Are Mitochondria a Potential Target for Treating β-Thalassemia?
Abstract
1. Introduction
2. Mitochondria in Physiology
3. Mitochondria in Pathology
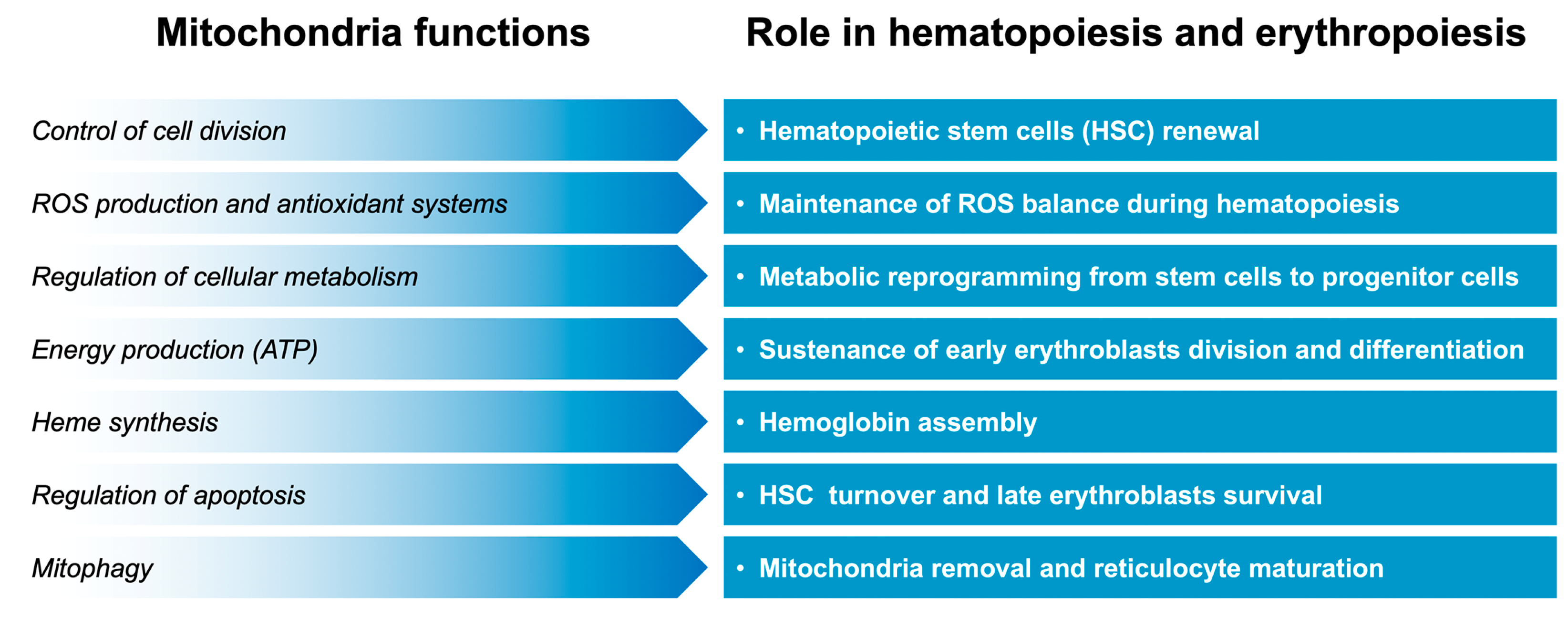
4. Mitochondria in Hematopoiesis and Erythropoiesis
5. Mitochondria in Sickle Cell Disease
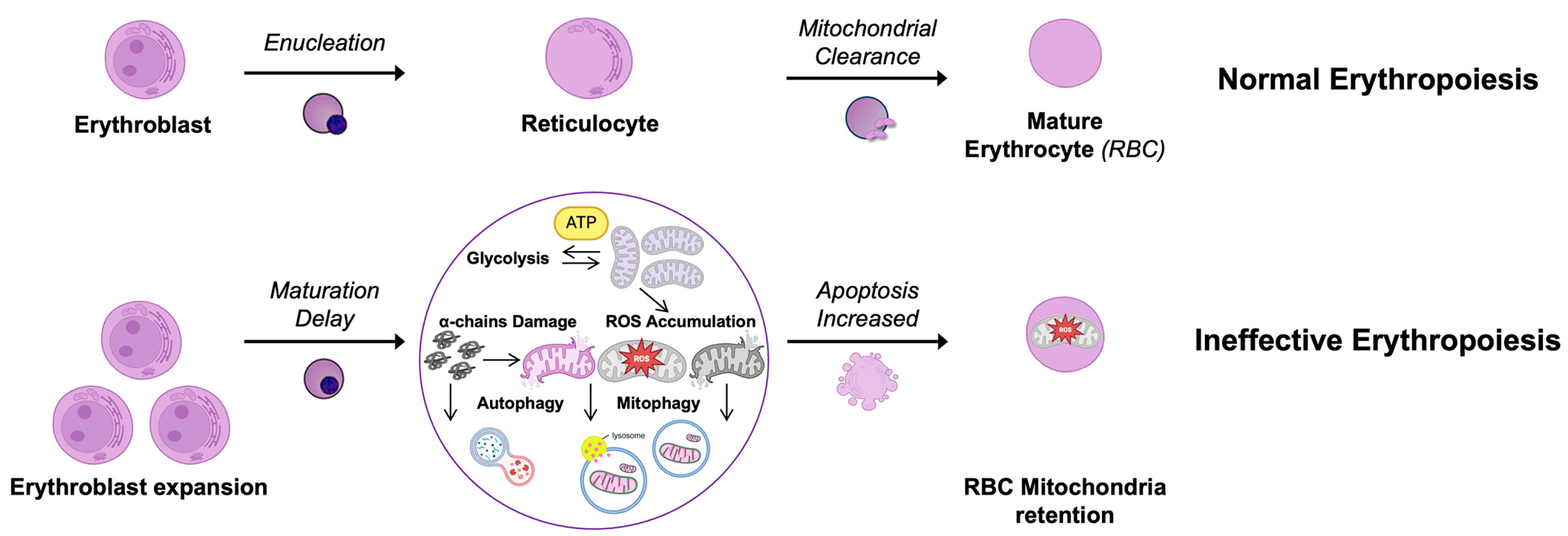
6. Mitochondria in β-Thalassemia
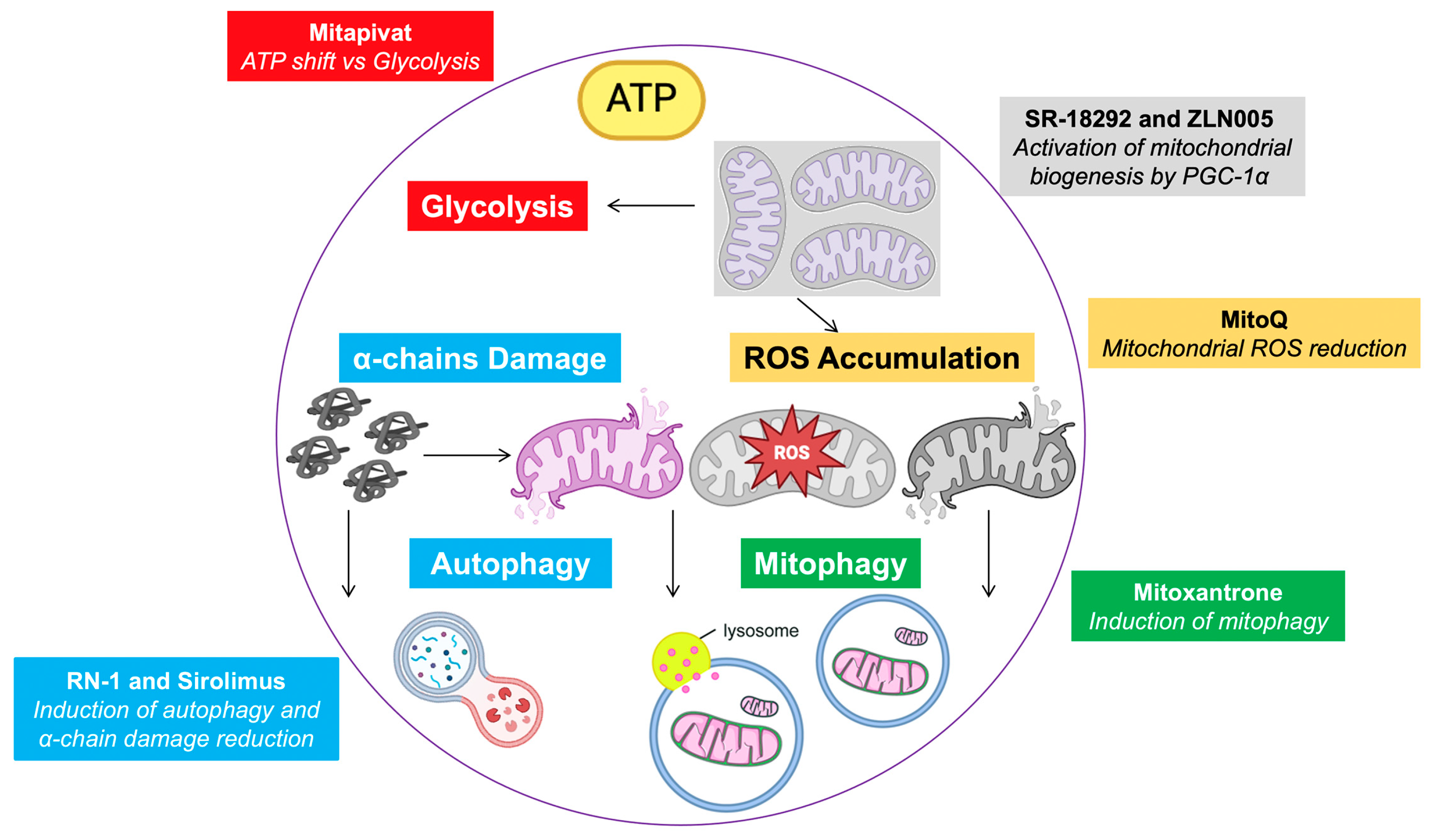
7. Mitochondria-Targeting Therapy
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weatherall, D.J. Thalassemia as a global health problem: Recent progress toward its control in the developing countries. Ann. N. Y. Acad. Sci. 2010, 1202, 17–23. [Google Scholar] [CrossRef]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull. World Health Organ. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Kattamis, A.; Forni, G.L.; Aydinok, Y.; Viprakasit, V. Changing patterns in the epidemiology of beta-thalassemia. Eur. J. Haematol. 2020, 105, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Taher, A.T.; Musallam, K.M.; Cappellini, M.D. beta-Thalassemias. N. Engl. J. Med. 2021, 384, 727–743. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.R.; Engel, J.D.; Stamatoyannopoulos, G. Thalassaemia. Lancet 2012, 379, 373–383. [Google Scholar] [CrossRef]
- Taher, A. Iron overload in thalassemia and sickle cell disease. Semin. Hematol. 2005, 42, S5–S9. [Google Scholar] [CrossRef]
- Musallam, K.M.; Cappellini, M.D.; Viprakasit, V.; Kattamis, A.; Rivella, S.; Taher, A.T. Revisiting the non-transfusion-dependent (NTDT) vs. transfusion-dependent (TDT) thalassemia classification 10 years later. Am. J. Hematol. 2021, 96, E54–E56. [Google Scholar] [CrossRef]
- Dadheech, S.; Jain, S.; Madhulatha, D.; Sharma, V.; Joseph, J.; Jyothy, A.; Munshi, A. Association of Xmn1 -158 gammaG variant with severity and HbF levels in beta-thalassemia major and sickle cell anaemia. Mol. Biol. Rep. 2014, 41, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Thein, S.L. Genetic association studies in beta-hemoglobinopathies. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Giardine, B.; Borg, J.; Viennas, E.; Pavlidis, C.; Moradkhani, K.; Joly, P.; Bartsakoulia, M.; Riemer, C.; Miller, W.; Tzimas, G.; et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014, 42, D1063–D1069. [Google Scholar] [CrossRef]
- Sankaran, V.G.; Weiss, M.J. Anemia: Progress in molecular mechanisms and therapies. Nat. Med. 2015, 21, 221–230. [Google Scholar] [CrossRef]
- Weatherall, D.J. Phenotype-genotype relationships in monogenic disease: Lessons from the thalassaemias. Nat. Rev. Genet. 2001, 2, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.G.; Huisman, T.H. DNA sequence variation associated with elevated fetal G gamma globin production. Blood 1985, 66, 783–787. [Google Scholar] [CrossRef]
- Bianchi, N.; Cosenza, L.C.; Lampronti, I.; Finotti, A.; Breveglieri, G.; Zuccato, C.; Fabbri, E.; Marzaro, G.; Chilin, A.; De, A.G.; et al. Structural and Functional Insights on an Uncharacterized Agamma-Globin-Gene Polymorphism Present in Four beta0-Thalassemia Families with High Fetal Hemoglobin Levels. Mol. Diagn. Ther. 2016, 20, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Farrell, J.J.; Alsultan, A.; Wang, S.; Edward, H.L.; Shappell, H.; Bae, H.; Milton, J.N.; Baldwin, C.T.; Al-Rubaish, A.M.; et al. BCL11A enhancer haplotypes and fetal hemoglobin in sickle cell anemia. Blood Cells Mol. Dis. 2015, 54, 224–230. [Google Scholar] [CrossRef]
- Lai, Y.; Zhou, L.; Yi, S.; Chen, Y.; Tang, Y.; Yi, S.; Yang, Z.; Wei, H.; Zheng, C.; He, S. The association between four SNPs (rs7482144, rs4671393, rs28384513 and rs4895441) and fetal hemoglobin levels in Chinese Zhuang beta-thalassemia intermedia patients. Blood Cells Mol. Dis. 2017, 63, 52–57. [Google Scholar] [CrossRef]
- Stadhouders, R.; Aktuna, S.; Thongjuea, S.; Aghajanirefah, A.; Pourfarzad, F.; van, I.W.; Lenhard, B.; Rooks, H.; Best, S.; Menzel, S.; et al. HBS1L-MYB intergenic variants modulate fetal hemoglobin via long-range MYB enhancers. J. Clin. Investig. 2014, 124, 1699–1710. [Google Scholar] [CrossRef]
- Thein, S.L.; Menzel, S.; Peng, X.; Best, S.; Jiang, J.; Close, J.; Silver, N.; Gerovasilli, A.; Ping, C.; Yamaguchi, M.; et al. Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 influencing fetal hemoglobin levels in adults. Proc. Natl. Acad. Sci. USA 2007, 104, 11346–11351. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, X.; Yu, L.; Cai, R.; Ma, X.; Zheng, C.; Zhou, Y.; Liu, Q.; Wei, X.; Lin, L.; et al. KLF1 mutations are relatively more common in a thalassemia endemic region and ameliorate the severity of beta-thalassemia. Blood 2014, 124, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Uda, M.; Galanello, R.; Sanna, S.; Lettre, G.; Sankaran, V.G.; Chen, W.; Usala, G.; Busonero, F.; Maschio, A.; Albai, G.; et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc. Natl. Acad. Sci. USA 2008, 105, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Mejri, A.; Mansri, M.; Hadj, F.S.; Ouali, F.; Bibi, A.; Hafsia, R.; Messaoud, T.; Siala, H. First description of the rs45496295 polymorphism of the C/EBPE gene in beta-thalassemia intermedia patients. Hemoglobin 2016, 40, 411–416. [Google Scholar] [CrossRef]
- Sherva, R.; Sripichai, O.; Abel, K.; Ma, Q.; Whitacre, J.; Angkachatchai, V.; Makarasara, W.; Winichagoon, P.; Svasti, S.; Fucharoen, S.; et al. Genetic modifiers of Hb E/beta0 thalassemia identified by a two-stage genome-wide association study. BMC Med. Genet. 2010, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Habara, A.; Le, C.Q.; Nguyen, N.; Chen, R.; Murphy, G.J.; Chui, D.H.K.; Steinberg, M.H.; Cui, S. Pharmacologic induction of PGC-1alpha stimulates fetal haemoglobin gene expression. Br. J. Haematol. 2022, 197, 97–109. [Google Scholar] [CrossRef]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Balas, B.; Tantiwong, P.; Dube, J.; Goodpaster, B.H.; O’Doherty, R.M.; DeFronzo, R.A.; Richardson, A.; Musi, N.; Ward, W.F. Whole body overexpression of PGC-1alpha has opposite effects on hepatic and muscle insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E945–E954. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Tanabe, O.; Lim, K.C.; Xu, H.E.; Zhou, X.E.; Lin, J.D.; Shi, L.; Schmidt, L.; Campbell, A.; Shimizu, R.; et al. PGC-1 coactivator activity is required for murine erythropoiesis. Mol. Cell Biol. 2014, 34, 1956–1965. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 2011, 1813, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Chen, J.; Singbrant, S. Decreased PGC1beta expression results in disrupted human erythroid differentiation, impaired hemoglobinization and cell cycle exit. Sci. Rep. 2021, 11, 17129. [Google Scholar] [CrossRef] [PubMed]
- Siekevitz, P. Powerhouse of the Cell. Sci. Am. 1957, 197, 131–144. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de, C.R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; De, S.D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- de, M.L.; Arruda, A.P.; da Costa, R.M.; Benchimol, M. Identification of a Ca2+-ATPase in brown adipose tissue mitochondria: Regulation of thermogenesis by ATP and Ca2+. J. Biol. Chem. 2006, 281, 16384–16390. [Google Scholar]
- Yau, W.W.; Yen, P.M. Thermogenesis in Adipose Tissue Activated by Thyroid Hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef] [PubMed]
- Melchinger, P.; Garcia, B.M. Mitochondria are midfield players in steroid synthesis. Int. J. Biochem. Cell Biol. 2023, 160, 106431. [Google Scholar] [CrossRef] [PubMed]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial Dynamics in Stem Cells and Differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Roy, M.; Reddy, P.H.; Iijima, M.; Sesaki, H. Mitochondrial division and fusion in metabolism. Curr. Opin. Cell Biol. 2015, 33, 111–118. [Google Scholar] [CrossRef]
- Mishra, P.; Chan, D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016, 212, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, Z.; Min, W. Mitochondria, Oxidative Stress and Innate Immunity. Front. Physiol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liao, Z.; Xu, P. Mitochondrial control of innate immune responses. Front. Immunol. 2023, 14, 1166214. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.G.; Pearson, A.G.; Pullar, J.M.; Jonsson, T.J.; Lowther, W.T.; Winterbourn, C.C.; Hampton, M.B. Mitochondrial peroxiredoxin 3 is more resilient to hyperoxidation than cytoplasmic peroxiredoxins. Biochem. J. 2009, 421, 51–58. [Google Scholar] [CrossRef]
- Cardenas-Rodriguez, M.; Chatzi, A.; Tokatlidis, K. Iron-sulfur clusters: From metals through mitochondria biogenesis to disease. J. Biol. Inorg. Chem. 2018, 23, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Belot, A.; Puy, H.; Hamza, I.; Bonkovsky, H.L. Update on heme biosynthesis, tissue-specific regulation, heme transport, relation to iron metabolism and cellular energy. Liver Int. 2024, 44, 2235–2250. [Google Scholar] [CrossRef]
- La Morgia, C.; Maresca, A.; Caporali, L.; Valentino, M.L.; Carelli, V. Mitochondrial diseases in adults. J. Intern. Med. 2020, 287, 592–608. [Google Scholar] [CrossRef]
- Kozhukhar, N.; Alexeyev, M.F. 35 Years of TFAM Research: Old Protein, New Puzzles. Biology 2023, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Tuppen, H.A.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta 2010, 1797, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, J.; Ye, C.; Pang, L.; Zhang, X.; Luk, A.; Du, Y.; Fan, K.Y.; Zhang, X.; Li, B.; et al. Leber’s Hereditary Optic Neuropathy with Mitochondrial DNA Mutation G11778A. A Systematic Literature Review and Meta-Analysis. Biomed. Res. Int. 2023, 2023, 1107866. [Google Scholar] [CrossRef]
- Gao, R.; Gu, L.; Zuo, W.; Wang, P. Long-term prognostic factors and outcomes in mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes: A clinical and biochemical marker analysis. Front. Neurol. 2024, 15, 1491283. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hou, Y.; Zhao, X.; Liufu, T.; Yu, M.; Zhang, W.; Xie, Z.; Zhang, V.W.; Yuan, Y.; Wang, Z. The clinical, myopathological, and genetic analysis of 155 Chinese mitochondrial ophthalmoplegia patients with mitochondrial DNA single large deletions. Mol. Genet. Genom. Med. 2024, 12, e2328. [Google Scholar] [CrossRef]
- Pyle, A.; Anugrha, H.; Kurzawa-Akanbi, M.; Yarnall, A.; Burn, D.; Hudson, G. Reduced mitochondrial DNA copy number is a biomarker of Parkinson’s disease. Neurobiol. Aging 2016, 38, 216. [Google Scholar] [CrossRef] [PubMed]
- Podlesniy, P.; Figueiro-Silva, J.; Llado, A.; Antonell, A.; Sanchez-Valle, R.; Alcolea, D.; Lleo, A.; Molinuevo, J.L.; Serra, N.; Trullas, R. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann. Neurol. 2013, 74, 655–668. [Google Scholar] [CrossRef]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao, C.R.; Nemade, L.S.; Kishor, K.N.; Borah, S.; Shrikant, D.S.; et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and Amyotrophic Lateral Sclerosis—An updated review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef]
- Xu, M.; Li, T.; Liu, X.; Islam, B.; Xiang, Y.; Zou, X.; Wang, J. Mechanism and Clinical Application Prospects of Mitochondrial DNA Single Nucleotide Polymorphism in Neurodegenerative Diseases. Neurochem. Res. 2024, 50, 61. [Google Scholar] [CrossRef]
- He, Y.H.; Lu, X.; Wu, H.; Cai, W.W.; Yang, L.Q.; Xu, L.Y.; Sun, H.P.; Kong, Q.P. Mitochondrial DNA content contributes to healthy aging in Chinese: A study from nonagenarians and centenarians. Neurobiol. Aging 2014, 35, 1779.e1–1779.e4. [Google Scholar] [CrossRef]
- Gao, X.; Campian, J.L.; Qian, M.; Sun, X.F.; Eaton, J.W. Mitochondrial DNA damage in iron overload. J. Biol. Chem. 2009, 284, 4767–4775. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Chen, G.; He, J.; Zhang, F.; Li, M.; Wang, Q.; Zhou, H.; Lyu, J.; Bai, Y. Mitochondrial common deletion is elevated in blood of breast cancer patients mediated by oxidative stress. Mitochondrion 2016, 26, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Hanke, T.; Erasmi, A.W.; Bechtel, M.J.; Scharfschwerdt, M.; Meissner, C.; Sievers, H.H.; Gosslau, A. Mitochondrial DNA deletions and the aging heart. Exp. Gerontol. 2006, 41, 508–517. [Google Scholar] [CrossRef]
- Mbiandjeu, S.C.T.; Siciliano, A.; Matte, A.; Federti, E.; Perduca, M.; Melisi, D.; Andolfo, I.; Amoresano, A.; Iolascon, A.; Valenti, M.T.; et al. Nrf2 Plays a Key Role in Erythropoiesis during Aging. Antioxidants 2024, 13, 454. [Google Scholar] [CrossRef]
- Chien, M.C.; Huang, W.T.; Wang, P.W.; Liou, C.W.; Lin, T.K.; Hsieh, C.J.; Weng, S.W. Role of mitochondrial DNA variants and copy number in diabetic atherogenesis. Genet. Mol. Res. 2012, 11, 3339–3348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Qu, Y.; Dang, S.; Yang, Q.; Shi, B.; Hou, P. Variable copy number of mitochondrial DNA (mtDNA) predicts worse prognosis in advanced gastric cancer patients. Diagn. Pathol. 2013, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, W.; Fang, W.; Dong, Y.; Zhang, H.; Luo, Q. Tumor energy metabolism: Implications for therapeutic targets. Mol. Biomed. 2024, 5, 63. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, T.; Huang, S.; Zhang, Z. Mitochondrial Damage-Associated Molecular Patterns and Metabolism in the Regulation of Innate Immunity. J. Innate Immun. 2023, 15, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.D.; Ghaffari, S. Mitochondria in the maintenance of hematopoietic stem cells: New perspectives and opportunities. Blood 2019, 133, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Papoin, J.; Yan, H.; Leduc, M.; Le, G.M.; Narla, A.; Palis, J.; Steiner, L.A.; Gallagher, P.G.; Hillyer, C.D.; Gautier, E.F.; et al. Phenotypic and proteomic characterization of the human erythroid progenitor continuum reveal dynamic changes in cell cycle and in metabolic pathways. Am. J. Hematol. 2024, 99, 99–112. [Google Scholar] [CrossRef]
- Lyu, J.; Ni, M.; Weiss, M.J.; Xu, J. Metabolic regulation of erythrocyte development and disorders. Exp. Hematol. 2024, 131, 104153. [Google Scholar] [CrossRef]
- Fontenay, M.; Cathelin, S.; Amiot, M.; Gyan, E.; Solary, E. Mitochondria in hematopoiesis and hematological diseases. Oncogene 2006, 25, 4757–4767. [Google Scholar] [CrossRef]
- Simsek, T.; Kocabas, F.; Zheng, J.; DeBerardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, M.P.; Hoi, K.; Chan, V.; Abraham, B.J.; Yang, S.; Mullahoo, J.; Papanastasiou, M.; Wang, Y.; Elia, I.; Perlin, J.R.; et al. Cell-specific transcriptional control of mitochondrial metabolism by TIF1gamma drives erythropoiesis. Science 2021, 372, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Anso, E.; Weinberg, S.E.; Diebold, L.P.; Thompson, B.J.; Malinge, S.; Schumacker, P.T.; Liu, X.; Zhang, Y.; Shao, Z.; Steadman, M.; et al. The mitochondrial respiratory chain is essential for haematopoietic stem cell function. Nat. Cell Biol. 2017, 19, 614–625. [Google Scholar] [CrossRef]
- Luis, T.C.; Lawson, H.; Kranc, K.R. Divide and Rule: Mitochondrial Fission Regulates Quiescence in Hematopoietic Stem Cells. Cell Stem Cell 2020, 26, 299–301. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, W.; Qiu, S. Mitochondrial genetic variations in leukemia: A comprehensive overview. Blood Sci. 2024, 6, e00205. [Google Scholar] [CrossRef] [PubMed]
- Samimi, A.; Khodayar, M.J.; Alidadi, H.; Khodadi, E. The Dual Role of ROS in Hematological Malignancies: Stem Cell Protection and Cancer Cell Metastasis. Stem Cell Rev. Rep. 2020, 16, 262–275. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Ni, M.; Cao, H.; Signer, R.A.J.; Li, D.; Li, M.; Gu, Z.; Hu, Z.; Dickerson, K.E.; et al. Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation. Nat. Cell Biol. 2017, 19, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ibanez, A.M.; Ruiz, L.M.; Jensen, E.; Echeverria, C.A.; Romero, V.; Stiles, L.; Shirihai, O.S.; Elorza, A.A. Erythroid Differentiation and Heme Biosynthesis Are Dependent on a Shift in the Balance of Mitochondrial Fusion and Fission Dynamics. Front. Cell Dev. Biol. 2020, 8, 592035. [Google Scholar] [CrossRef]
- Finsterer, J. Hematological manifestations of primary mitochondrial disorders. Acta Haematol. 2007, 118, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Chaichompoo, P.; Svasti, S.; Smith, D.R. The Roles of Mitophagy and Autophagy in Ineffective Erythropoiesis in beta-Thalassemia. Int. J. Mol. Sci. 2022, 23, 10811. [Google Scholar] [CrossRef]
- Sandoval, H.; Thiagarajan, P.; Dasgupta, S.K.; Schumacher, A.; Prchal, J.T.; Chen, M.; Wang, J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008, 454, 232–235. [Google Scholar] [CrossRef]
- Kundu, M.; Lindsten, T.; Yang, C.Y.; Wu, J.; Zhao, F.; Zhang, J.; Selak, M.A.; Ney, P.A.; Thompson, C.B. Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 2008, 112, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Chaichompoo, P.; Nithipongvanitch, R.; Kheansaard, W.; Tubsuwan, A.; Srinoun, K.; Vadolas, J.; Fucharoen, S.; Smith, D.R.; Winichagoon, P.; Svasti, S. Increased autophagy leads to decreased apoptosis during beta-thalassaemic mouse and patient erythropoiesis. Sci. Rep. 2022, 12, 18628. [Google Scholar] [CrossRef]
- Mortensen, M.; Ferguson, D.J.; Edelmann, M.; Kessler, B.; Morten, K.J.; Komatsu, M.; Simon, A.K. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Slavinsky, M.; Hermine, O.; Ghaffari, S. Mitochondrial regulation of erythropoiesis in homeostasis and disease. Br. J. Haematol. 2024, 205, 429–439. [Google Scholar] [CrossRef]
- Piel, F.B.; Steinberg, M.H.; Rees, D.C. Sickle Cell Disease. N. Engl. J. Med. 2017, 377, 305. [Google Scholar] [CrossRef]
- Akhter, M.S.; Hamali, H.A.; Rashid, H.; Dobie, G.; Madkhali, A.M.; Mobarki, A.A.; Oldenburg, J.; Biswas, A. Mitochondria: Emerging Consequential in Sickle Cell Disease. J. Clin. Med. 2023, 12, 765. [Google Scholar] [CrossRef]
- Moriconi, C.; Dzieciatkowska, M.; Roy, M.; D’Alessandro, A.; Roingeard, P.; Lee, J.Y.; Gibb, D.R.; Tredicine, M.; McGill, M.A.; Qiu, A.; et al. Retention of functional mitochondria in mature red blood cells from patients with sickle cell disease. Br. J. Haematol. 2022, 198, 574–586. [Google Scholar] [CrossRef]
- Martino, S.; Arlet, J.B.; Odievre, M.H.; Jullien, V.; Moras, M.; Hattab, C.; Lefebvre, T.; Gouya, L.; Ostuni, M.A.; Lefevre, S.D.; et al. Deficient mitophagy pathways in sickle cell disease. Br. J. Haematol. 2021, 193, 988–993. [Google Scholar] [CrossRef]
- Gallivan, A.; Alejandro, M.; Kanu, A.; Zekaryas, N.; Horneman, H.; Hong, L.K.; Vinchinsky, E.; Lavelle, D.; Diamond, A.M.; Molokie, R.E.; et al. Reticulocyte mitochondrial retention increases reactive oxygen species and oxygen consumption in mouse models of sickle cell disease and phlebotomy-induced anemia. Exp. Hematol. 2023, 122, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nohl, H.; Gille, L.; Staniek, K. Intracellular generation of reactive oxygen species by mitochondria. Biochem. Pharmacol. 2005, 69, 719–723. [Google Scholar] [CrossRef]
- Esperti, S.; Nader, E.; Stier, A.; Boisson, C.; Carin, R.; Marano, M.; Robert, M.; Martin, M.; Horand, F.; Cibiel, A.; et al. Increased retention of functional mitochondria in mature sickle red blood cells is associated with increased sickling tendency, hemolysis and oxidative stress. Haematologica 2023, 108, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Tumburu, L.; Ghosh-Choudhary, S.; Seifuddin, F.T.; Barbu, E.A.; Yang, S.; Ahmad, M.M.; Wilkins, L.H.W.; Tunc, I.; Sivakumar, I.; Nichols, J.S.; et al. Circulating mitochondrial DNA is a proinflammatory DAMP in sickle cell disease. Blood 2021, 137, 3116–3126. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Yin, P.H.; Lu, C.Y.; Chi, C.W.; Wei, Y.H. Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells. Biochem. J. 2000, 348 Pt 2, 425–432. [Google Scholar] [CrossRef]
- Khungwanmaythawee, K.; Sornjai, W.; Paemanee, A.; Jaratsittisin, J.; Fucharoen, S.; Svasti, S.; Lithanatudom, P.; Roytrakul, S.; Smith, D.R. Mitochondrial Changes in beta0-Thalassemia/Hb E Disease. PLoS ONE 2016, 11, e0153831. [Google Scholar] [CrossRef]
- Leecharoenkiat, A.; Wannatung, T.; Lithanatudom, P.; Svasti, S.; Fucharoen, S.; Chokchaichamnankit, D.; Srisomsap, C.; Smith, D.R. Increased oxidative metabolism is associated with erythroid precursor expansion in beta0-thalassaemia/Hb E disease. Blood Cells Mol. Dis. 2011, 47, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Mathias, L.A.; Fisher, T.C.; Zeng, L.; Meiselman, H.J.; Weinberg, K.I.; Hiti, A.L.; Malik, P. Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp. Hematol. 2000, 28, 1343–1353. [Google Scholar] [CrossRef]
- Lithanatudom, P.; Wannatung, T.; Leecharoenkiat, A.; Svasti, S.; Fucharoen, S.; Smith, D.R. Enhanced activation of autophagy in beta-thalassemia/Hb E erythroblasts during erythropoiesis. Ann. Hematol. 2011, 90, 747–758. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Xu, L.; Kong, Q.; Fang, J. Mitophagy is increased during erythroid differentiation in beta-thalassemia. Int. J. Hematol. 2017, 105, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Suriyun, T.; Winichagoon, P.; Fucharoen, S.; Sripichai, O. Impaired Terminal Erythroid Maturation in beta(0)-Thalassemia/HbE Patients with Different Clinical Severity. J. Clin. Med. 2022, 11, 1755. [Google Scholar] [CrossRef]
- Liu, C.S.; Tsai, C.S.; Kuo, C.L.; Chen, H.W.; Lii, C.K.; Ma, Y.S.; Wei, Y.H. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic. Res. 2003, 37, 1307–1317. [Google Scholar] [CrossRef]
- Lal, A.; Gomez, E.; Calloway, C. Increased mitochondrial DNA deletions and copy number in transfusion-dependent thalassemia. JCI Insight 2016, 1, e88150. [Google Scholar] [CrossRef] [PubMed]
- Suragani, R.N.; Cadena, S.M.; Cawley, S.M.; Sako, D.; Mitchell, D.; Li, R.; Davies, M.V.; Alexander, M.J.; Devine, M.; Loveday, K.S.; et al. Transforming growth factor-beta superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat. Med. 2014, 20, 408–414. [Google Scholar] [CrossRef]
- Dussiot, M.; Maciel, T.T.; Fricot, A.; Chartier, C.; Negre, O.; Veiga, J.; Grapton, D.; Paubelle, E.; Payen, E.; Beuzard, Y.; et al. An activin receptor IIA ligand trap corrects ineffective erythropoiesis in beta-thalassemia. Nat. Med. 2014, 20, 398–407. [Google Scholar] [CrossRef]
- Matte, A.; Wilson, A.B.; Gevi, F.; Federti, E.; Recchiuti, A.; Ferri, G.; Brunati, A.M.; Pagano, M.A.; Russo, R.; Leboeuf, C.; et al. Mitapivat reprograms the RBC metabolome and improves anemia in a mouse model of hereditary spherocytosis. JCI Insight 2023, 8, e172656. [Google Scholar] [CrossRef] [PubMed]
- Matte, A.; Federti, E.; Kung, C.; Kosinski, P.A.; Narayanaswamy, R.; Russo, R.; Federico, G.; Carlomagno, F.; Desbats, M.A.; Salviati, L.; et al. The pyruvate kinase activator mitapivat reduces hemolysis and improves anemia in a beta-thalassemia mouse model. J. Clin. Investig. 2021, 131, e144206. [Google Scholar] [CrossRef]
- Quezado, Z.M.N.; Kamimura, S.; Smith, M.; Wang, X.; Heaven, M.R.; Jana, S.; Vogel, S.; Zerfas, P.; Combs, C.A.; Almeida, L.E.F.; et al. Mitapivat increases ATP and decreases oxidative stress and erythrocyte mitochondria retention in a SCD mouse model. Blood Cells Mol. Dis. 2022, 95, 102660. [Google Scholar] [CrossRef] [PubMed]
- Jagadeeswaran, R.; Vazquez, B.A.; Thiruppathi, M.; Ganesh, B.B.; Ibanez, V.; Cui, S.; Engel, J.D.; Diamond, A.M.; Molokie, R.E.; DeSimone, J.; et al. Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp. Hematol. 2017, 50, 46–52. [Google Scholar] [CrossRef]
- Lechauve, C.; Keith, J.; Khandros, E.; Fowler, S.; Mayberry, K.; Freiwan, A.; Thom, C.S.; Delbini, P.; Romero, E.B.; Zhang, J.; et al. The autophagy-activating kinase ULK1 mediates clearance of free alpha-globin in beta-thalassemia. Sci. Transl. Med. 2019, 11, eaav4881. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, R.; Fang, Z.; Nie, L.; Ma, Y.; Sun, F.; Mei, J.; Song, Z.; Ginzburg, Y.Z.; Liu, J.; et al. Mitoxantrone ameliorates ineffective erythropoiesis in a beta-thalassemia intermedia mouse model. Blood Adv. 2024, 8, 4017–4024. [Google Scholar] [CrossRef]
- Sighinolfi, S.; Aprile, A.; Beretta, S.; Storto, M.; Merelli, I.; Ferrari, G. Intracellular Iron Controls HSC Metabolism By Affecting Mitochondrial Fitness. Blood 2023, 142, 2470. [Google Scholar] [CrossRef]
- Sun, Y.; Benmhammed, H.; Al, A.S.; Habara, A.; Fu, E.; Brady, J.; Williams, C.; Ilinski, A.; Sharma, A.; Mahdaviani, K.; et al. PGC-1alpha agonism induces fetal hemoglobin and exerts antisickling effects in sickle cell disease. Sci. Adv. 2024, 10, eadn8750. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Pierro, E.; Di Stefano, V.; Migone De Amicis, M.; Graziadei, G. Are Mitochondria a Potential Target for Treating β-Thalassemia? J. Clin. Med. 2025, 14, 1095. https://doi.org/10.3390/jcm14041095
Di Pierro E, Di Stefano V, Migone De Amicis M, Graziadei G. Are Mitochondria a Potential Target for Treating β-Thalassemia? Journal of Clinical Medicine. 2025; 14(4):1095. https://doi.org/10.3390/jcm14041095
Chicago/Turabian StyleDi Pierro, Elena, Valeria Di Stefano, Margherita Migone De Amicis, and Giovanna Graziadei. 2025. "Are Mitochondria a Potential Target for Treating β-Thalassemia?" Journal of Clinical Medicine 14, no. 4: 1095. https://doi.org/10.3390/jcm14041095
APA StyleDi Pierro, E., Di Stefano, V., Migone De Amicis, M., & Graziadei, G. (2025). Are Mitochondria a Potential Target for Treating β-Thalassemia? Journal of Clinical Medicine, 14(4), 1095. https://doi.org/10.3390/jcm14041095






