; Hasanen, E.; Elqusi, K.; ElTanbouly, S.; Gamal, S.; Hussin, A.G.; AlKhader, H.; Zaki, H. Reproductive outcomes of different sperm selection techniques for ICSI patients with abnormal sperm DNA fragmentation: A randomized controlled trial. Reprod. Sci. 2022, 29, 220–228. [Google Scholar] [CrossRef]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative stress in an assisted reproductive techniques setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, F.Y.; He, X.J.; Tang, S.H.; Long, T.; Peng, L.; Zhang, H.M.; Zou, Z.Z.; Xiong, Z.; Zhang, X.P. The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology. Open Life Sci. 2023, 18, 20220597. [Google Scholar] [CrossRef]
- Braga, D.P.A.F.; Setti, A.; Morishima, C.; Provenza, R.R.; Iaconelli, A., Jr.; Borges, E., Jr. The effect of sperm DNA fragmentation on ICSI outcomes depending on oocyte quality. Andrology 2023, 11, 1682–1693. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, C.; Franchi, A.; Duran, H.; Oehninger, S. DNA fragmentation of normal spermatozoa negatively impacts embryo quality and intracytoplasmic sperm injection outcome. Fertil. Steril. 2010, 94, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Siddhartha, N.; Reddy, N.S.; Pandurangi, M.; Muthusamy, T.; Vembu, R.; Kasinathan, K. The effect of sperm DNA fragmentation index on the outcome of intrauterine insemination and intracytoplasmic sperm injection. J. Hum. Reprod. Sci. 2019, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhu, L.; Gao, L.; Wang, J.; Fang, J.; Chen, L.; Wang, S. What sperm parameters affect blastocyst formation and quality during ICSI with severe male infertility. Syst. Biol. Reprod. Med. 2024, 70, 218–227. [Google Scholar] [CrossRef]
- Tarozzi, N.; Nadalini, M.; Borini, A. Effect on Sperm DNA Quality Following Sperm Selection for ART: New Insights. Adv. Exp. Med. Biol. 2019, 1166, 169–187. [Google Scholar] [CrossRef]
- Mendizabal-Ruiz, G.; Paredes, O.; Álvarez, Á.; Acosta-Gómez, F.; Hernández-Morales, E.; González-Sandoval, J.; Mendez-Zavala, C.; Borrayo, E.; Chavez-Badiola, A. Artificial Intelligence in Human Reproduction. Arch. Med. Res. 2024, 55, 103131. [Google Scholar] [CrossRef]
- Tsutsui Rao, M.; Tang, L.; Wang, L.; Chen, M.; Yan, G.; Zhao, S. Cumulative live birth rates after IVF/ICSI cycles with sperm prepared by density gradient centrifugation vs. swim-up: A retrospective study using a propensity score-matching analysis. Reprod. Biol. Endocrinol. 2022, 20, 60. [Google Scholar] [CrossRef]
- You, J.B.; McCallum, C.; Wang, Y.; Riordon, J.; Nosrati, R.; Sinton, D. Machine learning for sperm selection. Nat. Rev. Urol. 2021, 18, 387–403. [Google Scholar] [CrossRef]
- Keskin, M.; Pabuçcu, E.G.; Arslanca, T.; Demirkıran, Ö.D.; Pabuçcu, R. Does microfluidic sperm sorting affect embryo euploidy rates in couples with high sperm DNA fragmentation? Reprod. Sci. 2022, 29, 1801–1808. [Google Scholar] [CrossRef]
- Godiwala, P.; Kwieraga, J.; Almanza, E.; Neuber, E.; Grow, D.; Benadiva, C.; Makhijani, R.; DiLuigi, A.; Schmidt, D.; Bartolucci, A.; et al. The impact of microfluidics sperm processing on blastocyst euploidy rates compared with density gradient centrifugation: A sibling oocyte double-blinded prospective randomized clinical trial. Fertil. Steril. 2024, 122, 85–94. [Google Scholar] [CrossRef]
- Gavriil, E.; Desli, A.; Geladaris, V.; Kachpani, E.; Neofytou, E.; Tatsi, P.; Dovas, D. Embryology outcomes of a device-based sperm separation technique compared to density gradient centrifugation using thawed spermatozoa—A sibling donor oocyte study. J. Assist. Reprod. Genet. 2024, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lara-Cerrillo, S.; Jiménez Macedo, R.; Hortal, O.; Rosado Iglesias, C.; Lacruz Ruiz, T.; Carrera, J.; García Peiró, A. Impact of microfluidic sperm sorting on embryonic euploidy in infertile patients with sperm DNA damage: A retrospective study. Int. J. Fertil. Steril. 2024, 18, 417–423. [Google Scholar] [CrossRef]
- Lara-Cerrillo, S.; Muñoz, C.U.; Heras, M.d.l.C.; Fernández-Pacheco, S.C.; de la Santa, J.G.; Lacruz-Ruiz, T.; Rosado-Iglesias, C.; Gonçalves-Aponte, V.; Liébana, V.B.; García-Peiró, A. Microfluidic sperm sorting improves ICSI outcomes in patients with increased values of Double-Strand Breaks in sperm DNA. Rev. Int. Andrología 2023, 21, 100338. [Google Scholar] [CrossRef]
- Norozi-Hafshejani, M.; Tavalaee, M.; Najafi, M.H.; Shapour, F.; Arbabian, M.; Nasr-Esfahani, M.H. MACS-DGC versus DGC sperm wash procedure: Comparing clinical outcomes in couples with male factor infertility undergoing ICSI: A clinical trial study. Int. J. Fertil. Steril. 2022, 16, 17–22. [Google Scholar] [CrossRef]
- Hasanen, E.; Elqusi, K.; ElTanbouly, S.; Hussin, A.E.; AlKhadr, H.; Zaki, H.; Henkel, R.; Agarwal, A. PICSI vs. MACS for abnormal sperm DNA fragmentation ICSI cases: A prospective randomized trial. J. Assist. Reprod. Genet. 2020, 37, 2605–2613. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, H.; Kim, T.H.; Jeong, J.; Lee, W.S.; Lyu, S.W. Effect of sperm selection using hyaluronan on fertilization and quality of cleavage-stage embryos in intracytoplasmic sperm injection (ICSI) cycles of couples with severe teratozoospermia. Gynecol. Endocrinol. 2020, 36, 456–459. [Google Scholar] [CrossRef]
- Shivhare, S.; Karunakaran, S.; Bose, A.S.; Goel, R.; Ananthakrishnan, R. Does physiological intracytoplasmic sperm injection improve outcome in men with abnormal semen parameters: A retrospective cohort study. J. Hum. Reprod. Sci. 2024, 17, 200–206. [Google Scholar] [CrossRef]
- Scaruffi, P.; Bovis, F.; Casciano, I.; Maccarini, E.; De Leo, C.; Gazzo, I.; Massarotti, C.; Sozzi, F.; Stigliani, S.; Anserini, P. Hyaluronic acid-sperm selection significantly improves the clinical outcome of couples with previous ICSI cycles failure. Andrology 2022, 10, 677–685. [Google Scholar] [CrossRef]
- Novoselsky Persky, M.; Hershko-Klement, A.; Solnica, A.; Bdolah, Y.; Hurwitz, A.; Ketzin El Gilad, M.; Nefesh, I.; Esh-Broder, E. Conventional ICSI vs. physiological selection of spermatozoa for ICSI (PICSI) in sibling oocytes. Andrology 2021, 9, 873–877. [Google Scholar] [CrossRef]
- Inoue, T.; Iwayama, H.; Uemura, M.; Taguchi, S.; Yamashita, Y. A hyaluronic acid-containing reagent compatible with glass-bottom dishes and capable of sustained binding of human spermatozoa. Andrology 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, H.T.T.; Van Nguyen, T.; Nguyen, T.T.T.; Dang, H.N.T.; Dang, T.C.; Nguyen, Q.H.V. Physiological intracytoplasmic sperm injection does not improve the quality of embryos: A cross-sectional investigation on sibling oocytes. Clin. Exp. Reprod. Med. 2023, 50, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Emirdar, V.; Karatasli, V.; Tamer, B.; Pala, I.; Gunturkun, F.; Ozbaykus, C.; Işık, A.Z.; Gode, F. Influence of a hyaluronan-binding system for sperm selection in intracytoplasmic sperm injection cycles on embryo morphokinetic parameters and in vitro fertilization cycle outcomes. Arch. Gynecol. Obstet. 2023, 307, 1633–1639. [Google Scholar] [CrossRef]
- Anifandis, G.; Bounartzi, T.; Messini, C.I.; Dafopoulos, K.; Markandona, R.; Sotiriou, S.; Tzavella, A.; Messinis, I.E. Sperm DNA fragmentation measured by Halosperm does not impact on embryo quality and ongoing pregnancy rates in IVF/ICSI treatments. Andrologia 2016, 47, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Mehrjerd, A.; Dehghani, T.; Jajroudi, M.; Eslami, S.; Rezaei, H.; Ghaebi, N.K. Ensemble machine learning models for sperm quality evaluation concerning success rate of clinical pregnancy in assisted reproductive techniques. Sci. Rep. 2024, 14, 24283. [Google Scholar] [CrossRef]
- Buchanan, A.; Brock, D.W.; Daniels, N.; Wikler, D. From Chance to Choice: Genetics and Justice; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Spar, D. The Baby Business: How Money, Science, and Politics Drive the Commerce of Conception; Harvard Business Press: Boston, MA, USA, 2006. [Google Scholar]
- Glover, J. Choosing Children: Genes, Disability, and Design; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Darnovsky, M. The ethics of human genetic enhancement. Hastings Cent. Rep. 2015, 45, 1–3. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiptiri-Kourpeti, A.; Asimakopoulos, B.; Nikolettos, N. A Narrative Review on the Sperm Selection Methods in Assisted Reproductive Technology: Out with the New, the Old Is Better? J. Clin. Med. 2025, 14, 1066. https://doi.org/10.3390/jcm14041066
Tiptiri-Kourpeti A, Asimakopoulos B, Nikolettos N. A Narrative Review on the Sperm Selection Methods in Assisted Reproductive Technology: Out with the New, the Old Is Better? Journal of Clinical Medicine. 2025; 14(4):1066. https://doi.org/10.3390/jcm14041066
Chicago/Turabian StyleTiptiri-Kourpeti, Angeliki, Byron Asimakopoulos, and Nikolaos Nikolettos. 2025. "A Narrative Review on the Sperm Selection Methods in Assisted Reproductive Technology: Out with the New, the Old Is Better?" Journal of Clinical Medicine 14, no. 4: 1066. https://doi.org/10.3390/jcm14041066
APA StyleTiptiri-Kourpeti, A., Asimakopoulos, B., & Nikolettos, N. (2025). A Narrative Review on the Sperm Selection Methods in Assisted Reproductive Technology: Out with the New, the Old Is Better? Journal of Clinical Medicine, 14(4), 1066. https://doi.org/10.3390/jcm14041066






