Abstract
Coronary artery disease is one of the leading public health problems in the world in terms of mortality and economic burden from the disease. Traditionally, the focus of research and clinical pathways leading to the diagnosis and treatment of coronary artery disease was on the more common variant of the disease resulting from atherosclerosis in the epicardial coronary arteries. However, coronary microvasculature, representing the vast majority of the total heart circulation, has the greatest influence on overall coronary resistance and, therefore, blood flow. Coronary microvascular dysfunction (CMD), characterized by structural or functional abnormalities in the microvasculature, significantly impacts myocardial perfusion. Endothelial dysfunction results in inadequate coronary dilation during exercise or spontaneous spasm in the microvasculature or epicardial arteries. A significant proportion of people presenting for coronary angiography in the context of angina have unobstructed epicardial coronary arteries yet are falsely reassured about the benign nature of their condition. Meanwhile, increasing evidence indicates that patients diagnosed with CMD as well as vasospastic angina (VSA) face an increased risk of Major Adverse Cardiovascular Events (MACEs), including death. The aim of this review is to outline the current practice with regard to invasive and non-invasive methods of CMD and VSA diagnosis and assess the evidence supporting the existing treatment strategies. These include endotype-specific pharmacological therapies, a holistic approach to lifestyle modifications and risk factor management and novel non-pharmacological therapies. Furthermore, the review highlights critical gaps in research and suggests potential areas for future investigation, to improve understanding and management of these conditions.
1. Introduction
Ischaemic heart disease is a leading cause of global mortality and morbidity. Research has traditionally focused on epicardial coronary artery stenosis due to atherosclerosis. However, large studies show that only 35–60% of patients with angina symptoms have obstructive coronary artery disease detected via elective angiography [,]. The European Society of Cardiology’s (ESC) updated definition of Chronic Coronary Syndromes (CCSs) emphasizes recognizing the impact that structural and functional alterations to the microvasculature may have on transient episodes of myocardial hypoperfusion, which may manifest as angina or angina-equivalent symptoms [].
Patients with angina and unobstructed coronary arteries (ANOCAs) have previously been reassured about their condition’s benign nature [], but evidence now indicates an unfavourable prognosis, including a higher risk of Major Adverse Cardiovascular Events (MACEs) and mortality [,,,,]. The condition not only has a great impact on health resource utilisation, with increased costs relating to repeat hospitalisations, invasive catheterisation and anti-ischaemia drug treatment [], but also has a large functional and macroeconomic impact relating to reduced quality of life of the affected patients [,].
Despite international guidelines recommending invasive coronary functional testing for persistently symptomatic ANOCA patients [,], barriers to adoption remain, such as the need for procedural expertise, potential complications, additional time required to undertake the procedure and its costs. However, there is compelling evidence as to the cost-effectiveness of this testing [], and the benefits that stratified medical therapy according to the endotype of the disease has on patients’ symptom burden and quality of life []. The purpose of this paper is to summarise the existing evidence with regard to the diagnosis and management of coronary microvascular dysfunction and vasospastic angina as important ANOCA endotypes.
2. Normal Coronary Vascular Structure and Function
The coronary vascular tree consists of two compartments: the epicardial, which includes arteries and veins, and the microvascular, comprising arterioles, capillaries and venules. Epicardial coronary arteries are conduit vessels that contribute less than 5% resistance to blood flow. Coronary arterioles, which account for about 60% of resting vascular resistance, are crucial for regulating coronary blood flow. These arterioles branch into smaller ones that penetrate the myocardium and lead to capillaries. While capillaries represent only 25% of resting vascular resistance (with venules and veins making up the remaining 10%), they contain 90% of myocardial blood volume and are key sites for metabolic exchange with myocardial tissue [].
Large arterioles and coronary arteries are primarily controlled by the endothelium. Shear stress from blood flow triggers nitric oxide (NO) release, causing vascular smooth muscle cells (VSMCs) to relax in a process called flow-mediated vasodilation []. Medium-sized arterioles respond to changes in blood pressure through Ca2+-mediated VSMC vasoconstriction, an endothelium-independent myogenic response []. Small arterioles are influenced by local metabolites, like adenosine and carbon dioxide, which diffuse into VSMCs and cause vasodilation and increased perfusion []. Additionally, the autonomic nervous system affects coronary blood flow by altering metabolite release and directly stimulating alpha and beta-adrenoceptors on VSMCs. Together, these mechanisms regulate vasomotor tone to align myocardial perfusion with demand [].
3. Classification and Pathophysiology of ANOCA
The term ANOCA is used to describe patients who present with angina in the context of non-obstructive coronary arteries. Of these, depending on the type of the test performed, only 10–30% of patients have demonstrable ischaemia on stress testing (termed Ischaemia in Non-Obstructed Coronary Arteries (INOCA)) [,,]. ANOCA/INOCA can be further subdivided into endotypes: (i) coronary microvascular dysfunction (CMD), which can be further subdivided into structural and functional CMD (either endothelium-dependent or independent); (ii) vasospastic angina (VSA), a result of epicardial coronary spasm or microvascular spasm; (iii) both CMD and VSA; (vi) non-cardiac chest pain []. A recent meta-analysis has shown that, amongst patients presenting with cardiac chest pain and unobstructed coronary arteries, the prevalence of CMD was 41% (with women more likely to be affected), and the overall prevalence of epicardial or microvascular spasm was 49%, with both sexes equally affected. Interestingly, in three studies within the meta-analysis, which evaluated a total of 541 patients for both CMD and spasm, the prevalence of CMD alone was 23%, coronary spasm alone (either epicardial or microvascular) 19%, and coexistent CMD and coronary vasospasm 23%, which further supports the need for complete functional assessment of coronary physiology in patients presenting with ANOCA [].
3.1. Structural CMD
Structural CMD comprises a disorder of microvascular circulation defined as reduced coronary flow reserve (CFR) in association with an increased minimal microvascular resistance (MMR) []. The proposed pathophysiology includes changes to vessel architecture, such as capillary rarefaction or arteriolar obliteration []. However, patients with structural CMD have also been shown to exhibit endothelial dysfunction, which leads to diminished peak coronary blood flow augmentation and increased demand during exercise []. Patients with structural CMD often have established coronary disease risk factors, such as diabetes or hypertension, and show an augmented blood pressure response to exercise [], which further increases myocardial oxygen demand and predisposes them to ischaemia. It has been proposed that those patients benefit from lifestyle modification (e.g., weight loss or smoking cessation) and treatment leading to afterload reduction and vascular remodelling, for example, with angiotensin-converting enzyme inhibitors (ACEis) or statins [,].
3.2. Functional CMD
Historically, the pathophysiology of CMD was considered to result from high vascular resistance during increased demand for vasodilation (which nowadays is termed structural CMD). Interestingly though, these patients represent less than 50% of those with impaired CFR []. Functional CMD appears to result from increased demand for myocardial oxygenation at rest or disordered coronary autoregulation. This leads to sub-maximal vasodilation in resting conditions, with the inability of those patients to further augment coronary blood flow in periods of stress, leading to an overall reduced CFR in the presence of normal or decreased MMR []. This still has important clinical significance due to the impact of reduced CFR on prognosis [,]. It has been hypothesised that functional CMD can act as a precursor to structural CMD, as chronically raised coronary blood flow may precipitate structural vascular changes, a process common in other vascular beds, such as renal or pulmonary []. This association has not been proven and no disease-modifying therapies have been developed specifically for functional CMD, although, in theory, therapies modulating cardiac metabolism could be of benefit [].
3.3. Vasospastic Angina (VSA)
In contrast to CMD, which results from impaired vasodilation of the microvasculature, vasospastic angina is a disorder resulting from vasoconstriction of the coronary arterial system. VSA typically involves the epicardial coronary arteries, but microvascular spasm, resulting from vasoconstriction of pre-arterioles and arterioles, has also been described [], and both entities can co-exist. VSA typically occurs at rest and in a circadian pattern but may be triggered by catecholamine surges after exercise or stress []. VSA occurs spontaneously, but it can also be induced through acetylcholine (ACh) provocation testing in the cardiac catheterisation laboratory. Epicardial VSA has been attributed to abnormal reactivity of the epicardial arteries to vasoconstrictive stimuli affecting the VSMCs []. This is especially profound in the context of endothelial dysfunction—in a normally functioning endothelium, vasoactive agents, like ACh or histamine, lead to vasodilation through NO release, whereas, in dysfunctional endothelium, these agents lead to vasoconstriction through activation of VSMCs []. In microvascular spasms, there is an increased local release of vasoconstrictive substances, associated with an increased susceptibility of VSMCs, or an abnormal activity of sympathetic tone []. Cigarette smoking is the primary risk factor for VSA, with hypertension, diabetes and hypercholesterolemia being less common contributors [].
4. Risk Factors and Associations with Other Cardiovascular and Systemic Conditions
All common cardiovascular risk factors, including smoking, age, hypertension and dyslipidaemia have been found to be associated with CMD [], and are especially prevalent in patients with structural CMD. Not accidentally, these risk factors, in addition to diabetes, chronic kidney disease and obesity, are also associated with heart failure with preserved ejection fraction (HFpEF). In recent years, research has found links between HFpEF and CMD, although the cause-and-effect relationship between both entities has not yet been established []. The PROMIS-HFpEF study confirmed the presence of CMD in 75% of HFpEF patients and its association with markers of HF severity [], with other studies highlighting >5-fold risk of HFpEF hospitalization and a higher rate of MACE and mortality in patients with co-existent CMD []. A cohort study by Rush et al. assessing the prevalence of endothelium-dependent and independent CMD in HFpEF has found a predominance of the latter type, which suggests that factors such as abnormal vascular remodelling, extrinsic vascular compression and microvascular rarefaction are more likely to be contributory than endothelial and vascular smooth muscle dysfunction [], although endothelial dysfunction is associated with worse cardiovascular prognosis [].
Importantly, the co-existence of CMD and obstructive coronary artery disease (CAD) in both chronic and acute scenarios must be recognised. Both CMD and CAD exert a synergistic effect on the myocardium, compounding myocardial ischaemia []. For example, epicardial CAD leads to reduced blood flow distal to stenosis, resulting in abnormal microvascular remodelling and vasodilator capacity, hindering collateral flow formation and contributing to stress-induced ischaemia []. Moreover, co-existent CMD may lead to an underestimation of the degree of severity of stenosis as measured by fractional flow reserve (FFR) []. The presence of CMD in patients with CAD may also explain why some patients do not derive symptomatic benefit from an apparently successful PCI. Importantly, raised index of microvascular resistance (IMR) values following revascularisation procedures despite an improvement in FFR were associated with adverse clinical outcomes []. Coronary microvascular obstruction (CMVO), an acute variant of CMD seen in patients with ST-elevation myocardial infarction, is still poorly understood but is associated with increased risk of left ventricular (LV) remodelling, heart failure and death [,,]. Similarly, CMD was also found in >20% of cases of myocardial infarction with unobstructed coronary arteries (MINOCAs) []. It is unclear whether CMD has prognostic or causative implications or is a clinically insignificant bystander—in one stress perfusion cardiac magnetic resonance (CMR) study, abnormal stress perfusion was found in 63% of patients with MINOCA, but the areas corresponded with myocardial scar only in 75% of cases [].
CMD presence has also been confirmed in studies of patients with hypertrophic cardiomyopathy (HCM), with evidence of impaired myocardial blood flow in both hypertrophied and non-hypertrophied myocardium [] and reduced CFR in the subendocardium due to presumed extravascular compression []. Not only was severe CMD proven to exist in many asymptomatic HCM patients, but it has also been found to be important prognostically, with the degree of microvascular dysfunction being a strong and independent predictor of clinical deterioration and death []. Research utilising contemporary non-invasive imaging has also found an association between CMD and infiltrative cardiomyopathies, such as cardiac sarcoidosis, amyloidosis and Anderson–Fabry disease, with evidence of CMD being an early marker of disease in the latter two, which might present an optimal window for early treatment in these conditions [,,,].
In addition, inflammatory conditions, such as systemic lupus erythematosus or rheumatoid arthritis have also been found to be associated with microvascular angina, and the higher prevalence of these conditions in postmenopausal women than men might at least partly explain sex differences in CMD []. While the prevalence of CMD in women and men varies significantly between large cohort studies (34–66% in women and 14–60% in men) [], it is uniformly associated with an increased risk of MACE in both sexes []. Interestingly, the commonest MACE seen in men is cardiovascular death, whereas heart failure-related hospital admission is most frequently seen in women, suggesting a different underlying pathophysiology []. A study assessing stress CMR-derived Myocardial Perfusion Reserve Index (MPRI)’s association with traditional cardiovascular risk factors has found that men with CMD tend to fit an atherosclerotic risk profile more so than women []. Indeed, a study comparing women suffering from angina with or without obstructive coronary disease has found that women with ANOCA are less likely to demonstrate conventional cardiovascular risk factors but more likely to report pre-menopausal migraines or rheumatic conditions []. The relationship between menopause and the prevalence of CMD among women has been frequently reported [,]. Studies have shown that oestrogen plays a protective role in coronary microvascular function by regulating endothelial and smooth muscle cells, indicating that changing oestrogen levels at the time of perimenopause may play an important role in CMD progression [,].
5. Diagnosis of Coronary Microvascular Dysfunction and Vasospastic Angina
5.1. Diagnostic Algorithm for Patients Presenting with ANOCA
Both the European Association of Percutaneous Coronary Interventions (EAPCI) Expert Consensus Document [] and the ESC guidelines [] suggest a step-wise diagnostic approach to a patient presenting with anginal chest pain. The initial evaluation includes a clinical review of patient symptoms, physical examination and an electrocardiogram (ECG), with the aim of assessing risk factors for coronary disease and excluding non-cardiac disease or other alternative explanations for presentation. If clinical suspicion of angina is maintained, a non-invasive assessment should follow. This includes computed tomography coronary angiography (CTCA) or functional stress imaging. These tests can be performed in any sequence, depending on local availability and patient factors. In patients with no significant obstructive disease and/or no regional reversible ischaemia on functional testing, who have a significant burden of disease persistent despite medical treatment, invasive functional coronary assessment is recommended (ESC Class I recommendation) [,]. This process is summarised in Figure 1. Functional coronary assessment may allow identification of the underlying endotype of ANOCA, enabling stratified medical therapy as well as targeting modifiable risk factors, leading to improvement in symptoms and quality of life []. The clinical criteria for diagnosing both microvascular and vasospastic angina have been defined by the Coronary Vasomotor Disorders International Study (COVADIS) group and are presented in Table 1. These allow improvements in clinical diagnosis of affected patients and appropriate stratification of endotypes for subsequent research trials [,].
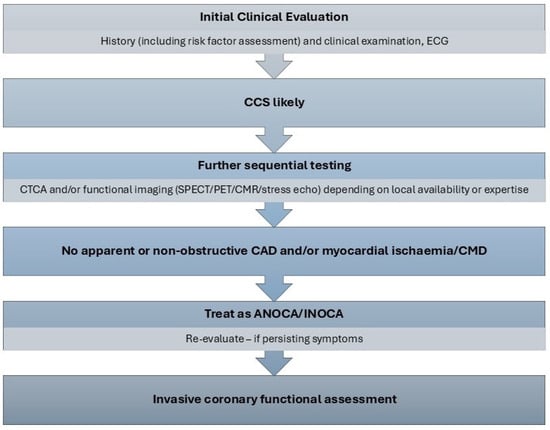
Figure 1.
Diagnostic algorithm for ANOCA []. ANOCA—angina with no obstructive coronary artery disease, CAD—coronary artery disease, CCS—chronic coronary syndrome, CMD—coronary microvascular dysfunction, CMR—cardiac magnetic resonance imaging, CTCA—computed tomography coronary angiography, ECG—electrocardiogram, INOCA—ischaemia with no obstructive coronary artery disease, SPECT—single photon emission computed tomography, PET—positron emission tomography.

Table 1.
Standardised diagnostic criteria for microvascular angina and vasospastic angina, derived by COVADIS working group [,].
5.2. Invasive Methods for Diagnosis of CMD and VSA
Functional invasive coronary assessment provides accurate and reproducible evaluation of microvascular dysfunction with minimal additional resource use and at low risk of complications []. A complete study will involve the assessment of epicardial disease, as well as endothelium-dependent and independent CMD and VSA. Initial assessment of the coronary anatomy will involve a visual assessment of any epicardial disease and should include a physiological assessment of any equivocal epicardial stenoses (angiographic stenosis degree between 40 and 90%) with the use of FFR, or alternative resting indices (for example instantaneous wave-free ratio or resting full cycle ratio (iFR/RFR)) []. Patients with INOCA who have non-obstructive coronary atheroma are at increased risk of cardiovascular events, in addition to the risk conferred from microvascular myocardial ischaemia, and, thus, should receive intensive risk-reduction therapies, including lipid-lowering agents []. Absence of obstructive coronary atheroma with an FFR value < 0.8 in the presence of angina symptoms, especially but not necessarily in the context of ischaemia found on non-invasive testing, should prompt further investigation of coronary physiology. Microvascular coronary function testing can be performed utilising Doppler or thermodilution-based techniques.
5.2.1. Doppler-Based Techniques
In the absence of obstructive coronary artery disease, reduced CFR is indicative of endothelium-independent coronary microvascular dysfunction. CFR is defined as a ratio of coronary blood flow (CBF) at hyperaemia versus rest. Historically, CBF was difficult to measure clinically, but Doppler-based techniques utilising a dual Doppler and pressure sensor coronary wire derive averaged peak flow velocity (APV) at rest and hyperaemia, which act as surrogate measurements of coronary blood flow []. Adenosine is selected as the most appropriate hyperaemic agent as it does not significantly influence the diameter of coronary arteries at peak hyperaemia [] (albeit limiting this technique to the detection of endothelium-independent CMD only). Doppler-based CFR is, therefore, described as follows:
CFR = APVhyperaemia/APVrest.
Values above 2.5 are indicative of normal microvascular function, whereas values below 2.0 are defined as abnormal. Reduced CFR can however result from coronary atherosclerosis not immediately apparent as functionally significant, and, thus, microvascular resistance (MR) indices have been developed which may differentiate between diffuse coronary atheroma and microvascular dysfunction []. MR is defined as the ratio between myocardial perfusion pressure (which approximates distal coronary pressure (Pd)) and coronary blood flow. In the Doppler-based technique, the resulting index is called hyperaemic microvascular resistance (hMR) []:
hMR = Pd/APV.
Abnormal values are defined as hMR > 2.4 mmHg/(cm/s). A finding of isolated raised MR is still significant as those patients might benefit from CMD treatment, including statin therapy [].
The doppler-based technique also allows the assessment of endothelial function by measurement of acetycholine flow reserve (AChFR). As described previously, the effect of ACh on coronary diameter depends on endothelial integrity and may result in either vasodilation or vasoconstriction—thus, volumetric coronary blood flow needs to be calculated, incorporating quantitative coronary angiography (QCA), and this equates to the following []:
volumetricCBF = 0.5 × π(APV)(vessel diameter/2)2.
ACh is infused either directly through a coronary guide catheter or through a dedicated infusion microcatheter, with the latter option being preferable as continuous aortic pressure measurements are available throughout the duration of ACh infusion. Various infusion protocols are in use—most commonly, either an infusion of three sequential concentrations, 0.182, 1.82 and 18.2 µg/mL at 1 mL/min for 2 min infusion periods, or an infusion of 18.2 µg/mL at 1 mL/min for 2 min followed by 2 mL/min for 2 min (when applied to LCA—for RCA the infusion dose or rate should be halved due to atrioventricular (AV) block concerns) []. Cine images are taken before and after each infusion and QCA is used to measure coronary diameter 5 mm distal to Doppler wire tip. AChFR is calculated as follows:
with values < 1.5 suggestive of coronary endothelial dysfunction and poorer long-term prognosis [].
AchFR = volumetricCBFACh/volumetric CBFrest,
However, currently, Doppler sensor coronary wires are commercially unavailable and thus thermodilution techniques have emerged as the more commonly adopted.
5.2.2. Thermodilution Techniques
A coronary microvascular study involving the thermodilution technique utilises a coronary wire with dual pressure and temperature sensors. Two approaches have been described—bolus and continuous thermodilution. Both techniques have been validated in the assessment of endothelium-independent coronary microvascular dysfunction [,].
Bolus thermodilution, a simpler and readily available method in most cardiac catheter laboratories, involves rapid sequential injections of 3 mL of room-temperature saline through a coronary guiding catheter. Mean transit time (Tmn) is calculated using dedicated software. An average of three values with less than 10% variation between them denotes resting mean transit time (Tmnrest). Hyperaemia is then induced using adenosine and hyperaemic mean transit time is derived (Tmnhyperaemia) in the same way. Coronary blood flow is defined as 1/Tmn, thus, from earlier equations, CFR is derived as:
CFR = Tmn(rest)/Tmn(hyperaemia).
A value < 2.5 is considered abnormal and suggestive of endothelium-independent coronary microvascular dysfunction. The bolus thermodilution technique also allows measurement of microvascular resistance, termed IMR, defined as Pd/Tmn, with values > 25 being abnormal [].
The bolus thermodilution technique has, however, been criticised as subject to substantial intrinsic variability (between 15 and 20%) [], as well as inter- and intraoperator variability, and inferior correlation of CFR with the gold standard for myocardial perfusion quantification ([15O]H2O positron emission tomography (PET)) as compared to Doppler derived methods []. Bolus thermodilution methods also rely on infusion of adenosine, which may be contraindicated in some patients. The continuous thermodilution method allows direct measurement of absolute coronary flow (Q) at rest and hyperaemia and has been validated against PET imaging []. It provides a means of reducing operator dependence, with improved reproducibility of results as compared to bolus thermodilution [,]. This method involves the infusion of room-temperature saline at varying rates through a dedicated infusion microcatheter with four side ports. Infusion of saline at room temperature at a rate of 20 mL/min induces steady-state maximal hyperaemia equivalent to that obtained with adenosine [], while infusion at 10 mL/min denotes absolute flow at rest []. CFR is thus derived as follows:
and is a measure of endothelium-independent CMD.
CFR = Qhyperaemia/Qrest,
Neither bolus nor continuous thermodilution methods allow assessment of endothelium-dependent CMD, although some centres are reporting the use of the continuous thermodilution method in the assessment of AChFR, expressed by the ratio of absolute flow under continuous infusion of varying concentrations of ACh vs. absolute flow at rest []. This method, however, requires further validation.
5.2.3. Vasospasm Provocation
A complete invasive assessment of coronary vasomotor function also involves vasospasm assessment by intracoronary ACh bolus injections. This can be achieved by injecting incremental boluses of ACh up to 100 mcg into LCA over 20 s (or 50 mcg into RCA over 20 s), while closely monitoring the patient for signs and symptoms of ischaemia. Epicardial spasm is confirmed by observing a >90% reduction in coronary artery diameter on coronary angiography, associated with ischaemic ECG changes and symptoms []. Microvascular spasms can be diagnosed if typical symptoms and ECG change are seen without evidence of epicardial coronary artery spasm as per COVADIS criteria. However, continuous blood flow velocity assessment by coronary Doppler wire interrogation may allow for a more sensitive assessment of coronary microvascular spasm (with AChFR < 1.0, signifying reduced blood flow versus rest state), as alterations in coronary blood flow occur earlier in the ischaemic cascade than ECG change []. Coronary vasospasm should be reversed with the use of intracoronary nitrates to avoid prolonged ischaemia.
5.2.4. Cardiac Catheter Laboratory Protocol for Invasive Coronary Function Assessment
The British Heart Foundation/National Institute for Health Research CMD (BHF/NIHR CMD) workgroup has standardised the cardiac catheter laboratory protocol (Figure 2), although variations of this protocol are also used in clinical practice.
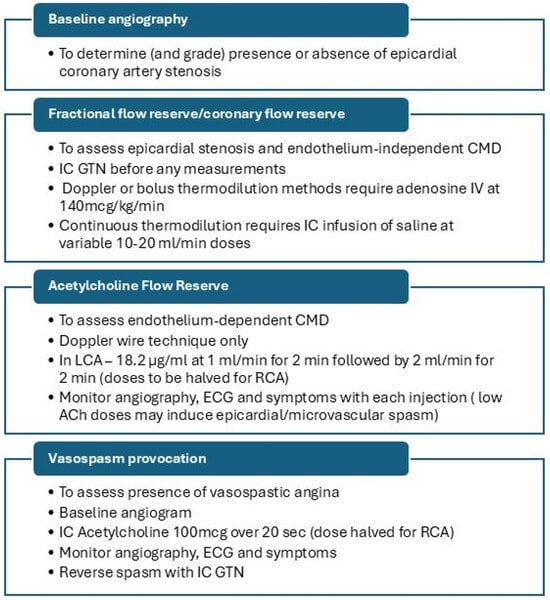
Figure 2.
Invasive coronary function testing protocol. ACh—acetylcholine, CMD—coronary microvascular dysfunction, ECG—electrocardiogram, GTN—glyceryl trinitrate, IC—intracoronary, IV—intravenous, LCA—left coronary artery, RCA—right coronary artery.
Due to the unavailability of coronary Doppler wires, AChFR assessment is frequently omitted from this protocol, and some centres use both bolus and continuous thermodilution methods for endothelium-dependent CMD. Regardless of the protocol used, reporting of findings should be produced in a standardised manner. This should include a comment on epicardial coronary anatomy, coronary microvascular endothelium-dependent and independent function assessment, and vasospasm provocation results. A sample report is presented in Figure 3.
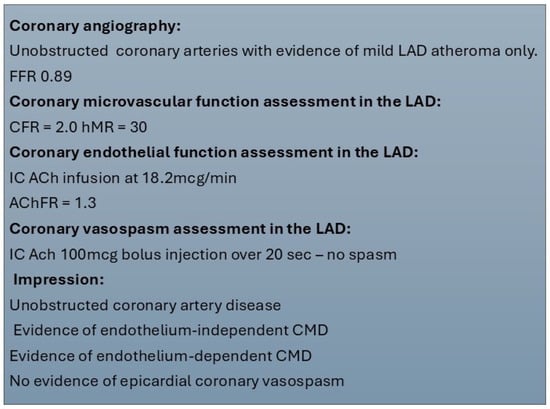
Figure 3.
Sample report for invasive coronary function testing. ACh—acetylcholine, AChFR—acetylcholine flow reserve, CFR—coronary flow reserve, CMD—coronary microvascular dysfunction, FFR—fractional flow reserve, hMR—hyperaemic microvascular resistance, IC—intracoronary, LAD—left anterior descending.
5.3. Non-Invasive Methods for CMD Diagnosis
In some patients, for example, in those for whom invasive testing may not be acceptable due to safety concerns or further rule-in tests following the discovery of non-obstructive coronary artery disease on CT coronary angiography is required, non-invasive imaging can be performed. Various methods have been described, including PET, CMR and Doppler echocardiography, each with their own advantages and disadvantages. These functional tests are currently awarded a class IIb recommendation in ESC guidelines for the investigation of persistently symptomatic patients with suspected ANOCA/INOCA [].
Cardiac PET myocardial perfusion imaging at rest and hyperaemia, utilising endothelium-independent vasodilators, allows measurement of regional myocardial blood flow (MBF) in absolute terms. The quantification of MBF, and the derived myocardial flow reserve (MFR), extended the scope of myocardial perfusion imaging from assessment of ischaemia due to flow-limiting epicardial coronary disease to assessment of microvascular dysfunction, with evidence that impaired MBF and MPR as determined by PET in the absence of obstructive CAD is predictive of increased risk of MACE or death []. This method is considered the “gold standard” against which other non-invasive and invasive methods of coronary blood flow have been evaluated []. It has the benefit of assessing all myocardial territories simultaneously but is associated with significant radiation burden and is expensive and time-consuming.
Visual interpretation of myocardial perfusion defects on stress CMR is limited in the detection of CMD []; however, the development of the quantitative assessment of MBF or MPR by stress CMR now also provides diagnostic and prognostic value in patients with CMD [,,]. Several studies have attempted validation of quantitative and semi-quantitative CMR against invasive methods of CMD assessment with various MBF and MPR cut-off values required for diagnosis [,,]. CMR allows tissue characterisation as well as the assessment of all myocardial segments at once, and its high spatial resolution allows for the assessment of myocardial blood flow within specific layers of the myocardium, offering greater specificity for CMD []. The clinical use of quantitative stress CMR is, however, limited due to incomplete validation, the presence of imaging artefacts, a high cost and contraindications in specific patient populations [].
Transthoracic Doppler echocardiography (TTDE) is another applicable method to assess coronary flow velocity reserve. This imaging technique, which utilises pulsed wave Doppler imaging of the proximal left anterior descending (LAD) segment at rest and with vasodilator hyperaemia, is widely available but hampered by technical difficulty and is limited to assessment of the LAD []. The data on the use of TTDE in non-obstructive CAD, although limited, show the reproducibility of this method in patients with CMD [].
The invasive and non-invasive methods of CMD and VSA assessment are summarised in Table 2.

Table 2.
Invasive and non-invasive methods of CMD and VSA diagnosis.
Novel Directions in Non-Invasive Assessment
One of the drawbacks of using non-invasive imaging techniques to diagnose CMD stems from the fact that the vasodilators used to achieve hyperaemia (adenosine or regadenoson) act only on the endothelium-independent pathways. Currently, endothelium-dependent CMD or vasospastic angina can only be diagnosed with intracoronary acetylcholine infusion; thus, complete coronary functional assessment necessitates invasive methods []. This is associated with a small risk of complications and increased initial cost. Thus, research focus is required to develop techniques which allow a complete, physiological assessment of all pathways involved in CMD and VSA pathophysiology.
Recently, there has been a resurgence of interest in exercise stress testing (EST). EST, historically validated against the presence of obstructive coronary artery disease, has been declassified in international guidelines due to apparent high false positive rates for obstructive CAD. However, as myocardial ischaemia can be present in the absence of obstructive CAD, recent studies have shown that, in patients with ANOCA, ischaemia seen on EST was highly specific for underlying ischaemic substrate, as determined by invasive functional coronary assessment [,]. While EST had poor sensitivity for the detection of both endothelium-dependent and independent CMD, it had excellent specificity for endothelium-dependent CMD, with low AChFR being the strongest predictor of ischaemia on EST []. The low sensitivity and high specificity of EST in detecting CMD as determined by invasive coronary blood flow measurements may be related to the fact that ECG changes occur further down the ischaemic cascade than changes in coronary blood flow. In the context of ANOCA, a negative EST does not rule out CMD, but a positive EST is highly suggestive of CMD (both endothelium and non-endothelium dependent) and thus could be used as a rule-in test in the diagnostic pathways.
Another novel technique focusing on physiological stress which is currently under investigation includes Oxygenation-Sensitive CMR (OS-CMR). In a healthy myocardium, stress decreases myocardial deoxyhaemoglobin concentration and increases OS-CMR signal intensity. Endothelium-dependent vasodilation can be induced using respiratory manoeuvres, such as hyperventilation followed by a breath-hold, and through the actions of carbon dioxide, a known physiological vasodilator. An inadequate response of coronary vasculature to vasodilatory stimuli (either adenosine or carbon dioxide) results in a higher concentration of deoxyhaemoglobin in the myocardium, detectable on OS-CMR []. In patients with ANOCA, OS-CMR has recently shown a heterogeneous coronary vascular response to a standardized vasoactive breathing manoeuvre with regionally reduced myocardial oxygenation, which may explain the presence of ischaemic symptoms []. This technique needs further validation but shows early promise in terms of the ability to non-invasively demonstrate and potentially differentiate between endothelium-dependent and independent CMD, allowing for precise non-invasive endotyping. The use of OS-CMR in non-invasive diagnosis of CMD is currently being investigated in a pilot CONCORD study (NCT06070662).
6. Management of Coronary Microvascular Dysfunction and Vasospastic Angina
Despite ongoing improvements in technology allowing invasive and non-invasive diagnostic options for CMD, robust data on therapeutic options is lacking, largely due to the paucity of large randomised controlled trials in patient groups clearly characterised by the ANOCA endotype. The majority of the trials of various therapeutic strategies have either taken place in the era before invasive testing for ANOCA was commonplace or simply included patients with evidence of chest pain with unobstructed coronaries, which contributes to heterogeneity of data and poor external validity of the results to current populations. A recent systematic review of all treatment studies in patients with ANOCA or CMD revealed that only a quarter of the studies enrolled patients who met contemporary COVADIS criteria for microvascular angina []. Sceptics of coronary vasoreactivity testing argue that subjecting patients to invasive diagnostics carries limited additional value over empirical antianginal therapy. However, as many as half of all patients with vasomotor angina proven on invasive testing are misdiagnosed as non-cardiac chest pain []. Arguably, the biggest contributor to poor patient outcomes and increased cardiovascular risk stems from inappropriate reassurance of patients with ANOCA and discontinuation of antianginal and prognostic therapies, such as statins or ACEi. Cross-over randomised controlled trial of ranolazine and amlodipine in patients with ANOCA (ChaMP-CMD) showed that only patients with impaired CFR derive benefit from anti-ischaemia therapy, which further supports the use of invasive diagnostic tools to avoid unnecessary treatments []. While no specific disease-modifying agents for CMD have been developed, evidence from CorMicA randomised controlled trial suggests that a stratified therapy based on specific ANOCA endotypes improves angina burden and quality of life [], with invasive testing showing favourable cost-effectiveness []. A recent qualitative study addressed the psychological impact that the journey to diagnosis can have on patients with ANOCA []. International guidelines advocate a holistic approach to managing patients with suspected vasomotor angina, focusing on lifestyle factor modification, risk factor reduction and personalised antianginal therapy, as summarised in Table 3.

Table 3.
International guideline-directed strategies for holistic management of ANOCA/INOCA.
6.1. Lifestyle Factors Modification
Lifestyle advice is considered the cornerstone of management of CMD and VSA by international guidelines [,,]. However, in practice, the delivery of lifestyle advice to patients with cardiovascular conditions both in primary and secondary care settings can be inconsistent, and the importance of lifestyle factors on disease management and progression is often overlooked by both the clinician and the patient [,,,]. Patients with CMD or VSA often have established coronary atherosclerosis and endothelial dysfunction where personalised counselling on lifestyle factors, including exercise, diet, weight management, smoking cessation and stress management strategies is mandated [].
Exercise appears to enhance endothelium-dependent vasodilation of the microvasculature, improving myocardial perfusion, reducing anginal burden and improving functional capacity [,,,]. This effect appears most pronounced with high-intensity interval training (HIIT) []. Dietary interventions, particularly the Mediterranean diet, likewise have proven positive effects on the endothelium []. However, prior published work on diet and exercise interventions in CMD is very heterogeneous [] and tested individual elements of lifestyle interventions separately. A recent pilot study of intense medical therapy and a supervised exercise regime lasting 12 weeks showed improvement in SAQ scores and MPR but was hampered by a significant drop-out rate (47%), highlighting challenges with cardiac rehabilitation adherence in this patient group []. A feasibility randomised controlled trial of a personalised rehabilitation programme combining diet and structured HIIT exercise in patients with CMD (MICROFIT) is currently underway and will assess the effects of such a programme on improvement in angina burden as well as functional capacity and myocardial perfusion (NCT06681896).
Psychological disorders may also play a significant role in vasomotor angina. Studies show a correlation between the prevalence of anxiety and depression in patients with ANOCA [,,]. While a definite cause-and-effect relationship between mental state and angina is yet to be fully evaluated, the concept of Mental Stress-Induced Myocardial Ischaemia (MSIMI) has an established evidence base []. Patients with MSIMI appear to have impaired vasodilatory response to acetylcholine, implying endothelial dysfunction []. Importantly, in patients with stable coronary heart disease, MSIMI has been found to be associated with an increased risk of cardiovascular death or non-fatal myocardial infarction []. Psychotherapy or behavioural techniques targeting a patient’s response to mental stress and emotional regulation may, therefore, prove beneficial in angina management. There is some evidence that mindfulness-based stress reduction therapy can improve endothelial function in women with microvascular angina [], although the evidence base in this area is generally sparse, with a Cochrane review of a highly heterogeneous sample of studies suggesting a modest to moderate effect of psychological interventions on patients with non-specific cardiac chest pain, which appeared to be limited to three months after the intervention [].
Additionally, the impact of this chronic condition on patients’ quality of life, social interactions, employment and perception of control cannot be underestimated []. The maladaptive psychological response to chronic pain can potentiate pain perception and perpetuate potentially harmful behaviours, such as avoidance of exercise, obesity secondary to a sedentary lifestyle or smoking. In patients with chronic refractory angina, psychoeducational interventions focusing on challenging patients’ health beliefs and pragmatic goal setting have shown improvements in quality of life as well as a reduction in anxiety and depression scores and improved perceived control over anginal symptoms [,].
6.2. Risk Factor Reduction
Structural and functional changes in the coronary vasculature of patients with ANOCA are driven by the same cardiovascular risk factors as conventional coronary artery disease, such as hypertension, dyslipidaemia and diabetes. In patients with ANOCA, the presence of atherosclerosis is linked to an increased risk of MACE. In male patients with ANOCA, cardiovascular death is the most commonly occurring MACE [], likely due to poorly managed risk factors. Therefore, it is essential to identify and manage these risk factors to improve patient outcomes.
The goal of antihypertensive therapy is to reduce microvascular remodelling. Blood pressure control can often be achieved through lifestyle changes (e.g., weight loss, exercise) and endotype-specific antianginal therapies (e.g., calcium channel blockers, beta-blockers). If needed, adding ACE inhibitors (ACEis) can be effective and well tolerated. ACEis may also benefit normotensive CMD patients by improving endothelial NO bioavailability and modulating microvascular tone [,]. A recent RCT in 61 women with CMD showed that quinapril improved both angina scores and coronary flow reserve, with the effect more pronounced in those with severe perfusion impairment []. This small trial forms the basis of the current ESC IIa recommendation to consider ACEis for symptom control in patients with endothelial dysfunction [].
Importantly, statin therapy is known to reduce cardiovascular risk not only through direct reduction of LDL cholesterol but also through reduction of vascular inflammation and improvement of endothelial function []. It is known to be beneficial in patients with non-obstructive CAD [], but there is also some evidence that it may confer a modest improvement in CFR in patients with CMD [] and exercise tolerance, especially if used in combination with diltiazem [].
Trials of both ACEi and statin use in patients with unobstructed coronary arteries have been small and heterogeneous, although both ACEi and statin therapy have been found to improve CFR in a recent meta-analysis []. Both statins and ACEis/ARBs have been shown to be associated with improved long-term clinical outcomes in observational trials of patients with VSA [,]. The hypothesis that a combination of ACEi/ARB and intensive statin therapy can reduce the risk of cardiovascular events, specifically in the cohort of female patients with ANOCA, will be tested in the randomized-controlled WARRIOR trial (NCT03417388) and provide much-needed high-quality evidence for use of these drugs in patients with ANOCA [].
CMD is an early sign of diabetic cardiomyopathy, with microvascular dysfunction occurring before ventricular dysfunction. Functional CMD is more prevalent in non-insulin-dependent patients and the evidence seems to suggest that this disease can evolve into structural CMD as diabetes advances []. Poor glycaemic control is associated with CMD amongst women with diabetes and ANOCA []. Optimisation of diabetic control is, therefore, crucial in this cohort of patients. Interestingly, a small randomised controlled trial showed that metformin may improve endothelial function as well as exercise tolerance also in non-diabetic women with ANOCA [], but the use of oral antiglycaemic agents, including sodium–glucose co-transporter 2 (SGLT2) inhibitors, in ANOCA populations needs to be further evaluated.
6.3. Pharmacological Therapies
Treatment of angina in ANOCA patients is challenging due to the lack of high-quality randomized trials addressing the heterogeneity of treated patients. Traditional antianginal medications are often ineffective, with many patients experiencing residual symptoms. Response to treatment varies by endotype—for example, beta-blockers are considered first-line for CMD, but may worsen vasospastic angina, while nitrates can be ineffective or exacerbate symptoms in CMD but are recommended as third-line therapy in VSA. These differences highlight the need for invasive endotyping in patients with refractory angina, where a personalised, stepwise approach is essential for optimal angina control.
6.3.1. Beta-Blockers
Beta-blockers (BBs) decrease myocardial oxygen demand by reducing myocardial contractility and increase oxygen supply by increasing coronary perfusion during diastole. Early-generation BBs (e.g., propranolol) cause beta-mediated vasodilation whilst leaving unopposed alpha-mediated vasoconstriction, which may potentiate coronary vasospasm []. Third-generation BBs (e.g., carvedilol and nebivolol) exert an additional effect on CBF through NO-mediated vasodilation [,], an effect proven in hypertensive patients and thus potentially beneficial in patients with structural CMD [,]. Although some evidence seems to suggest that nebivolol can be beneficial in vasospastic angina [], the international guidelines recommend vasodilatory BB use as a first-line therapy in CMD only [,]. This endorsement, however, is supported by surprisingly scanty and outdated evidence. A small cross-over randomised controlled trial (RCT) showed atenolol to significantly reduce chest pain episodes (over amlodipine and long-acting nitrates) in patients with cardiac syndrome X []. A small observational trial suggested that BB use was most associated with improved quality of life in patients with microvascular angina []. However, a most recent meta-analysis showed that BBs did not improve CFR in patients with unobstructed coronary arteries []. Further research is thus required to evaluate the role of BBs in symptom control as well as the prognosis of patients with ANOCA.
6.3.2. Calcium Channel Blockers
Calcium channel blockers (CCBs) exert their antianginal effect through the reduction of oxygen consumption by negative inotropy, as well as a vasodilatory effect on VSMCs, which are implicated in the pathophysiology of coronary vasospasm []. There is conflicting evidence on the benefit of CCBs in CMD. An early case-control trial of 16 patients with microvascular angina did not show an improvement in CFR in patients who were administered intravenous (IV) diltiazem []. The ChaMP-CMD cross-over RCT did show an improvement in CFR with amlodipine in patients with CMD, but this was not reflected in an improvement in angina scores []. Interestingly, a recent meta-analysis showed CCBs to improve CFR in studies with a more prolonged (>6 months) follow-up period, suggesting that a longer duration of treatment may be required to exert effect []. The EDIT-CMD RCT, investigating a six-week-long course of diltiazem, did not find an improvement in CFR, anginal symptoms or quality of life of patients with ANOCA, but did show a reduction in the prevalence of epicardial coronary spasm through progression to microvascular spasm or no spasm []. CCBs have been extensively investigated at standard therapeutic doses and found to be effective in upwards of 80% of VSA patients []. Importantly, in observational studies, the use of CCBs in VSA has been associated with an improved prognosis [,], especially with benipidine []. The ESC guidelines give a Class I recommendation for the use of CCBs in VSA as the first line for symptom control and prevention of ischaemia and fatal complications and suggest the use of CCBs as the second line in CMD []. High doses of non-DHP CCBs may be required to control symptoms in severe VSA, with or without combination with DHP CCBs []; however, side effects may limit their use.
6.3.3. Ranolazine
Ranolazine’s mode of action is incompletely understood, but it is believed to inhibit the late sodium current, reducing sodium and calcium overload in cardiomyocytes, which, in turn, improves myocardial relaxation and diastolic function. The largest RCT investigating a two-week course of ranolazine in 153 patients with CMD did not show an improvement in SAQ scores []. Ranolazine has since been investigated in a recent ChaMP-CMD RCT trial, showing improvement in both CFR and angina scores over 6 weeks [], as well as the MARINA trial, which, in contrast, did not demonstrate an improvement in SAQ angina frequency score, microvascular function or functional capacity compared with placebo at 3 months []. The use of ranolazine has been evaluated in a 2024 meta-analysis and systematic review comprising RCTs, suggesting that it is associated with improvements in CFR, myocardial perfusion and angina scores in patients with CMD []. The study suggested that an extended duration of therapy may be needed for improvement in quality-of-life indices. The definite effect of ranolazine remains to be established through high-quality, large randomised controlled trials of sufficient therapy duration; however, it is nonetheless recommended as a third-line treatment for CMD in the international guidelines [].
6.3.4. Other (Trimetazidine, Ivabradine, Nicorandil, Nitrates)
Trimetazidine’s unusual action focuses on altering metabolism within cardiomyocytes by inhibiting fatty acid metabolism and stimulating myocardial glucose utilisation. This appears to have a cytoprotective effect on the myocardium during ischaemia []. The evidence for its use in CMD is limited—two placebo-controlled small RCTs showed an improvement in exercise time and degree of ischaemia on the exercise tolerance test following trimetazidine [,]. Nonetheless, as this drug does not exert any effect on a patient’s haemodynamic state, it is recommended as a further-line therapy for patients with CMD in the guidelines [,].
Ivabradine, a selective pacemaker channel blocker in the sinoatrial node, produces bradycardia without having an impact on myocardial contractility. This effect leads to increased coronary blood flow in diastole at rest and on exercise. The use of ivabradine in CMD remains poorly investigated, with one small RCT reporting improvement in SAQ angina scores and functional capacity [], but no effect on myocardial perfusion in a meta-analysis [].
Nicorandil exhibits a pleiotropic effect through dual properties of a nitrate and adenosine triphosphate (ATP)-sensitive potassium channel agonist, resulting in vasodilation due to NO synthesis as well as activation of the cyclic guanosine monophosphate (cGMP) signalling pathway in VSMCs []. Nicorandil may be beneficial in CMD due to cardiovascular protection from oxidative injury and systemic inflammation, as well as improved endothelial function []. A recent meta-analysis appears to suggest a positive effect of nicorandil on angina scores in patients with cardiac syndrome X, but a definite judgement could not be made due to publication bias and low quality of evidence []. Guidelines suggest the use of nicorandil as a further-line therapy in VSA and CMD [].
Despite their effectiveness in patients with obstructive coronary artery disease, nitrates are poorly tolerated [] and have a disappointing effect in CMD, which is thought to be secondary to a blood steal phenomenon where vasodilatation of the vessels within normally functioning myocardium reduces blood flow to dysfunctional territories []. This effect appears to extend to both long- and short-acting nitrate preparations. In VSA, however, short-acting nitrate plays an important role, although it appears to exhibit a greater effect in epicardial rather than microvascular spasms []. Observational data seem to suggest the prognostic disadvantage of nitrate therapy in addition to CCB [], but it is unclear whether this represents a true effect or if long-acting nitrate use in VSA as an adjunct to CCBs is simply a marker of more severe disease. Long-acting nitrates are recommended as second-line therapy in epicardial coronary spasms, but tolerance as well as rebound vasoconstriction in therapy discontinuation may limit their use [].
6.4. Non-Pharmacological Therapies
6.4.1. Coronary Sinus Reducer
The coronary sinus reducer (CSR) device has emerged as a mechanical therapy for patients with refractory angina. This hourglass-shaped stent, implanted over a coronary sinus valve with 20% oversizing, exerts its anti-anginal effect after endothelialisation []. The exact mechanism of action of this device is not entirely known. It has been hypothesized to raise coronary venous pressure, which dilates subendocardial vessels, recruits capillaries, reduces microvascular resistance and redistributes blood from non-ischemic to ischaemic myocardium [].
The CSR has primarily been studied in patients with left-sided epicardial coronary disease who are unsuitable for further revascularisation []. Clinical trials and multicentre registries demonstrate its efficacy in reducing angina severity and improving quality of life [,], earning it a Class IIb/Level of Evidence B indication in recent ESC guidelines [].
The use of CSR for microvascular angina is less established, though case reports suggest it may reduce ischaemic burden, alleviate symptoms and improve microvascular function []. A recent blinded, sham-controlled crossover randomized trial of 20 patients with moderate/severe angina due to CMD showed that increased coronary venous pressure reduced microvascular resistance []. This was, however, a mechanistic study not designed to assess clinical outcomes. Additionally, in a group of eight patients with previous PCI and refractory angina with unobstructed coronaries, CSR implantation led to a significant improvement of symptoms and exercise tolerance, alongside evidence of ischemia reduction in a subgroup of these patients who underwent quantitative stress perfusion CMR []. These findings can only be interpreted as hypothesis-generating and will need further validation by adequately powered randomized trials.
6.4.2. External Enhanced Counter Pulsation (EECP) Therapy
EECP is a non-invasive medical device the use of which has largely been tested in refractory angina secondary to obstructive coronary artery disease []. There is limited, although positive, evidence for its use in patients with CMD, including a single-centre observational trial which showed an improvement in exercise and functional capacity [], and an RCT demonstrating an improvement in CFR and angina status in patients treated with EECP []. The use of these devices is, however, limited by patient discomfort, cost, accessibility and patient acceptability. Further research in this area is required, although a consideration to use this technique is recommended by the EAPCI consensus document in patients with refractory symptoms [].
6.5. Other Therapies
Multiple other treatment strategies have been investigated previously, including tricyclic antidepressants [], neuromodulation [,,], rho-kinase inhibitors [,], hormone therapy [,], endothelin receptor antagonists [], stem cell therapy [,] and phosphodiesterase inhibitors [], some of which have shown promising effects in small trials. These treatment strategies are however not recommended in international guidelines as further research is required to provide evidence in terms of efficacy as well as the risk vs. benefit ratio of these therapies in patients with ANOCA.
7. Conclusions
Coronary microcirculation dysfunction, as fascinating an entity as it may be, is a major global health problem which impacts both patients and health systems. Current technology allows us to better study the disease, both via invasive and non-invasive modalities. As our knowledge expands and we better understand the pathophysiologic pathways of the disease, we will be able to provide patients with a more personalized treatment. The current pharmacological therapies have a limited evidence base but with improvements in diagnostic rates and endotype characterisation; well-designed and targeted randomised controlled trials can now be undertaken to help guide treatment. Novel interventional therapies are still under investigation and might be an addition to established pharmacotherapies in the cardiologist’s armamentarium.
Author Contributions
Conceptualisation, J.A. and I.F.; writing—original draft preparation J.A.; visualisation, J.A.; writing—review and editing, J.A., M.M. and I.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACh | Acetylcholine |
| AChFR | Acetylcholine Flow Reserve |
| ACEi | Angiotensin Converting Enzyme inhibitor |
| ANOCA | Angina with Non-Obstructed Coronary Arteries |
| APV | Averaged Pulsed Velocities |
| ARB | Angiotensin II Receptor Blocker |
| ATP | Adenosine Triphosphate |
| AV | Atrio-ventricular |
| BB | Beta-Blocker |
| BHF | British Heart Foundation |
| CAD | Coronary Artery Disease |
| CBF | Coronary Blood Flow |
| CCB | Calcium Channel Blocker |
| CCS | Chronic Coronary Syndromes |
| CFR | Coronary Flow Reserve |
| cGMP | Cyclic Guanosine Monophosphate |
| CMD | Coronary Microvascular Dysfunction |
| CMVO | Coronary Microvascular Obstruction |
| COVADIS | Coronary Vascular Disorders International Study Group |
| CSR | Coronary Sinus Reducer |
| CT | Computed Tomography |
| DHP | Dihydropyridine |
| EAPCI | European Association of Percutaneous Coronary Intervention |
| ECG | Electrocardiogram |
| EECP | Enhanced External Counterpulsation |
| ESC | European Society of Cardiology |
| EST | Exercise Stress Test |
| FFR | Fractional Flow Reserve |
| GTN | Glyceryl Trinatrate |
| HCM | Hypertrophic Cardiomyopathy |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HIIT | High Intensity Interval Training |
| hMR | Hyperaemic Microvascular Resistance |
| IC | Intra-coronary |
| iFR | Instantaneous wave-free ratio |
| IMR | Index of Microvascular Resistance |
| INOCA | Ischaemia with Non-Obstructed Coronary Arteries |
| IV | Intravenous |
| MACE | Major Adverse Cardiovascular Events |
| MBF | Myocardial Blood Flow |
| MINOCA | Myocardial Infarction with Non-Obstructed Coronary Arteries |
| MMR | Minimal Microvascular Resistance |
| MPR | Myocardial Perfusion Reserve |
| MR | Microvascular Resistance |
| MRI/CMR | Magnetic Resonance Imaging/Cardiac Magnetic Resonance Imaging |
| MSIMI | Mental State Induced Myocardial Ischaemia |
| NIHR | National Institute of Health Research |
| LAD | Left Anterior Descending |
| LCA | Left Coronary Artery |
| LDL | Low Density Lipoprotein |
| LV | Left Ventricle |
| NO | Nitric Oxide |
| PCI | Percutaneous Coronary Intervention |
| PET | Positron Emission Tomography |
| OS-CMR | Oxygenation-Sensitive Cardiac Magnetic Resonance Imaging |
| Q | Absolute Coronary Flow |
| QCA | Quantitative Coronary Angiography |
| RCA | Right Coronary Artery |
| RCT | Randomised Controlled Trial |
| RFR | Resting full cycle Ratio |
| SAQ | Seattle Angina Questionnaire |
| SGLT2 | Sodium-Glucose Cotransporter 2 |
| SPECT | Single Photon Emission Computed Tomography |
| TIMI | Thrombolysis in Myocardial Infarction |
| Tmn | Mean transit time |
| TTDE | Transthoracic Tissue Doppler Echocardiography |
| VSA | Vasospastic Angina |
| VSMC | Vascular Smooth Muscle Cells |
References
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low Diagnostic Yield of Elective Coronary Angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbæk, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery dis-ease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2012, 33, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar]
- Bugiardini, R.; Bairey Merz, C.N. Angina with “Normal” Coronary Arteries. JAMA 2005, 293, 477. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.M.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse Cardiovascular Outcomes in Women with Nonobstructive Coronary Artery Disease. Arch. Intern. Med. 2009, 169, 843. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, D.; Lee, J.M.; van de Hoef, T.P.; Hong, D.; Choi, K.H.; Hwang, D.; Boerhout, C.K.M.; de Waard, G.A.; Jung, J.H.; et al. ILIAS Registry Investigators. Clinical Relevance of Ischemia with Nonobstructive Coronary Arteries According to Coronary Microvascular Dysfunction. J. Am. Heart Assoc. 2022, 11, e025171. [Google Scholar] [CrossRef]
- Boerhout, C.K.M.; de Waard, G.A.; Lee, J.M.; Mejia-Renteria, H.; Lee, S.H.; Jung, J.H.; Hoshino, M.; Echavar-ria-Pinto, M.; Meuwissen, M.; Matsuo, H.; et al. Prognostic value of structural and functional coronary microvascular dysfunction in patients with non-obstructive coronary artery disease; from the multicentre international ILIAS registry. EuroIntervention 2022, 18, 719–728. [Google Scholar] [CrossRef]
- Gdowski, M.A.; Murthy, V.L.; Doering, M.; Monroy-Gonzalez, A.G.; Slart, R.; Brown, D.L. Association of Isolated Coronary Microvascular Dysfunction with Mortality and Major Adverse Cardiac Events: A Systematic Review and Meta-Analysis of Aggregate Data. J. Am. Heart Assoc. 2020, 9, e014954. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.J.; Merz, C.N.B.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kip, K.E.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; et al. The economic burden of angina in women with suspected ischemic heart disease: Results from the National Institutes of Health-National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation. Circulation 2006, 114, 894–904. [Google Scholar] [CrossRef]
- Schumann, C.L.; Mathew, R.C.; Dean, J.H.L.; Yang, Y.; Balfour, P.C.; Shaw, P.W.; Robinson, A.A.; Salerno, M.; Kramer, C.M.; Bourque, J.M. Functional and Economic Impact of INOCA and Influence of Coro-nary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 1369–1379. [Google Scholar] [CrossRef]
- Gulati, M.; Khan, N.; George, M.; Berry, C.; Chieffo, A.; Camici, P.G.; Crea, F.; Kaski, J.C.; Marzilli, M.; Merz, C.N.B. Ischemia with no obstructive coronary artery disease (INOCA): A patient self-report quality of life survey from INOCA international. Int. J. Cardiol. 2023, 371, 28–39. [Google Scholar] [CrossRef]
- Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bull-ock-Palmer, R.P.; Conejo, T.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/ SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 144, E368–E454. [Google Scholar] [PubMed]
- Ahmad, A.; Corban, M.T.; Moriarty, J.P.; Kanaji, Y.; Rosedahl, J.K.; Gulati, R.; Rihal, C.S.; Prasad, A.; Sara, J.D.; Toya, T.; et al. Coronary Reactivity Assessment Is Associated with Lower Health Care-Associated Costs in Patients Presenting with Angina and Nonobstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2023, 16, e012387. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K. Stratified Medical Therapy Using Invasive Coronary Function Testing in Angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- De Waard, G.A.; Cook, C.M.; Van Royen, N.; Davies, J.E. Coronary autoregulation and assessment of stenosis severity without pharmacological vasodilation. Eur. Heart J. 2018, 39, 4062–4071. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.; Chilian, W.M.; Davis, M.J. Coronary arteriolar myogenic response is independent of endothelium. Circ. Res. 1990, 66, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Gorman, M.W.; Feigl, E.O. Skeletal and Cardiac Muscle Blood Flow Matching coronary blood flow to myocardial oxygen consumption. J. Appl. Physiol. 2004, 97, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Ryan, M.; Lumley, M.; Modi, B.; Mcconkey, H.; Ellis, H.; Scannell, C.; Clapp, B.; Marber, M.; Webb, A.; et al. Coronary Microvascular Dysfunction Is Associated with Myocardial Ischemia and Abnormal Coronary Perfusion During Exercise. Circulation 2019, 140, 1805–1816. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Diaz, A.; Cyr, D.D.; Shaw, L.J.; Mancini, G.B.J.; Leipsic, J.; Budoff, M.J.; Min, J.K.; Hague, C.J.; Ber-man, D.S.; et al. Ischemia with Nonobstructive Coronary Arteries: Insights from the ISCHEMIA Trial. JACC Cardiovasc. Imaging 2023, 16, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. EuroIntervention 2021, 16, 1049–1069. [Google Scholar]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients with Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023207. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Demir, O.M.; Khan, F.; Ryan, M.; Ellis, H.; Mills, M.T.; Chiribiri, A.; Webb, A.; Perera, D. Physiological Stratification of Patients with Angina Due to Coronary Microvascular Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 2538–2549. [Google Scholar] [CrossRef]
- Jenkins, K.; Pompei, G.; Ganzorig, N.; Brown, S.; Beltrame, J.; Kunadian, V. Vasospastic angina: A review on diagnostic approach and management. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241230400. [Google Scholar] [CrossRef] [PubMed]
- Prinzmetal, M.; Kennamer, R.; Merliss, R.; Wada, T.; Bor, N. Angina pectoris I. A variant form of angina pectoris. Am. J. Med. 1959, 27, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of coronary artery spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Taqueti, V.R.; Di Carli, M.F. Coronary Microvascular Disease Pathogenic Mechanisms and Therapeutic Options. J. Am. Coll. Cardiol. 2018, 72, 2625–2641. [Google Scholar] [CrossRef]
- Rehan, R.; Yong, A.; Ng, M.; Weaver, J.; Puranik, R. Coronary microvascular dysfunction: A review of recent progress and clinical implications. Front. Cardiovasc. Med. 2023, 10, 1111721. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.-S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A.P.; Nathan, S.; Butler, J.; Davidson, C.J.; Fearon, W.F.; et al. Coronary microvascular dysfunction in patients with heart failure with pre-served ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, 1033–1042. [Google Scholar] [CrossRef]
- Rush, C.J.; Berry, C.; Oldroyd, K.G.; Rocchiccioli, J.P.; Lindsay, M.M.; Touyz, R.M.; Murphy, C.L.; Ford, T.J.; Sidik, N.; McEntegart, M.B.; et al. Prevalence of Coronary Artery Disease and Coronary Microvascular Dys-function in Patients with Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2021, 6, 1130. [Google Scholar] [CrossRef]
- Zuchi, C.; Tritto, I.; Carluccio, E.; Mattei, C.; Cattadori, G.; Ambrosio, G. Role of endothelial dysfunction in heart failure. Heart Fail. Rev. 2020, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Duncker, D.J.; Koller, A.; Merkus, D.; Canty, J.M. Regulation of Coronary Blood Flow in Health and Ischemic Heart Disease. Prog. Cardiovasc. Dis. 2015, 57, 409–422. [Google Scholar] [CrossRef]
- Nishi, T.; Murai, T.; Ciccarelli, G.; Shah, S.V.; Kobayashi, Y.; Derimay, F.; Waseda, K.; Moonen, A.; Hoshino, M.; Hirohata, A.; et al. Prognostic Value of Coronary Microvascular Function Measured Immediately after Percutaneous Coronary Intervention in Stable Coronary Artery Disease: An International Multicenter Study. Circ. Cardiovasc. Interv. 2019, 12, e007889. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Tiroch, K.; Fusaro, M.; Keta, D.; Seyfarth, M.; Byrne, R.A.; Pache, J.; Alger, P.; Mehilli, J.; Schömig, A.; et al. 5-Year Prognostic Value of No-Reflow Phenomenon After Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2010, 55, 2383–2389. [Google Scholar] [CrossRef]
- Henriques, J.P.S.; Zijlstra, F.; van ‘t Hof, A.W.J.; de Boer, M.-J.; Dambrink, J.-H.E.; Gosselink, M.; Hoorntje, J.C.A.; Suryapranata, H. Angiographic Assessment of Reperfusion in Acute Myocardial Infarction by Myocardial Blush Grade. Circulation 2003, 107, 2115–2119. [Google Scholar] [CrossRef]
- Bax, M.; De Winter, R.J.; Schotborgh, C.E.; Koch, K.T.; Meuwissen, M.; Voskuil, M.; Adams, R.; Mulder, K.J.J.; Tijs-sen, J.G.P.; Piek, J.J. Short- and Long-Term recovery of left ventricular function predicted at the time of primary percutaneous coronary intervention in anterior myocardial infarction. J. Am. Coll. Cardiol. 2004, 43, 534–541. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Vancheri, S.; Henein, M. Coronary Microvascular Dysfunction. J. Clin. Med. 2020, 9, 2880. [Google Scholar] [CrossRef]
- Mauricio, R.; Srichai, M.B.; Axel, L.; Hochman, J.S.; Reynolds, H.R. Stress Cardiac MRI in Women with Myocardial Infarction and Nonobstructive Coronary Artery Disease. Clin. Cardiol. 2016, 39, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.; Chiriatti, G.; Lorenzoni, R.; Bellina, R.C.; Gistri, R.; Italiani, G.; Parodi, O.; Salvadori, P.A.; Nista, N.; Papi, L.; et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: A study with nitrogen-13 ammonia and positron emission tomography. J. Am. Coll. Cardiol. 1991, 17, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Olivotto, I.; Gistri, R.; Lorenzoni, R.; Chiriatti, G.; Camici, P.G. Coronary Microvascular Dysfunction and Prognosis in Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2003, 349, 1027–1035. [Google Scholar] [CrossRef]
- Tomberli, B.; Cecchi, F.; Sciagrà, R.; Berti, V.; Lisi, F.; Torricelli, F.; Morrone, A.; Castelli, G.; Yacoub, M.H.; Olivotto, I. Coronary microvascular dysfunction is an early feature of cardiac involve-ment in patients with Anderson–Fabry disease. Eur. J. Heart Fail. 2013, 15, 1363–1373. [Google Scholar] [CrossRef]
- Kruse, M.J.; Kovell, L.; Kasper, E.K.; Pomper, M.G.; Moller, D.R.; Solnes, L.; Chen, E.S.; Schindler, T.H. Myocardial Blood Flow and Inflammatory Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Suwaidi, J.A.; Velianou, J.L.; Gertz, M.A.; Cannon, R.O.; Higano, S.T.; Holmes, D.R.; Lerman, A. Systemic Amyloidosis Presenting with Angina Pectoris. Ann. Intern. Med. 1999, 131, 838. [Google Scholar] [CrossRef] [PubMed]
- Kalliokoski, R.J.; Kalliokoski, K.K.; Sundell, J.; Engblom, E.; Penttinen, M.; Kantola, I.; Raitakari, O.T.; Knuuti, J.; Nuutila, P. Impaired myocardial perfusion reserve but preserved peripheral endothelial function in patients with Fabry disease. J. Inherit. Metab. Dis. 2005, 28, 563–573. [Google Scholar] [CrossRef]
- Kwan, A.C.; Wei, J.; Ouyang, D.; Ebinger, J.E.; Merz, C.N.B.; Berman, D.; Cheng, S. Sex differences in contributors to coronary microvascular dysfunction. Front. Cardiovasc. Med. 2023, 10, 1085914. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Konst, R.E.; Elias-Smale, S.E.; Lier, A.; Bode, C.; Maas, A.H.E.M. Different cardiovascular risk factors and psychosocial burden in symptomatic women with and without obstructive coronary artery disease. Eur. J. Prev. Cardiol. 2019, 26, 657–659. [Google Scholar] [CrossRef]
- Reis, S.E.; Holubkov, R.; Smith, A.J.C.; Kelsey, S.F.; Sharaf, B.L.; Reichek, N.; Rogers, W.J.; Merz, C.N.B.; Sopko, G.; Pepine, C.J. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. Am. Heart J. 2001, 141, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction Among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary Microvascular Reactivity to Adenosine Predicts Adverse Outcome in Women Evaluated for Suspected Ischemia. Results From the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef]
- Shaw, L.J.; Bairey Merz, C.N.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights From the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006, 47, S4–S20. [Google Scholar] [CrossRef]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Perera, D.; Berry, C.; Hoole, S.P.; Sinha, A.; Rahman, H.; Morris, P.D.; Kharbanda, R.K.; Petraco, R.; Channon, K. Invasive coronary physiology in patients with angina and non-obstructive coronary artery disease: A consensus document from the coronary microvascular dysfunction workstream of the British Heart Foundation/National Institute for Health Research Partnership. Heart 2022, 109, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Corcoran, D.; Aetesam-Ur-Rahman, M.; Hoole, S.P.; Berry, C.; Perera, D. Diagnosis of patients with angina and non-obstructive coronary disease in the catheter laboratory. Heart 2019, 105, 1536–1542. [Google Scholar] [CrossRef]
- Radico, F.; Zimarino, M.; Fulgenzi, F.; Ricci, F.; Di Nicola, M.; Jespersen, L.; Chang, S.M.; Humphries, K.H.; Mar-zilli, M.; De Caterina, R. Determinants of long-term clinical outcomes in patients with angina but without obstructive coronary artery disease: A systematic review and meta-analysis. Eur. Heart J. 2018, 39, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Echavarria-Pinto, M.; Escaned, J.; Macías, E.; Medina, M.; Gonzalo, N.; Petraco, R.; Sen, S.; Jimenez-Quevedo, P.; Hernandez, R.; Mila, R.; et al. Disturbed Coronary Hemodynamics in Vessels with Intermediate Stenoses Evaluated with Fractional Flow Reserve. Circulation 2013, 128, 2557–2566. [Google Scholar] [CrossRef]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A. Long-Term Follow-Up of Patients with Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef]
- Everaars, H.; de Waard, G.A.; Schumacher, S.P.; Zimmermann, F.M.; Bom, M.J.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; Götte, M.J.; van Rossum, A.C.; et al. Continuous thermodilution to assess absolute flow and micro-vascular resistance: Validation in humans using [15O]H2O positron emission tomography. Eur. Heart J. 2019, 40, 2350–2359. [Google Scholar] [CrossRef]
- Pijls, N.H.J.; De Bruyne, B.; Smith, L.; Aarnoudse, W.; Barbato, E.; Bartunek, J.; Bech, G.J.W.; Van De Vosse, F. Coronary Thermodilution to Assess Flow Reserve. Circulation 2002, 105, 2482–2486. [Google Scholar] [CrossRef]
- Gutiérrez-Barrios, A.; Izaga-Torralba, E.; Rivero Crespo, F.; Gheorghe, L.; Cañadas-Pruaño, D.; Gómez-Lara, J.; Silva, E.; Noval-Morillas, I.; Zayas Rueda, R.; Calle-Pérez, G.; et al. Continuous Thermodilution Method to Assess Coronary Flow Reserve. Am. J. Cardiol. 2021, 141, 31–37. [Google Scholar] [CrossRef]
- Everaars, H.; de Waard, G.A.; Driessen, R.S.; Danad, I.; van de Ven, P.M.; Raijmakers, P.G.; Lammertsma, A.A.; van Rossum, A.C.; Knaapen, P.; van Royen, N. Doppler Flow Velocity and Thermodilution to Assess Coronary Flow Reserve. JACC Cardiovasc. Interv. 2018, 11, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Gallinoro, E.; Bertolone, D.T.; Fernandez-Peregrina, E.; Paolisso, P.; Bermpeis, K.; Esposito, G.; Gomez-Lopez, A.; Candreva, A.; Mileva, N.; Belmonte, M.; et al. Reproducibility of bolus versus continuous thermodilution for assessment of coronary microvascular function in patients with ANOCA. EuroIntervention 2023, 19, E155–E166. [Google Scholar] [CrossRef]
- De Bruyne, B.; Adjedj, J.; Xaplanteris, P.; Ferrara, A.; Mo, Y.; Penicka, M.; Floré, V.; Pellicano, M.; Toth, G.; Barbato, E.; et al. Saline-Induced Coronary Hyperemia: Mechanisms and Effects on Left Ventricular Function. Circ. Cardiovasc. Interv. 2017, 10, e004719. [Google Scholar] [CrossRef]
- Ibrahim, A.; Fawaza, S.; Sajjada, U.; Colletc, C.; De Bruyne, B.; Perera, D.; Davies, J.R.; Keeble, T.R.; Carson, K.; Konstantinou, K. Coronary Endothelial Function: A Novel Acetylcholine Infusion Protocol with Continuous Thermodilution. JACC, 2025; in press. [Google Scholar]
- Schindler, T.H.; Schelbert, H.R.; Quercioli, A.; Dilsizian, V. Cardiac PET Imaging for the Detection and Monitoring of Coronary Artery Disease and Microvascular Health. JACC Cardiovasc. Imaging 2010, 3, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Scannell, C.M.; Demir, O.M.; Ryan, M.; McConkey, H.; Ellis, H.; Masci, P.G.; Perera, D.; Chiribiri, A. High-Resolution Cardiac Magnetic Resonance Imaging Techniques for the Identification of Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2021, 14, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Thomson, L.E.J.; Wei, J.; Agarwal, M.; Haft-Baradaran, A.; Shufelt, C.; Mehta, P.K.; Gill, E.B.; Johnson, B.D.; Kenkre, T.; Handberg, E.M.; et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction: A National Heart, Lung, and Blood Institute-sponsored study from the Women’s Ischemia Syndrome Evaluation. Circ. Cardiovasc. Imaging 2015, 8, e002481. [Google Scholar] [CrossRef]
- Sharrack, N.; Chiribiri, A.; Schwitter, J.; Plein, S. How to do quantitative myocardial perfusion cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 315–318. [Google Scholar] [CrossRef]
- Kotecha, T.; Martinez-Naharro, A.; Boldrini, M.; Knight, D.; Hawkins, P.; Kalra, S.; Patel, D.; Coghlan, G.; Moon, J.; Plein, S.; et al. Automated Pixel-Wise Quantitative Myocardial Perfusion Mapping by CMR to Detect Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction. JACC Cardiovasc. Imaging 2019, 12, 1958–1969. [Google Scholar] [CrossRef]
- Galiuto, L.; Sestito, A.; Barchetta, S.; Sgueglia, G.A.; Infusino, F.; La Rosa, C.; Lanza, G.; Crea, F. Noninvasive Evaluation of Flow Reserve in the Left Anterior Descending Coronary Artery in Patients with Cardiac Syndrome X. Am. J. Cardiol. 2007, 99, 1378–1383. [Google Scholar] [CrossRef]
- Sinha, A.; Dutta, U.; Demir, O.M.; De Silva, K.; Ellis, H.; Belford, S.; Ogden, M.; Li Kam Wa, M.; Morgan, H.P.; Shah, A.M.; et al. Rethinking False Positive Exercise Electrocardiographic Stress Tests by Assessing Coronary Microvascular Function. J. Am. Coll. Cardiol. 2024, 83, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Cassar, A.; Chareonthaitawee, P.; Rihal, C.S.; Prasad, A.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Lack of Correlation Between Noninvasive Stress Tests and Invasive Coronary Vasomotor Dysfunction in Patients with Nonobstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2009, 2, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hillier, E.; Friedrich, M.G. The Potential of Oxygenation-Sensitive CMR in Heart Failure. Curr. Heart Fail. Rep. 2021, 18, 304–314. [Google Scholar] [CrossRef]
- Hillier, E.; Elharram, M.; White, J.A.; Anderson, T.; Luu, J.; Labib, D.; Alhussein, M.; Friedrich, M.G.; Pilote, L. Heterogeneity of coronary vascular function and myocardial oxygenation in women with angina and non-obstructive coronary artery disease. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Haley, M.; Chiew, K.; Ahmed-Jushuf, F.; Rajkumar, C.A.; Foley, M.J.; Simader, F.A.; Chotai, S.; Shun-Shin, M.J.; Al-Lamee, R. A systematic review of enrolment criteria and treatment efficacy for microvascular angina. EuroIntervention 2025, 21, 46–57. [Google Scholar] [CrossRef]
- Sidik, N.P.; Stanley, B.; Sykes, R.; Morrow, A.J.; Bradley, C.P.; McDermott, M.; Ford, T.J.; Roditi, G.; Hargreaves, A.; Stobo, D.; et al. Invasive Endotyping in Patients with Angina and No Obstructive Coronary Artery Disease: A Randomized Controlled Trial. Circulation 2024, 149, 7–23. [Google Scholar] [CrossRef]
- Sinha, A.; Rahman, H.; Douiri, A.; Demir, O.M.; De Silva, K.; Clapp, B.; Webb, I.; Gulati, A.; Pinho, P.; Dutta, U.; et al. ChaMP-CMD: A Phenotype-Blinded, Randomized Controlled, Cross-Over Trial. Circulation 2024, 149, 36–47. [Google Scholar] [CrossRef]
- Heggie, R.; Briggs, A.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. Stratified medicine using invasive coronary function testing in angina: A cost-effectiveness analysis of the British Heart Foundation CorMicA trial. Int. J. Cardiol. 2021, 337, 44–51. [Google Scholar] [CrossRef]
- Humphreys, H.; Paddock, D.; Brown, S.; Berry, C.; Cowie, A.; Dawkes, S.; Nichols, S. Living with myocardial ischaemia and no obstructive coronary arteries: A qualitative study. Open Heart 2024, 11, e002569. [Google Scholar] [CrossRef] [PubMed]
- Silagy, C.; Muir, J.; Coulter, A.; Thorogood, M.; Yudkin, P.; Roe, L. Lifestyle advice in general practice: Rates recalled by patients. BMJ 1992, 305, 871–874. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arber, S.; McKinlay, J.; Adams, A.; Marceau, L.; Link, C.; O’Donnell, A. Influence of patient characteristics on doctors’ questioning and lifestyle advice for coronary heart disease: A UK/US video experiment. Br. J. Gen. Pract. 2004, 54, 673–678. [Google Scholar]
- Hivert, M.-F.; Arena, R.; Forman, D.E.; Kris-Etherton, P.M.; McBride, P.E.; Pate, R.R.; Spring, B.; Trilk, J.; Van Horn, L.V.; Kraus, W.E. Medical Training to Achieve Competency in Lifestyle Counselling: An Essential Foundation for Prevention and Treatment of Cardiovascular Diseases and Other Chronic Medical Conditions: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e308–e327. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Jankowski, P.; De Bacquer, D.; Kotseva, K. Dietary measures among patients with coronary heart disease in Europe. ESC EORP Euroaspire V. Int. J. Cardiol. 2020, 302, 5–14. [Google Scholar] [CrossRef]
- Larsen, A.I.; Sæland, C.; Vegsundvåg, J.; Skadberg, M.S.; Nilsen, J.; Butt, N.; Ushakova, A.; Valborgland, T.; Munk, P.S.; Isaksen, K. Aerobic high-intensity interval exercise training in patients with angina and no obstructive coronary artery disease: Feasibility and physiological effects. Eur. Heart J. Open 2023, 3, oead030. [Google Scholar] [CrossRef]
- Carvalho, E.E.; Crescêncio, J.C.; Santi, G.L.; Oliveira, L.F.; Schwartzmann, P.V.; Gallo-Junior, L.; Marin-Neto, J.A.; Simões, M.V. Physical training improves myocardial perfusion but not left ventricular function response to exercise in patients with microvascular angina. Q. J. Nucl. Med. Mol. Imaging 2019, 63, 302–310. [Google Scholar] [CrossRef]
- Kissel, C.K.; Nikoletou, D. Cardiac Rehabilitation and Exercise Prescription in Symptomatic Patients with Non-Obstructive Coronary Artery Disease—A Systematic Review. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 78. [Google Scholar] [CrossRef]
- Long, L.; Anderson, L.; He, J.; Gandhi, M.; Dewhirst, A.; Bridges, C.; Taylor, R. Exercise-based cardiac rehabilitation for stable angina: Systematic review and meta-analysis. Open Heart 2019, 6, e000989. [Google Scholar] [CrossRef] [PubMed]
- Hannan, A.; Hing, W.; Simas, V.; Climstein, M.; Coombes, J.; Jayasinghe, R.; Byrnes, J.; Furness, J. High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: A systematic review and meta-analysis. Open Access J. Sports Med. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Shannon, O.M.; Mendes, I.; KÖchl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.-M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Schumann, C.L.; Nealy, Z.B.; Mathew, R.C.; Yang, Y.; Balfour, P.C.; Shaw, P.W.; Salerno, M.; Kramer, C.M.; Bour-que, J.M. Pilot Study of Supervised Exercise and Intensive Medical Therapy in Patients with Ischemia with No Evidence of Obstructive Coronary Artery Disease and Coronary Microvascular Dysfunction. Am. J. Cardiol. 2024, 214, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Rutledge, T.; Kenkre, T.S.; Bittner, V.; Krantz, D.S.; Thompson, D.V.; Linke, S.E.; Eastwood, J.A.; Eteiba, W.; Cornell, C.E.; Vaccarino, V.; et al. Anxiety associations with cardiac symptoms, angiographic disease sever-ity, and healthcare utilization: The NHLBI-sponsored Women’s Ischemia Syndrome Evaluation. Int. J. Cardiol. 2013, 168, 2335–2340. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Guo, L. Anxiety is associated with coronary microvascular dysfunction: Results from the CAMADA study. Microcirculation 2023, 30, e12798. [Google Scholar] [CrossRef]
- Handberg, E.M.; Eastwood, J.A.; Eteiba, W.; Johnson, B.D.; Krantz, D.S.; Thompson, D.V.; Vaccarino, V.; Bittner, V.; Sopko, G.; Pepine, C.J.; et al. Clinical implications of the Women’s Ischemia Syndrome Evaluation: Inter-relationships between symptoms, psychosocial factors and cardiovascular outcomes. Women’s Health 2013, 9, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Cappola, T.P. Mental Stress-Induced Myocardial Ischemia: When the Mind Controls the Fate of the Heart. JAMA 2021, 326, 1803–1804. [Google Scholar] [CrossRef] [PubMed]
- Hammadah, M.; Kim, J.H.; Al Mheid, I.; Samman Tahhan, A.; Wilmot, K.; Ramadan, R.; Alkhoder, A.; Khayata, M.; Mekonnen, G.; Levantsevych, O.; et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. J. Am. Heart Assoc. 2018, 7, e008532. [Google Scholar] [CrossRef]
- Vaccarino, V.; Almuwaqqat, Z.; Kim, J.H.; Hammadah, M.; Shah, A.J.; Ko, Y.-A.; Elon, L.; Sullivan, S.; Shah, A.; Alkhoder, A.; et al. Association of Mental Stress-Induced Myocardial Ischemia with Cardiovascular Events in Patients with Coronary Heart Disease. JAMA 2021, 326, 1818–1828. [Google Scholar] [CrossRef]
- Kim, B.J.; Cho, I.S.; Cho, K.I. Impact of Mindfulness Based Stress Reduction Therapy on Myocardial Function and Endothelial Dysfunction in Female Patients with Microvascular Angina. J. Cardiovasc. Ultrasound 2017, 25, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kisely, S.R.; Campbell, L.A.; Yelland, M.J.; Paydar, A. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst. Rev. 2012, 13, CD004101. [Google Scholar]
- McGillion, M.; Arthur, H.; Victor, J.; Watt-Watson, J.; Cosman, T. Effectiveness of Psychoeducational Interventions for Improving Symptoms, Health-Related Quality of Life, and Psychological well Being in Patients with Stable Angina. Curr. Cardiol. Rev. 2008, 4, 1–11. [Google Scholar] [CrossRef][Green Version]
- Khan, M.; Thappar, S.; Taylor, S.; Scally, A.; Sainsbury, P. The impact of a short psychological intervention on quality of life and angina control in patients with chronic refractory angina. Eur. Heart J. 2013, 34, 2265. [Google Scholar] [CrossRef]
- Chen, J.-W.; Hsu, N.-W.; Wu, T.-C.; Lin, S.-J.; Chang, M.-S. Long-term angiotensin-converting enzyme inhibition reduces plasma asymmetric dimethylarginine and improves endothelial nitric oxide bioavailability and coronary microvascular function in patients with syndrome X. Am. J. Cardiol. 2002, 90, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Kaski, J.C.; Rosano, G.; Gavrielides, S.; Chen, L. Effects of angiotensin-converting enzyme inhibition on exercise-induced angina and ST segment depression in patients with microvascular angina. J. Am. Coll. Cardiol. 1994, 23, 652–657. [Google Scholar] [CrossRef]
- Pauly, D.F.; Johnson, B.D.; Anderson, R.D.; Handberg, E.M.; Smith, K.M.; Cooper-DeHoff, R.M.; Sopko, G.; Sharaf, B.M.; Kelsey, S.F.; Merz, C.N.B.; et al. In women with symptoms of cardiac ischemia, nonobstructive coro-nary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with im-proved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood In-stitute Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart. J. 2011, 162, 678–684. [Google Scholar]
- Ong, P.; Athanasiadis, A.; Sechtem, U. Pharmacotherapy for coronary microvascular dysfunction. Eur. Heart J. Cardiovasc. Pharmacother. 2015, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.-C.; Jeon, J.-Y.; Kim, Y.; Kim, H.M.; Yoon, Y.E.; Lee, S.-P.; Kim, H.-K.; Sohn, D.-W.; Sung, J.; Kim, Y.-J. Statin therapy is associated with lower all-cause mortality in patients with non-obstructive coronary artery disease. Atherosclerosis 2015, 239, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Eshtehardi, P.; McDaniel, M.C.; Dhawan, S.S.; Binongo, J.N.G.; Krishnan, S.K.; Golub, L.; Corban, M.T.; Raggi, P.; Quyyumi, A.A.; Samady, H. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J. Invasive Cardiol. 2012, 24, 522–529. [Google Scholar]
- Zhang, X.; Li, Q.; Zhao, J.; Li, X.; Sun, X.; Yang, H.; Wu, Z.; Yang, J. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron. Artery Dis. 2014, 25, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Tian, J.; Yang, X.; Xing, H.; He, Y.; Song, X. Effects of Oral Drugs on Coronary Microvascular Function in Patients Without Significant Stenosis of Epicardial Coronary Arteries: A Systematic Review and Meta-Analysis of Coronary Flow Reserve. Front. Cardiovasc. Med. 2020, 7, 580419. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kaikita, K.; Sato, K.; Yamanaga, K.; Miyazaki, T.; Akasaka, T.; Tabata, N.; Arima, Y.; Sueta, D.; Sakamoto, K.; et al. Impact of Statin Therapy on Clinical Outcome in Patients with Coronary Spasm. J. Am. Heart Assoc. 2016, 5, e003426. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Jeon, S.Y.; Rha, S.; Park, S.; Shim, M.S.; Choi, S.Y.; Byun, J.K.; Li, H.; Choi, J.Y.; Park, E.J.; et al. Impact of Renin-Angiotensin System Inhibitors on Long-Term Clinical Outcomes of Patients with Coronary Artery Spasm. J. Am. Heart Assoc. 2016, 5, e003217. [Google Scholar] [CrossRef]
- Handberg, E.M.; Merz, C.N.B.; Cooper-Dehoff, R.M.; Wei, J.; Conlon, M.; Lo, M.C.; Boden, W.; Frayne, S.M.; Villines, T.; Spertus, J.A.; et al. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am. Heart J. 2021, 237, 90–103. [Google Scholar] [CrossRef]
- Gallinoro, E.; Paolisso, P.; Bertolone, D.T.; Belmonte, M.; Esposito, G.; Munhoz, D.; Sakai, K.; Bermpeis, K.; Ohashi, H.; Storozhenko, T.; et al. Endotypes of coronary microvascular dysfunction in patients with and without Diabetes Mellitus. Eur. Heart J. 2023, 44, 2. [Google Scholar] [CrossRef]
- Sara, J.D.; Taher, R.; Kolluri, N.; Vella, A.; Lerman, L.O.; Lerman, A. Coronary microvascular dysfunction is associated with poor glycemic control amongst female diabetics with chest pain and non-obstructive coronary artery disease. Cardiovasc. Diabetol. 2019, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Ferrell, W.; Greer, I.A.; Petrie, J.R.; Cobbe, S.M.; Sattar, N. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: A randomized, double-blind, placebo-controlled study. J. Am. Coll. Cardiol. 2006, 48, 956–963. [Google Scholar] [CrossRef]
- Yasue, H.; Omote, S.; Takizawa, A.; Nagao, M. Coronary arterial spasm in ischemic heart disease and its pathogenesis. A review. Circ. Res. 1983, 52, I147–I152. [Google Scholar] [PubMed]
- Galderisi, M.; D’Errico, A. Beta-blockers and coronary flow reserve: The importance of a vasodilatory action. Drugs 2008, 68, 579–590. [Google Scholar] [CrossRef]
- Kalinowski, L.; Dobrucki, L.W.; Szczepanska-Konkel, M.; Jankowski, M.; Martyniec, L.; Angielski, S.; Malinski, T. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: A novel mechanism for antihypertensive action. Circulation 2003, 107, 2747–2752. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; D’Errico, A.; Sidiropulos, M.; Innelli, P.; de Divitiis, O.; de Simone, G. Nebivolol induces parallel improvement of left ventricular filling pressure and coronary flow reserve in uncomplicated arterial hypertension. J. Hypertens. 2009, 27, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cicala, S.; D’Errico, A.; de Divitiis, O.; de Simone, G. Nebivolol improves coronary flow reserve in hypertensive patients with-out coronary heart disease. J. Hypertens. 2004, 22, 2201–2208. [Google Scholar] [CrossRef]
- Kook, H.; Hong, S.J.; Yang, K.-S.; Lee, S.; Kim, J.-S.; Park, C.G. Comparison of nebivolol versus diltiazem in improving coronary artery spasm and quality of life in patients with hypertension and vasospastic angina: A prospective, randomized, double-blind pilot study. PLoS ONE 2020, 15, e0239039. [Google Scholar] [CrossRef]
- Lanza, G.A.; Colonna, G.; Pasceri, V.; Maseri, A. Atenolol versus amlodipine versus isosorbide-5-mononitrate on anginal symptoms in syndrome X. Am. J. Cardiol. 1999, 84, 854–856, A8. [Google Scholar] [CrossRef] [PubMed]
- Di Franco, A.; Villano, A.; Di Monaco, A.; Lamendola, P.; Russo, G.; Stazi, A.; Scavone, G.; Nerla, R.; Sestito, A.; Lanza, G.A.; et al. Correlation between coronary microvascular function and angina status in patients with stable microvascular angina. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 374–379. [Google Scholar] [PubMed]
- Sütsch, G.; Oechslin, E.; Mayer, I.; Hess, O.M. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int. J. Cardiol. 1995, 52, 135–143. [Google Scholar] [CrossRef]
- Jansen, T.P.J.; Konst, R.E.; de Vos, A.; Paradies, V.; Teerenstra, S.; van den Oord, S.C.H.; Dimitriu-Leen, A.; Maas, A.H.E.M.; Smits, P.C.; Damman, P.; et al. Efficacy of Diltiazem to Improve Coronary Vasomotor Dysfunction in ANOCA. JACC Cardiovasc. Imaging 2022, 15, 1473–1484. [Google Scholar] [CrossRef]
- Lanza, G.A.; Shimokawa, H. Management of Coronary Artery Spasm. Eur. Cardiol. 2023, 18, e38. [Google Scholar] [CrossRef]
- Yasue, H.; Takizawa, A.; Nagao, M.; Nishida, S.; Horie, M.; Kubota, J.; Omote, S.; Takaoka, K.; Okumura, K. Long-term prognosis for patients with variant angina and influential factors. Circulation 1988, 78, 1–9. [Google Scholar] [CrossRef]
- Waters, D.D.; Miller, D.D.; Szlachcic, J.; Bouchard, A.; Méthé, M.; Kreeft, J.; Théroux, P. Factors influencing the long-term prognosis of treated patients with variant angina. Circulation 1983, 68, 258–265. [Google Scholar] [CrossRef]
- Nishigaki, K.; Inoue, Y.; Yamanouchi, Y.; Fukumoto, Y.; Yasuda, S.; Sueda, S.; Urata, H.; Shimokawa, H.; Mina-toguchi, S. Prognostic Effects of Calcium Channel Blockers in Patients with Vasospastic Angina—A Meta-Analysis. Circ. J. 2010, 74, 1943–1950. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Handberg, E.M.; Shufelt, C.L.; Mehta, P.K.; Minissian, M.B.; Wei, J.; Thomson, L.E.J.; Berman, D.S.; Shaw, L.J.; Petersen, J.W.; et al. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): Impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016, 37, 1504–1513. [Google Scholar] [CrossRef]
- Koh, J.-S.; Hung, O.Y.; Eshtehardi, P.; Kumar, A.; Rabah, R.; Raad, M.; Kumar, S.; Chaudhry, S.; Gupta, S.; Hosseini, H.; et al. Microvascular Assessment of Ranolazine in Non-Obstructive Atherosclerosis: The MARINA Randomized, Double-Blinded, Controlled Pilot Trial. Circ. Cardiovasc. Interv. 2020, 13, e008204. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Fu, S.; Xu, M.; Wang, B.; Li, B.; Li, Y.; Liu, X.; Zhang, X.; Wang, Q.; Li, A.; et al. Ranolazine for improving coronary microvascular function in patients with nonobstructive coronary artery disease: A systematic review and meta-analysis with a trial sequential analysis of randomized controlled trials. Quant. Imaging Med. Surg. 2024, 14, 1451–1465. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, A.A.; Giannoglou, G.D.; Chatzizisis, Y.S. Pharmacological approaches of refractory angina. Pharmacol. Ther. 2016, 163, 118–131. [Google Scholar] [CrossRef]
- Rogacka, D.; Guzik, P.; Wykretowicz, A.; Rzeźniczak, J.; Dziarmaga, M.; Wysocki, H. Effects of trimetazidine on clinical symptoms and tolerance of exercise of patients with syndrome X: A preliminary study. Coron. Artery Dis. 2000, 11, 171–177. [Google Scholar] [CrossRef]
- Nalbantgil, S.; Altintigbreve, A.; Yilmaz, H.; Nalbantgil, I.; Önder, R. The The Effect of Trimetazidine in the Treatment of Microvascular Angina. Int. J. Angiol. 1999, 8, 40–43. [Google Scholar] [CrossRef]
- Villano, A.; Di Franco, A.; Nerla, R.; Sestito, A.; Tarzia, P.; Lamendola, P.; Di Monaco, A.; Sarullo, F.M.; Lanza, G.A.; Crea, F. Effects of Ivabradine and Ranolazine in Patients with Microvascular An-gina Pectoris. Am. J. Cardiol. 2013, 112, 8–13. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, X.; Fang, X.; Zheng, J.; Zhao, Q.; Chen, T.; Huang, J. Effects of the Antianginal Drugs Ranolazine, Nicorandil, and Ivabradine on Coronary Microvascular Function in Patients with Nonobstructive Coronary Artery Disease: A Meta-analysis of Randomized Controlled Trials. Clin. Ther. 2019, 41, 2137–2152.e12. [Google Scholar] [CrossRef] [PubMed]
- Nakae, I.; Matsumoto, T.; Horie, H.; Yokohama, H.; Omura, T.; Minai, K.; Matsui, T.; Nozawa, M.; Takahashi, M.; Sugimoto, Y.; et al. Effects of intravenous nicorandil on coronary circulation in humans: Plasma concentration and action mechanism. J. Cardiovasc. Pharmacol. 2000, 35, 919–925. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Takahashi, N.; Tokumaru, A.; Karino, K.; Sugamori, T.; Sakane, T.; Yoshitomi, H.; Sato, H.; Oyake, N.; Murakami, Y.; et al. Effects of Long-term Nicorandil Administration on Endothelial Function, Inflammation, and Oxidative Stress in Patients Without Coronary Artery Disease. J. Cardiovasc. Pharma-Col. 2008, 51, 311–316. [Google Scholar] [CrossRef]
- Jia, Q.; Shi, S.; Yuan, G.; Shi, J.; Shi, S.; Wei, Y.; Hu, Y. The effect of nicorandil in patients with cardiac syndrome X. Medicine 2020, 99, e22167. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Villano, A.; Russo, G.; Di Franco, A.; Stazi, A.; Lauria, C.; Sestito, A.; Lanza, G.A.; Crea, F. Poor tolerance and limited effects of isosorbide-5-mononitrate in microvascular angina. Cardiology 2015, 130, 201–206. [Google Scholar] [CrossRef]
- Russo, G.; Di Franco, A.; Lamendola, P.; Tarzia, P.; Nerla, R.; Stazi, A.; Villano, A.; Sestito, A.; Lanza, G.A.; Crea, F. Lack of Effect of Nitrates on Exercise Stress Test Results in Patients with Microvascular Angina. Cardiovasc. Drugs Ther. 2013, 27, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Seitz, A.; Feenstra, R.; Konst, R.E.; Martínez Pereyra, V.; Beck, S.; Beijk, M.; van de Hoef, T.; van Royen, N.; Bekere-djian, R.; Sechtem, U.; et al. Acetylcholine Rechallenge: A First Step Toward Tailored Treatment in Patients with Coronary Artery Spasm. JACC Cardiovasc. Interv. 2022, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Nihei, T.; Takagi, Y.; Miyata, S.; Odaka, Y.; Tsunoda, R.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: Multicentre registry study of the Japanese coronary spasm association. Eur. Heart J. 2015, 36, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, N.R.; Prasad, M.; Widmer, R.J.; Toleva, O.; Quesada, O.; Sutton, N.R.; Lerman, A.; Reynolds, H.R.; Kesarwani, M.; Savage, M.P.; et al. Comprehensive Management of ANOCA, Part 2—Program Development, Treatment, and Research Initiatives. J. Am. Coll. Cardiol. 2023, 82, 1264–1279. [Google Scholar] [CrossRef]
- Giannini, F.; Tzanis, G.; Ponticelli, F.; Baldetti, L.; Demir, O.M.; Mitomo, S.; Gallone, G.; Banai, S.; Colombo, A. Technical aspects in coronary sinus Reducer implantation. EuroIntervention 2020, 15, 1269–1277. [Google Scholar] [CrossRef]
- Servoz, C.; Verheye, S.; Giannini, F.; Banai, S.; Fradi, M.; Cuenin, L.; Bellemain-Appaix, A.; Gilard, M.; Benamer, H.; Adjedj, J. Impact of coronary sinus reducer on absolute coronary blood flow and microvascular resistance. Catheter. Cardiovasc. Interv. 2024, 104, 58–60. [Google Scholar] [CrossRef]
- Verheye, S.; Jolicœur, E.M.; Behan, M.W.; Pettersson, T.; Sainsbury, P.; Hill, J.; Vrolix, M.; Agostoni, P.; Engstrom, T.; Labinaz, M.; et al. Efficacy of a Device to Narrow the Coronary Sinus in Refractory Angina. N. Engl. J. Med. 2015, 372, 519–527. [Google Scholar] [PubMed]
- Foley, M.J.; Rajkumar, C.A.; Ahmed-Jushuf, F.; Simader, F.A.; Chotai, S.; Pathimagaraj, R.H.; Mohsin, M.; Salih, A.; Wang, D.; Dixit, P.; et al. Coronary sinus reducer for the treatment of refractory angina (ORBITA-COSMIC): A randomised, placebo-controlled trial. Lancet 2024, 403, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Konigstein, M.; Ponticelli, F.; Zivelonghi, C.; Merdler, I.; Revivo, M.; Verheye, S.; Giannini, F.; Banai, S. Long. Long-term outcomes of patients undergoing coronary sinus reducer implantation—A multicenter study. Clin. Cardiol. 2021, 44, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Keramida, G.; Baksi, A.J.; de Silva, R. Implantation of the coronary sinus reducer for refractory angina due to coronary microvascular dysfunction in the context of apical hypertrophic cardiomyopathy—A case report. Eur. Heart J. Case Rep. 2022, 6, ytac440. [Google Scholar] [CrossRef]
- Ullrich, H.; Hammer, P.; Olschewski, M.; Münzel, T.; Escaned, J.; Gori, T. Coronary Venous Pressure and Microvascular Hemodynamics in Patients with Microvascular Angina. JAMA Cardiol. 2023, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Giannini, F.; Baldetti, L.; Ielasi, A.; Ruparelia, N.; Ponticelli, F.; Latib, A.; Mitomo, S.; Esposito, A.; Palmisano, A.; Chieffo, A.; et al. First Experience with the Coronary Sinus Reducer System for the Management of Refractory Angina in Patients Without Obstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2017, 10, 1901–1903. [Google Scholar] [CrossRef] [PubMed]
- Caceres, J.; Atal, P.; Arora, R.; Yee, D. Enhanced external counterpulsation: A unique treatment for the “No-Option” refractory angina patient. J. Clin. Pharm. Ther. 2021, 46, 295–303. [Google Scholar] [CrossRef]
- Lee, J.; Oh, J.; Kim, I.-C.; Lee, S.; Kim, S.-E.; Lee, C.J.; Kim, H.; Kang, S.-M. Prospective Cohort Study for Evaluating the Safety and Efficacy of Mobile, Motorized Enhanced Extracorporeal Counterpulsation in Patients with Refractory Angina. Am. J. Cardiol. 2024, 213, 106–109. [Google Scholar] [CrossRef]
- Wang, Q.; Hao, J.; Jiang, W.; Tan, Q. Enhanced external counterpulsation increases coronary flow reserve in coronary microvascular disease. Saudi Med. J. 2023, 44, 1277–1282. [Google Scholar] [CrossRef]
- Cox, I. Low dose imipramine improves chest pain but not quality of life in patients with angina and normal coronary angiograms. Eur. Heart J. 1998, 19, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Jessurun, G.A.J.; Hautvast, R.W.M.; Tio, R.A.; DeJongste, M.J.L. Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: Fad or future? Eur. J. Pain 2003, 7, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Sestito, A.; Sgueglia, G.A.; Infusino, F.; Papacci, F.; Visocchi, M.; Ierardi, C.; Meglio, M.; Bellocci, F.; Crea, F. Effect of spinal cord stimulation on spontaneous and stress-induced angina and ‘ischemia-like’ ST-segment depression in patients with cardiac syndrome X. Eur. Heart J. 2005, 26, 983–989. [Google Scholar] [CrossRef][Green Version]
- Eliasson, T.; Albertsson, P.; Hårdhammar, P.; Emanuelsson, H.; Augustinsson, L.E.; Mannheimer, C. Spinal cord stimulation in angina pectoris with normal coronary arteriograms. Coron. Artery Dis. 1993, 4, 819–827. [Google Scholar] [PubMed]
- Masumoto, A.; Mohri, M.; Shimokawa, H.; Urakami, L.; Usui, M.; Takeshita, A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002, 105, 1545–1547. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Ibuki, C.; Suzuki, T.; Ishii, K.; Yoshida, H.; Kodani, E.; Kusama, Y.; Atarashi, H.; Kishida, H.; Takano, T.; et al. Administration of the Rho-kinase inhibitor, fasudil, following nitroglycerin additionally dilates the site of coronary spasm in patients with vasospastic angina. Coron. Artery Dis. 2008, 19, 105–110. [Google Scholar] [CrossRef]
- English, K.M.; Steeds, R.P.; Jones, T.H.; Diver, M.J.; Channer, K.S. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina: A randomized, double-blind, placebo-controlled study. Circulation 2000, 102, 1906–1911. [Google Scholar] [CrossRef]
- Merz, C.N.B.; Olson, M.B.; McClure, C.; Yang, Y.-C.; Symons, J.; Sopko, G.; Kelsey, S.F.; Handberg, E.; Johnson, B.D.; Cooper-DeHoff, R.M.; et al. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: Results from the National Institutes of Health/National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J. 2010, 159, 987.e1–987.e7. [Google Scholar] [PubMed]
- Morrow, A.; Young, R.; Abraham, G.R.; Hoole, S.; Greenwood, J.P.; Arnold, J.R.; El Shibly, M.; Shanmuganathan, M.; Ferreira, V.; Rakhit, R.; et al. Zibotentan in Microvascular Angina: A Randomized, Placebo-Controlled, Crossover Trial. Circulation. 2024, 150, 1671–1683. [Google Scholar] [CrossRef]
- Henry, T.D.; Bairey Merz, C.N.; Wei, J.; Corban, M.T.; Quesada, O.; Joung, S.; Kotynski, C.L.; Wang, J.; Lewis, M.; Schumacher, A.M.; et al. Autologous CD34+ Stem Cell Therapy Increases Coronary Flow Reserve and Reduces Angina in Patients with Coronary Microvascular Dysfunction. Circ. Cardiovasc. Interv. 2022, 15, e010802. [Google Scholar] [CrossRef]
- Corban, M.T.; Toya, T.; Albers, D.; Sebaali, F.; Lewis, B.R.; Bois, J.; Gulati, R.; Prasad, A.; Best, P.J.M.; Bell, M.R.; et al. IMPROvE-CED Trial: Intracoronary Autologous CD34+ Cell Therapy for Treatment of Coronary Endothelial Dysfunction in Patients with Angina and Nonobstructive Coronary Arteries. Circ. Res. 2022, 130, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Denardo, S.J.; Wen, X.; Handberg, E.M.; Bairey Merz, C.N.; Sopko, G.S.; Cooper-DeHoff, R.M.; Pepine, C.J. Effect of Phosphodiesterase Type 5 Inhibition on Microvascular Coronary Dysfunction in Women: A Women’s Ischemia Syndrome Evaluation (WISE) Ancillary Study. Clin. Cardiol. 2011, 34, 483–487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).