Clinical Outcomes of Machine Perfusion and Temperature Control Systems in Heart Transplantation: Where We Stand
Abstract
1. Introduction
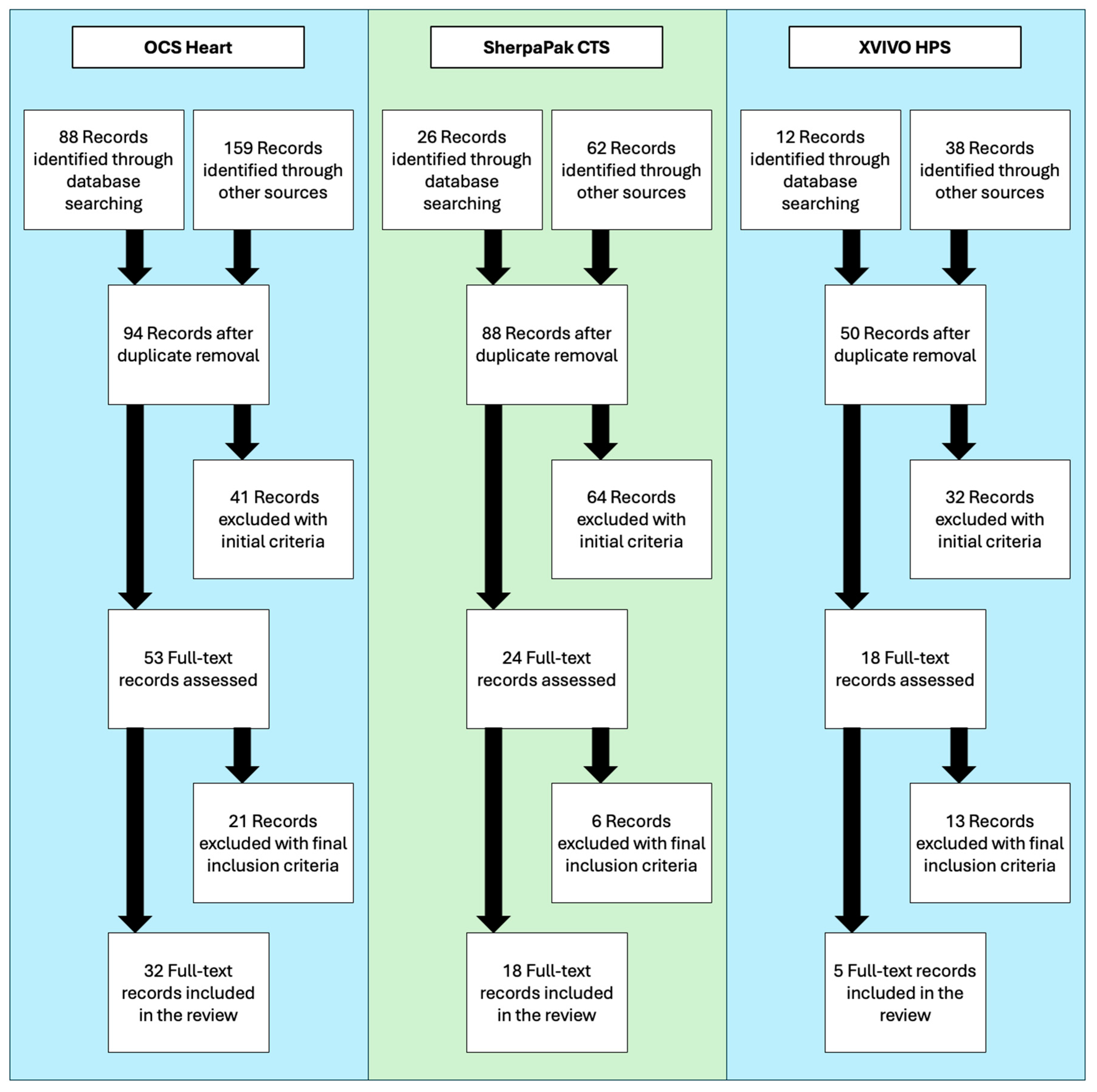
2. Methods
2.1. Literature Search
2.2. Selection Criteria
3. TransMedics Organ Care System Heart
3.1. Clinical Trials Evaluating OCS Heart
3.1.1. PROCEED II Trial
3.1.2. EXPAND and EXPAND-CAP Trial
3.1.3. DCD Heart Trial
3.1.4. Limitations of the OCS Heart Clinical Trials
3.2. Single-Center Experience with the OCS Heart
3.2.1. High-Risk Donor and Recipient Profiles
3.2.2. DCD Heart Transplantation
3.2.3. Advanced Use of the OCS Heart
3.3. Conclusions and Remaining Questions with the OCS Heart
4. SherpaPak Cardiac Transport System
4.1. GUARDIAN-Heart Registry
4.1.1. Primary Outcomes of the GUARDIAN-Heart Registry
4.1.2. High-Risk Donor and Recipient Profiles
4.1.3. Limitations of the SCTS GUARDIAN-Heart Registry
4.2. Single-Center Experience with the SCTS
4.3. Conclusion and Remaining Questions with the SCTS
5. XVIVO Heart Preservation System
5.1. Clinical Trials Evaluating the XHPS
5.1.1. NIHP Trial
5.1.2. HOPE Trial
5.1.3. NIHP 2019 Trial
5.1.4. Ongoing Clinical Trials
5.1.5. Limitations of the XHPS Clinical Trials
5.2. Single Center Experiences with the XHPS
5.3. Conclusion and Remaining Questions with the XHPS
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AOR | Adjusted odds ratio |
| BTT | Bridge to transplant |
| CAV | Cardiac allograft vasculopathy |
| CFDD-DNA | Cell-free donor-derived deoxyribonucleic acid |
| CE | European Conformity |
| CI | Confidence interval |
| DBD | Donation after brain death |
| DCD | Donation after circulatory death |
| ECD | Extended criteria donors |
| ECMO | Extracorporeal membrane oxygenation |
| EXPAND | Expanded Criteria Donor Hearts for Transplantation |
| EXPAND-CAP | Expanded Criteria Donor Hearts for Transplantation Continued Access Protocol |
| FDA | Food and Drug Administration |
| GUARDIAN | Global Utilization And Registry Database for Improved heArt preservation |
| HOPE | Hypothermic oxygenation machine perfusion |
| IABP | Intra-aortic balloon pump |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| IVUS | Intravascular ultrasound |
| ISHLT | International Society of Heart and Lung Transplantation |
| LVAD | Left ventricular assist device |
| MCS | Mechanical circulatory support |
| MRI | Magnetic resonance imaging |
| NF-MACE | non-fatal major adverse cardiovascular event |
| NIHP | Nonischemic heart preservation |
| OCS | Organ care system |
| OR | Odds ratio |
| PGD | Primary graft dysfunction |
| PROCEED-II | Ex vivo Perfusion of Donor Hearts for Human Heart Transplantation |
| RR | Risk ratio |
| SCS | Static cold storage |
| SCTS | SherpaPak cardiac transport system |
| TA-NRP | Thoracoabdominal normothermic regional perfusion |
| UNOS | United Network for Organ Sharing |
| VAD | Ventricular assist device |
| XHPS | XVIVO Heart Preservation System |
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Truby, L.K.; Rogers, J.G. Advanced Heart Failure: Epidemiology, Diagnosis, and Therapeutic Approaches. JACC Heart Fail. 2020, 8, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.M.; Smith, J.M.; Ahn, Y.S.; Handarova, D.K.; Martinez, A.C.; Lindblad, K.A.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2022 Annual Data Report: Heart. Am. J. Transpl. 2024, 24, S305–S393. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.J.; Chen, J.M.; Sorabella, R.A.; Martens, T.P.; Garrido, M.; Davies, R.R.; George, I.; Cheema, F.H.; Mosca, R.S.; Mital, S.; et al. The effect of ischemic time on survival after heart transplantation varies by donor age: An analysis of the United Network for Organ Sharing database. J. Thorac. Cardiovasc. Surg. 2007, 133, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.R.; Ziegler, L.A.; Kaczorowski, D.J. Heart Donation and Preservation: Historical Perspectives, Current Technologies, and Future Directions. J. Clin. Med. 2022, 11, 5762. [Google Scholar] [CrossRef]
- Tenderich, G.; Zittermann, A.; Schulz, U.; Schulte-Eistrup, S.; Schleithoff, S.S.; Wlost, S.; Korfer, R. Heart transplantation at the Heart Center North Rhine-Westfalia. Clin. Transpl. 2008, 151–161. [Google Scholar]
- Pahuja, M.; Case, B.C.; Molina, E.J.; Waksman, R. Overview of the FDA’s Circulatory System Devices Panel virtual meeting on the TransMedics Organ Care System (OCS) Heart—Portable extracorporeal heart perfusion and monitoring system. Am. Heart J. 2022, 247, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ardehali, A.; Esmailian, F.; Deng, M.; Soltesz, E.; Hsich, E.; Naka, Y.; Mancini, D.; Camacho, M.; Zucker, M.; Leprince, P.; et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): A prospective, open-label, multicentre, randomised non-inferiority trial. Lancet 2015, 385, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Egle, M.; Longnus, S.; Yildiz, M.; Siepe, M.; Reineke, D. Surgical techniques for cardiac procurement, preparation and perfusion using the Organ Care System. Multimed. Man. Cardiothorac. Surg. 2024, 2024. [Google Scholar] [CrossRef]
- Schroder, J.N.; Patel, C.B.; DeVore, A.D.; Casalinova, S.; Koomalsingh, K.J.; Shah, A.S.; Anyanwu, A.C.; D’Alessandro, D.A.; Mudy, K.; Sun, B.; et al. Increasing Utilization of Extended Criteria Donor Hearts for Transplantation: The OCS Heart EXPAND Trial. JACC Heart Fail. 2024, 12, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.N.; Patel, C.B.; DeVore, A.D.; Bryner, B.S.; Casalinova, S.; Shah, A.; Smith, J.W.; Fiedler, A.G.; Daneshmand, M.; Silvestry, S.; et al. Transplantation Outcomes with Donor Hearts after Circulatory Death. N. Engl. J. Med. 2023, 388, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Garcia Saez, D.; Zych, B.; Sabashnikov, A.; Bowles, C.T.; De Robertis, F.; Mohite, P.N.; Popov, A.F.; Maunz, O.; Patil, N.P.; Weymann, A.; et al. Evaluation of the organ care system in heart transplantation with an adverse donor/recipient profile. Ann. Thorac. Surg. 2014, 98, discussion 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Sponga, S.; Bonetti, A.; Ferrara, V.; Beltrami, A.P.; Isola, M.; Vendramin, I.; Finato, N.; Ortolani, F.; Livi, U. Preservation by cold storage vs ex vivo normothermic perfusion of marginal donor hearts: Clinical, histopathologic, and ultrastructural features. J. Heart Lung Transpl. 2020, 39, 1408–1416. [Google Scholar] [CrossRef]
- Sponga, S.; Vendramin, I.; Salman, J.; Ferrara, V.; De Manna, N.D.; Lechiancole, A.; Warnecke, G.; Dralov, A.; Haverich, A.; Ius, F.; et al. Heart Transplantation in High-Risk Recipients Employing Donor Marginal Grafts Preserved with Ex-Vivo Perfusion. Transpl. Int. 2023, 36, 11089. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.V.; Avsar, M.; Ius, F.; Schibilsky, D.; Kaufeld, T.; Benk, C.; Maeding, I.; Berchtold-Herz, M.; Bara, C.; Beyersdorf, F.; et al. Ex-Vivo Preservation with the Organ Care System in High Risk Heart Transplantation. Life 2022, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Sponga, S.; Benedetti, G.; de Manna, N.D.; Ferrara, V.; Vendramin, I.; Lechiancole, A.; Maiani, M.; Nalon, S.; Nalli, C.; Di Nora, C.; et al. Heart transplant outcomes in patients with mechanical circulatory support: Cold storage versus normothermic perfusion organ preservation. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Kaliyev, R.; Lesbekov, T.; Bekbossynov, S.; Nurmykhametova, Z.; Bekbossynova, M.; Novikova, S.; Medressova, A.; Smagulov, N.; Faizov, L.; Samalavicius, R.; et al. Heart transplantation of patients with ventricular assist devices: Impact of normothermic ex-vivo preservation using organ care system compared with cold storage. J. Cardiothorac. Surg. 2020, 15, 323. [Google Scholar] [CrossRef]
- Stamp, N.L.; Shah, A.; Vincent, V.; Wright, B.; Wood, C.; Pavey, W.; Cokis, C.; Chih, S.; Dembo, L.; Larbalestier, R. Successful Heart Transplant after Ten Hours Out-of-body Time using the TransMedics Organ Care System. Heart Lung Circ. 2015, 24, 611–613. [Google Scholar] [CrossRef]
- Medressova, A.; Faizov, L.; Kuanyshbek, A.; Kaliyev, R.; Myrzakhmetova, G.; la Fleur, P.; Pya, Y. Successful heart transplantation after 17 h ex vivo time using the Organ Care System-3 years follow-up. J. Card. Surg. 2021, 36, 2592–2595. [Google Scholar] [CrossRef] [PubMed]
- Dang Van, S.; Gaillard, M.; Laverdure, F.; Thes, J.; Venhard, J.C.; Fradi, M.; Vallee, A.; Ramadan, R.; Hebert, G.; Tamarat, R.; et al. Ex vivo perfusion of the donor heart: Preliminary experience in high-risk transplantations. Arch. Cardiovasc. Dis. 2021, 114, 715–726. [Google Scholar] [CrossRef]
- Fleck, T.P.K.; Ayala, R.; Kroll, J.; Siepe, M.; Schibilsky, D.; Benk, C.; Maier, S.; Reineker, K.; Hoehn, R.; Humburger, F.; et al. Ex Vivo Allograft Perfusion for Complex Pediatric Heart Transplant Recipients. Ann. Thorac. Surg. 2021, 112, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Verzelloni Sef, A.; Sef, D.; Garcia Saez, D.; Trkulja, V.; Walker, C.; Mitchell, J.; McGovern, I.; Stock, U. Heart Transplantation in Adult Congenital Heart Disease with the Organ Care System Use: A 4-Year Single-Center Experience. ASAIO J. 2021, 67, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Isath, A.; Ohira, S.; Levine, A.; Pan, S.; Aggarwal-Gupta, C.; Lanier, G.M.; Wolfe, K.; Spielvogel, D.; Gass, A.; Kai, M. Ex Vivo Heart Perfusion for Cardiac Transplantation Allowing for Prolonged Perfusion Time and Extension of Distance Traveled for Procurement of Donor Hearts: An Initial Experience in the United States. Transpl. Direct 2023, 9, e1455. [Google Scholar] [CrossRef] [PubMed]
- Pizanis, N.; Dimitriou, A.M.; Koch, A.; Luedike, P.; Papathanasiou, M.; Rassaf, T.; Ruhparwar, A.; Schmack, B.; Weymann, A.; Ferenz, K.B.; et al. Introduction of machine perfusion of donor hearts in a single center in Germany. Int. J. Cardiol. Heart Vasc. 2023, 47, 101233. [Google Scholar] [CrossRef]
- Medina, C.K.; Aykut, B.; Parker, L.E.; Prabhu, N.K.; Kang, L.; Beckerman, Z.; Schroder, J.N.; Overbey, D.M.; Turek, J.W. Early Single Center Experience with an Ex Vivo Organ Care System in Pediatric Heart Transplantation. J. Heart Lung Transplant. 2024, in press. [CrossRef]
- Dhital, K.K.; Iyer, A.; Connellan, M.; Chew, H.C.; Gao, L.; Doyle, A.; Hicks, M.; Kumarasinghe, G.; Soto, C.; Dinale, A.; et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: A case series. Lancet 2015, 385, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Taylor, M.; Hasan, J.; Dimarakis, I.; Barnard, J.; Callan, P.; Shaw, S.; Venkateswaran, R.V. Establishing a heart transplant programme using donation after circulatory-determined death donors: A United Kingdom based single-centre experience. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Cernic, S.; Page, A.; Messer, S.; Bhagra, S.; Pettit, S.; Dawson, S.N.; McKie, M.A.; Osman, M.; Nachum, E.; White, D.; et al. Lactate during ex-situ heart perfusion does not predict the requirement for mechanical circulatory support following donation after circulatory death (DCD) heart transplants. J. Heart Lung Transpl. 2022, 41, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Coniglio, A.C.; Kim, H.W.; Alenezi, F.; Schroder, J.N.; Bryner, B.S.; Agarwal, R.; Patel, C.B.; DeVore, A.D. The association with organ procurement techniques and early cardiac transplant outcomes using cardiac MRI. Clin. Transpl. 2023, 37, e14959. [Google Scholar] [CrossRef]
- Ghodsizad, A.; Bordel, V.; Ungerer, M.; Karck, M.; Bekeredjian, R.; Ruhparwar, A. Ex vivo coronary angiography of a donor heart in the organ care system. Heart Surg. Forum 2012, 15, E161–E163. [Google Scholar] [CrossRef] [PubMed]
- Meredith, T.; Scheuer, S.; Hoffman, M.; Joshi, Y.; Kathir, K.; Gunalingam, B.; Roy, D.; Wilson, S.; Jansz, P.; Macdonald, P.; et al. Coronary angiography of the ex-situ beating donor heart in a portable organ care system. Catheter. Cardiovasc. Interv. 2022, 100, 1252–1260. [Google Scholar] [CrossRef]
- Krishnan, A.; Elde, S.; Ruaengsri, C.; Guenthart, B.A.; Zhu, Y.; Fawad, M.; Lee, A.; Currie, M.; Ma, M.R.; Hiesinger, W.; et al. Survival, function, and immune profiling after beating heart transplantation. J. Thorac. Cardiovasc. Surg. 2024, in press. [CrossRef] [PubMed]
- Chan, J.L.; Kobashigawa, J.A.; Reich, H.J.; Ramzy, D.; Thottam, M.M.; Yu, Z.; Aintablian, T.L.; Liou, F.; Patel, J.K.; Kittleson, M.M.; et al. Intermediate outcomes with ex-vivo allograft perfusion for heart transplantation. J. Heart Lung Transpl. 2017, 36, 258–263. [Google Scholar] [CrossRef]
- Chen, Q.; Singer-Englar, T.; Kobashigawa, J.A.; Roach, A.; Emerson, D.; Megna, D.; Ramzy, D.; Catarino, P.; Patel, J.K.; Kittleson, M.; et al. Long-term outcomes after heart transplantation using ex vivo allograft perfusion in standard risk donors: A single-center experience. Clin. Transpl. 2022, 36, e14591. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Azarbal, B.; Cheng, R.; Esmailian, F.; Patel, J.; Kittleson, M.; Czer, L.; Thottam, M.; Levine, R.; Dimbil, S.; et al. Does ex vivo perfusion lead to more or less intimal thickening in the first-year post-heart transplantation? Clin. Transpl. 2019, 33, e13648. [Google Scholar] [CrossRef]
- Jawitz, O.K.; Devore, A.D.; Patel, C.B.; Bryner, B.S.; Schroder, J.N. EXPANDing the Donor Pool: Quantifying the Potential Impact of a Portable Organ-Care System for Expanded Criteria Heart Donation. J. Card. Fail. 2021, 27, 1462–1465. [Google Scholar] [CrossRef] [PubMed]
- Shudo, Y.; Benjamin-Addy, R.; Koyano, T.K.; Hiesinger, W.; MacArthur, J.W.; Woo, Y.J. Donors after circulatory death heart trial. Future Cardiol. 2021, 17, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Kasinpila, P.; Wang, H.; Ruaengsri, C.; Shudo, Y.; Jackson, E.; Woo, Y.J. First-in-human beating-heart Transpl. JTCVS Tech. 2023, 19, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Guenthart, B.A.; Ruaengsri, C.; Elde, S.; Zhu, Y.; MacArthur, J.W.; Woo, Y.J. Beating Heart Transplantation: How to Do It. Innovations 2024, 19, 88–91. [Google Scholar] [CrossRef]
- Krishnan, A.; Ruaengsri, C.; Guenthart, B.A.; Shudo, Y.; Wang, H.; Ma, M.R.; MacArthur, J.W.; Hiesinger, W.; Woo, Y.J. Beating Heart Transplant Procedures Using Organs From Donors with Circulatory Death. JAMA Netw. Open 2024, 7, e241828. [Google Scholar] [CrossRef] [PubMed]
- Naito, N.; Funamoto, M.; Pierson, R.N.; D’Alessandro, D.A. First clinical use of a novel hypothermic storage system for a long-distance donor heart procurement. J. Thorac. Cardiovasc. Surg. 2020, 159, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- Silvestry, S.; Meyer, D.; Pham, S.; Jacobs, J.P.; Shudo, Y.; Schroder, J.; Leacche, M.; Sciortino, C.M.; Copeland, H.; Rodrigo, M.E.; et al. Improved 2-Year Heart Transplant Survival with Moderate Hypothermic Donor Heart Preservation in the Guardian Heart Registry. J. Heart Lung Transpl. 2024, 43, S67–S68. [Google Scholar] [CrossRef]
- D’Alessandro, D.; Schroder, J.; Meyer, D.M.; Vidic, A.; Shudo, Y.; Silvestry, S.; Leacche, M.; Sciortino, C.M.; Rodrigo, M.E.; Pham, S.M.; et al. Impact of controlled hypothermic preservation on outcomes following heart transplantation. J. Heart Lung Transpl. 2024, 43, 1153–1161. [Google Scholar] [CrossRef]
- Voigt, J.D.; Leacche, M.; Copeland, H.; Wolfe, S.B.; Pham, S.M.; Shudo, Y.; Molina, E.; Jacobs, J.P.; Stukov, Y.; Meyer, D.; et al. Multicenter Registry Using Propensity Score Analysis to Compare a Novel Transport/Preservation System to Traditional Means on Postoperative Hospital Outcomes and Costs for Heart Transplant Patients. ASAIO J. 2023, 69, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Shudo, Y.; Leacche, M.; Copeland, H.; Silvestry, S.; Pham, S.M.; Molina, E.; Schroder, J.N.; Sciortino, C.M.; Jacobs, J.P.; Kawabori, M.; et al. A Paradigm Shift in Heart Preservation: Improved Post-transplant Outcomes in Recipients of Donor Hearts Preserved with the SherpaPak System. ASAIO J. 2023, 69, 993–1000. [Google Scholar] [CrossRef]
- Moayedifar, R.; Shudo, Y.; Kawabori, M.; Silvestry, S.; Schroder, J.; Meyer, D.M.; Jacobs, J.P.; D’Alessandro, D.; Zuckermann, A. Recipient Outcomes with Extended Criteria Donors Using Advanced Heart Preservation: An Analysis of the GUARDIAN-Heart Registry. J. Heart Lung Transpl. 2024, 43, 673–680. [Google Scholar] [CrossRef]
- Silvestry, S.; Leacche, M.; Meyer, D.M.; Shudo, Y.; Kawabori, M.; Mahesh, B.; Zuckermann, A.; D’Alessandro, D.; Schroder, J. Outcomes in Heart Transplant Recipients by Bridge to Transplant Strategy When Using the SherpaPak Cardiac Transport System. ASAIO J. 2024, 70, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lerman, J.B.; Patel, C.B.; Casalinova, S.; Nicoara, A.; Holley, C.L.; Leacche, M.; Silvestry, S.; Zuckermann, A.; D’Alessandro, D.A.; Milano, C.A.; et al. Early Outcomes in Patients with LVAD Undergoing Heart Transplant via Use of the SherpaPak Cardiac Transport System. Circ. Heart Fail. 2024, 17, e010904. [Google Scholar] [CrossRef] [PubMed]
- Farhoud, H.; Shah, Z.; Dalia, T.; Silvestry, S.; Shah, H.; Meyer, D.; D’Alessandro, D.; Vidic, A. Chilling Choices: Heart Transplant Outcomes Using SherpaPak with Long Ischemic Time Versus Traditional Ice Storage with Short Ischemic Time. ASAIO J. 2024, in press. [Google Scholar] [CrossRef]
- Radakovic, D.; Karimli, S.; Penov, K.; Schade, I.; Hamouda, K.; Bening, C.; Leyh, R.G.; Aleksic, I. First clinical experience with the novel cold storage SherpaPak system for donor heart transportation. J. Thorac. Dis. 2020, 12, 7227–7235. [Google Scholar] [CrossRef] [PubMed]
- Guenthart, B.A.; Krishnan, A.; Koyano, T.; La Francessca, S.; Chan, J.; Alassar, A.; Macarthur, J.W.; Shudo, Y.; Hiesinger, W.; Woo, Y.J. Extended Static Hypothermic Preservation In Cardiac Transplantation: A Case Report. Transpl. Proc. 2021, 53, 2509–2511. [Google Scholar] [CrossRef]
- Schmiady, M.O.; Graf, T.; Ouda, A.; Aser, R.; Flammer, A.J.; Vogt, P.R.; Wilhelm, M.J. An innovative cold storage system for donor heart transportation-lessons learned from the first experience in Switzerland. J. Thorac. Dis. 2021, 13, 6790–6799. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Posawatz, D.A.; Chen, F.Y.; Couper, G.S. A Simple Technique for Reliable Donor Organ Temperature Management with the SherpaPak System. ASAIO J. 2022, 68, e134–e135. [Google Scholar] [CrossRef] [PubMed]
- Bitargil, M.; Haddad, O.; Pham, S.M.; Garg, N.; Jacob, S.; El-Sayed Ahmed, M.M.; Landolfo, K.; Patel, P.C.; Goswami, R.M.; Leoni Moreno, J.C.; et al. Packing the donor heart: Is SherpaPak cold preservation technique safer compared to ice cold storage. Clin. Transpl. 2022, 36, e14707. [Google Scholar] [CrossRef]
- Lechiancole, A.; Sponga, S.; Gliozzi, G.; Martin-Suarez, S.; Visentin, P.; Botta, L.; Copetti, S.; Dralov, A.; Benedetti, G.; Finato, N.; et al. Ice-cold storage or controlled hypothermia to preserve marginal grafts in high-risk heart transplantation. Artif. Organs 2025, 49, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; van Zyl, J.S.; Afzal, A.; Felius, J.; Hall, S.A.; Meyer, D.M.; Carey, S.A. Early elevated donor-derived cell-free DNA levels in heart transplant recipients following precision-controlled cardiac transport system or ice-cooled organ transport. Clin. Transpl. 2023, 37, e15151. [Google Scholar] [CrossRef] [PubMed]
- Lotan, D.; Moeller, C.M.; Rahman, A.; Rubinstein, G.; Oren, D.; Mehlman, Y.; Valledor, A.F.; DeFilippis, E.M.; Raikhelkar, J.; Clerkin, K.; et al. Comparative Analysis of Ischemia-Reperfusion Injury in Heart Transplantation: A Single-Center Study Evaluating Conventional Ice-Cold Storage versus the Paragonix SherpaPak Cardiac Transport System. Clin. Transpl. 2024, 38, e15397. [Google Scholar] [CrossRef]
- Urban, M.; Castleberry, A.W.; Siddique, A.; Lowes, B.D.; Stoller, D.A.; Lundgren, S.W.; Um, J.Y. Utilization of Paragonix Sherpapak Cardiac Transport System for the Preservation of Donor Hearts After Circulatory Death. Transpl. Proc. 2023, 55, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shudo, Y.; He, H.; Kim, J.Y.; Elde, S.; Williams, K.M.; Walsh, S.K.; Koyano, T.K.; Guenthart, B.; Woo, Y.J. Outcomes of Heart Transplantation Using a Temperature-controlled Hypothermic Storage System. Transplantation 2023, 107, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Jernryd, V.; Qin, G.; Paskevicius, A.; Metzsch, C.; Sjoberg, T.; Steen, S. A nonrandomized open-label phase 2 trial of nonischemic heart preservation for human heart transplantation. Nat. Commun. 2020, 11, 2976. [Google Scholar] [CrossRef]
- McGiffin, D.C.; Kure, C.E.; Macdonald, P.S.; Jansz, P.C.; Emmanuel, S.; Marasco, S.F.; Doi, A.; Merry, C.; Larbalestier, R.; Shah, A.; et al. Hypothermic oxygenated perfusion (HOPE) safely and effectively extends acceptable donor heart preservation times: Results of the Australian and New Zealand trial. J. Heart Lung Transpl. 2024, 43, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Rega, F.; Lebreton, G.; Para, M.; Michel, S.; Schramm, R.; Begot, E.; Vandendriessche, K.; Kamla, C.; Gerosa, G.; Berman, M.; et al. Hypothermic oxygenated perfusion of the donor heart in heart transplantation: The short-term outcome from a randomised, controlled, open-label, multicentre clinical trial. Lancet 2024, 404, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Joshi, Y.; MacLean, C.; Emmanuel, S.; Wang, K.; Soto, C.; Villanueva, J.; Gao, L.; Doyle, A.; Dutta, S.; Wu, J.; et al. Australian outcomes from heart transplantation in the machine perfusion era. Ann. Cardiothorac. Surg. 2024, 13, 502–512. [Google Scholar] [CrossRef]
- Brouckaert, J.; Vandendriessche, K.; Degezelle, K.; Van de Voorde, K.; De Burghgraeve, F.; Desmet, L.; Vlasselaers, D.; Ingels, C.; Dauwe, D.; De Troy, E.; et al. Successful clinical transplantation of hearts donated after circulatory death using direct procurement followed by hypothermic oxygenated perfusion: A report of the first 3 cases. J. Heart Lung Transpl. 2024, 43, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Brouckaert, J.; Dellgren, G.; Wallinder, A.; Rega, F. Non-ischaemic preservation of the donor heart in heart transplantation: Protocol design and rationale for a randomised, controlled, multicentre clinical trial across eight European countries. BMJ Open 2023, 13, e073729. [Google Scholar] [CrossRef]
- Première Evaluation Clinique de la Transplantation Cardiaque de Greffons préservés à l’Aide d’un Système de Perfusion Ex-vivo prolongée (PEGASE). Available online: https://clinicaltrials.gov/study/NCT06035991 (accessed on 10 January 2025).
- PRESERVE Heart Study: A Prospective, Multi-Center, Single-Arm, Open-Label Study of Hearts Transplanted After Non-Ischemic Heart PRESERVation from Extended Donors. Available online: https://clinicaltrials.gov/study/NCT05881278 (accessed on 10 January 2025).
- Direct Procurement (DP) and Hypothermic Oxygenated Perfusion (HOPE) of Donor Hearts After Circulatory Death (DCD) Using the XVIVO Heart Assist Transport System. Available online: https://clinicaltrials.gov/study/NCT06485596 (accessed on 10 January 2025).
- Jain, P.; Prichard, R.A.; Connellan, M.B.; Dhital, K.K.; Macdonald, P.S. Long distance heart transplantation: A tale of two cities. Intern. Med. J. 2017, 47, 1202–1205. [Google Scholar] [CrossRef]
- Hong, Y.; Hess, N.R.; Dorken-Gallastegi, A.; Abdullah, M.; Iyanna, N.; Nasim, U.; Sultan, I.; Hickey, G.W.; Keebler, M.E.; Kaczorowski, D.J. Effects of Nighttime Procurement and Transplantation on Outcomes Following Heart Transplantation. Clin. Transpl. 2025, 39, e70093. [Google Scholar] [CrossRef] [PubMed]
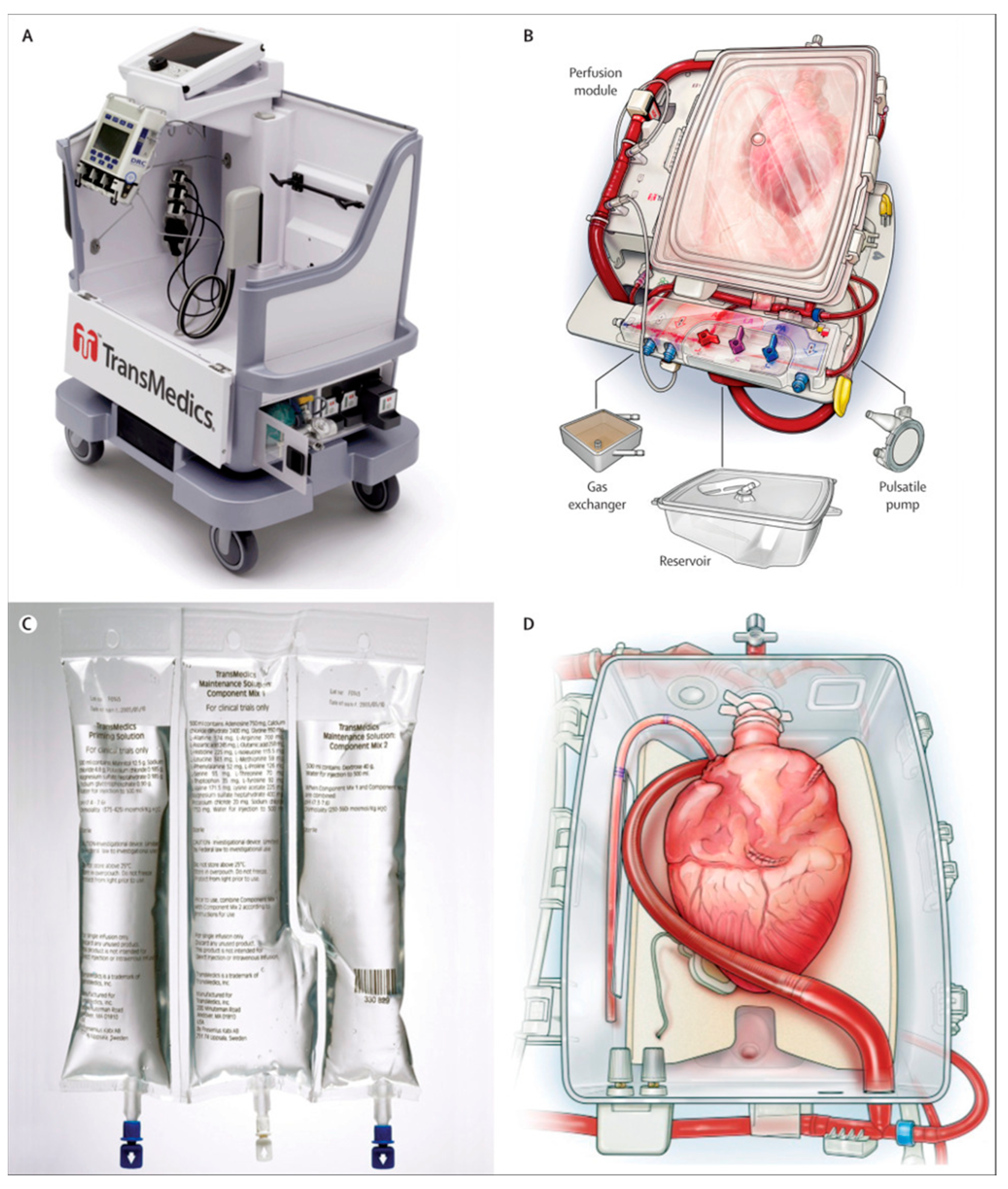
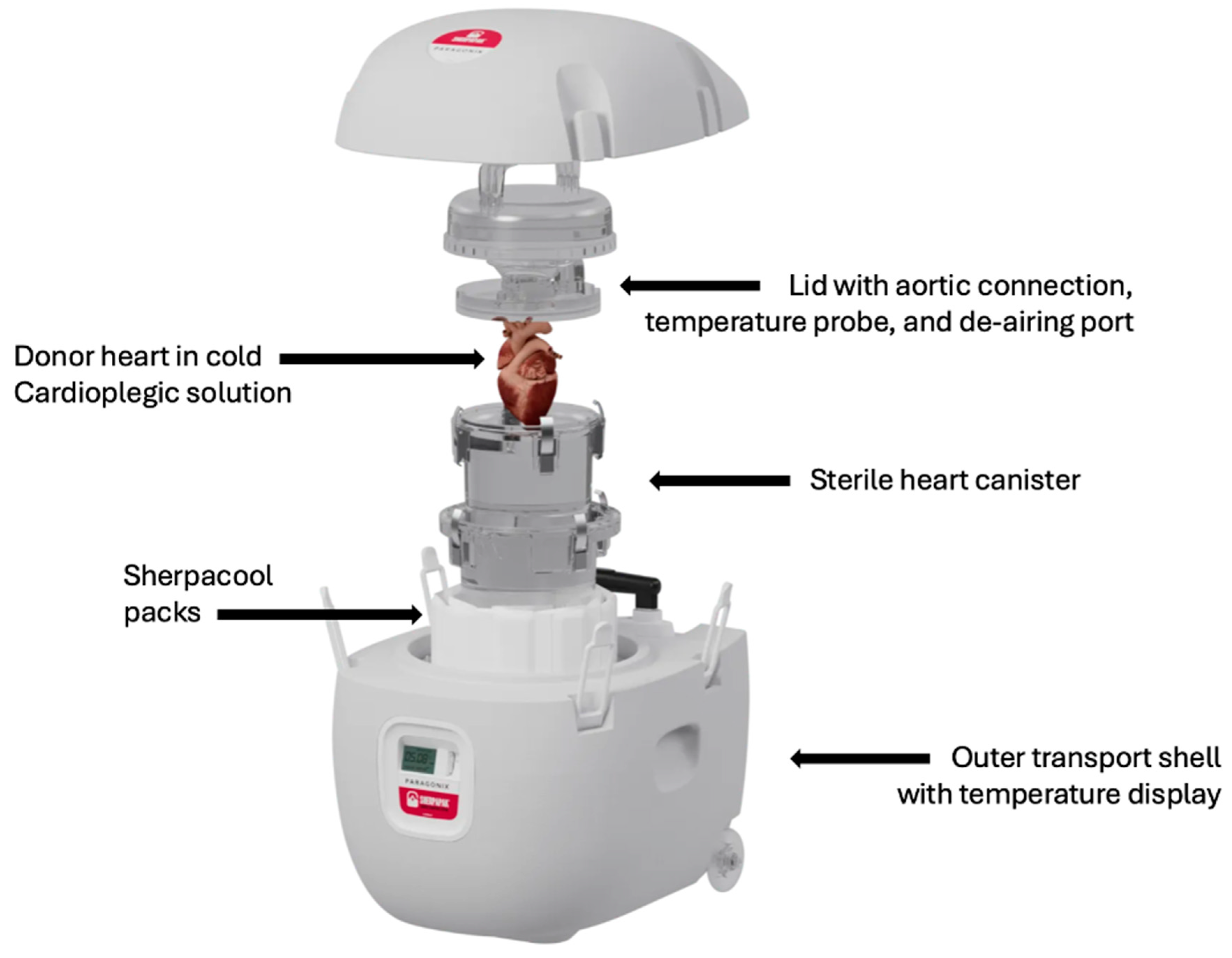
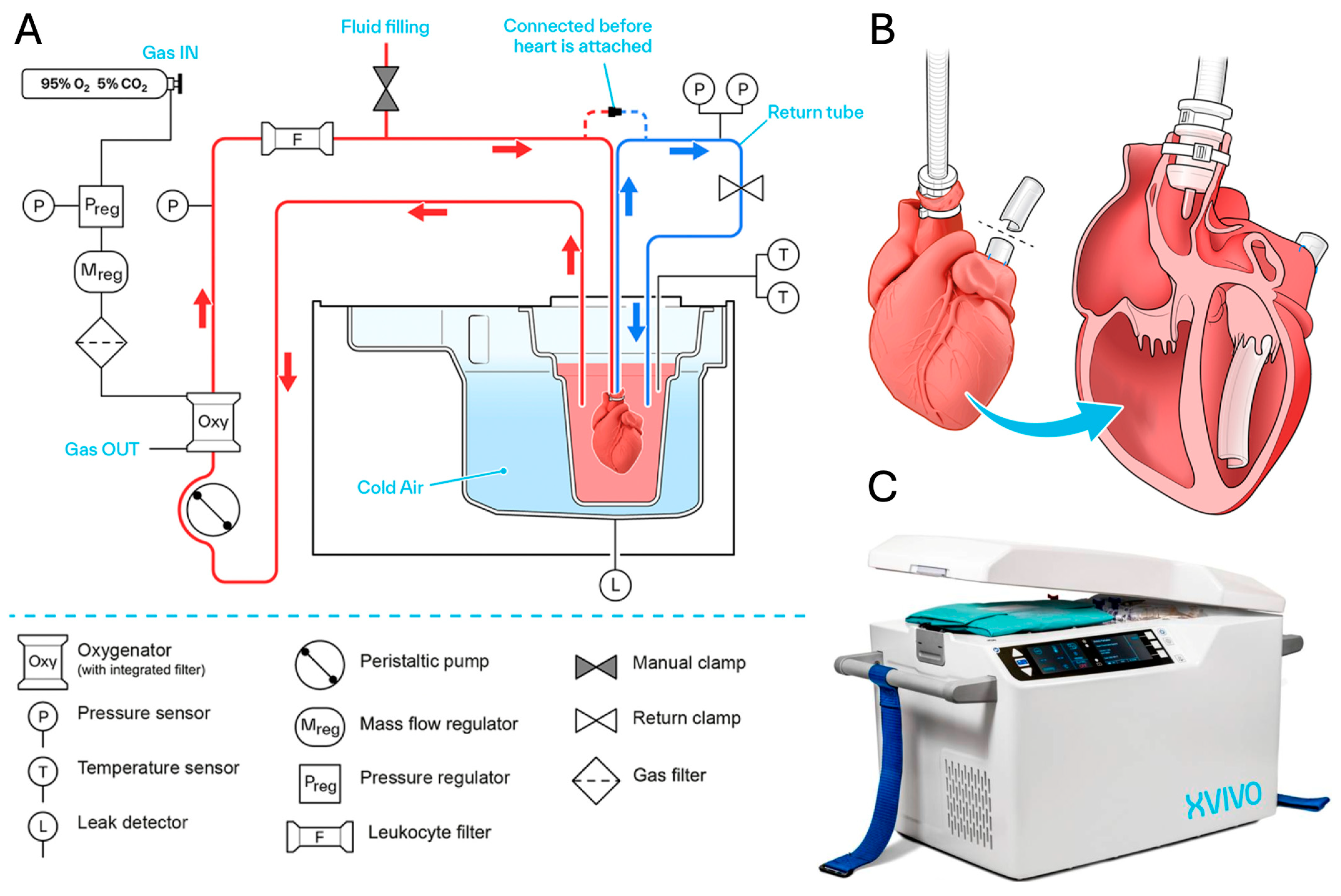
| TransMedics Organ Care System Heart | ||||
|---|---|---|---|---|
| Clinical Trials | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary Outcome | Secondary Outcome |
| Ardehali, et al. 2015 PROCEED-II Trial [9] | Prospective, open-label, multicenter randomized clinical trial | OCS (n = 63) versus SCS (n = 67) with standard criteria donors | Non-inferior 30-day survival (OCS 94% vs. SCS 97%, p = 0.36). | No difference in cardiac adverse events, severe rejection, or ICU length of stay (all p > 0.05). |
| Schroder, et al. 2024 EXPAND-CAP Trial [11] | Prospective, single-arm, multicenter clinical trial | OCS (n = 173) with ECDs | 30-day survival free of severe PGD achieve in 92% of grafts ( > 65%). | Donor heart utilization rate (87%), severe graft-related events (17%), and severe PGD (16%). |
| Schroder, et al. 2023 DCD Heart [12] | Prospective, open-label, multicenter, randomized clinical trial | DCD-OCS (n = 90) versus DBD-SCS (n = 90) | 6-month survival (DCD-OCS 94% vs. DBD-SCS 90%, p < 0.001 for non-inferiority). | Moderate or severe PGD (DCD-OCS 22% vs. DBD-SCS 10%). |
| Single-Center Experiences: High Risk Donor & Recipient Populations | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary Outcome | Secondary Outcome |
| Garcia Saez et al., 2014, United Kingdom [13] | Descriptive case series | OCS (n = 26) with ECDs and/or high-risk recipients (MCS or elevated PVR) | 100% 1-month survival and 96% survival at 257 ± 116 days of follow-up | Post-transplant IABP (11%), moderate right ventricular failure (19.2%). |
| Sponga et al., 2020, Italy [14] | Retrospective cohort | OCS (n = 21) versus SCS (n = 79) with ECDs (EXPAND trial criteria) | Post-transplant 30-day survival comparable between (OCS 100% vs. SCS 95%, p = 0.58), and estimated 5-year survival higher for OCS (73% vs. 100%, p: 0.04), with incomplete follow-up (median follow-up CSS: 32 and OCS: 19). | Lower incidence of early post-transplant complications (PGD, need for ECMO, atrial fibrillation, renal or respiratory insufficiency, acute rejection) for OCS (67% vs 87%, p: 0.04) |
| Sponga et al., 2023, Italy [15] | Case series | OCS (n = 80) with ECDs and high-risk recipients | Post-transplant 1-year survival: 83 ± 4%, 3-year survival: 72 ± 7% (median follow-up: 16 months). | Rate of in-hospital mortality 9 (11%), moderate to severe PGD: 13 (16%). |
| Rojas et al., 2022, Germany [16] | Retrospective cohort | OCS (n = 68) versus SXS (n = 51 with ECDs and/or high-risk recipients | Difference in 30-day (OCS: 92.4%, CSS: 90.2%) and 1-year (OCS:89%, CSS: 85%) post-transplant survival not statistically significant between OCS and CSS. | Lower incidence of post-transplant dialysis in the OCS group (4.4% vs 27.5%). Comparable incidence of PGD requiring ECMO between OCS and CSS. |
| Sponga et al., 2021, Italy [17] | Retrospective cohort | OCS (n = 14) versus SCS (n = 24) with recipients MCS | Post-transplant 30-day mortality not significantly different between CSS (13%) and OCS (0%), p = 0.28. | PGD higher in CSS (42%) vs OCS (7%), p = 0.03. |
| Kaliyev et al., Kazakhstan, 2020 [18] | Retrospective cohort | OCS (n = 25) versus SCS (n = 10) with recipients on MCS | Post-transplant 30-day mortality not significantly different between CSS (100%) and OCS (96%), p = 0.5. | |
| Stamp et al., 2015, Australia [19] | Case report | Heart transplant after 10-hour preservation with OCS. | Discharged from hospital alive | Need for ECMO post-transplant |
| Medressova et al., 2021, Kazakhstan [20] | Case report | Heart transplant after 17-hour preservation with OCS. | Discharged from hospital alive | Need for ECMO post-transplant |
| Dang Van et al., 2021, France [21] | Descriptive case series | DBD-OCS (n = 4) with ECDs and DCD-OCS (n = 1) OCS | Post-transplant 3-month survival: 75%. | Post transplant ECMO: 3 (60%). |
| Fleck et al., 2021, Germany [22] | Retrospective cohort | OCS (n = 8) versus SCS (n = 13) for pediatric heart transplantation | Post-transplant 1-year survival similar between CSS (85%) and OCS (88%). | Incidence of PGD, renal failure, and hepatic failure similar between OCS and CSS. |
| Verzelloni et al., 2021, United Kingdom [23] | Descriptive case series | OCS (n = 9) for recipients with adult congenital heart disease undergoing heart transplantation | Post-transplant 30-day survival: 89%, 1-year survival: 78%. | Post-transplant ECMO in 44%. |
| Isath et al., 2023, United States [24] | Retrospective cohort | OCS (n = 8) versus SCS (n = 13) with expected transport time >4 h. | Post-transplant in-hospital survival similar between OCS (100%) and CSS (92%), p = 0.85. | PGD similar between OCS (12%) and CSS (15%), p = 0.85. |
| Pizanis et al., 2023, Germany [25] | Descriptive case series | OCS (n = 12) with ECDs | Post-transplant 30-day survival: 100%. | Post-transplant graft LVEF >50% in all cases, no cases of rejection on endomyocardial biopsies. |
| Medina, et al. 2024, United States [26] | Descriptive case series | OCS (n = 8) for pediatric heart transplantation | 100% survival (median follow-up 11.9 months) | 50% (n = 4) required either post-operative VAD or ECMO support. |
| Single-Center Experiences: DCD Heart Transplantation | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary Outcome | Secondary Outcome |
| Dhital et al., 2015, Australia [27] | Case series | OCS (n = 3) for DCD heart transplantation | All patients survived past 2-months postop. | Post-transplant mechanical circulatory support: 2 (66%). |
| Mehta et al., 2019, United Kingdom [28] | Case series | OCS (n = 7) for DCD heart transplantation | Post-transplant 30-day survival: 100%, 90-day survival: 86%. | Post-transplant ECMO: 3 (43%) Initiation of the OCS DCD program resulted in 23% increase in heart transplant volume. |
| Cernic et al., 2022, United Kingdom [29] | Case-control | OCS (n = 51) for DCD heart transplantation stratified by need for post-transplant MCS (MCS n = 20 versus No MCS n = 31) | OCS lactate levels, arteriovenous lactate gradient, or the percentage of patients with lactate >5 mmol/L at 3 h were significantly associated with the need for post-transplant MCS. | |
| Coniglio et al., 2023, United States [30] | Retrospective cohort | DCD-OCS (n = 31) versus DBD-OCS (n = 16) versus DBD-SCS (n = 38) | No difference in post-transplant 6-month survival between the transplant types. | Higher incidence of PGD requiring MCS in the DCD group. No difference in cardiac MRI findings between the transplant types. |
| Single-Center Experiences: Advanced Uses | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary Outcome | Secondary Outcome |
| Ghodsizad et al., 2012, Germany [31] | Case report | DBD heart evaluated with coronary angiography during OCS perfusion | Successful diagnostic ex-vivo coronary angiography. | |
| Meredith et al., 2022, Australia [32] | Case series | DCD-OCS (n = 8) undergoing coronary angiography during OCS support | Successful ex-vivo coronary angiography in all cases without injury to the graft. | Six hearts (75%) proceeded to transplantation. Severe multivessel disease found in one graft (12.5%), all other hearts were found to have <30% coronary stenosis. |
| Krishnan et al., 2024, United States [33] | Retrospective cohort | OCD Beating Heart DCD (n = 21) and DBD (n = 3) versus OCD-DCD (n = 22) | Post-transplant survival 95.8% at median 192-day follow-up in the beating heart transplant group. | Lower rate of post-transplant mechanical circulatory support in beating heart transplant vs. non-beating heart (0% vs. 36.4%, p < 0.005). |
| Paragonix SherpaPak Cardiac Transport System | ||||
|---|---|---|---|---|
| GURADIAN-Heart Registry: Primary Outcomes | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design * | Patient Population | Post-transplant Survival | Severe PGD |
| Shudo, et al., 2023 [46] | Unmatched | SCS (n = 314) versus SCTS (n = 255) | SCS (90.6%) versus SCTS (92.6%, p = 0.37) after 1 year. | SCS (10.2%) versus SCTS (5.4%, p = 0.03). |
| Propensity score-matched | SCS (n = 150) versus SCTS (n = 150) | SCS (88.7%) versus SCTS (94.0%, p = 0.10) after 1 year. | SCS (12.0%) versus SCTS (4.0%, p = 0.011). | |
| Voigt, et al., 2023 [24] | Propensity score-matched | SCS (n = 87) versus SCTS (n = 87) | SCS (86.2%) versus SCTS (92.0%, p = 0.23) after 1 year. | SCS (16.1%) versus SCTS (5.7%, p = 0.03). |
| D’Alessandro, et al., 2024 [44] | Propensity score-matched | SCS (n = 281) versus SCTS (n = 281) | SCS (92.1%) versus SCTS (95.9%, p = 0.07) after 1 year. | SCS (12.1%) versus SCTS (6.0%, p = 0.018). |
| Silvestry, et al., 2024 [43] | Propensity score-matched | SCS (n = 353) versus SCTS (n = 353) | SCS (89.9%) versus SCTS (94.9%, p = 0.021) after 2 years. | SCS (11.0%) versus SCTS (5.4%, p = 0.009). |
| GUARDIAN-Heart Registry: Donor and Recipient Risk Stratification | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design * | Patient Population | Post-transplant Survival | Severe PGD |
| Moayedifar, et al., 2024 [47] | Extended criteria donors, unmatched | SCS (n = 137) versus SCTS (n = 193) | SCS (89.6%) versus SCTS (92.9%, p = 0.41) after 1 year. | SCS (13.9%) versus SCTS (6.2%, p = 0.022). |
| Silvestry, et al., 2024 [48] | MCS BTT, unmatched | SCS (n = 354) versus SCTS (n = 422) | SCS (91.5%) versus SCTS (93.5%, p = 0.18) after 1 year. | SCS (10.2%) versus SCTS (6.2%, p = 0.046). |
| MCS BTT, PSM | SCS (n = 216) versus SCTS (n = 216) | SCS (92.5%) versus SCTS (93.2%, p = 0.757) after 1 year. | SCS (10.2%) versus SCTS (5.1%, p = 0.069). | |
| Lerman, et al., 2024 [49] | LVAD BTT, unmatched | SCS (n = 178) versus SCTS (n = 149) | SCS (94.4%) versus SCTS (96.6%, p = 0.492) after 1 year. | SCS (14.0%) versus SCTS (5.4%, p = 0.010) |
| LVAD BTT, PSM | SCS (n = 216) versus SCTS (n = 216) | SCS (93.2%) versus SCTS (90.2%, p = 0.57) after 1 year. | SCS (15.5%) versus SCTS (3.6%, p = 0.016). | |
| Farhoud, et al., 2024 [50] | SCS < 3 hours versus SCTS > 4 hours, unmatched | SCS (n = 183) versus SCTS (n = 148) | SCS (100.0%) versus SCTS (98.6%, p = 0.20) after 30 days | SCS (10.4%) versus SCTS (8.1%, p = 0.57) |
| SCS < 3 hours versus SCTS > 4 hours, PSM | SCS (n = 85) versus SCTS (n = 85) | SCS (100.0%) versus SCTS (98.8%, p > 0.99) after 30 days | SCS (11.8%) versus SCTS (5.9%, p = 0.28) | |
| Single-Center Experiences | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary Outcomes | Secondary Outcomes |
| Naito, et al., 2020 [42] | Case report | SCTS (n = 1) | Discharged to home after 17 days with left ventricular ejection fraction of 72%. | |
| Radakovic, et. al., 2020 [51] | Case control, retrospective analysis | SCTS (n = 7) versus SCS (n = 14) | 1 year survival (SCTS 71.4% vs. SCS 78.6%, p = 0.717). | Improved right heart function after 30-dau follow up (tricuspid annular plane systolic excursion DBD-SCTS 17.83 ± 2.71 vs. DBD-SCS 14.52 ± 2.61 mm, p = 0.02). |
| Guenthart, et al., 2021 [52] | Case report | SCTS (n = 1) | Discharged to home after 10 days, and 3-month complication-free survival | |
| Schmiady, et al., 2021 [53] | Single-arm, retrospective analysis | SCTS (n = 4) | All patients survived to the end of follow up (average 303 days). One replicate was notable for warm preservation (>10 C) during preservation due to high initial temperature. | |
| Kawabori, et al., 2022 [54] | Case series | SCTS (n = 15) | SCTS (n = 3) notable for warm preservation (>10 C) due to high initial preservation solution temperature. | |
| Bitargil, et al., 2022 [55] | Retrospective analysis | SCTS (n = 34) versus SCS (n = 47) | No difference in 30-day survival (SCTS 100% vs. SCS 98%, p = 0.7). | No significant difference in groups regarding VIS, PGD, or the need for a temporary pacer. |
| Lechiancole et. al., 2024 [56] | Retrospective Analysis | SCTS (n = 30) versus SCS (n = 60) | 1-year survival (SCTS 90% vs. SCS (88%, p = 0.89). | Rate of moderate to severe was similar (SCTS 7% vs. SCS 20%, p = 0.08). |
| Alam et. al., 2023 [57] | Retrospective analysis | SCTS (n = 35) versus SCS (n = 30) | Higher risk of elevated dd-cfDNA with SCS compared to SCTS (AOR 4.9, o5% CI [1.08, 22.5]. p = 0.046). | |
| Lotan et al., 2024 [58] | Single-blinded, retrospective analysis | STCS (n = 57) versus SCS (n = 33) | No significant differences in ischemic reperfusion injuries were observed between groups at weeks 1, 4, and 8 post-transplant. | A 59.3% reduction in coagulative myocyte necrosis occurred from weeks 1 to 4 with SCTS but not with SCS. Similar survival and rejection rate after 1 year. |
| Urban et al., 2023 [59] | Single-arm, retrospective analysis | DCD-SCTS (n = 12) | 83% survival to discharge (n = 10) with one death from graft dysfunction (8%). | Median length of ICU stay for hospital survivors was 5 days and hospital stay 17 days. |
| XVIVO Heart Preservation System | ||||
|---|---|---|---|---|
| Clinical Trials | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary | Secondary |
| Nilsson, et al., 2020 NIHP Trial [61] | Prospective, open-label, multicenter, non-randomized clinical trial | XHPS (n = 6) versus SCS (n = 353) with standard criteria donors | 6-month survival without severe PGD, ECMO, or acute cellular rejection (XHPS 100% vs. SCS 72%, RR 1.4 95% CI [1.1–1.8]). | No significant differences: IR-injury, Immediate graft function, and 6-month adverse events. |
| McGiffin, et al., 2024 HOPE Trial [62] | Prospective, single-arm, multicenter, non-randomized clinical trial | XHPS (n = 7) short preservation (<6 h) versus XHPS (n = 29) long preservation (6–9 h) | 100% survival in both cohorts at 30 days. | One instance of PGD in the long preservation cohort. |
| Rega, et al., 2024 NIHP2019 [63] | Prospective, open-label, multicenter, randomized, clinical trial | XHPS (n = 101) versus SCS (n = 103) | Composite cardiac-related death, PGD, rejection, or graft failure (XHPS 19% vs. SCS 30%, p = 0.059). | No difference observed in the rate of serious adverse events. |
| Single Center Experiences | ||||
| Study Information | Outcomes | |||
| Author, Year | Study Design | Patient Population | Primary | Secondary |
| Joshi, et al., 2024 [64] | Retrospective analysis | DCD-NMP (n = 44) versus BD-HMP (n = 38) versus BD-SCS (n = 78) | No difference in severe PGD or 30-day survival (DCD-NMP 100% vs. BD-HMP 97% vs. BD-SCS 100%). | |
| Brouckaert et al., 2024 [65] | Prospective, single arm case series | DCD-XHPS donors (n = 3) | No severe PGD in any recipients and 100% survival at 30 days. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasim, U.; Dorken-Gallastegi, A.; Dadson, P.; Hong, Y. Clinical Outcomes of Machine Perfusion and Temperature Control Systems in Heart Transplantation: Where We Stand. J. Clin. Med. 2025, 14, 1152. https://doi.org/10.3390/jcm14041152
Nasim U, Dorken-Gallastegi A, Dadson P, Hong Y. Clinical Outcomes of Machine Perfusion and Temperature Control Systems in Heart Transplantation: Where We Stand. Journal of Clinical Medicine. 2025; 14(4):1152. https://doi.org/10.3390/jcm14041152
Chicago/Turabian StyleNasim, Umar, Ander Dorken-Gallastegi, Peter Dadson, and Yeahwa Hong. 2025. "Clinical Outcomes of Machine Perfusion and Temperature Control Systems in Heart Transplantation: Where We Stand" Journal of Clinical Medicine 14, no. 4: 1152. https://doi.org/10.3390/jcm14041152
APA StyleNasim, U., Dorken-Gallastegi, A., Dadson, P., & Hong, Y. (2025). Clinical Outcomes of Machine Perfusion and Temperature Control Systems in Heart Transplantation: Where We Stand. Journal of Clinical Medicine, 14(4), 1152. https://doi.org/10.3390/jcm14041152







