Detailed Insights into the Relationship Between Three-Dimensional Speckle-Tracking Echocardiography-Derived Systolic Left Atrial Global Strains and Left Ventricular Volumes in Healthy Adults from the MAGYAR-Healthy Study
Abstract
:1. Introduction
2. Methods
2.1. Subject Population
2.2. Two-Dimensional Doppler Echocardiography
2.3. Three-Dimensional Speckle-Tracking Echocardiography--Derived Data Acquisition
2.4. Three-Dimensional Speckle-Tracking Echocardiography-Derived LV Volumetric Measurements
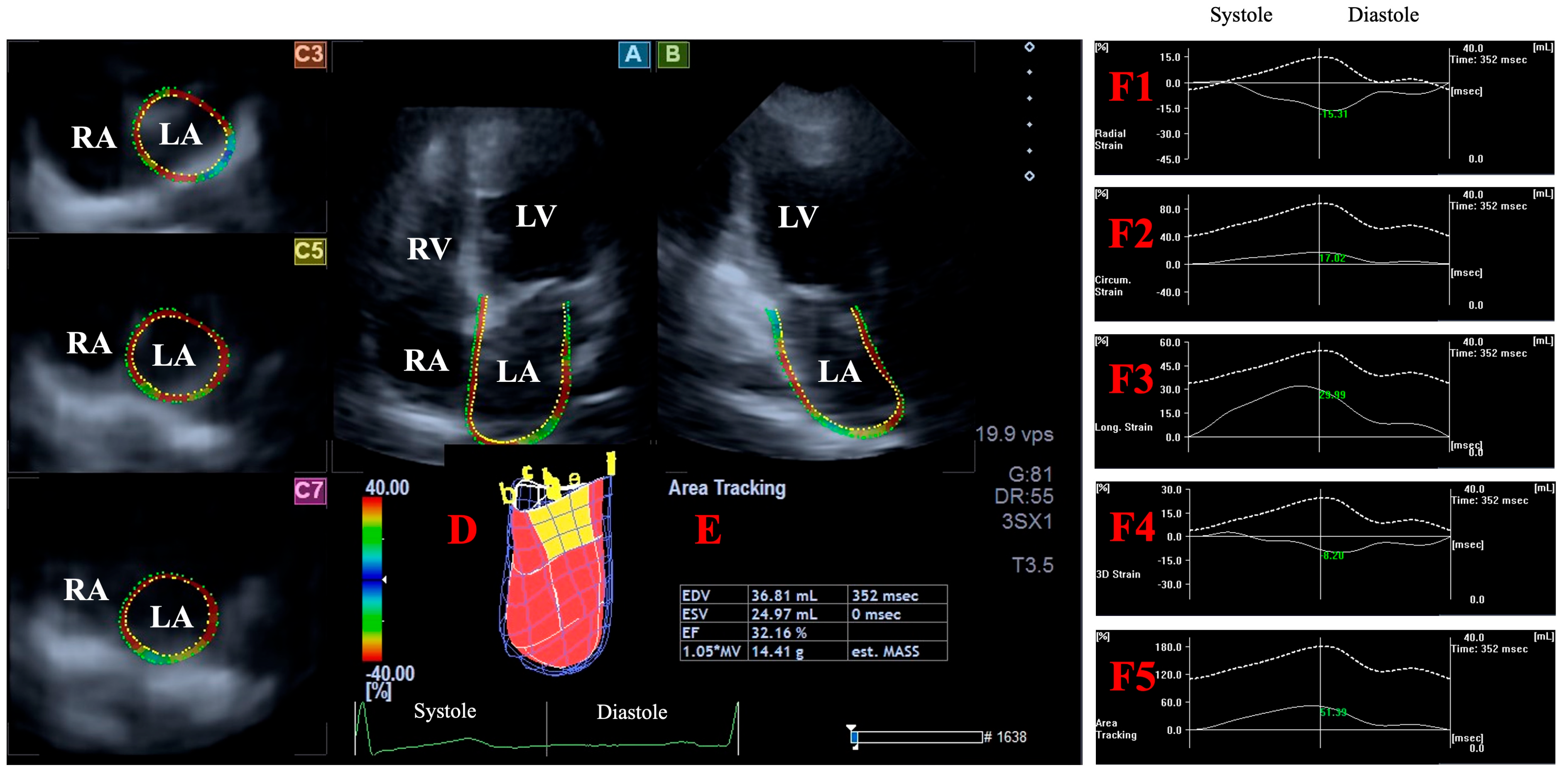
2.5. Three-Dimensional Speckle-Tracking Echocardiography-Derived Assessment of Peak LA Global Strains
- LA-Vmax—maximum LA volume at end-systole;
- LA-VpreA—pre-atrial contraction LA volume at early diastole;
- LA-Vmin—minimum LA volume at end-diastole;
- LA-GRS—LA global radial strain representing LA thinning/thickening;
- LA-GCS—LA global circumferential strain representing LA widening/narrowing;
- LA-GLS—LA global longitudinal strain representing LA lengthening/shortening;
- LA-G3DS—LA global 3D strain, combination of all unidirectional LA strains;
- LA-GAS—LA global area strain, combination of LA circumferential and longitudinal strains.
2.6. Statistical Analysis
3. Results
3.1. Two-Dimensional Echocardiography with Doppler
3.2. Classification of Subjects
3.3. Higher LV Volumes vs. Other LV and LA Volumetric Parameters
3.4. Higher LV Volumes vs. Peak LA Global Strains
3.5. Higher Peak LA Global Strains vs. LV and LA Volumes
3.6. Higher Peak LA Global Strains vs. Other Peak LA Global Strains
3.7. Reproducibility of 3DSTE-Derived Peak LA Strains and LV Volumes
4. Discussion
Limitations Section
- − For optimal image quality, six wedge-shaped subvolumes during six cardiac cycles were acquired. However, this technical requirement required great care, which may have affected the image quality.
- − All the subjects were considered to be healthy, but it could not be excluded with 100 percent certainty that the examined adults did not have an undiagnosed latent disease. Further examinations could have strengthened our findings.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dell’Angela, L.; Nicolosi, G.L. From ejection fraction, to myocardial strain, and myocardial work in echocardiography: Clinical impact and controversies. Echocardiography 2024, 41, e15758. [Google Scholar] [CrossRef] [PubMed]
- Akbalaeva, B.; Raiimbek Uulu, N.; Gulamov, I.; Abylov, K.; Pershukov, I. Speckle-tracking echocardiography: A tool for early detection of cardiotoxicity in cancer patients after chemotherapy. Acta Cardiol. 2024, 79, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kagami, K.; Kato, T.; Obokata, M. Echocardiography in the diagnostic evaluation and phenotyping of heart failure with preserved ejection fraction. J. Cardiol. 2022, 79, 679–690. [Google Scholar] [CrossRef]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial mechanics: Understanding and applying three-dimensional speckle tracking echocardiography in clinical practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef]
- Urbano-Moral, J.A.; Patel, A.R.; Maron, M.S.; Arias-Godinez, J.A.; Pandian, N.G. Three-dimensional speckle-tracking echocardiography: Methodological aspects and clinical potential. Echocardiography 2012, 29, 997–1010. [Google Scholar] [CrossRef]
- Muraru, D.; Niero, A.; Rodriguez-Zanella, H.; Cherata, D.; Badano, L. Three-dimensional speckle-tracking echocardiography: Benefits and limitations of integrating myocardial mechanics with three-dimensional imaging. Cardiovasc. Diagn. Ther. 2018, 8, 101–117. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Y.; Ji, M.; Wu, W.; Li, H.; Qian, M.; Zhang, L.; Xie, M.; Li, Y. Clinical Utility of Three-Dimensional Speckle-Tracking Echocardiography in Heart Failure. J. Clin. Med. 2022, 11, 6307. [Google Scholar] [CrossRef]
- Franke, A.; Kuhl, H.P. Second-generation real-time 3D echocardiography: A revolutionary new technology. MedicaMundi 2003, 47, 34–40. [Google Scholar]
- Nemes, A.; Kormányos, Á.; Ruzsa, Z.; Achim, A.; Ambrus, N.; Lengyel, C. Simultaneously Assessed Three-Dimensional Speckle-Tracking Echocardiography-Derived Left Ventricular and Left Atrial Volumes Are Associated in Healthy Adults-Findings from the MAGYAR-Healthy Study. J. Clin. Med. 2023, 12, 4153. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Gyenes, N.; Lengyel, C. Correlations between left atrial volumes and strains in healthy adults: Detailed analysis from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. J. Clin. Ultrasound 2021, 49, 650–658. [Google Scholar] [CrossRef]
- Blume, G.G.; Mcleod, C.J.; Barnes, M.E.; Seward, J.B.; Pellikka, P.A.; Bastiansen, P.M.; Tsang, T.S.M. Left atrial function: Physiology, assessment, and clinical implications. Eur. J. Echocardiogr. 2011, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Hoit, B.D. Left atrial size and function: Role in prognosis. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.J.; Teixeira, R.; Golçalves, L.; Gersch, B.J. Left atrial mechanics: Echocardiographic assessment and clinical implications. J. Am. Soc. Echocardiogr. 2014, 27, 463–478. [Google Scholar] [CrossRef]
- Narang, A.; Addetia, K. An introduction to left ventricular strain. Curr. Opin. Cardiol. 2018, 33, 455–463. [Google Scholar] [CrossRef]
- Berman, M.N.; Tupper, C.; Bhardwaj, A. Physiology, Left Ventricular Function. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1-39.e14. [Google Scholar] [CrossRef]
- Kormányos, Á.; Kalapos, A.; Domsik, P.; Gyenes, N.; Lengyel, C.; Nemes, A. Normal reference values of left ventricular volumetric parameters in healthy adults-real-life single-center experience from the three-dimensional speckle-tracking echocardiographic MAGYAR-Healthy Study. Quant. Imaging Med. Surg. 2021, 11, 1496–1503. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Ambrus, N.; Lengyel, C. Normal reference values of left atrial volumes and volume-based functional properties using three-dimensional speckle-tracking echocardiography in healthy adults (Insights from the MAGYAR-Healthy Study). J. Clin. Ultrasound 2021, 49, 49–55. [Google Scholar] [CrossRef]
- Nemes, A.; Kormányos, Á.; Domsik, P.; Kalapos, A.; Lengyel, C.; Forster, T. Normal reference values of three-dimensional speckle-tracking echocardiography-derived left atrial strain parameters (results from the MAGYAR-Healthy Study). Int. J. Cardiovasc. Imaging 2019, 35, 991–998. [Google Scholar] [CrossRef]
- Badano, L.P.; Nour, A.; Muraru, D. Left atrium as a dynamic three-dimensional entity: Implications for echocardiographic assessment. Rev. Esp. Cardiol. 2013, 66, 1–4. [Google Scholar] [CrossRef]
- O’Neill, T.; Kang, P.; Hagendorff, A.; Tayal, B. The Clinical Applications of Left Atrial Strain: A Comprehensive Review. Medicina 2024, 60, 693. [Google Scholar] [CrossRef]
- Han, P.L.; Shen, M.T.; Jiang, Y.; Jiang, Z.K.; Li, K.; Yang, Z.G. Prognostic Value of Left Atrial Reservoir Strain in Left Ventricular Myocardial Noncompaction: A 3.0 T Cardiac Magnetic Resonance Feature Tracking Study. J. Magn. Reson. Imaging 2023, 57, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Nesser, H.J.; Mor-Avi, V.; Gorissen, W.; Weinert, L.; Steringer-Mascherbauer, R.; Niel, J.; Sugeng, L.; Lang, R.M. Quantification of left ventricular volumes using three-dimensional echocardiographic speckle tracking: Comparison with MRI. Eur. Heart J. 2009, 30, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, S.A.; Brouwer, W.P.; Aly, M.F.A.; Rüssel, I.K.; de Roest, G.J.; Beek, A.M.; van Rossum, A.C.; Kamp, O. Comparison between three-dimensional speckle-tracking echocardiography and cardiac magnetic resonance imaging for quantification of left ventricular volumes and function. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Comparison between direct volumetric and speckle tracking methodologies for left ventricular and left atrial chamber quantification by three-dimensional echocardiography. Am. J. Cardiol. 2011, 108, 1038–1044. [Google Scholar] [CrossRef]
- Kleijn, S.A.; Aly, M.F.A.; Terwee, C.B.; van Rossum, A.C.; Kamp, O. Reliability of left ventricular volumes and function measurements using three-dimensional speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 159–168. [Google Scholar] [CrossRef]
- Nagaya, M.; Kawasaki, M.; Tanaka, R.; Onishi, N.; Sato, N.; Ono, K.; Watanabe, T.; Minatoguchi, S.; Miwa, H.; Goto, Y.; et al. Quantitative validation of left atrial structure and function by two-dimensional and three-dimensional speckle tracking echocardiography: A comparative study with three-dimensional computed tomography. J. Cardiol. 2013, 62, 188–194. [Google Scholar] [CrossRef]
- Mochizuki, A.; Yuda, S.; Oi, Y.; Kawamukai, M.; Nishida, J.; Kouzu, H.; Muranaka, A.; Kokubu, N.; Shimoshige, S.; Hashimoto, A.; et al. Assessment of left atrial deformation and synchrony by three-dimensional speckle-tracking echocardiography: Comparative studies in healthy subjects and patients with atrial fibrillation. J. Am. Soc. Echocardiogr. 2013, 26, 165–174. [Google Scholar] [CrossRef]
- Dini, F.L.; Cameli, M.; Stefanini, A.; Aboumarie, H.O.; Lisi, M.; Lindqvist, P.; Henein, M.Y. Echocardiography in the Assessment of Heart Failure Patients. Diagnostics 2024, 14, 2730. [Google Scholar] [CrossRef]
- O’Donnell, C.; Sanchez, P.A.; Celestin, B.; McConnell, M.V.; Haddad, F. The Echocardiographic Evaluation of the Right Heart: Current and Future Advances. Curr. Cardiol. Rep. 2023, 25, 1883–1896. [Google Scholar] [CrossRef]
- Tadic, M. The right atrium, a forgotten cardiac chamber: An updated review of multimodality imaging. J. Clin. Ultrasound 2015, 43, 335–345. [Google Scholar] [CrossRef]
- Xu, N.; Cheng, X.; Ren, L.; Yuan, Q. Application prospect of speckle tracking echocardiography in pacemaker implantation. Front. Cardiovasc. Med. 2025, 11, 1484520. [Google Scholar] [CrossRef]

| Parameters | Measures |
|---|---|
| Left ventricular volumes | |
| end-diastolic left ventricular volume (LV-EDV) | 86.0 ± 22.8 |
| end-systolic left ventricular volume (LV-ESV) | 36.2 ± 10.4 |
| left ventricular ejection fraction (LV-EF) | 58.2 ± 5.6 |
| left ventricular mass (g) | 158.9 ± 32.0 |
| Peak left atrial (reservoir) global strains | |
| peak left atrial global radial strain (LA-GRS, %) | −14.8 ± 8.1 |
| peak left atrial global circumferential strain (LA-GCS, %) | 33.6 ± 15.3 |
| peak left atrial global longitudinal strain (LA-GLS, %) | 26.3 ± 9.1 |
| peak left atrial global 3D strain (LA-G3DS, %) | −7.5 ± 5.9 |
| peak left atrial global area strain (LA-GAS, %) | 67.5 ± 28.1 |
| LV-EDV ≤ 63.2 mL (n = 19) | 63.2 mL < LV-EDV < 108.8 mL (n = 128) | 108.8 mL ≤ LV-EDV (n = 18) | LV-ESV ≤ 25.8 mL (n = 22) | 25.8 mL < LV-ESV < 46.6 mL (n = 121) | 46.6 mL ≤ LV-ESV (n = 22) | |
|---|---|---|---|---|---|---|
| LV-EDV (mL) | 53.6 ± 12.3 | 84.7 ± 11.9 * | 131.2 ± 19.6 *† | 64.1 ± 14.2 | 83.6 ± 12.8 # | 125.0 ± 22.1 #‡ |
| LV-ESV (mL) | 23.9 ± 5.7 | 35.4 ± 6.6 * | 56.1 ± 8.9 *† | 21.8 ± 3.3 | 35.4 ± 5.5 # | 54.9 ± 8.1 #‡ |
| LV-EF (%) | 58.7 ± 8.0 | 58.2 ± 5.4 | 57.1 ± 4.3 | 65.4 ± 6.1 | 57.4 ± 5.0 # | 55.7 ± 3.9 # |
| LV mass (g) | 133.3 ± 23.7 | 157.6 ± 28.8 * | 196.2 ± 29.5 *† | 134.5 ± 25.2 | 157.2 ± 28.3 # | 191.9 ± 29.4 #‡ |
| LA-Vmax (mL) | 33.2 ± 9.8 | 40.7 ± 12.7 * | 49.0 ± 14.0 *† | 34.1 ± 12.0 | 40.7 ± 12.1 # | 50.0 ± 15.0 #‡ |
| LA-VpreA (mL) | 24.3 ± 9.0 | 27.3 ± 11.6 | 33.3 ± 13.9 *† | 23.8 ± 9.5 | 27.4 ± 11.3 | 34.5 ± 14.2 #‡ |
| LA-Vmin (mL) | 18.1 ± 6.4 | 19.2 ± 8.3 | 21.9 ± 8.2 | 16.5 ± 5.2 | 19.1 ± 7.8 | 23.7 ± 10.0 #‡ |
| LA-GRS (%) | −10.0 ± 5.7 | −15.5 ± 8.3 * | −15.3 ± 7.4 * | −12.2 ± 9.3 | −15.5 ± 8.1 | −14.7 ± 7.3 |
| LA-GCS (%) | 29.1 ± 16.0 | 33.3 ± 14.4 | 41.2 ± 18.2 | 32.5 ± 22.2 | 33.6 ± 13.7 | 36.2 ± 18.6 |
| LA-GLS (%) | 21.2 ± 6.0 | 26.9 ± 9.3 * | 30.1 ± 7.1 * | 25.4 ± 10.1 | 26.5 ± 8.7 | 26.3 ± 10.6 |
| LA-G3DS (%) | −3.4 ± 2.7 | −7.9 ± 6.0 * | −8.3 ± 6.0 * | −4.6 ± 4.3 | −7.9 ± 6.1# | −8.4 ± 5.9 # |
| LA-GAS (%) | 58.2 ± 29.4 | 67.0 ± 26.7 | 79.1 ± 34.8 * | 65.9 ± 38.3 | 67.1 ± 25.3 | 74.3 ± 35.9 |
| LA-GRS ≤ −6.7% (n = 24) | −6.7% < LA-GRS < −22.9% (n = 121) | −22.9% ≤ LA-GRS (n = 20) | LA-GCS ≤ 18.3% (n = 25) | 18.3% < LA-GCS < 48.9% (n = 112) | 48.9% ≤ LA-GCS (n = 28) | LA-GLS ≤ 17.2% (n = 25) | 17.2% < LA-GLS < 35.4% (n = 115) | 35.4% ≤ LA-GLS (n = 25) | |
|---|---|---|---|---|---|---|---|---|---|
| LV-EDV (mL) | 82.3 ± 25.3 | 86.1 ± 21.2 | 93.3 ± 22.1 | 79.5 ± 24.8 | 86.7 ± 21.0 | 91.5 ± 22.5 | 86.3 ± 24.5 | 86.1 ± 22.6 | 88.2 ± 16.4 |
| LV-ESV (mL) | 34.4 ± 12.3 | 36.0 ± 9.5 | 39.6 ± 12.6 | 32.1 ± 11.3 | 37.0 ± 9.5 ‡ | 36.7 ± 12.2 | 37.6 ± 11.5 | 35.8 ± 10.6 | 37.0 ± 8.2 |
| LV-EF (%) | 58.9 ± 5−7 | 58.1 ± 5.2 | 57.8 ± 7.9 | 60.2 ± 5.6 | 57.2 ± 5.0 | 60.1 ± 7.0 | 57.1 ± 5.9 | 58.4 ± 5.8 | 58.1 ± 4.8 |
| LV mass (g) | 154.9 ± 31.3 | 157.0 ± 31.5 | 175.1 ± 32.9 *† | 156.8 ± 25.2 | 159.5 ± 32.4 | 158.2 ± 36.5 | 166.0 ± 29.9 | 159.6 ± 31.5 | 148.5 ± 35.1 $ |
| LA-Vmax (mL) | 35.5 ± 13.2 | 41.5 ± 12.9 * | 42.8 ± 13.2 | 38.4 ± 11.7 | 41.6 ± 13.0 | 39.8 ± 14.5 | 39.8 ± 15.6 | 41.1 ± 13.1 | 40.0 ± 10.0 |
| LA-VpreA (mL) | 26.1 ± 11.3 | 27.6 ± 12.1 | 29.3 ± 10.5 | 30.1 ± 10.4 | 28.0 ± 11.6 | 23.8 ± 13.2 ‡ | 31.2 ± 14.3 | 27.7 ± 11.5 | 23.4 ± 8.8 $ |
| LA-Vmin (mL) | 20.2 ± 8.7 | 19.1 ± 8.3 | 19.0 ± 6.5 | 24.5 ± 8.3 | 19.6 ± 7.9 ‡ | 13.7 ± 5.2 ‡# | 24.0 ± 11.6 | 19.2 ± 7.2 $ | 14.9 ± 5.1 $& |
| LA-GRS (%) | −3.0 ± 2.3 | −14.8 ± 4.7 * | −28.9 ± 6.6 *† | −10.0 ± 7.0 | −15.1 ± 8.0 ‡ | −18.2 ± 7.7 ‡# | −10.2 ± 8.8 | −15.6 ± 8.1 $ | −15.7 ± 5.6 $ |
| LA-GCS (%) | 24.2 ± 10.6 | 34.7 ± 15.3 * | 37.6 ± 16.4 * | 13.6 ± 3.6 | 31.7 ± 7.8 ‡ | 59.0 ± 10.6 ‡# | 21.9 ± 11.9 | 33.9 ± 14.3 $ | 43.6 ± 15.5 $& |
| LA-GLS (%) | 19.3 ± 8.6 | 27.6 ± 8.8 * | 26.2 ± 8.0 * | 17.8 ± 5.5 | 26.7 ± 8.2 ‡ | 31.9 ± 9.7 ‡# | 13.2 ± 3.3 | 25.9 ± 4.8 $ | 41.0 ± 6.1 $& |
| LA-G3DS (%) | −0.9 ± 1.1 | −7.5 ± 4.3 * | −14.9 ± 8.2 *† | −4.4 ± 4.1 | −8.1 ± 6.4 ‡ | −7.7 ± 4.2 ‡ | −4.3 ± 5.5 | −7.8 ± 6.0 $ | −9.0 ± 4.4 $ |
| LA-GAS (%) | 48.0 ± 25.1 | 70.9 ± 26.9 * | 69.8 ± 30.5 * | 31.7 ± 8.4 | 65.1 ± 17.9 ‡ | 109.1 ± 20.4 ‡# | 35.2 ± 16.6 | 67.3 ± 22.1 $ | 100.5 ± 24.0 $& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes, A.; Ambrus, N.; Lengyel, C. Detailed Insights into the Relationship Between Three-Dimensional Speckle-Tracking Echocardiography-Derived Systolic Left Atrial Global Strains and Left Ventricular Volumes in Healthy Adults from the MAGYAR-Healthy Study. J. Clin. Med. 2025, 14, 1143. https://doi.org/10.3390/jcm14041143
Nemes A, Ambrus N, Lengyel C. Detailed Insights into the Relationship Between Three-Dimensional Speckle-Tracking Echocardiography-Derived Systolic Left Atrial Global Strains and Left Ventricular Volumes in Healthy Adults from the MAGYAR-Healthy Study. Journal of Clinical Medicine. 2025; 14(4):1143. https://doi.org/10.3390/jcm14041143
Chicago/Turabian StyleNemes, Attila, Nóra Ambrus, and Csaba Lengyel. 2025. "Detailed Insights into the Relationship Between Three-Dimensional Speckle-Tracking Echocardiography-Derived Systolic Left Atrial Global Strains and Left Ventricular Volumes in Healthy Adults from the MAGYAR-Healthy Study" Journal of Clinical Medicine 14, no. 4: 1143. https://doi.org/10.3390/jcm14041143
APA StyleNemes, A., Ambrus, N., & Lengyel, C. (2025). Detailed Insights into the Relationship Between Three-Dimensional Speckle-Tracking Echocardiography-Derived Systolic Left Atrial Global Strains and Left Ventricular Volumes in Healthy Adults from the MAGYAR-Healthy Study. Journal of Clinical Medicine, 14(4), 1143. https://doi.org/10.3390/jcm14041143







