Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives
Abstract
1. Introduction
2. The Biological Therapy in Selected Allergic Diseases: Current Standards and Future Perspectives
2.1. Asthma
2.1.1. Epidemiology and Current Landscape
2.1.2. Asthma Phenotypes: T2 vs. Non-T2
2.1.3. Biologic Therapies for T2-High Asthma
2.1.4. Biologic Therapies for Non-T2 Asthma
2.1.5. Emerging Biologics in Asthma
2.1.6. Role of Biomarkers in the Biological Treatment of Asthma
2.2. Atopic Dermatitis
2.2.1. Epidemiology, Clinics, and Current Standard of Care
2.2.2. Emerging Biologic Agents for Atopic Dermatitis
| Drug | Molecular Target | Study Register | Study Type | Phase | Main Findings/Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Asthma | ||||||
| Depemokimab | IL-5 | NCT04719832 NCT04718103 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 3a | Reduced AER; no improvement in QoL (SGRQ score) | [43] |
| Lebrikizumab | IL-13 | NCT01875003 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 3 | Lebrikizumab (37.5 and 125 mg) reduced exacerbation rates by 40–51%, with greater effect in patients with higher eosinophil counts (≥300 cells/μL). | [50] |
| Tozorakimab (MEDI3506) | IL-33 | NCT04570657 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 2a | Tozorakimab improved prebronchodilator FEV1 in patients with ≥2 exacerbations but showed no overall improvement at week 16 compared to placebo. | [54] |
| Astegolimab | ST2/IL-33R | NCT02918019 | Randomized, double-blind, placebo-controlled, multicenter, multi-arm study | 2b | Astegolimab (490 mg) reduced AER by 43% overall and 54% in the eosinophil-low subgroup compared to placebo. | [61] |
| Itepekimab | IL-33 | NCT03387852 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 2 | Itepekimab monotherapy improved FEV1, asthma control, and QoL compared to placebo, with lower asthma control loss (22% vs. 41%). Adverse events were mild-to-moderate and similar across groups. | [56] |
| Melrilimab (CNTO 7160) | IL33R | NCT03393806 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 2a | The study terminated early due to high screen failure and low enrollment; melrilimab reduced free sST2 concentration but showed no change in eosinophil count compared to placebo at week 12. | [57] |
| Melrilimab (CNTO 7160) | IL33R | NCT03207243 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 2a | Melrilimab reduced asthma control loss by 18% over 16 weeks but had higher TRAE incidence (10% vs. 4%), primarily cardiac and musculoskeletal disorders. | [59] |
| Risankizumab | IL-23 | NCT02443298 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 2a | Risankizumab was well-tolerated but associated with a shorter time to asthma worsening (HR 1.46) and higher annualized worsening rates compared to placebo. | [65] |
| Ecleralimab (CSJ117) | TSLP | NCT04410523 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter, multi-national study | 2 | Only the 0.5 mg dose of Ecleralimab achieved a 12% FEV1 improvement at week 8; no significant changes in FeNO were observed for any dose. | NA |
| REGN1908-190 (1:1 cocktail of monoclonal antibodies) | Fel d 1 | NCT03838731 | Randomized, double-blind, placebo-controlled, parallel-group, single-center study | 2 | A single dose of REGN1908/1909 prevented cat allergen-induced FEV1 decline (days 8–85) and early asthmatic response, increasing allergen tolerance on day 85 (54 vs. 12.9 ng). | [69] |
| MTPS9579A | Tryptase | NCT04092582 | Randomized, double-blind, placebo-controlled, multicenter study | 2a | MTPS9579A failed to meet the primary or secondary endpoints, showing no significant difference from placebo over 48 weeks. | [74] |
| Atopic dermatitis | ||||||
| Bermekimab | IL-1α | NCT03496974 NCT04021862 NCT04791319 NCT04990440 | (1) Open-label, dose-escalation study (2) Double-blind, placebo-controlled, randomized study (3) Double-blind, placebo- and active-comparator-controlled, randomized study (4) Double-blind, placebo-controlled, randomized study | 2 | No efficacy in larger controlled trials. | [99] |
| Lebrikizumab | IL-13 | NCT04146363 NCT04178967 | Randomized, double-blind, placebo-controlled study | 3 | Lebrikizumab maintained IGA and EASI-75 improvements (71.2–81.7%) with Q2W and Q4W dosing; 63% of patients reported mild-to-moderate TEAEs. | [100] |
| Stapokibart (CM310) | IL-4 | NCT04893707 | Multicenter, open-label, nonrandomized study | 2 | High clinical efficacy of stapokibart treatment with acceptable safety profile of the drug. | [84] |
| Itepekimab | IL-33 | NCT03738423 NCT03736967 | (1) Dose-ranging, randomized, double-blind, placebo-controlled, parallel-group study (2) Proof-of-concept randomized, double-blind, placebo-controlled study | 2 | Trials were prematurely terminated due to a lack of clinical benefits with itepekimab. | [106] |
| Nemolizumab | IL-31Rα | NCT03985943 NCT03989349 | Replicate, randomized, double-blind, placebo-controlled, multicenter, parallel-group study | 3 | Nemolizumab significantly reduced pruritus (VAS) and improved EASI scores, with no increase in adverse event incidence compared to placebo. | [110] |
| Cendakimab | IL-13Rα1 and IL-13Rα2 | NCT04800315 | Randomized, double-blind, placebo-controlled, parallel-group, dose-ranging study | 2 | Cendakimab was effective, well-tolerated, and generally safe. | [102] |
| Rademikibart (CBP-201) | IL-4Rα | NCT04444752 NCT05017480 | Randomized, double-blind, placebo-controlled, multi-centered study | 2 | Studies showed sustained efficacy, continued improvement in outcomes, and meaningful benefits in pruritus and QoL. | [104,105] |
| Benralizumab | IL-5Rα | NCT04605094 | Randomized, double-blind, placebo-controlled, parallel-group, multinational study | 2 | Benralizumab showed no differences from the placebo in primary (IGA response) or secondary endpoints, including EASI scores, itch improvement, symptom control, and HRQoL. | [114] |
| Eblasakimab | IL-13Rα1 | NCT05158023 | Randomized, double-blind, placebo-controlled study | 2b | Eblasakimab improved EASI scores (−65% vs. −27%); 71% of patients reported mild-to-moderate TEAEs compared to 47% in the placebo group. | [103] |
| PF-06817924 | IL-33 | NCT02743871 | Randomized, placebo-controlled study | 1 | The drug was well tolerated, exhibited linear pharmacokinetics, and showed the lowest anti-drug antibody production in AD patients. | [107] |
| Ligelizumab | IgE | EudraCT Number 2011-002112-84 | Randomized, double-blind, placebo-controlled, parallel-group, proof-of-concept study | 2 | Ligelizumab efficacy was not superior to placebo but showed better responses in patients with high baseline IgE levels. | [115] |
| Amlitelimab | OX40 | NCT03754309 | Multi-center, randomized, double-blind, placebo-controlled, parallel-group study | 2a | Amlitelimab was well tolerated with no hypersensitivity events and showed the greatest EASI score improvements at weeks 2–16. | [117] |
| Rocatinlimab | OX40 | NCT03703102 | Multicenter, randomized, placebo-controlled, double-blind, parallel-group study | 2b | Rocatinlimab improved AD symptoms, with benefits maintained after discontinuation, and was well tolerated. | [118] |
| Spesolimab | IL-36R | NCT03822832 | Multicenter, randomized, double-blind, placebo-controlled | 2a | The study showed that the drug was well tolerated, and at week 16, the EASI score decreased. | [120] |
| Astegolimab | IL-33/ST2 | NCT03747575 | Randomized, double-blind, placebo-controlled multicenter | 2 | Despite the good tolerability of the drug, astegolimab did not show clinical efficacy in patients with AD. | [108] |
| Melrilimab (CNTO 7160) | IL-33R | NCT02345928. | Randomized, double-blind, placebo-controlled study | 1 | The drug was well tolerated, with one severe adverse event, but showed no clear clinical activity in asthma or AD. | [58] |
| YH35324 | IgE | NCT05061524 | Randomized, double-blind, placebo/active-controlled, single ascending dose study | 1 | YH35324 demonstrated a good safety profile and reduced serum-free IgE levels in subjects with atopic conditions, including AD. | [121] |
| Tezepelumab | TSLP | NCT02525094 | Randomized, double-blind, placebo-control, multicenter study | 2a | Tezepelumab plus TCS increased EASI50 achievement (64.7% vs. 48.2%) without statistical significance; TEAE incidence was similar between groups. | [123] |
| Tezepelumab | TSLP | NCT03809663 | Randomized, double-blind, placebo-controlled, dose-ranging study | 2b | The study failed to meet primary endpoints, including an IGA score of 0/1 and a 75% reduction in EASI75 at week 16. | NA |
| Solrikitug (MK-8226) | TSLP | NCT01732510 | Randomized, double-blind, placebo-controlled, multiple rising dose study | 1b | Solrikitug (3 mg/kg) significantly reduced EASI scores after 12 weeks; adverse events were common (10/13 vs. 7/8) but did not lead to discontinuation. | NA |
| Drug | Molecular Target | Study Register | Study Type | Phase | Status |
|---|---|---|---|---|---|
| Asthma | |||||
| MG-K10 | IL-4Rα | NCT05382910 | Randomized, placebo-controlled study | 1b/2 | Recruiting |
| Telikibart (GR1802) | IL-4Rα | NCT06642961 | Randomized, double-blind, placebo-controlled, multicenter study | 2 | Recruiting |
| FB825 | CεmX domain of membrane IgE (mIgE) | NCT05008965 | Randomized, double-blind, placebo-controlled study | 2 | Recruiting |
| Stapokibart (CM310) | IL-4Rα | NCT05761028 | Randomized, double-blind, placebo-controlled, multicenter study | 2/3 | Recruiting |
| Solrikitug (MK-8226) | IlL-4Rα | NCT06496607 | Randomized, double-blind, placebo-controlled, multiple dose-ranging study | 2a | Recruiting |
| Depemokimab vs. mepolizumab or benralizumab | IL-5 | NCT04718389 | Randomized, double-blind, double-dummy, parallel-group, multicenter, non-inferiority study | 3 | Active, not recruiting |
| Depemokimab | IL-5 | NCT05243680 | Open-label, single-arm, multicenter, extension study | 3 | Active, not recruiting |
| IBI3002 | IL-4Rα, TSLP | NCT06213844 | Randomized, double-blind, placebo-controlled, single-center, single-ascending dose study | 1 | Recruiting |
| Lunsekimig (SAR443765) | IL-13/TSLP | NCT06676319 | Randomized, double-blind, placebo-controlled, parallel-group, two-arm study | 2 | Recruiting |
| Lunsekimig (SAR443765) | IL-13/TSLP | NCT06102005 | Randomized, double-blind, placebo-controlled, parallel-group, dose-ranging, multicenter study | 2 | Recruiting |
| Atopic dermatitis | |||||
| Lebrikizumab | IL-13 | NCT05916365 | Interventional, single-group assignment, open-label study | 3 | Active, not recruiting |
| Lebrikizumab | IL-13 | NCT06526182 | Interventional, single-group assignment, open-label study | 3 | Recruiting |
| Stapokibart (CM310) | IL-4Rα | NCT06277765 | Multi-center, randomized, double-blind, placebo-controlled study | 3 | Not yet recruiting |
| Telikibart (GR1802) | IL-4Rα | NCT06216392 | Randomized, double-blind, placebo-controlled, multicenter study | 3 | Not yet recruiting |
| Nemolizumab | IL-31Rα | NCT03989206 | Interventional, prospective, multicenter, long-term study | 3 | Active, not recruiting |
| Rademikibart (CBP-201) | IL-4Rα | NCT05905133 | Interventional, single-arm, open-label, multicenter study | 2 | Active, not recruiting |
| Eblasakimab (ASLAN004) | IL-13Rα1 | NCT05694884 | Multicenter, randomized, double-blind, placebo-controlled, parallel-arm study | 2 | Recruiting |
| Amlitelimab | OX40 | NCT06241118 | Parallel-group, multinational, multicenter, randomized, double-blind, placebo-controlled study | 3 | Recruiting |
| Amlitelimab | OX40 | NCT06407934 | Multinational, multicenter, randomized, double-blind, placebo-controlled, parallel-group study | 3 | Recruiting |
| Amlitelimab | OX40 | NCT06181435 | Parallel-group, multinational, multicenter, randomized, double-blind, placebo-controlled, three-arm study | 3 | Recruiting |
| Rocatinlimab | OX40 | NCT05633355 | Interventional, single-group assignment, open-label study | 3 | Active, not recruiting |
| Rocatinlimab | OX40 | NCT05724199 | Interventional, randomized, placebo-controlled, double-blind study | 3 | Active, not recruiting |
| YH35324 | IgETrap-Fc | NCT05564221 | Randomized, double-blind, placebo/active-controlled, multiple ascending dose study | 1 | Active, not recruiting |
2.2.3. Role of Biomarkers in Biological Treatment of Atopic Dermatitis
2.3. Chronic Spontaneous Urticaria
2.3.1. Epidemiology, Symptoms, and Current Treatment Options
2.3.2. Biologics in CSU-Current Recommendations and Future Perspectives
2.4. Eosinophilic Gastritis, Enteritis, and Colitis
2.4.1. Classification, Prevalence, Symptoms, and Treatment
2.4.2. The Role of Biologics in Non-EoE-EGIDs-Current State and Perspectives
2.5. Chronic Rhinosinusitis with Nasal Polyps
2.5.1. Symptoms and Treatment Strategies
2.5.2. Biological Treatment-Approved and Investigational Agents
2.6. Allergic Rhinitis
2.6.1. Clinical Presentation and Treatment Strategies
2.6.2. The Role of Biologics in AR-Efficiency of Available Agents and Current Investigations
| Drug | Molecular Target | Study Register | Study Type | Phase | Main Findings/Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Chronic spontaneous urticaria | ||||||
| Dupilumab | IL-4Rα | NCT04180488 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 3 | In study A, UAS7 and ISS7 improved significantly vs. placebo, while study B showed minor, non-significant improvements. | [139] |
| YH35324 | IgE | NCT05061524 | Randomized, double-blind, placebo/active-controlled, single ascending dose study | 1 | YH35324 showed no serious AEs, discontinuations, or anaphylaxis and suppressed serum-free IgE longer than omalizumab. | [121] |
| Benralizumab | IL-5Rα | NCT04612725 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 2b | Benralizumab showed no changes in ISS7 or UAS7 scores at week 12 but significantly depleted blood eosinophil levels at week 24. | [142] |
| Ligelizumab | IgE | NCT03580369 NCT03580356 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 3 | Both studies demonstrated significant improvement in UAS7 scores compared to placebos but not in omalizumab. | [146] |
| Lirentelimab | Siglec-8 | NCT03436797 | Open-label study | 2a | Lirentelimab reduced disease activity (73% in omalizumab-naive; 47% in refractory) with UAS7 responses of 77% and 45% and no serious AEs. | [151] |
| Canakinumab | IL-1β | NCT01635127 | Randomized, double-blind, placebo-controlled, single-center study | 2 | Canakinumab showed no significant improvements in UAS7 or clinical outcomes compared to placebo but was well tolerated with mild AEs. | [153] |
| Non-esophageal eosinophilic gastrointestinal disorders | ||||||
| Lirentelimab (AK002) | Siglec-8 | NCT04322604 | Multi-center, randomized, double-blind, placebo-controlled | 3 | Lirentelimab met the primary endpoint for gastric eosinophils but failed to alleviate patient-reported symptoms. | NA |
| Lirentelimab (AK002) | Siglec-8 | NCT04856891 | Multi-center, randomized, double-blind, placebo-controlled | 3 | The trial met the histologic endpoint for duodenal eosinophils (≤15 eos/hpf) but failed to achieve significance in patient-reported symptoms. | NA |
| Lirentelimab (AK002) | Siglec-8 | NCT04620811 | Multi-center, open-label, extension study | 3 | Extended lirentelimab treatment improved TSS but showed lower eosinophil response compared to the placebebo+lirentelimab group (96.3% vs. 92.3%), with lower TAEA incidence compared to the placebo group (34.5 vs. 44%). | NA |
| Benralizumab | IL-5Rα | NCT03473977 | Randomized, double-blind, placebo-controlled | 2 | Benralizumab improved remission rates (77% vs. 8%), EoG histology, inflammatory scores, and eosinophil levels, with no significant changes in SODA or PROMIS scores. | [170] |
| Chronic rhinosinusitis with nasal polyps | ||||||
| Dupilumab | IL-4Rα | NCT04181190 | Real-life, observational, multicenter study | 4 | Dupilumab reduced polyp size and improved QoL, severity of symptoms, nasal congestion, and smell. | [186] |
| Dupilumab | IL-4Rα | NCT02912468 NCT02898454 | Randomized, multicenter, double-blind, placebo-controlled, parallel-group studies | 3 | Dupilumab improved NC, NPS, and LMK scores, particularly in patients with recent sinonasal surgery (<3 years), and reduced SCS use and sinonasal surgery rates compared to placebo. | [188] |
| Mepolizumab | IL-5 | NCT04607005 | Randomized, double-blind, placebo-controlled, parallel-group | 3 | Mepolizumab significantly improved nasal obstruction and ENPS scores and was well-tolerated with no serious adverse events in CRSwNP/ECRS patients. | [194] |
| Mepolizumab | IL-5 | NCT03085797 | Randomized, double-blind, placebo-controlled, parallel-group, multi-center trial | 3 | Mepolizumab improved nasal polyp size, obstruction, and smell/taste loss over 52 weeks, reduced SCS use (37.5% vs. 25.4%), and lowered prednisolone-equivalent doses regardless of prior sinus surgeries or eosinophilia. | [193,195] |
| Mepolizumab | IL-5 | NCT03085797 | Randomized, double-blind, placebo-controlled study | 3 | Mepolizumab provided partially sustained clinical benefits in symptoms, QoL, and corticosteroid use up to 24 weeks post-discontinuation compared to placebo. | [197] |
| Benralizumab | IL-5Rα | NCT03170271 | Randomized, double-blind, placebo-controlled, parallel-group, multicenter study | 3b | Benralizumab improved nasal symptoms in patients with high SNOT-22 scores, reduced AER by 69%, and enhanced SGRQ, FEV1, and ACQ-6 scores, with similar adverse event incidence to placebo. | [201] |
| Benralizumab | IL-5Rα | NCT03401229 | Randomized, double-blind, placebo-controlled, parallel-group, international, multicenter study | 3 | Benralizumab nearly eliminated blood eosinophils (weeks 16–56) and reduced nasal polyp tissue eosinophils to 0 cells/mm2 at week 56 compared to placebo. | [198] |
| Benralizumab | IL-5Rα | NCT03401229 | Randomized, double-blind, placebo-controlled, parallel-group, multi-center trial | 3 | Over 40 weeks, benralizumab improved NPS, nasal blockage, and sense of smell scores compared to placebo and was well tolerated, with mostly mild-to-moderate adverse events in both groups (77.3% vs. 78.8%). | [199] |
| PF-06817024 | IL-33 | NCT02743871 | Randomized, double-blind, third-party open, placebo-controlled, dose-escalating study | 1 | Of 20 randomized CRSwNP patients, 16 completed treatment; 45.5% experienced mild-to-moderate TEAEs, primarily general disorders and administration site conditions. | [107] |
| Allergic rhinitis | ||||||
| Dupilumab | IL-4Rα | NCT02912468 NCT02898454 | Randomized, double-blind, placebo-controlled, parallel assignment study | 3 | Dupilumab improved CRSwNP measures and reduced steroid use and surgical treatment compared to placebo. | [209] |
| Tezepelumab | TSLP | NCT02237196 | Randomized, triple-masking, placebo-controlled, parallel assignment study | 2 | Tezepelumab enhanced SCIT effectiveness and reduced clinical responses up to one-year post-therapy in AR patients. | [210] |
| REGN1908-1909 | Fel d 1 | NCT02127801 | Randomized, double-blind, placebo-controlled study | 1b | A single dose of REGN1908-1909 reduced nasal symptoms and inhibited FcεRI-, FcεRII-, and T-cell-mediated allergic responses. | [211] |
| Stapokibart (CM310) | IL-4Rα | NCT05470647 | Randomized, double-blind, placebo-controlled, parallel assignment study | 2 | Administration of stapokibart did not significantly improve the clinical status of patients with uncontrolled seasonal AR. | [212] |
| REGN5713-5714-5715 | Bet v 1 | NCT04709575 | Randomized, double-blind, placebo-controlled, parallel-group study | 3 | A single dose of REGN5713-5714-5715 improved symptom control compared to the placebo group. | NA |
| Drug | Molecular Target | Study Register | Study Type | Phase | Status |
|---|---|---|---|---|---|
| Chronic spontaneous urticaria | |||||
| Dupilumab | IL-4Rα | NCT05526521 | Single-arm, multi-center study | 3 | Recruiting |
| YH35324 | IgE | NCT05564221 | Randomized, double-blind, placebo/active-controlled, multiple ascending dose | 1b | Active, not recruiting |
| UB-221 | IgE | NCT04175704 | Randomized, single-blind, placebo-controlled, parallel-group, single ascending dose study | 1 | Not yet recruiting |
| CMAB007 | IgE | NCT06365879 | Randomized, double-blind, positive parallel controlled, multicenter study | 3 | Recruiting |
| Non-esophageal eosinophilic gastrointestinal disorders | |||||
| Dupilumab | IL-4Rα | NCT03678545 | Multi-center, randomized, double-blind, placebo-controlled trial | 2 | Active, not recruiting |
| Dupilumab | IL-4Rα | NCT05831176 | Randomized, double-blind,placebo-controlled, 3-part study | 2/3 | Recruiting |
| Cendakimab | anti-IL-13Rα1 and α2 | NCT05214768 | Multicenter, randomized, double-blind, placebo-controlled trial | 3 | Active, not recruiting |
| Chronic rhinosinusitis with nasal polyps | |||||
| Dupilumab vs. Omalizumab | IL-4Rα | NCT04998604 | Multicenter, randomized, double-blind, active-controlled trial | 4 | Active, not recruiting |
| TQC2731 | anti-TSLP | NCT06451640 NCT06036927 | Multicenter, randomized, double-blind, placebo-controlled | 2 | Recruiting |
| Calpurbatug (TRL1068) | Pathogen-protecting biofilm | NCT05355207 | Interventional study | 1 | Not yet recruiting |
| Tezepelumab | anti-TSLP | NCT04851964 | Multicentre, randomized, double-blind, placebo-controlled, parallel-group study | 3 | Active, not recruiting |
| TQH2722 | IL-4Rα | NCT06439381 NCT06089278 | Multicenter, randomized, continuing trial | 2 | Recruiting |
| Telikibart (GR1802) | IL-4Rα | NCT05873803 | Randomized, double-blind, placebo-controlled, multicenter study | 2 | Active, not recruiting |
| Telikibart (GR1802) | IL-4Rα | NCT06516302 | Randomized, double-blind, placebo-controlled, multicenter study | 3 | Not yet recruiting |
| Mepolizumab | IL-5 | NCT05923047 | Randomized, controlled multicenter trial | 4 | Not yet recruiting |
| Mepolizumab | IL-5 | NCT05598814 | Randomized, two-arm trial | 4 | Recruiting |
| Allergic rhinitis | |||||
| Tezepelumab | TSLP | NCT06189742 | Non-randomized, single-group assignment, open-label study | 2 | Active, recruiting |
| Stapokibart (CM310) | IL-4Rα | NCT06300203 | Randomized, double-blind, placebo-controlled study | 2 | Not yet recruiting |
| Stapokibart (CM310) | IL-4Rα | NCT06525597 | Randomized, double-blind, placebo-controlled study | 2 | Recruiting |
| Telikibart (GR1802) | IL-4Rα | NCT06315426 | Randomized, double-blind, placebo-controlled, multicenter study | 2 | Not yet recruiting |
| Telikibart (GR1802) | IL-4Rα | NCT06028490 | Randomized, double-blind, placebo-controlled study | 2 | Recruiting |
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; GBD 2019 Allergic Disorders Collaborators; Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; et al. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Dierick, B.J.H.; van der Molen, T.; Flokstra-de Blok, B.M.J.; Muraro, A.; Postma, M.J.; Kocks, J.W.H.; van Boven, J.F. Burden and socioeconomics of asthma, allergic rhinitis, atopic dermatitis and food allergy. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 20, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Adeloye, D.; Salim, H.; Dos Santos, J.P.; Campbell, H.; Sheikh, A.; Rudan, I. Global, regional, and national prevalence of asthma in 2019: A systematic analysis and modelling study. J. Glob. Health 2022, 12, 04052. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global epidemiology of atopic dermatitis: A comprehensive systematic analysis and modelling study. Br. J. Dermatol. 2024, 190, 55–61. [Google Scholar] [CrossRef]
- Rank, M.A.; Wonnaparhown, A.M.; Freeman, C.M. Recent guidelines addressing chronic rhinosinusitis with nasal polyps: Practical aspects. Pol. Arch. Intern. Med. 2023, 133, 16611. [Google Scholar] [CrossRef]
- Raciborski, F.; Arcimowicz, M.; Samolinski, B.; Pinkas, W.; Samel-Kowalik, P.; Śliwczyński, A. Recorded prevalence of nasal polyps increases with age. Adv. Dermatol. Allergol. 2020, 38, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Fricke, J.; Ávila, G.; Keller, T.; Weller, K.; Lau, S.; Maurer, M.; Zuberbier, T.; Keil, T. Prevalence of chronic urticaria in children and adults across the globe: Systematic review with meta-analysis. Allergy 2020, 75, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Papaiakovou, G.; Papageorgiou, A.; Bakakos, A.; Sinaniotis, A.C.; Rovina, N. Eosinophilic gastrointestinal diseases: Current perspectives on pathogenesis and management. Explor. Asthma Allergy 2024, 2, 205–218. [Google Scholar] [CrossRef]
- Alnahas, S.; Abouammoh, N.; Althagafi, W.; Abd-Ellatif, E.E. Prevalence, severity, and risk factors of allergic rhinitis among schoolchildren in Saudi Arabia: A national cross-sectional study, 2019. World Allergy Organ. J. 2023, 16, 100824. [Google Scholar] [CrossRef]
- Wong, A.; Tavakoli, H.; Sadatsafavi, M.; Carlsten, C.; FitzGerald, J.M. Asthma control and productivity loss in those with work-related asthma: A population-based study. J. Asthma 2017, 54, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Rönnebjerg, L.; Axelsson, M.; Kankaanranta, H.; Backman, H.; Rådinger, M.; Lundbäck, B.; Ekerljung, L. Severe Asthma in a General Population Study: Prevalence and Clinical Characteristics. J. Asthma Allergy 2021, 14, 1105–1115. [Google Scholar] [CrossRef]
- Fuxench, Z.C.C.; Block, J.K.; Boguniewicz, M.; Boyle, J.; Fonacier, L.; Gelfand, J.M.; Grayson, M.H.; Margolis, D.J.; Mitchell, L.; Silverberg, J.I.; et al. Atopic Dermatitis in America Study: A Cross-Sectional Study Examining the Prevalence and Disease Burden of Atopic Dermatitis in the US Adult Population. J. Investig. Dermatol. 2019, 139, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Soong, W.; Patil, D.; Pivneva, I.; Signorovitch, J.; Wells, M.A.; Balp, M.-M.; Kuruvilla, M. Disease burden and predictors associated with non-response to antihistamine-based therapy in chronic spontaneous urticaria. World Allergy Organ. J. 2023, 16, 100843. [Google Scholar] [CrossRef] [PubMed]
- Savouré, M.; Bousquet, J.; Leynaert, B.; Renuy, A.; Siroux, V.; Goldberg, M.; Zins, M.; Jacquemin, B.; Nadif, R. Rhinitis phenotypes and multimorbidities in the general population: The CONSTANCES cohort. Eur. Respir. J. 2023, 61, 2200943. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Jiménez, F.; Pavón-Romero, G.F.; Velásquez-Rodríguez, J.M.; López-Garza, M.I.; Lazarini-Ruiz, J.F.; Gutiérrez-Quiroz, K.V.; Teran, L.M. Biologic Therapies for Asthma and Allergic Disease: Past, Present, and Future. Pharmaceuticals 2023, 16, 270. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; Hearn, A.P.; Jackson, D.J. A pragmatic guide to choosing biologic therapies in severe asthma. Breathe 2022, 17, 210144. [Google Scholar] [CrossRef] [PubMed]
- Dubini, M.; Benzecry, V.; Rivolta, F.; Sangalli, A.; Marzano, A.V.; Pravettoni, V.; Tavecchio, S.; Ferrucci, S.M. Asthma improvement in patients treated with dupilumab for severe atopic dermatitis. Front. Allergy 2023, 4, 1223657. [Google Scholar] [CrossRef]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Sanuki, T. Review of Non-Eosinophilic Esophagitis-Eosinophilic Gastrointestinal Disease (Non-EoE-EGID) and a Case Series of Twenty-Eight Affected Patients. Biomolecules 2023, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Xu, S.; Lou, H.; Zhang, L. Comparison of Different Biologics for Treating Chronic Rhinosinusitis With Nasal Polyps: A Network Analysis. J. Allergy Clin. Immunol. Pract. 2022, 10, 1876–1886.e7. [Google Scholar] [CrossRef] [PubMed]
- Azzano, P.; Dufresne, É.; Poder, T.; Bégin, P. Economic considerations on the usage of biologics in the allergy clinic. Allergy 2021, 76, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Sitek, A.N.; Li, J.T.; Pongdee, T. Risks and safety of biologics: A practical guide for allergists. World Allergy Organ. J. 2023, 16, 100737. [Google Scholar] [CrossRef] [PubMed]
- Runnstrom, M.; Pitner, H.; Xu, J.; Lee, F.E.-H.; Kuruvilla, M. Utilizing Predictive Inflammatory Markers for Guiding the Use of Biologicals in Severe Asthma. J. Inflamm. Res. 2022, 15, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Usuba, K.; Zhang, L.; Liu, X.; Han, T.; Nightingale, N.; Tehrani, A.; Zhang, S.; Howarth, P.; Alfonso-Cristancho, R. Predicting real-world response to mepolizumab in severe asthma using machine learning. Eur. Respir. J. 2024, 64 (Suppl. 68), PA448. Available online: https://publications.ersnet.org/content/erj/64/suppl68/pa448 (accessed on 18 December 2024).
- Bonini, S.; Bonini, M. Biosimilars and drug development in allergic and immunologic diseases. J. Allergy Clin. Immunol. 2017, 139, 1461–1464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, R.L.; Grayson, M.H.; Strothman, K. Advances in asthma: New understandings of asthma’s natural history, risk factors, underlying mechanisms, and clinical management. J. Allergy Clin. Immunol. 2021, 148, 1430–1441. [Google Scholar] [CrossRef]
- Chung, K.F.; Dixey, P.; Abubakar-Waziri, H.; Bhavsar, P.; Patel, P.H.; Guo, S.; Ji, Y. Characteristics, phenotypes, mechanisms and management of severe asthma. Chin. Med. J. 2022, 135, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Vitale, C.; Molino, A.; Stanziola, A.; Sanduzzi, A.; Vatrella, A.; Mormile, M.; Lanza, M.; Calabrese, G.; Antonicelli, L.; et al. Asthma-related deaths. Multidiscip. Respir. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.A.; Brightling, C. Biologics for severe asthma-Which, when and why? Respirol. Carlton. Vic. 2023, 28, 709–721. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.-H.; Lee, G.B. Understanding Asthma Phenotypes, Endotypes, and Mechanisms of Disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, J.; Hoyte, F.C.; Phipatanakul, W.; Silver, J.; Howarth, P.; Lugogo, N.L. Allergic and eosinophilic asthma in the era of biomarkers and biologics: Similarities, differences and misconceptions. Ann. Allergy Asthma Immunol. 2022, 129, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, C.; Gogali, A.; Bartziokas, K.; Kostikas, K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021, 7, 00309–02020. [Google Scholar] [CrossRef] [PubMed]
- Hudey, S.N.; Ledford, D.K.; Cardet, J.C. Mechanisms of non-type 2 asthma. Curr. Opin. Immunol. 2020, 66, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Adrish, M.; Akuthota, P. Approach to non-type 2 asthma. Respir. Med. 2023, 216, 107327. [Google Scholar] [CrossRef]
- Liu, T.; Woodruff, P.G.; Zhou, X. Advances in non-type 2 severe asthma: From molecular insights to novel treatment strategies. Eur. Respir. J. 2024, 64, 2300826. [Google Scholar] [CrossRef] [PubMed]
- GINA. Difficult-to-Treat & Severe Asthma Guide. Available online: https://ginasthma.org/wp-content/uploads/2024/11/GINA-Severe-Asthma-Guide-2024-WEB-WMS.pdf (accessed on 13 December 2024).
- Loureiro, C.C.; Amaral, L.; Ferreira, J.A.; Lima, R.; Pardal, C.; Fernandes, I.; Semedo, L.; Arrobas, A. Omalizumab for Severe Asthma: Beyond Allergic Asthma. BioMed Res. Int. 2018, 2018, 3254094. [Google Scholar] [CrossRef]
- Cushen, B.; Menzies-Gow, A. Benralizumab: An updated treatment of eosinophilic asthma. Expert Rev. Respir. Med. 2020, 14, 435–444. [Google Scholar] [CrossRef]
- Sardon-Prado, O.; Diaz-Garcia, C.; Corcuera-Elosegui, P.; Korta-Murua, J.; Valverde-Molina, J.; Sanchez-Solis, M. Severe Asthma and Biological Therapies: Now and the Future. J. Clin. Med. 2023, 12, 5846. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Katelaris, C.H. Dupilumab in the Treatment of Asthma. Immunotherapy 2019, 11, 859–872. [Google Scholar] [CrossRef]
- Panettieri, R., Jr.; Lugogo, N.; Corren, J.; Ambrose, C.S. Tezepelumab for Severe Asthma: One Drug Targeting Multiple Disease Pathways and Patient Types. J. Asthma. Allergy 2024, 17, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Jackson, D.J.; Bernstein, D.; Korn, S.; Pfeffer, P.E.; Chen, R.; Saito, J.; Martinez, G.d.L.; Dymek, L.; Jacques, L.; et al. Twice-Yearly Depemokimab in Severe Asthma with an Eosinophilic Phenotype. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef]
- Seluk, L.; Davis, A.E.; Rhoads, S.; Wechsler, M.E. Novel asthma treatments: Advancing beyond approved novel step-up therapies for asthma. Ann. Allergy Asthma Immunol. 2024, 134, 9–18. [Google Scholar] [CrossRef]
- Singh, D.; Fuhr, R.; Bird, N.P.; Mole, S.; Hardes, K.; Man, Y.L.; Cahn, A.; Yancey, S.W.; Pouliquen, I.J. A Phase 1 study of the long-acting anti-IL-5 monoclonal antibody GSK3511294 in patients with asthma. Br. J. Clin. Pharmacol. 2022, 88, 702–712. [Google Scholar] [CrossRef]
- Antoniu, S.A. Lebrikizumab for the treatment of asthma. Expert Opin. Investig. Drugs 2016, 25, 1239–1249. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.D.; Gonzalez Edick, M.; Ferrando, R.E.; Solon, M.; Baca, M.; Mesh, K.; Bradding, P.; Gauvreau, G.M.; Sumino, K.; FitzGerald, J.M.; et al. A randomized, placebo-controlled trial evaluating effects of lebrikizumab on airway eosinophilic inflammation and remodelling in uncontrolled asthma (CLAVIER). Clin. Exp. Allergy 2020, 50, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Korenblat, P.; Chapman, K.R.; Bateman, E.D.; Kopecky, P.; Paggiaro, P.; Yokoyama, A.; Olsson, J.; Gray, S.; Holweg, C.T.J.; et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): Replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir. Med. 2016, 4, 781–796. [Google Scholar] [CrossRef]
- Szefler, S.; Roberts, G.; Rubin, A.; Zielen, S.; Kuna, P.; Alpan, O.; Anzures-Cabrera, J.; Chen, Q.; Holweg, C.; Kaminski, J.; et al. Efficacy, safety, and tolerability of lebrikizumab in adolescent patients with uncontrolled asthma (ACOUSTICS). Clin. Transl. Allergy 2022, 12, e12176. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Szefler, S.J.; Sher, E.; Korenblat, P.; Soong, W.; Hanania, N.A.; Berman, G.; Brusselle, G.; Zitnik, R.; Natalie, C.R.; et al. Lebrikizumab in Uncontrolled Asthma: Reanalysis in a Well-Defined Type 2 Population. J. Allergy Clin. Immunol. Pract. 2024, 12, 1215–1224. [Google Scholar] [CrossRef]
- England, E.; Rees, D.G.; Scott, I.C.; Carmen, S.; Chan, D.T.Y.; Huntington, C.E.C.; Houslay, K.F.; Erngren, T.; Penney, M.; Majithiya, J.B.; et al. Tozorakimab (MEDI3506): An anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction. Sci. Rep. 2023, 13, 9825. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zou, G.-L.; Zhang, W.; Lai, X.-N.; Chen, H.-W.; Xiong, L.-X. Interleukin-33: Its Emerging Role in Allergic Diseases. Molecules 2018, 23, 1665. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Reid, F.; Moate, R.; Jimenez, E.; Sadiq, M.; Williams, A.; Rytelewski, M.; Muthas, D.; Brooks, D.; Lindqvist, E.; et al. S90 FRONTIER-3: A randomized, phase 2a study to evaluate the efficacy and safety of tozorakimab (an anti-interleukin-33 monoclonal antibody) in early-onset asthma. Thorax 2024, 79 (Suppl. 2), A65–A66. [Google Scholar]
- Wechsler, M.E.; Ruddy, M.K.; Pavord, I.D.; Israel, E.; Rabe, K.F.; Ford, L.B.; Maspero, J.F.; Abdulai, R.M.; Hu, C.-C.; Martincova, R.; et al. Efficacy and Safety of Itepekimab in Patients with Moderate-to-Severe Asthma. N. Engl. J. Med. 2021, 385, 1656–1668. [Google Scholar] [CrossRef]
- Kosloski, M.P.; Kalliolias, G.D.; Xu, C.R.; Harel, S.; Lai, C.H.; Zheng, W.; Davis, J.D.; Kamal, M.A. Pharmacokinetics and pharmacodynamics of itepekimab in healthy adults and patients with asthma: Phase I first-in-human and first-in-patient trials. Clin. Transl. Sci. 2022, 15, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Akinseye, C.; Crim, C.; Newlands, A.; Fairman, D. Efficacy and safety of GSK3772847 in participants with moderate-to-severe asthma with allergic fungal airway disease: A phase IIa randomized, multicenter, double-blind, sponsor-open, comparative trial. PLoS ONE 2023, 18, e0281205. [Google Scholar] [CrossRef]
- Nnane, I.; Frederick, B.; Yao, Z.; Raible, D.; Shu, C.; Badorrek, P.; Boer, M.v.D.; Branigan, P.; Duffy, K.; Baribaud, F.; et al. The first-in-human study of CNTO 7160, an anti-interleukin-33 receptor monoclonal antibody, in healthy subjects and patients with asthma or atopic dermatitis. Br. J. Clin. Pharmacol. 2020, 86, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Crim, C.; Stone, S.; Millar, V.; Lettis, S.; Bel, E.H.; Menzies-Gow, A.; Chanez, P.; Wenzel, S.; Lugogo, N.; Bleecker, E.R. IL-33 receptor inhibition in subjects with uncontrolled asthma: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. Glob. 2022, 1, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Bagnasco, D.; Testino, E.; Nicola, S.; Melissari, L.; Russo, M.; Canevari, R.F.; Brussino, L.; Passalacqua, G. Specific Therapy for T2 Asthma. J. Pers. Med. 2022, 12, 593. [Google Scholar] [CrossRef]
- Kelsen, S.G.; Agache, I.O.; Soong, W.; Israel, E.; Chupp, G.L.; Cheung, D.S.; Theess, W.; Yang, X.; Staton, T.L.; Choy, D.F.; et al. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: A randomized clinical trial. J. Allergy Clin. Immunol. 2021, 148, 790–798. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; Duggan, S. Risankizumab: First Global Approval. Drugs 2019, 79, 893–900. [Google Scholar] [CrossRef]
- Li, Y.; Hua, S. Mechanisms of pathogenesis in allergic asthma: Role of interleukin-23. Respirology 2014, 19, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Wakashin, H.; Hirose, K.; Maezawa, Y.; Kagami, S.-I.; Suto, A.; Watanabe, N.; Saito, Y.; Hatano, M.; Tokuhisa, T.; Iwakura, Y.; et al. IL-23 and Th17 Cells Enhance Th2-Cell–mediated Eosinophilic Airway Inflammation in Mice. Am. J. Respir. Crit. Care Med. 2008, 178, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Brightling, C.E.; Nair, P.; Cousins, D.J.; Louis, R.; Singh, D. Risankizumab in Severe Asthma—A Phase 2a, Placebo-Controlled Trial. N. Engl. J. Med. 2021, 385, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Que, L.G.; Ingram, J.L. Effects of biological therapies on patients with Type-2 high asthma and comorbid obesity. Front. Pharmacol. 2024, 14, 1315540. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Hohlfeld, J.M.; FitzGerald, J.M.; Boulet, L.-P.; Cockcroft, D.W.; Davis, B.E.; Korn, S.; Kornmann, O.; Leigh, R.; Mayers, I.; et al. Inhaled anti-TSLP antibody fragment, ecleralimab, blocks responses to allergen in mild asthma. Eur. Respir. J. 2023, 61, 2201193. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Dingman, R.; Wang, C.Q.; Lai, C.; Rajadhyaksha, M.; DeVeaux, M.; Orengo, J.M.; Radin, A.; Davis, J.D. REGN1908-1909 monoclonal antibodies block Fel d 1 in cat allergic subjects: Translational pharmacokinetics and pharmacodynamics. Clin. Transl. Sci. 2021, 14, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- de Blay, F.J.; Gherasim, A.; Domis, N.; Meier, P.; Shawki, F.; Wang, C.Q.; Orengo, J.M.; DeVeaux, M.; Ramesh, D.; Jalbert, J.J.; et al. REGN1908/1909 prevented cat allergen-induced early asthmatic responses in an environmental exposure unit. J. Allergy Clin. Immunol. 2022, 150, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Rymut, S.; Yoshida, K.; Sukumaran, S.; Cai, F.; Sperinde, G.; Sverkos, V.; Banerjee, P.; Belloni, P.; Lin, J. Local Airway Concentration of Anti-Tryptase Antibody (MTPS9579A) Predicts Extent of Tryptase Disruption. J. Allergy Clin. Immunol. 2020, 145, AB172. [Google Scholar] [CrossRef]
- Vitte, J. Human mast cell tryptase in biology and medicine. Mol. Immunol. 2015, 63, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Rymut, S.M.; Sukumaran, S.; Sperinde, G.; Bremer, M.; Galanter, J.; Yoshida, K.; Smith, J.; Banerjee, P.; Sverkos, V.; Cai, F.; et al. Dose-dependent inactivation of airway tryptase with a novel dissociating anti-tryptase antibody (MTPS9579A) in healthy participants: A randomized trial. Clin. Transl. Sci. 2022, 15, 451–463. [Google Scholar] [CrossRef]
- Rymut, S.M.; Henderson, L.M.; Poon, V.; Staton, T.L.; Cai, F.; Sukumaran, S.; Rhee, H.; Owen, R.; Ramanujan, S.; Yoshida, K. A mechanistic PK/PD model to enable dose selection of the potent anti-tryptase antibody (MTPS9579A) in patients with moderate-to-severe asthma. Clin. Transl. Sci. 2023, 16, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Henderson, L.M.; Bauer, R.N.; Wong, K.; Staton, T.L.; Choy, D.F.; Banerjee, P.; Poon, V.; Yoshida, K.; Chen, C.; et al. Airway tryptase levels inform the lack of clinical efficacy of the tryptase inhibitor MTPS9579A in asthma. Allergy 2024, 79, 2993–3004. [Google Scholar] [CrossRef]
- Corren, J.; Castro, M.; O’Riordan, T.; Hanania, N.A.; Pavord, I.D.; Quirce, S.; Chipps, B.E.; Wenzel, S.E.; Thangavelu, K.; Rice, M.S.; et al. Dupilumab Efficacy in Patients with Uncontrolled, Moderate-to-Severe Allergic Asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Diver, S.; Khalfaoui, L.; Emson, C.; Wenzel, S.E.; Menzies-Gow, A.; Wechsler, M.E.; Johnston, J.; Molfino, N.; Parnes, J.R.; Megally, A.; et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021, 9, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Corren, J.; Menzies-Gow, A.; Chupp, G.; Israel, E.; Korn, S.; Cook, B.; Ambrose, C.S.; Hellqvist, Å.; Roseti, S.L.; Molfino, N.A.; et al. Efficacy of Tezepelumab in Severe, Uncontrolled Asthma: Pooled Analysis of the PATHWAY and NAVIGATOR Clinical Trials. Am. J. Respir. Crit. Care Med. 2023, 208, 13–24. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Carriero, V.; Bertolini, F. Which Therapy for Non-Type(T)2/T2-Low Asthma. J. Pers. Med. 2021, 12, 10. [Google Scholar] [CrossRef]
- Hernandez, M.L.; Mills, K.; Almond, M.; Todoric, K.; Aleman, M.M.; Zhang, H.; Zhou, H.; Peden, D.B. IL-1 receptor antagonist reduces endotoxin-induced airway inflammation in healthy volunteers. J. Allergy Clin. Immunol. 2015, 135, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.-Y.; Yang, B.; Ding, Y.-F.; Wu, L.-M.; Zhang, L.-T.; Wang, J.-Y.; Lu, Q.-J.; Zhang, C.-L.; Zhang, F.-R.; et al. Long-Term Efficacy and Safety of Stapokibart in Adults with Moderate-to-Severe Atopic Dermatitis: An Open-Label Extension, Nonrandomized Clinical Trial. BioDrugs 2024, 38, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, G.M.; Harris, J.M.; Boulet, L.-P.; Scheerens, H.; Fitzgerald, J.M.; Putnam, W.S.; Cockcroft, D.W.; Davis, B.E.; Leigh, R.; Zheng, Y.; et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci. Transl. Med. 2014, 6, 243ra85. [Google Scholar] [CrossRef] [PubMed]
- Solrikitug Phase II Clinical Trial|ATS 2024. Available online: https://www.delveinsight.com/ats-conference/ats-2024/solrikitug-phase-ii-clinical-trial (accessed on 12 December 2024).
- Innovent Announces First Participant Dosed in a Phase I Study of IBI3002 (an Anti-IL-4Rα/TSLP Bispecific Antibody) in Australia. Available online: https://pharmashots.com/press-releases/innovent-announces-first-participant-dosed-in-a-phase-i-study-of-ibi3002-an-anti-il-4r%CE%B1tslp-bispecific-antibody-in-australia (accessed on 12 December 2024).
- Deiteren, A.; Bontinck, L.; Conickx, G.; Vigan, M.; Dervaux, N.; Gassiot, M.; Bas, S.; Suratt, B.; Staudinger, H.; Krupka, E. A first-in-human, single and multiple dose study of lunsekimig, a novel anti-TSLP/anti-IL-13 NANOBODY® compound, in healthy volunteers. Clin. Transl. Sci. 2024, 17, e13864. [Google Scholar] [CrossRef]
- Sun, B.; Shen, K.; Zhao, R.; Li, Y.; Xiang, M.; Lin, J. Precision medicine for severe asthma—Biological targeted therapy. Int. Immunopharmacol. 2024, 134, 112189. [Google Scholar] [CrossRef]
- Suzaki, I.; Hirano, K.; Maruyama, Y.; Kobayashi, H.; Rubin, B.K. IL-13-induced periostin production from human bronchial epithelial cells and human nasal epithelial cells. World Allergy Organ. J. 2020, 13, 100274. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Sjöbring, U.; Péterffy, A.; Wessman, P.; Bowen, K.; Piper, E.; Colice, G.; Brightling, C.E. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): Two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir. Med. 2018, 6, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; O’Byrne, P.M. The interleukin-13 paradox in asthma: Effective biology, ineffective biologicals. Eur. Respir. J. 2019, 53, 1802250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Shi, Y.; Liu, N.; Li, B. Elevated levels of interleukin-33 are associated with asthma: A meta-analysis. Immun. Inflamm. Dis. 2023, 11, e842. [Google Scholar] [CrossRef] [PubMed]
- Morikubo, H.; Tojima, R.; Maeda, T.; Matsuoka, K.; Matsuura, M.; Miyoshi, J.; Tamura, S.; Hisamatsu, T. Machine learning using clinical data at baseline predicts the medium-term efficacy of ustekinumab in patients with ulcerative colitis. Sci. Rep. 2024, 14, 4386. [Google Scholar] [CrossRef] [PubMed]
- Arrais, M.; Lulua, O.; Quifica, F.; Rosado-Pinto, J.; Gama, J.M.R.; Taborda-Barata, L. Prevalence of asthma, allergic rhinitis and eczema in 6-7-year-old schoolchildren from Luanda, Angola. Allergol. Immunopathol. (Madr) 2019, 47, 523–534. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2019, 8, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.M.; Baghoomian, W.B.; Grinich, E.; Leshem, Y.A.M.; Jacobson, M.B.; Simpson, E.L.M. The Eczema Area and Severity Index—A Practical Guide. Dermatitis 2022, 33, 187–192. [Google Scholar] [CrossRef]
- Davis, D.M.; Drucker, A.M.; Alikhan, A.; Bercovitch, L.; Cohen, D.E.; Darr, J.M.; Eichenfield, L.F.; Frazer-Green, L.; Paller, A.S.; Schwarzenberger, K.; et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J. Am. Acad. Dermatol. 2024, 90, e43–e56. [Google Scholar] [CrossRef] [PubMed]
- Dupilumab Label, FDA-Approved Drugs Database. Drugs@FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761055s064lbl.pdf (accessed on 11 November 2024).
- Tralokinumab Label, FDA-Approved Drugs Database. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761180s001lbl.pdf (accessed on 18 December 2024).
- Simpson, E.L.; Guttman-Yassky, E.; Pawlikowski, J.; Ghorayeb, E.G.; Ota, T.; Lebwohl, M.G. Interleukin-1α inhibitor bermekimab in patients with atopic dermatitis: Randomized and nonrandomized studies. Arch. Dermatol. Res. 2024, 316, 589. [Google Scholar] [CrossRef]
- Blauvelt, A.; Thyssen, J.P.; Guttman-Yassky, E.; Bieber, T.; Serra-Baldrich, E.; Simpson, E.; Rosmarin, D.; Elmaraghy, H.; Meskimen, E.; Natalie, C.R.; et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br. J. Dermatol. 2023, 188, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Lam, M.; Prieto-Merino, D.; Malek, R.; Ellis, A.G.; Yiu, Z.Z.; Rochwerg, B.; Di Giorgio, S.; Arents, B.W.; Mohan, T.; et al. Systemic Immunomodulatory Treatments for Atopic Dermatitis: Living Systematic Review and Network Meta-Analysis Update. JAMA Dermatol. 2024, 160, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Guttman-Yassky, E.; Lynde, C.; Khattri, S.; Schlessinger, J.; Imafuku, S.; Tada, Y.; Morita, A.; Wiseman, M.; Kwiek, B.; et al. Cendakimab in Patients With Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol. 2024, 160, 856–864. [Google Scholar] [CrossRef]
- Simpson, E.; Gooderham, M.; Kircik, L.; Armstrong, A.W.; Schlesinger, T.; Del Rosso, J.Q.; Murrell, D.F.; Thng, S.; Szepietowski, J.C.; Veverka, K.A.; et al. 53652 Efficacy and safety of eblasakimab in adults with severe atopic dermatitis: A posthoc analysis of the Phase 2b TREK-AD trial. J. Am. Acad. Dermatol. 2024, 91, AB194. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Strober, B.; Feinstein, B.; Xu, J.; Guttman-Yassky, E.; Simpson, E.L.; Li, P.; Longphre, M.; Song, J.; Guo, J.; et al. Efficacy and safety of rademikibart (CBP-201), a next-generation mAb targeting IL-4Rα, in adults with moderate to severe atopic dermatitis: A phase 2 randomized trial (CBP-201-WW001). J. Allergy Clin. Immunol. 2023, 153, 1040–1049.e12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Silverberg, J.I.; Guo, J.; Yun, J.; Pan, W.; Wei, Z.; Collazo, R. 712-Positive 52-week maintenance data observed with rademikibart in patients with moderate-to-severe atopic dermatitis (SEASIDE CHINA). Br. J. Dermatol. 2024, 191, ljae266.086. [Google Scholar] [CrossRef]
- Kosloski, M.P.; Guttman-Yassky, E.; Cork, M.J.; Worm, M.; Nahm, D.; Zhu, X.; Ruddy, M.K.; Harel, S.; Kamal, M.A.; Goulaouic, H.; et al. Pharmacokinetics and pharmacodynamics of itepekimab in adults with moderate-to-severe atopic dermatitis: Results from two terminated phase II trials. Clin. Transl. Sci. 2024, 17, e13874. [Google Scholar] [CrossRef] [PubMed]
- Danto, S.I.; Tsamandouras, N.; Reddy, P.; Gilbert, S.; Mancuso, J.; Page, K.; Peeva, E.; Vincent, M.S.; Beebe, J.S. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of PF-06817024 in Healthy Participants, Participants with Chronic Rhinosinusitis with Nasal Polyps, and Participants with Atopic Dermatitis: A Phase 1, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Pharmacol. 2024, 64, 529–543. [Google Scholar] [PubMed]
- Maurer, M.; Cheung, D.S.; Theess, W.; Yang, X.; Dolton, M.; Guttman, A.; Choy, D.F.; Dash, A.; Grimbaldeston, M.A.; Soong, W. Phase 2 randomized clinical trial of astegolimab in patients with moderate to severe atopic dermatitis. J. Allergy Clin. Immunol. 2022, 150, 1517–1524. [Google Scholar] [CrossRef]
- Kabashima, K.; Irie, H. Interleukin-31 as a Clinical Target for Pruritus Treatment. Front. Med. 2021, 8, 638325. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Wollenberg, A.; Reich, A.; Thaçi, D.; Legat, F.J.; Papp, K.A.; Gold, L.S.; Bouaziz, J.D.; Pink, A.E.; Carrascosa, J.M.; et al. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): Results from two replicate, double-blind, randomised controlled phase 3 trials. Lancet 2024, 404, 445–460. [Google Scholar] [CrossRef]
- Liang, J.; Hu, F.; Dan, M.; Sang, Y.; Abulikemu, K.; Wang, Q.; Hong, Y.; Kang, X. Safety and Efficacy of Nemolizumab for Atopic Dermatitis With Pruritus: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Front. Immunol. 2022, 13, 825312. [Google Scholar] [CrossRef]
- Labib, A.; Does, A.V.; Yosipovitch, G. Nemolizumab for atopic dermatitis. Drugs Today 2022, 58, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.J.; Wechsler, M.E.; Brusselle, G.; Buhl, R. Targeting the IL-5 pathway in eosinophilic asthma: A comparison of anti-IL-5 versus anti-IL-5 receptor agents. Allergy 2024, 79, 2943–2952. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bahadori, L.; Brooks, L.; Clark, K.L.; Grindebacke, H.; Ho, C.N.; Katial, R.; Pham, T.H.; Walton, C.; Datto, C.J.; et al. Lack of effect of benralizumab on signs and symptoms of moderate-to-severe atopic dermatitis: Results from the phase 2 randomized, double-blind, placebo-controlled HILLIER trial. J. Eur. Acad. Dermatol. Venereol. JEADV. 2023, 37, e1211–e1214. [Google Scholar] [CrossRef] [PubMed]
- Bangert, C.; Loesche, C.; Skvara, H.; Fölster-Holst, R.; Lacour, J.-P.; Jones, J.; Burnett, P.; Novak, N.; Stingl, G. IgE Depletion with Ligelizumab Does Not Significantly Improve Clinical Symptoms in Patients with Moderate-to-Severe Atopic Dermatitis. J. Investig. Dermatol. 2023, 143, 1896–1905.e8. [Google Scholar] [CrossRef]
- Croft, M.; Esfandiari, E.; Chong, C.; Hsu, H.; Kabashima, K.; Kricorian, G.; Warren, R.B.; Wollenberg, A.; Guttman-Yassky, E. OX40 in the Pathogenesis of Atopic Dermatitis—A New Therapeutic Target. Am. J. Clin. Dermatol. 2024, 25, 447–461. [Google Scholar] [CrossRef]
- Weidinger, S.; Bieber, T.; Cork, M.J.; Reich, A.; Wilson, R.; Quaratino, S.; Stebegg, M.; Brennan, N.; Gilbert, S.; O’malley, J.T.; et al. Safety and efficacy of amlitelimab, a fully human nondepleting, noncytotoxic anti-OX40 ligand monoclonal antibody, in atopic dermatitis: Results of a phase IIa randomized placebo-controlled trial. Br. J. Dermatol. 2023, 189, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Simpson, E.L.; Reich, K.; Kabashima, K.; Igawa, K.; Suzuki, T.; Mano, H.; Matsui, T.; Esfandiari, E.; Furue, M. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: A multicentre, double-blind, placebo-controlled phase 2b study. Lancet Lond. Engl. 2023, 401, 204–214. [Google Scholar] [CrossRef]
- Fukaura, R.; Akiyama, M. Targeting IL-36 in Inflammatory Skin Diseases. BioDrugs 2023, 37, 279–293. [Google Scholar] [CrossRef]
- Bissonnette, R.; Abramovits, W.; Saint-Cyr Proulx, É.; Lee, P.; Guttman-Yassky, E.; Zovko, E.; Sigmund, R.; Willcox, J.; Bieber, T. Spesolimab, an anti-interleukin-36 receptor antibody, in patients with moderate-to-severe atopic dermatitis: Results from a multicentre, randomized, double-blind, placebo-controlled, phase IIa study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 549–557. [Google Scholar] [CrossRef]
- Ye, Y.M.; Park, J.W.; Kim, S.H.; Cho, Y.S.; Lee, S.Y.; Lee, S.Y.; Sim, S.; Song, E.; Kim, B.; Lee, J.; et al. Safety, Tolerability, Pharmacokinetics, and pharmacodynamics of YH35324, a novel Long-Acting High-Affinity IgETrap-Fc protein in subjects with Atopy: Results from the First-in-Human study. Int. Immunopharmacol. 2024, 130, 111706. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M. Turning off the alarm—Targeting alarmins and other epithelial mediators of allergic inflammation with biologics. Allergologie 2021, 5, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef]
- Chokevittaya, P.; Jirattikanwong, N.; Thongngarm, T.; Phinyo, P.; Wongsa, C. Factors Associated With Dupilumab Response in Atopic Dermatitis: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2024, 12, 3044–3056. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, C.; Wang, S.; Yin, H.; Qiu, Z.; Luo, Y.; Li, Z.; Wang, C.; Yao, X.; Li, W. Serum biomarker-based endotypes of atopic dermatitis in China and prediction for efficacy of dupilumab. Br. J. Dermatol. 2023, 188, 649–660. [Google Scholar] [CrossRef]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Maintz, L.; Welchowski, T.; Herrmann, N.; Brauer, J.; Traidl-Hoffmann, C.; Havenith, R.; Müller, S.; Rhyner, C.; Dreher, A.; CK-CARE Study Group; et al. IL-13, periostin and dipeptidyl-peptidase-4 reveal endotype-phenotype associations in atopic dermatitis. Allergy 2023, 78, 1554–1569. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Alpizar, S.; Laquer, V.; Dhawan, S.; Abramovits, W.; Loprete, L.; Krishnaswamy, J.K.; Ahmad, F.; Jabbar-Lopez, Z.; Piketty, C. Pharmacokinetics, Safety, Efficacy, and Biomarker Profiles During Nemolizumab Treatment of Atopic Dermatitis in Adolescents. Dermatol. Ther. 2022, 12, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.-K.; Maurer, M. Autoimmune chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Peck, G.; Hashim, M.; Shaughnessy, C.; Muddasani, S.; Elsayed, N.; Fleischer, A. Global Epidemiology of Urticaria: Increasing Burden among Children, Females and Low-income Regions. Acta Dermato-Venereologica 2021, 101, adv00433. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Meguid, A.M.; Awad, S.M.; Noaman, M.; Abdel Gawad, A.M.; Abou-Taleb, D.A.E. Does chronic urticaria affect quality of sleep and quality of life? J. Public Health Res. 2024, 13, 22799036241243268. [Google Scholar] [CrossRef] [PubMed]
- Guillén-Aguinaga, S.; Presa, I.J.; Aguinaga-Ontoso, E.; Guillén-Grima, F.; Ferrer, M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: A systematic review and meta-analysis. Br. J. Dermatol. 2016, 175, 1153–1165. [Google Scholar] [CrossRef]
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bernstein, J.A.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef]
- Xu, L.; Yu, H.; Xu, S.; Wang, Y.; Cao, Y. Comparative efficacy and safety of the treatment by Omalizumab for chronic idiopathic urticaria in the general population: A systematic review and network meta-analysis. Ski. Res. Technol. 2024, 30, e13749. [Google Scholar] [CrossRef]
- Tharp, M.D.; Bernstein, J.A.; Kavati, A.; Ortiz, B.; MacDonald, K.; Denhaerynck, K.; Abraham, I.; Lee, C.S. Benefits and Harms of Omalizumab Treatment in Adolescent and Adult Patients With Chronic Idiopathic (Spontaneous) Urticaria: A Meta-analysis of “Real-world” Evidence. JAMA Dermatol. 2019, 155, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Agache, I.; Rocha, C.; Pereira, A.; Song, Y.; Alonso-Coello, P.; Solà, I.; Beltran, J.; Posso, M.; Akdis, C.A.; Akdis, M.; et al. Efficacy and safety of treatment with omalizumab for chronic spontaneous urticaria: A systematic review for the EAACI Biologicals Guidelines. Allergy 2021, 76, 59–70. [Google Scholar] [CrossRef]
- Keller, L.; Perera, E.K.; Bindon, B.; Khatiwada, A.; Stitt, J.M.; Dreskin, S.C. Total IgE as a biomarker of omalizumab response in chronic spontaneous urticaria: A meta-analysis. Allergy Asthma Proc. 2024, 45, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.M. Dupilumab: Human monoclonal antibody against IL-4Rα for moderate to severe atopic dermatitis. Drugs Today 2017, 53, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; Casale, T.B.; Saini, S.S.; Ben-Shoshan, M.; Giménez-Arnau, A.M.; Bernstein, J.A.; Yagami, A.; Stjepanovic, A.; Radin, A.; Staudinger, H.W.; et al. Dupilumab in patients with chronic spontaneous urticaria (LIBERTY-CSU CUPID): Two randomized, double-blind, placebo-controlled, phase 3 trials. J. Allergy Clin. Immunol. 2024, 154, 184–194. [Google Scholar] [CrossRef]
- Maurer, M.; Casale, T.B.; Saini, S.S.; Ben-Shoshan, M.; Laws, E.; Maloney, J.; Bauer, D.; Radin, A.; Makhija, M. Dupilumab Reduces Urticaria Activity, Itch, and Hives in Patients with Chronic Spontaneous Urticaria Regardless of Baseline Serum Immunoglobulin E Levels. Dermatol. Ther. 2024, 14, 2427–2441. [Google Scholar] [CrossRef] [PubMed]
- Benralizumab Label, FDA-Approved Drugs Database. Drugs@FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761070s021lbl.pdf (accessed on 11 November 2024).
- Altrichter, S.; Giménez-Arnau, A.M.; Bernstein, J.A.; Metz, M.; Bahadori, L.; Bergquist, M.; Brooks, L.; Ho, C.N.; Jain, P.; Lukka, P.B.; et al. Benralizumab does not elicit therapeutic effect in patients with chronic spontaneous urticaria: Results from the phase IIb multinational randomized double-blind placebo-controlled ARROYO trial. Br. J. Dermatol. 2024, 191, 187–199. [Google Scholar] [CrossRef]
- An, S.B.; Yang, B.-G.; Jang, G.; Kim, D.-Y.; Kim, J.; Oh, S.-M.; Oh, N.; Lee, S.; Moon, J.-Y.; Kim, J.-A.; et al. Combined IgE neutralization and Bifidobacterium longum supplementation reduces the allergic response in models of food allergy. Nat. Commun. 2022, 13, 5669. [Google Scholar] [CrossRef]
- Gasser, P.; Tarchevskaya, S.S.; Guntern, P.; Brigger, D.; Ruppli, R.; Zbären, N.; Kleinboelting, S.; Heusser, C.; Jardetzky, T.S.; Eggel, A. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020, 11, 165. [Google Scholar] [CrossRef]
- Maurer, M.; Giménez-Arnau, A.M.; Sussman, G.; Metz, M.; Baker, D.R.; Bauer, A.; Bernstein, J.A.; Brehler, R.; Chu, C.-Y.; Chung, W.-H.; et al. Ligelizumab for Chronic Spontaneous Urticaria. N. Engl. J. Med. 2019, 381, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Ensina, L.F.; Gimenez-Arnau, A.M.; Sussman, G.; Hide, M.; Grattan, C.; Fomina, D.; Rigopoulos, D.; Berard, F.; Canonica, G.W.; Rockmann, H.; et al. Efficacy and safety of ligelizumab in adults and adolescents with chronic spontaneous urticaria: Results of two phase 3 randomised controlled trials. Lancet 2024, 403, 147–159. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, K.; Wang, Z.; Ye, K.; Xu, Z.; Gong, X.; Zhu, G. Time-course and dose-effect of omalizumab in treating chronic idiopathic urticaria/chronic spontaneous urticaria. Eur. J. Clin. Pharmacol. 2024, 80, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Kuo, B.-S.; Li, C.-H.; Chen, J.-B.; Shiung, Y.-Y.; Chu, C.-Y.; Lee, C.-H.; Liu, Y.-J.; Kuo, J.-H.; Hsu, C.; Su, H.-W.; et al. IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms. J. Clin. Investig. 2022, 132, e157765. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, B.; Li, J.; Qian, W.; Hou, S.; Zhang, D.; Kou, G.; Li, B.; Wang, H.; Chen, Y.; et al. Tolerability, pharmacokinetics and pharmacodynamics of CMAB007, a humanized anti-immunoglobulin E monoclonal antibody, in healthy Chinese subjects. mAbs 2012, 4, 110–119. [Google Scholar] [CrossRef]
- Youngblood, B.A.; Leung, J.; Falahati, R.; Williams, J.; Schanin, J.; Brock, E.C.; Singh, B.; Chang, A.T.; O’sullivan, J.A.; Schleimer, R.P.; et al. Discovery, Function, and Therapeutic Targeting of Siglec-8. Cells 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Altrichter, S.; Staubach, P.; Pasha, M.; Singh, B.; Chang, A.T.; Bernstein, J.A.; Rasmussen, H.S.; Siebenhaar, F.; Maurer, M. An open-label, proof-of-concept study of lirentelimab for antihistamine-resistant chronic spontaneous and inducible urticaria. J. Allergy Clin. Immunol. 2022, 149, 1683–1690.e7. [Google Scholar] [CrossRef] [PubMed]
- Canakinumab Label, FDA-Approved Drugs Database. Drugs@FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/125319s110lbl.pdf (accessed on 11 November 2024).
- Maul, J.-T.; Distler, M.; Kolios, A.; Maul, L.V.; Guillet, C.; Graf, N.; Imhof, L.; Lang, C.; Navarini, A.A.; Schmid-Grendelmeier, P. Canakinumab Lacks Efficacy in Treating Adult Patients with Moderate to Severe Chronic Spontaneous Urticaria in a Phase II Randomized Double-Blind Placebo-Controlled Single-Center Study. J. Allergy Clin. Immunol. Pract. 2021, 9, 463–468.e3. [Google Scholar] [CrossRef]
- Redd, W.D.; Dellon, E.S. Eosinophilic Gastrointestinal Diseases Beyond the Esophagus: An Evolving Field and Nomenclature. Gastroenterol. Hepatol. 2022, 18, 522–528. [Google Scholar]
- Dellon, E.S.; Gonsalves, N.; Abonia, J.P.; Alexander, J.A.; Arva, N.C.; Atkins, D.; Attwood, S.E.; Auth, M.K.; Bailey, D.D.; Biederman, L.; et al. International Consensus Recommendations for Eosinophilic Gastrointestinal Disease Nomenclature. Clin. Gastroenterol. Hepatol. 2022, 20, 2474–2484.e3. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Amil-Dias, J.; Auth, M.K.; Chehade, M.; Collins, M.H.; Gupta, S.K.; Gutiérrez-Junquera, C.; Orel, R.; Vieira, M.C.; Zevit, N.; et al. Joint ESPGHAN/NASPGHAN Guidelines on Childhood Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis. J. Pediatr. Gastroenterol. Nutr. 2024, 78, 122–152. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Hottinger, S.K.; Gonsalves, N.; Furuta, G.T.; Collins, M.H.; Talley, N.J.; Peterson, K.; Menard-Katcher, C.; Smith, M.; Hirano, I.; et al. Impressions and aspirations from the FDA GREAT VI Workshop on Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis and Perspectives for Progress in the Field. J. Allergy Clin. Immunol. 2022, 149, 844–853. [Google Scholar] [CrossRef]
- Marasco, G.; Visaggi, P.; Vassallo, M.; Fiocca, M.; Cremon, C.; Barbaro, M.R.; De Bortoli, N.; Bellini, M.; Stanghellini, V.; Savarino, E.V.; et al. Current and Novel Therapies for Eosinophilic Gastrointestinal Diseases. Int. J. Mol. Sci. 2023, 24, 15165. [Google Scholar] [CrossRef] [PubMed]
- Sandström, T. Omalizumab in the management of patients with allergic (IgE-mediated) asthma. J. Asthma Allergy 2009, 2, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Losa, F.; Cingolani, A. Eosinophilic gastrointestinal disorders: New perspectives and the emerging role of biological therapies. Explor. Asthma Allergy 2023, 1, 60–72. [Google Scholar] [CrossRef]
- Egan, M.; Furuta, G.T. Eosinophilic gastrointestinal diseases beyond eosinophilic esophagitis. Ann. Allergy Asthma Immunol. 2018, 121, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Wang, Z.; Zhou, W.; Yin, J.; Zhi, Y. Eosinophilic gastritis and gluten-sensitive enteropathy manifested as hypoproteinemia and treated with omalizumab: A case report. Allergy Asthma Clin. Immunol. 2024, 20, 19. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Hew, G.S.Y.; Mehta, M.; Shukla, S.D.; Satija, S.; Khurana, N.; Anand, K.; Dureja, H.; Singh, S.K.; Mishra, V.; et al. Targeting eosinophils in respiratory diseases: Biological axis, emerging therapeutics and treatment modalities. Life Sci. 2021, 267, 118973. [Google Scholar] [CrossRef] [PubMed]
- Prussin, C.; James, S.; Huber, M.; Klion, A.; Metcalfe, D. Pilot study of anti-IL-5 in eosinophilic gastroenteritis. J. Allergy Clin. Immunol. 2003, 111, S275. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Prussin, C.; Martin, B.; Law, M.A.; Haverty, T.P.; Nutman, T.B.; Klion, A.D. Rebound eosinophilia after treatment of hypereosinophilic syndrome and eosinophilic gastroenteritis with monoclonal anti–IL-5 antibody SCH55700. J. Allergy Clin. Immunol. 2004, 114, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Pitlick, M.M.; Li, J.T.; Pongdee, T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ. J. 2022, 15, 100676. [Google Scholar] [CrossRef]
- Dellon, E.S.; Peterson, K.A.; Murray, J.A.; Falk, G.W.; Gonsalves, N.; Chehade, M.; Genta, R.M.; Leung, J.; Khoury, P.; Klion, A.D.; et al. Anti–Siglec-8 Antibody for Eosinophilic Gastritis and Duodenitis. N. Engl. J. Med. 2020, 383, 1624–1634. [Google Scholar] [CrossRef] [PubMed]
- Menzella, F.; Ruggiero, P.; Ghidoni, G.; Fontana, M.; Bagnasco, D.; Livrieri, F.; Scelfo, C.; Facciolongo, N. Anti-IL5 Therapies for Severe Eosinophilic Asthma: Literature Review and Practical Insights. J. Asthma Allergy 2020, 13, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Kuang, F.L.; Legrand, F.; Makiya, M.; Ware, J.; Wetzler, L.; Brown, T.; Magee, T.; Piligian, B.; Yoon, P.; Ellis, J.H.; et al. Benralizumab for PDGFRA-Negative Hypereosinophilic Syndrome. N. Engl. J. Med. 2019, 380, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, K.L.; Murray-Petzold, C.; Collins, M.H.; Abonia, J.P.; Bolton, S.M.; DiTommaso, L.A.; Martin, L.J.; Zhang, X.; Mukkada, V.A.; Putnam, P.E.; et al. Benralizumab for eosinophilic gastritis: A single-site, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 803–815. [Google Scholar] [CrossRef]
- Thomas, B.; Mathur, N. S4185 Beyond the Esophagus: Dupilumab’s Potential in Treatment of Eosinophilic Gastritis. Am. J. Gastroenterol. 2023, 118, S2648. [Google Scholar] [CrossRef]
- Sia, T.; Bacchus, L.; Tanaka, R.; Khuda, R.; Mallik, S.; Leung, J. Dupilumab Can Induce Remission of Eosinophilic Gastritis and Duodenitis: A Retrospective Case Series. Clin. Transl. Gastroenterol. 2024, 15, e00646. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Shibuya, T.; Nomura, K.; Haraikawa, M.; Kurosawa, T.; Haga, K.; Akazawa, Y.; Murakami, T.; Nomura, O.; Hojo, M.; et al. Successful Treatment of Steroid-resistant Eosinophilic Gastrointestinal Disease with Mepolizumab. Intern. Med. 2023, 62, 3461–3467. [Google Scholar] [CrossRef]
- Debnath, P.; Rathi, P.M. Vedolizumab in Inflammatory Bowel Disease: West versus East. Inflamm. Intest. Dis. 2021, 6, 1–17. [Google Scholar] [CrossRef]
- Grandinetti, T.; Biedermann, L.; Bussmann, C.; Straumann, A.; Hruz, P. Eosinophilic Gastroenteritis: Clinical Manifestation, Natural Course, and Evaluation of Treatment with Corticosteroids and Vedolizumab. Dig. Dis. Sci. 2019, 64, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Janssens, J.; Vanuytsel, T. Non-esophageal eosinophilic gastrointestinal diseases: A narrative review. Acta Gastro Enterol. Belg. 2023, 86, 449–459. [Google Scholar] [CrossRef]
- Kim, H.P.; Reed, C.C.; Herfarth, H.H.; Dellon, E.S. Vedolizumab Treatment May Reduce Steroid Burden and Improve Histology in Patients With Eosinophilic Gastroenteritis. Clin. Gastroenterol. Hepatol. 2018, 16, 1992–1994. [Google Scholar] [CrossRef]
- Laidlaw, T.M.; Buchheit, K.M. Biologics in chronic rhinosinusitis with nasal polyposis. Ann. Allergy Asthma Immunol. 2020, 124, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.R.; Kuan, E.C.; Scadding, G.K. Epidemiology of Chronic Rhinosinusitis: Prevalence and Risk Factors. J. Allergy Clin. Immunol. Pract. 2022, 10, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Rank, M.A.; Chu, D.K.; Bognanni, A.; Oykhman, P.; Bernstein, J.A.; Ellis, A.K.; Golden, D.B.; Greenhawt, M.; Horner, C.C.; Ledford, D.K.; et al. The Joint Task Force on Practice Parameters GRADE Guidelines for the Medical Management of Chronic Rhinosinusitis with Nasal Polyposis. J. Allergy Clin. Immunol. 2023, 151, 386–398. [Google Scholar] [CrossRef]
- Hellings, P.; Fokkens, W.; Orlandi, R.; Adriaensen, G.; Alobid, I.; Baroody, F.; Bjermer, L.; Senior, B.; Cervin, A.; Cohen, N.; et al. The EUFOREA pocket guide for chronic rhinosinusitis. Rhinology 2022, 61, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Masson, E. Comparative Efficacy and Safety of Monoclonal Antibodies and Aspirin Desensitization for Chronic Rhinosinusitis with Nasal Polyposis: A Systematic Review and Network Meta-Analysis. EM-Consulte. Available online: https://www.em-consulte.com/article/1509938/comparative-efficacy-and-safety-of-monoclonal-anti (accessed on 26 November 2024).
- Fokkens, W.J.; Viskens, A.-S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A.; et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yin, J.; Yang, Y.; Wang, G.; Zhang, Y.; Song, X. Dupilumab in chronic rhinosinusitis with nasal polyposis: Current status, challenges, and future perspectives. Expert Rev. Clin. Immunol. 2023, 19, 939–948. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Pasquini, E.; Trimarchi, M.; La Mantia, I.; Pagella, F.; Ottaviano, G.; Garzaro, M.; Pipolo, C.; Torretta, S.; Seccia, V.; et al. Dupilumab in the treatment of severe uncontrolled chronic rhinosinusitis with nasal polyps (CRSwNP): A multicentric observational Phase IV real-life study (DUPIREAL). Allergy 2023, 78, 2669–2683. [Google Scholar] [CrossRef]
- Geng, B.; Bachert, C.; Busse, W.W.; Gevaert, P.; Lee, S.E.; Niederman, M.S.; Chen, Z.; Lu, X.; Khokhar, F.A.; Kapoor, U.; et al. Respiratory Infections and Anti-Infective Medication Use From Phase 3 Dupilumab Respiratory Studies. J. Allergy Clin. Immunol. Pract. 2021, 10, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, C.; Wagenmann, M.; Bachert, C.; Desrosiers, M.; Han, J.K.; Hellings, P.W.; Lee, S.E.; Msihid, J.; Radwan, A.; Rowe, P.; et al. Efficacy of dupilumab in patients with a history of prior sinus surgery for chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2021, 11, 1087–1101. [Google Scholar] [CrossRef]
- Papacharalampous, G.X.; Constantinidis, J.; Fotiadis, G.; Zhang, N.; Bachert, C.; Katotomichelakis, M. Chronic rhinosinusitis with nasal polyps (CRSwNP) treated with omalizumab, dupilumab, or mepolizumab: A systematic review of the current knowledge towards an attempt to compare agents’ efficacy. Int. Forum Allergy Rhinol. 2024, 14, 96–109. [Google Scholar] [CrossRef] [PubMed]
- De Prado Gomez, L.; Khan Mbbs, A.H.; Peters, A.T.; Bachert, C.; Wagenmann, M.; Heffler, E.; Hopkins, C.; Hellings, P.W.; Zhang, M.; Xing, J.; et al. Efficacy and Safety of Dupilumab Versus Omalizumab in Chronic Rhinosinusitis With Nasal Polyps and Asthma: EVEREST Trial Design. Am. J. Rhinol. Allergy 2022, 36, 788–795. [Google Scholar] [CrossRef]
- Namazy, J.A.; Blais, L.; Andrews, E.B.; Scheuerle, A.E.; Cabana, M.D.; Thorp, J.M.; Umetsu, D.T.; Veith, J.H.; Sun, D.; Kaufman, D.G.; et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J. Allergy Clin. Immunol. 2020, 145, 528–536.e1. [Google Scholar] [CrossRef]
- Klimek, L.; Förster-Ruhrmann, U.; Olze, H.; Beule, A.G.; Chaker, A.M.; Hagemann, J.; Huppertz, T.; Hoffmann, T.K.; Dazert, S.; Deitmer, T.; et al. Monitoring mepolizumab treatment in chronic rhinosinusitis with nasal polyps (CRSwNP): Discontinue, change, continue therapy? Allergol. Sel. 2024, 8, 26–39. [Google Scholar] [CrossRef]
- Mullol, J.; Lund, V.J.; Wagenmann, M.; Han, J.K.; Sousa, A.N.; Smith, S.G.; Mayer, B.; Chan, R.H.; Fokkens, W.J. Mepolizumab improves sense of smell in severe chronic rhinosinusitis with nasal polyps: SYNAPSE. Rhinology 2024, 62, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Fujieda, F.; Wang, C.; Yoshikawa, M.; Asako, M.; Suzaki, I.; Bachert, C.; Han, J.K.; Fuller, A.; Baylis, L.; Su, L.; et al. Mepolizumab in CRSwNP/ECRS and NP: The phase III randomised MERIT trial in Japan, China, and Russia. Rhinology 2024, 62, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Chupp, G.; Alobid, I.; Lugogo, N.; Kariyawasam, H.; Bourdin, A.; Chaker, A.; Sousa, A.; Martin, N.; Yang, S.; Mayer, B.; et al. Mepolizumab Reduces Systemic Corticosteroid Use in Chronic Rhinosinusitis With Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2023, 11, 3504–3512.e2. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Mullol, J.; Kennedy, D.; Philpott, C.; Seccia, V.; Kern, R.C.; Coste, A.; Sousa, A.R.; Howarth, P.H.; Benson, V.S.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): In-depth sinus surgery analysis. Allergy 2023, 78, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, M.; Diamant, Z.; Castelnuovo, P.; Hellings, P.W.; Han, J.K.; Peters, A.T.; Silver, J.; Smith, S.G.; Fuller, A.; Sousa, A.R.; et al. Sustained efficacy of mepolizumab in patients with severe chronic rhinosinusitis with nasal polyps: SYNAPSE 24-week treatment-free follow-up. Int. Forum Allergy Rhinol. 2024, 14, 18–31. [Google Scholar] [CrossRef]
- Emson, C.; Han, J.K.; Hopkins, C.; Asimus, S.; Cann, J.A.; Chain, D.; Wu, Y.; Reddy, Y.; McCrae, C.; Cohen, D.; et al. Pharmacokinetics/pharmacodynamics of benralizumab in chronic rhinosinusitis with nasal polyps: Phase III, randomized, placebo-controlled OSTRO trial. Br. J. Clin. Pharmacol. 2024, 90, 1952–1963. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and Safety of Benralizumab in Chronic Rhinosinusitis with Nasal Polyps: A Randomized, Placebo-controlled Trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e12. [Google Scholar] [CrossRef] [PubMed]
- Takabayashi, T.; Asaka, D.; Okamoto, Y.; Himi, T.; Haruna, S.; Yoshida, N.; Kondo, K.; Yoshikawa, M.; Sakuma, Y.; Shibata, K.; et al. A Phase II, Multicenter, Randomized, Placebo-Controlled Study of Benralizumab, a Humanized Anti-IL-5R Alpha Monoclonal Antibody, in Patients With Eosinophilic Chronic Rhinosinusitis. Am. J. Rhinol. Allergy 2021, 35, 861–870. [Google Scholar] [CrossRef]
- Canonica, G.W.; Harrison, T.W.; Chanez, P.; Menzella, F.; Louis, R.; Cosio, B.G.; Lugogo, N.L.; Mohan, A.; Burden, A.; Gil, E.G. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy 2022, 77, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Parnes, J.R.; Molfino, N.A.; Colice, G.; Martin, U.; Corren, J.; Menzies-Gow, A. Targeting TSLP in Asthma. J. Asthma Allergy 2022, 15, 749–765. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Estellés, A.; Li, L.; Abdelhady, W.; Gonzales, R.; Bayer, A.S.; Tenorio, E.; Leighton, A.; Ryser, S.; Kauvar, L.M. A Human Biofilm-Disrupting Monoclonal Antibody Potentiates Antibiotic Efficacy in Rodent Models of both Staphylococcus aureus and Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2017, 61, e00904-17. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.; Delanois, R.E.; Mont, M.A.; Stavrakis, A.; McPherson, E.; Stolarski, E.; Incavo, S.; Oakes, D.; Salvagno, R.; Adams, J.S.; et al. Phase 1 study of the pharmacokinetics and clinical proof-of-concept activity of a biofilm-disrupting human monoclonal antibody in patients with chronic prosthetic joint infection of the knee or hip. Antimicrob. Agents Chemother. 2024, 68, e0065524. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Walker, A.; Pirwani, M.; Tahiri, M.; Syed, I. Allergic rhinitis: Diagnosis and management. Br. J. Hosp. Med. 2022, 83, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.I.; Lee, I.H.; Kim, M.; Ryu, G.; Kang, S.Y.; Kim, M.A.; Lee, S.M.; Kim, H.J.; Park, D.Y.; Lee, Y.J.; et al. KAAACI Allergic Rhinitis Guidelines: Part 1. Update in Pharmacotherapy. Allergy Asthma Immunol. Res. 2023, 15, 19–31. [Google Scholar] [CrossRef]
- Tidke, M.; Borghare, P.T.; Pardhekar, P.; Nasre, Y.; Gomase, K.; Chaudhary, M. Recent Advances in Allergic Rhinitis: A Narrative Review. Cureus 2024, 16, e68607. [Google Scholar] [CrossRef] [PubMed]
- Tsabouri, S.; Ntritsos, G.; Koskeridis, F.; Evangelou, E.; Olsson, P.; Kostikas, K. Omalizumab for the treatment of allergic rhinitis: A systematic review and meta-analysis. Rhinology 2021, 59, 501–510. [Google Scholar] [CrossRef]
- Peters, A.T.; Wagenmann, M.; Bernstein, J.A.; Khan, A.H.; Nash, S.; Jacob-Nara, J.A.; Siddiqui, S.; Rowe, P.J.; Deniz, Y. Dupilumab efficacy in patients with chronic rhinosinusitis with nasal polyps with and without allergic rhinitis. Allergy Asthma Proc. 2023, 44, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Larson, D.; Altman, M.C.; Segnitz, R.M.; Avila, P.C.; Greenberger, P.A.; Baroody, F.; Moss, M.H.; Nelson, H.; Burbank, A.J.; et al. Effects of combination treatment with tezepelumab and allergen immunotherapy on nasal responses to allergen: A randomized controlled trial. J. Allergy Clin. Immunol. 2022, 151, 192–201. [Google Scholar] [CrossRef]
- Shamji, M.H.; Singh, I.; Layhadi, J.A.; Ito, C.; Karamani, A.; Kouser, L.; Sharif, H.; Tang, J.; Handijiev, S.; Parkin, R.V.; et al. Passive Prophylactic Administration with a Single Dose of Anti-Fel d 1 Monoclonal Antibodies REGN1908-1909 in Cat Allergen-induced Allergic Rhinitis: A Randomized, Double-Blind, Placebo-controlled Clinical Trial. Am. J. Respir Crit. Care Med. 2021, 204, 23–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, B.; Zhu, Z.; Wang, X.; Song, X.; Zhu, D.; Ma, T.; Zhang, Y.; Meng, C.; Wang, G.; et al. Efficacy and safety of stapokibart (CM310) in uncontrolled seasonal allergic rhinitis (MERAK): An investigator-initiated, placebo-controlled, randomised, double-blind, phase 2 trial. eClinicalMedicine 2024, 69, 102467. [Google Scholar] [CrossRef] [PubMed]
- Atanasio, A.; Franklin, M.C.; Kamat, V.; Hernandez, A.R.; Badithe, A.; Ben, L.-H.; Jones, J.; Bautista, J.; Yancopoulos, G.D.; Olson, W.; et al. Targeting immunodominant Bet v 1 epitopes with monoclonal antibodies prevents the birch allergic response. J. Allergy Clin. Immunol. 2022, 149, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Rand, K.; Ramos-Goñi, J.M.; Akmaz, B.; Solé-Feu, L.; Armario-Hita, J.-C. Matching-Adjusted Indirect Comparison of the Long-Term Efficacy Maintenance and Adverse Event Rates of Lebrikizumab versus Dupilumab in Moderate-to-Severe Atopic Dermatitis. Dermatol. Ther. 2023, 14, 169–182. [Google Scholar] [CrossRef]
- Katoh, N.; Tsunemi, Y.; Kamata, M.; Dossenbach, M.; Hanada, T. An indirect comparison of lebrikizumab, dupilumab, and tralokinumab in Japanese patients with moderate to severe atopic dermatitis by network meta-analysis methodology. In Proceedings of the European Academy of Dermatology and Venerology Congress, Berlin, Germany, 11–14 October 2023; Available online: https://s3.eu-central-1.amazonaws.com/m-anage.com.storage.eadv/abstracts_congress2023/37622.pdf (accessed on 18 December 2024).
- Liu, W.; Zhao, Y.; He, Y.; Yan, X.; Yu, J.; Song, Q.; Zhang, L.; Dong, B.; Xu, G.; Wang, C.; et al. Stapokibart (CM310) targets IL-4Rα for the treatment of type 2 inflammation. iScience 2024, 27, 110721. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Zhang, J. Efficacy and safety of stapokibart (CM310) in adults with moderate-to-severe atopic dermatitis: A multicenter, randomized, double-blind, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2024, 91, 984–986. [Google Scholar] [CrossRef]
- Wang, J.; White, J.; Sansone, K.J.; Spelman, L.; Sinclair, R.; Yang, X.; Pan, W.; Wei, Z. Rademikibart (CBP-201), a next-generation monoclonal antibody targeting human IL-4Rα: Two phase I randomized trials, in healthy individuals and patients with atopic dermatitis. Clin. Transl. Sci. 2023, 16, 2614–2627. [Google Scholar] [CrossRef]
- Nishi, K.; Matsumoto, H.; Sunadome, H.; Nagasaki, T.; Oguma, T.; Tashima, N.; Hayashi, Y.; Terada, S.; Morita, K.; Yoshimura, C.; et al. IL1RL1variant may affect the response to type 2 biologics in patients with severe asthma. ERJ Open Res. 2024, 11, 00448-2024. [Google Scholar] [CrossRef] [PubMed]
- Porsbjerg, C.M.; Townend, J.; Bergeron, C.; Christoff, G.C.; Katsoulotos, G.P.; Larenas-Linnemann, D.; Tran, T.N.; Al-Lehebi, R.; Bosnic-Anticevich, S.Z.; Busby, J.; et al. Association between pre-biologic T2-biomarker combinations and response to biologics in patients with severe asthma. Front. Immunol. 2024, 15, 1361891. [Google Scholar] [CrossRef]
- Thomas, V.A.; Balthasar, J.P. Understanding Inter-Individual Variability in Monoclonal Antibody Disposition. Antibodies 2019, 8, 56. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Wollenberg, A.; Legat, F.J.; Laquer, V.T.; Armstrong, A.W.; Herranz, P.; Naldi, L.; Ahmad, F.; Ulianov, L.; Piketty, C. 667-Maintenance of efficacy and safety with nemolizumab at week 48: Results from two global phase 3 pivotal studies (ARCADIA-1 and ARCADIA-2) in patients with moderate-to-severe atopic dermatitis. Br. J. Dermatol. 2024, 191, ljae266.041. [Google Scholar] [CrossRef]
- Boada-Fernández-Del-Campo, C.; García-Sánchez-Colomer, M.; Fernández-Quintana, E.; Poza-Guedes, P.; Rolingson-Landaeta, J.L.; Sánchez-Machín, I.; González-Pérez, R. Real-World Safety Profile of Biologic Drugs for Severe Uncontrolled Asthma: A Descriptive Analysis from the Spanish Pharmacovigilance Database. J. Clin. Med. 2024, 13, 4192. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Burroni, A.G.; Chiricozzi, A.; Dapavo, P.; Ferrucci, S.M.; Gola, M.; Napolitano, M.; Ortoncelli, M.; et al. Management of Patients Affected by Moderate-to-Severe Atopic Dermatitis with JAK Inhibitors in Real-World Clinical Practice: An Italian Delphi Consensus. Dermatol. Ther. 2024, 14, 919–932. [Google Scholar] [CrossRef]
- Padilla-Galo, A.; García-Ruiz, A.J.; Abitbol, R.C.L.; Olveira, C.; Rivas-Ruiz, F.; Soler, N.G.-A.; Morales, M.P.; Azcona, B.V.; Tortajada-Goitia, B.; Moya-Carmona, I.; et al. Real-life cost-effectiveness of benralizumab in patients with severe asthma. Respir. Res. 2021, 22, 163. [Google Scholar] [CrossRef]
- Habash, M.; Guiang, H.; Mayers, I.; Quinton, A.; Vuong, V.; Dineen, A.; Singh, S.; Gibson, D.; Turner, A.P. Cost-effectiveness of tezepelumab in Canada for severe asthma. J. Med. Econ. 2023, 26, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Tugay, D.; Top, M.; Aydin, Ö.; Bavbek, S.; Damadoğlu, E.; Erkekol, F.Ö.; Kalkan, I.K.; Kalyoncu, A.F.; Karakaya, G.; Oğuzülgen, I.K.; et al. Real-world patient-level cost-effectiveness analysis of omalizumab in patients with severe allergic asthma treated in four major medical centers in Turkey. J. Med. Econ. 2023, 26, 720–730. [Google Scholar] [CrossRef]
- Anderson, W.C.; Szefler, S.J. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: To biologic or not to biologic? Ann. Allergy Asthma Immunol. 2019, 122, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Shamji, M.H.; Anto, J.M.; Schünemann, H.J.; Canonica, G.W.; Jutel, M.; Del Giacco, S.; Zuberbier, T.; Pfaar, O.; Fonseca, J.A.; et al. Patient-centered digital biomarkers for allergic respiratory diseases and asthma: The ARIA-EAACI approach—ARIA-EAACI Task Force Report. Allergy 2023, 78, 1758–1776. [Google Scholar] [CrossRef] [PubMed]

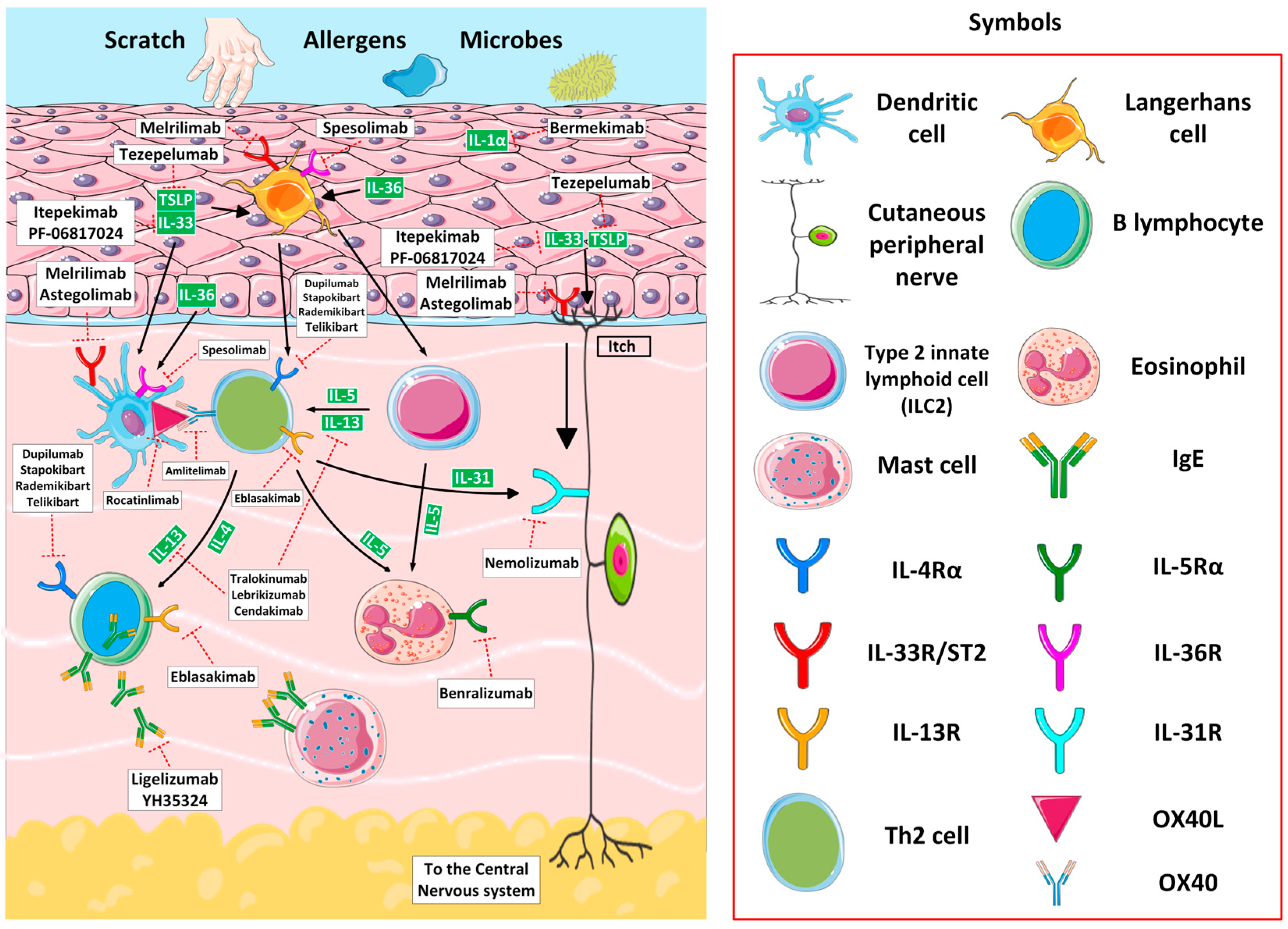
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alska, E.; Łaszczych, D.; Napiórkowska-Baran, K.; Szymczak, B.; Rajewska, A.; Rubisz, A.E.; Romaniuk, P.; Wrzesień, K.; Mućka, N.; Bartuzi, Z. Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives. J. Clin. Med. 2025, 14, 1079. https://doi.org/10.3390/jcm14041079
Alska E, Łaszczych D, Napiórkowska-Baran K, Szymczak B, Rajewska A, Rubisz AE, Romaniuk P, Wrzesień K, Mućka N, Bartuzi Z. Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives. Journal of Clinical Medicine. 2025; 14(4):1079. https://doi.org/10.3390/jcm14041079
Chicago/Turabian StyleAlska, Ewa, Dariusz Łaszczych, Katarzyna Napiórkowska-Baran, Bartłomiej Szymczak, Alicja Rajewska, Aleksandra Ewa Rubisz, Paulina Romaniuk, Katarzyna Wrzesień, Natalia Mućka, and Zbigniew Bartuzi. 2025. "Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives" Journal of Clinical Medicine 14, no. 4: 1079. https://doi.org/10.3390/jcm14041079
APA StyleAlska, E., Łaszczych, D., Napiórkowska-Baran, K., Szymczak, B., Rajewska, A., Rubisz, A. E., Romaniuk, P., Wrzesień, K., Mućka, N., & Bartuzi, Z. (2025). Advances in Biologic Therapies for Allergic Diseases: Current Trends, Emerging Agents, and Future Perspectives. Journal of Clinical Medicine, 14(4), 1079. https://doi.org/10.3390/jcm14041079







