Serum Spike Protein Persistence Post COVID Is Not Associated with ME/CFS
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Study Cohorts, and Symptom Assessment
2.2. Quantification of Serum Spike RBD and Anti-S1 IgG
2.3. Assessment of Routine Laboratory and Functional Parameters
2.4. Data Collection and Management
2.5. Statistical Analysis
3. Results
3.1. Spike Protein Is Detectable in Serum of Individuals After Acute SARS-CoV-2 Infection
3.2. Serum Spike Protein Concentration Is Independent of the Time Since Last SARS-CoV-2 Spike Protein Contact
3.3. Serum Spike Protein Is Not Associated with ME/CFS Disease and Symptom Severity
3.4. Serum Spike Protein Is Reduced by Immunoadsorption (IA) to Deplete Immunoglobulins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAB | Autoantibody |
| AU | Arbitrary units |
| BAU | Binding Antibody Unit |
| β2-AdR | β2 adrenergic receptor |
| CCC | Canadian Consensus Criteria |
| CDC | Centers for Disease Control and Prevention |
| COMPASS-31 | Composite Autonomic Symptom Score 31 |
| COVID-19 | Coronavirus disease 2019 |
| EBV | Epstein-Barr virus |
| GPCR | G protein-coupled receptor |
| IA | Immunoadsorption |
| IL-8 | Interleukin-8 |
| IOM | Institute of Medicine |
| IgG | Immunoglobulin G |
| IQR | Interquartile range |
| m | Months |
| ME/CFS | Myalgic encephalomyelitis/chronic fatigue syndrome |
| MPV | Mean platelet volume |
| NA | Not assessed |
| ns | Not significant |
| PCS | Post-COVID syndrome |
| PEM | Post-exertional malaise |
| RBD | Receptor-binding domain |
| RH-PAT | Reactive hyperemia peripheral arterial tonometry |
| RHI | Reactive hyperemia index |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus type 2 |
| SF-36 | Short Form Health Survey 36 |
| unvacc. | Unvaccinated |
| WHO | World Health Organization |
| y | Years |
| pcHC | Post-COVID healthy control |
| ppHC | Pre-pandemic healthy control |
References
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.J.; Williams, D.M.; Walker, A.J.; Mitchell, R.E.; Niedzwiedz, C.L.; Yang, T.C.; Huggins, C.F.; Kwong, A.S.F.; Silverwood, R.J.; Di Gessa, G.; et al. Long COVID burden and risk factors in 10 UK longitudinal studies and electronic health records. Nat. Commun. 2022, 13, 3528. [Google Scholar] [CrossRef]
- Ford, N.D.; Slaughter, D.; Edwards, D.; Dalton, A.; Perrine, C.; Vahratian, A.; Saydah, S. Long COVID and Significant Activity Limitation Among Adults, by Age—United States, June 1-13, 2022, to June 7-19, 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 866–870. [Google Scholar] [CrossRef]
- Iba, A.; Hosozawa, M.; Hori, M.; Muto, Y.; Muraki, I.; Masuda, R.; Tamiya, N.; Iso, H. Prevalence of and Risk Factors for Post-COVID-19 Condition during Omicron BA.5-Dominant Wave, Japan. Emerg. Infect. Dis. 2024, 30, 1380–1389. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Hanitsch, L.G.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat. Commun. 2022, 13, 5104. [Google Scholar] [CrossRef] [PubMed]
- Twomey, R.; DeMars, J.; Franklin, K.; Culos-Reed, S.N.; Weatherald, J.; Wrightson, J.G. Chronic Fatigue and Postexertional Malaise in People Living With Long COVID: An Observational Study. Phys. Ther. 2022, 102, pzac005. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.R.; Lin, J.S.; Wisk, L.E.; Yu, H.; L’Hommedieu, M.; Lavretsky, H.; Montoy, J.C.C.; Gottlieb, M.A.; Rising, K.L.; Gentile, N.L.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome After SARS-CoV-2 Infection. JAMA Netw. Open 2024, 7, e2423555. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Julg, B.; Mohandas, S.; Bradfute, S.B.; Force, R.M.P.T. Viral persistence, reactivation, and mechanisms of long COVID. eLife 2023, 12, e86015. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 746021. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, Q.; Yao, Q.; Wang, X.; Zhang, H.; Chen, R.; Ren, L.; Min, J.; Deng, F.; et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov. 2021, 7, 17. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.-Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Lütgehetmann, M.; Hagel, C.; Sperhake, J.P.; Schröder, A.S.; Edler, C.; Mushumba, H.; Fitzek, A.; Allweiss, L.; Dandri, M.; et al. Neuropathology of patients with COVID-19 in Germany: A post-mortem case series. Lancet Neurol. 2020, 19, 919–929. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Natarajan, A.; Zlitni, S.; Brooks, E.F.; Vance, S.E.; Dahlen, A.; Hedlin, H.; Park, R.M.; Han, A.; Schmidtke, D.T.; Verma, R.; et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med 2022, 3, 371–387.e9. [Google Scholar] [CrossRef]
- Peluso, M.J.; Ryder, D.; Flavell, R.R.; Wang, Y.; Levi, J.; LaFranchi, B.H.; Deveau, T.-M.; Buck, A.M.; Munter, S.E.; Asare, K.A.; et al. Tissue-based T cell activation and viral RNA persist for up to 2 years after SARS-CoV-2 infection. Sci. Transl. Med. 2024, 16, eadk3295. [Google Scholar] [CrossRef]
- Peluso, M.J.; Swank, Z.N.; Goldberg, S.A.; Lu, S.; Dalhuisen, T.; Borberg, E.; Senussi, Y.; Luna, M.A.; Chang Song, C.; Clark, A.; et al. Plasma-based antigen persistence in the post-acute phase of COVID-19. Lancet Infect. Dis. 2024, 24, e345–e347. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2023, 76, e487–e490. [Google Scholar] [CrossRef]
- Craddock, V.; Mahajan, A.; Spikes, L.; Krishnamachary, B.; Ram, A.K.; Kumar, A.; Chen, L.; Chalise, P.; Dhillon, N.K. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med. Virol. 2023, 95, e28568. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.S.; Morrison-Porter, A.; Quehl, H.; Capric, V.; Lamothe, P.A.; Anam, F.; Runnstrom, M.C.; Truong, A.D.; Dixit, A.N.; Woodruff, M.C.; et al. MENSA, a Media Enriched with Newly Synthesized Antibodies, to Identify SARS-CoV-2 Persistence and Latent Viral Reactivation in Long-COVID. medRxiv 2024. [Google Scholar] [CrossRef]
- Kanberg, N.; Grahn, A.; Stentoft, E.; Bremell, D.; Yilmaz, A.; Studahl, M.; Nilsson, S.; Schöll, M.; Gostner, J.M.; Blennow, K.; et al. COVID-19 Recovery: Consistent Absence of Cerebrospinal Fluid Biomarker Abnormalities in Patients With Neurocognitive Post-COVID Complications. J. Infect. Dis. 2023, 229, 493–501. [Google Scholar] [CrossRef]
- Zollner, A.; Koch, R.; Jukic, A.; Pfister, A.; Meyer, M.; Rössler, A.; Kimpel, J.; Adolph, T.E.; Tilg, H. Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology 2022, 163, 495–506.e8. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Heindrich, C.; Wittke, K.; Kedor, C.; Rust, R.; Freitag, H.; Sotzny, F.; Krüger, A.; Tölle, M.; Grabowski, P.; et al. Efficacy of repeated immunoadsorption in patients with post-COVID myalgic encephalomyelitis/chronic fatigue syndrome and elevated β2-adrenergic receptor autoantibodies: A prospective cohort study. Lancet Reg. Health—Eur. 2025, 49, 101161. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.C.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Jason, L.A.; Evans, M.; Porter, N.; Brown, M.; Brown, A.; Hunnell, J.; Anderson, V.; Lerch, A.; De Meirleir, K.; Friedberg, F. The Development of a Revised Canadian Myalgic Encephalomyelitis Chronic Fatigue Syndrome Case Definition. Am. J. Biochem. Biotechnol. 2010, 6, 120–135. [Google Scholar] [CrossRef]
- Cotler, J.; Holtzman, C.; Dudun, C.; Jason, L.A. A Brief Questionnaire to Assess Post-Exertional Malaise. Diagnostics 2018, 8, 66. [Google Scholar] [CrossRef]
- Bell, D.S. The Doctor’s Guide to Chronic Fatigue Syndrome: Understanding, Treating, and Living with Cfids; Addison-Wesley Pub. Co.: Reading, MA, USA, 1994. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Fors Connolly, A.M. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: Nationwide self-controlled cases series and matched cohort study. Bmj 2022, 377, e069590. [Google Scholar] [CrossRef]
- Swank, Z.; Borberg, E.; Chen, Y.; Senussi, Y.; Chalise, S.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Henrich, T.J.; et al. Measurement of circulating viral antigens post-SARS-CoV-2 infection in a multicohort study. Clin. Microbiol. Infect. 2024, 30, 1599–1605. [Google Scholar] [CrossRef]
- Rahmati, A.; Shahbaz, S.; Osman, M.; Tervaert, J.W.C.; Elahi, S. Blood transcriptomic analyses do not support SARS-CoV-2 persistence in patients with post-COVID-19 condition with chronic fatigue syndrome. Lancet Microbe 2025, 6, 101012. [Google Scholar] [CrossRef]
- Menezes, S.M.; Jamoulle, M.; Carletto, M.P.; Moens, L.; Meyts, I.; Maes, P.; Van Weyenbergh, J. Blood transcriptomic analyses reveal persistent SARS-CoV-2 RNA and candidate biomarkers in post-COVID-19 condition. Lancet Microbe 2024, 5, 100849. [Google Scholar] [CrossRef]
- Perico, L.; Morigi, M.; Pezzotta, A.; Locatelli, M.; Imberti, B.; Corna, D.; Cerullo, D.; Benigni, A.; Remuzzi, G. SARS-CoV-2 spike protein induces lung endothelial cell dysfunction and thrombo-inflammation depending on the C3a/C3a receptor signalling. Sci. Rep. 2023, 13, 11392. [Google Scholar] [CrossRef]
- Montezano, A.C.; Camargo, L.L.; Mary, S.; Neves, K.B.; Rios, F.J.; Stein, R.; Lopes, R.A.; Beattie, W.; Thomson, J.; Herder, V.; et al. SARS-CoV-2 spike protein induces endothelial inflammation via ACE2 independently of viral replication. Sci. Rep. 2023, 13, 14086. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Xie, Y.; Bowe, B.; Al-Aly, Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat. Commun. 2021, 12, 6571. [Google Scholar] [CrossRef]
- Hill, E.L.; Mehta, H.B.; Sharma, S.; Mane, K.; Singh, S.K.; Xie, C.; Cathey, E.; Loomba, J.; Russell, S.; Spratt, H.; et al. Risk factors associated with post-acute sequelae of SARS-CoV-2: An N3C and NIH RECOVER study. BMC Public Health 2023, 23, 2103. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment With Nirmatrelvir and the Risk of Post–COVID-19 Condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Lin, F.; Peyser, N.D.; Isasi, C.; Carton, T.W.; Henrich, T.J.; Deeks, S.G.; Olgin, J.E.; Pletcher, M.J.; et al. Association of nirmatrelvir for acute SARS-CoV-2 infection with subsequent Long COVID symptoms in an observational cohort study. J. Med. Virol. 2024, 96, e29333. [Google Scholar] [CrossRef]
- Congdon, S.; Narrowe, Z.; Yone, N.; Gunn, J.; Deng, Y.; Nori, P.; Cowman, K.; Islam, M.; Rikin, S.; Starrels, J. Nirmatrelvir/ritonavir and risk of long COVID symptoms: A retrospective cohort study. Sci. Rep. 2023, 13, 19688. [Google Scholar] [CrossRef]
- Geng, L.N.; Bonilla, H.; Hedlin, H.; Jacobson, K.B.; Tian, L.; Jagannathan, P.; Yang, P.C.; Subramanian, A.K.; Liang, J.W.; Shen, S.; et al. Nirmatrelvir-Ritonavir and Symptoms in Adults With Postacute Sequelae of SARS-CoV-2 Infection: The STOP-PASC Randomized Clinical Trial. JAMA Intern. Med. 2024, 184, 1024–1034. [Google Scholar] [CrossRef]
- Troyer, Z.; Alhusaini, N.; Tabler, C.O.; Sweet, T.; de Carvalho, K.I.L.; Schlatzer, D.M.; Carias, L.; King, C.L.; Matreyek, K.; Tilton, J.C. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J. Extracell. Vesicles 2021, 10, e12112. [Google Scholar] [CrossRef]
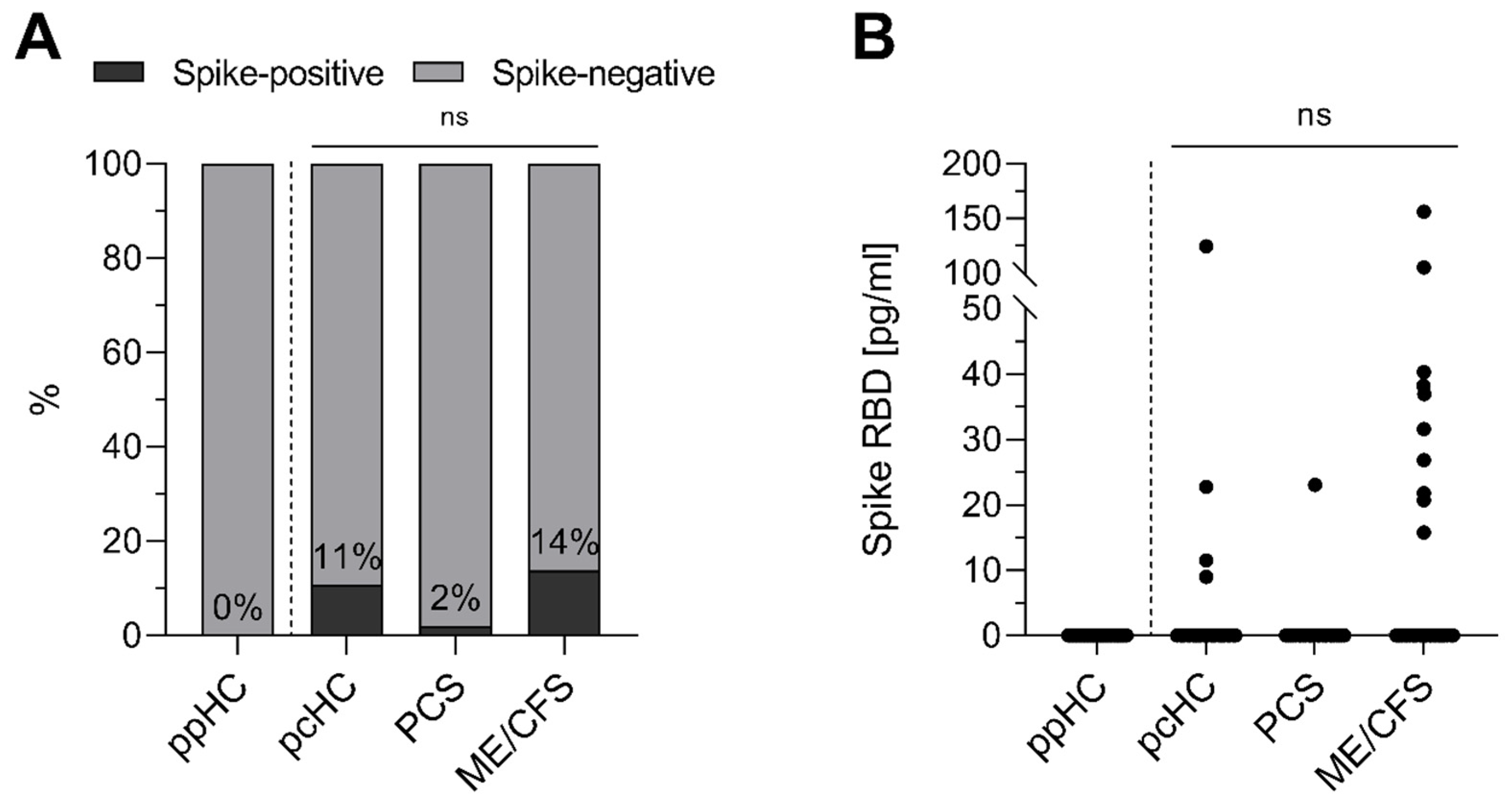
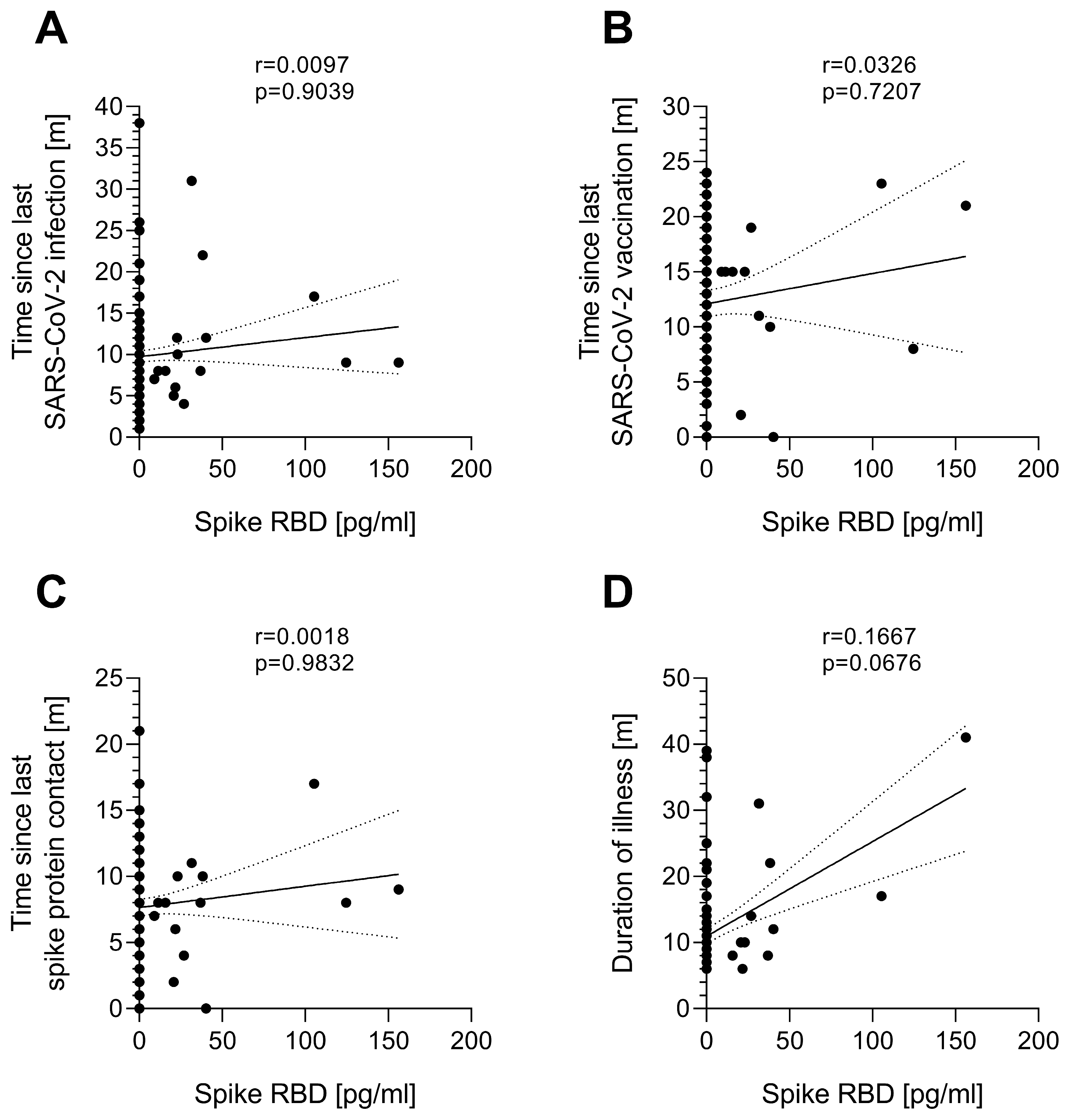

| Study Group | ppHC | pcHC | PCS | ME/CFS | IA-ME/CFS | p-Values |
|---|---|---|---|---|---|---|
| n | 32 | 37 | 49 | 50 | 22 | NA |
| Age [y], median (range) | 29 (21–54) | 34 (23–56) | 37 (23–62) | 40.5 (21–59) | 40 (31–59) | p1 = 0.2463 p2 = 0.1484 p3 = 0.0824 p4 = 0.6535 p5 = 0.3027 p6 = 0.4243 |
| Female sex [%] | 77.14 | 78.38 | 79.59 | 80.00 | 68.18 | p = 0.8230 |
| Duration of illness [m], median (range) | NA | NA | 9 (6–14) | 9 (6–17) | 20 (8–41) | p4 = 0.3443 p5 < 0.0001 p6 < 0.0001 |
| Time after last SARS-CoV-2 infection [m], median (range) | NA | 10 (5–12) | 8 (1–13) | 9 (5–12) | 14 (2–38) | p1 = 0.0679 p2 = 0.3034 p3 = 0.2233 p4 = 0.2819 p5 = 0.0055 p6 = 0.0504 |
| Time after last SARS-CoV-2 vaccination [m], median (range) | NA | 16 (4–22) 0 unvacc. n = 24 | 9 (0–20) 9 unvacc. n = 47 | 11.5 (0–24) 6 unvacc. n = 49 | 17 (0–24) 1 unvacc. n = 20 | p1 = 0.0032 p2 = 0.0955 p3 = 0.3700 p4 = 0.1049 p5 = 0.0004 p6 = 0.0133 |
| Bell Disability Scale, median (range) | NA | NA | 50 (30–90) | 30 (10–60) | 30 (20–40) | p4 < 0.0001 p5 < 0.0001 p6 = 0.4806 |
| Parameter | Spike+ (n = 10) Median (Range) n | Spike− (n = 62) Median (Range) n | p-Value |
|---|---|---|---|
| Severity of disability and PEM | |||
| Bell Disability Scale | 30.00 (20.00–40.00) n = 10 | 30.00 (10.00–60.00) n = 61 | 0.7380 |
| SF-36 Physical Functioning | 25.00 (5.00–50.00) n = 10 | 35.00 (0.00–70.00) n = 61 | 0.1021 |
| PEM score | 36.00 (29.00–46.00) n = 9 | 34.00 (19.00–46.00) n = 58 | 0.5707 |
| Symptom scores | |||
| Fatigue score | 8.25 (6.25–10.00) n = 9 | 8.00 (5.5–10.00) n = 59 | 0.8686 |
| Cognitive score | 6.67 (1.67–9.33) n = 9 | 7.00 (2.33–10.00) n = 60 | 0.9265 |
| Immune score | 2.67 (1.00–7.33) n = 8 | 3.67 (1.00–8.67) n = 58 | 0.2961 |
| Headache | 6.00 (1.00–10.00) n = 9 | 6.50 (1.00–10.00) n = 60 | 0.5623 |
| Muscle pain | 6.00 (1.00–10.00) n = 9 | 7.00 (1.00–10.00) n = 60 | 0.6602 |
| Joint pain | 6.00 (1.00–9.00) n = 9 | 5.00 (1.00–10.00) n = 60 | 0.8496 |
| Assessment of autonomic dysfunction | |||
| COMPASS-31 total | 41.51 (11.55–58.24) n = 10 | 38.25 (2.68–76.23) n = 59 | 0.4045 |
| Parameter | Reference Range | Spike+ (n = 10) Median (IQR) n (out of Reference Range)/n | Spike− (n = 62) Median (IQR) n (out of Reference Range)/n | p-Value |
|---|---|---|---|---|
| (A) | Thrombotic and Inflammatory Markers | |||
| IL-8 [pg/mL] | <150.00 | 126.80 (94.60–132.10) 1/7 | 131.00 (106.20–176.90) 18/55 | 0.2705 |
| D-Dimer [mg/L] | <0.50 | 0.21 (0.19–0.34) 0/5 | 0.23 (0.19–0.38) 3/39 | 0.5863 |
| Platelets [/nL] | 150.00–370.00 | 258.50 (244.50–277.00) 0/8 | 265.50 (220.75–308.50) 3/60 | 0.9513 |
| MPV [fL] | 7.00–12.00 | 10.90 (10.45–11.55) 1/8 | 10.70 (10.20–11.60) 4/60 | 0.5273 |
| (B) | β2-AdR-AAB and Anti-S1 IgG | |||
| β2-AdR-AAB [a.u.] | <14.00 | 24.15 (10.2–34.79) 6/10 | 15.63 (8.51–24.50) 31/59 | 0.2613 |
| Anti-S1 IgG [BAU/mL] median, (IQR), n | 1953.51 (1034.04–3265.13) n = 16 * | 838.33 (447.05–1957.15) n = 10 | 1185.01 (547.86–1944.94) n = 32 | 0.7382 |
| (C) | Markers of Endothelial Dysfunction | |||
| RHI | >1.67 | 1.78 (1.41–2.19) 2/4 | 2.08 (1.70–2.37) 4/17 | 0.4531 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehrer, A.; Sotzny, F.; Kim, L.; Kedor, C.; Freitag, H.; Heindrich, C.; Grabowski, P.; Babel, N.; Scheibenbogen, C.; Wittke, K. Serum Spike Protein Persistence Post COVID Is Not Associated with ME/CFS. J. Clin. Med. 2025, 14, 1086. https://doi.org/10.3390/jcm14041086
Fehrer A, Sotzny F, Kim L, Kedor C, Freitag H, Heindrich C, Grabowski P, Babel N, Scheibenbogen C, Wittke K. Serum Spike Protein Persistence Post COVID Is Not Associated with ME/CFS. Journal of Clinical Medicine. 2025; 14(4):1086. https://doi.org/10.3390/jcm14041086
Chicago/Turabian StyleFehrer, Annick, Franziska Sotzny, Laura Kim, Claudia Kedor, Helma Freitag, Cornelia Heindrich, Patricia Grabowski, Nina Babel, Carmen Scheibenbogen, and Kirsten Wittke. 2025. "Serum Spike Protein Persistence Post COVID Is Not Associated with ME/CFS" Journal of Clinical Medicine 14, no. 4: 1086. https://doi.org/10.3390/jcm14041086
APA StyleFehrer, A., Sotzny, F., Kim, L., Kedor, C., Freitag, H., Heindrich, C., Grabowski, P., Babel, N., Scheibenbogen, C., & Wittke, K. (2025). Serum Spike Protein Persistence Post COVID Is Not Associated with ME/CFS. Journal of Clinical Medicine, 14(4), 1086. https://doi.org/10.3390/jcm14041086





