Minimally Invasive Surgery for Spontaneous Intracerebral Hemorrhage: A Review
Abstract
1. Introduction
2. Rationale and Challenges for MIS
2.1. ICH Evacuation: Hitting the Target Before It Starts Moving
2.2. Main Challenge and Theoretical Advantages of MIS
3. Current State of Research and Perspectives
3.1. Techniques Used in Trials and Outcomes
3.2. Clot Evacuation, Complication, and Adverse Events in MIS vs. Other Management Methods
4. Technical Aspects of MIS Techniques
4.1. Common Aspects
4.1.1. Anesthetic Plan Consideration
4.1.2. Patient Position and MIS Technique Planning
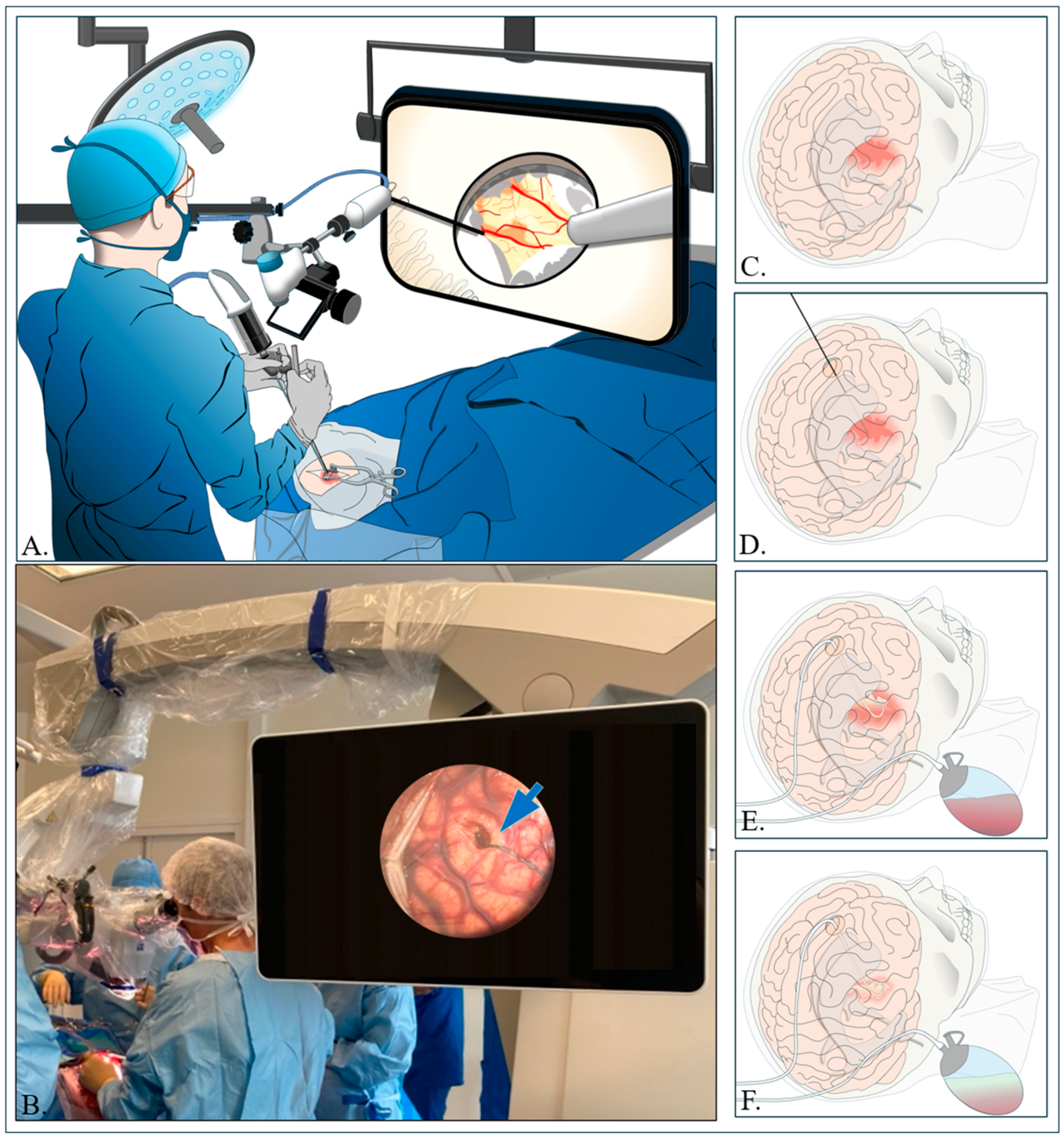
4.2. Small Stereotactic Craniotomy and Cannulation (CRANIO-CAN)
4.2.1. Small Stereotactic Craniotomy
4.2.2. Aspiration of Hematoma and Hemostasis
4.3. Aspiration with Drainage and Irrigation with Thrombolysis Agent Injection (ADIWIT)
4.3.1. Catheter Insertion Procedure
4.3.2. Aspiration Process and Drainage
4.3.3. Catheter Irrigation with rt-PA Administration
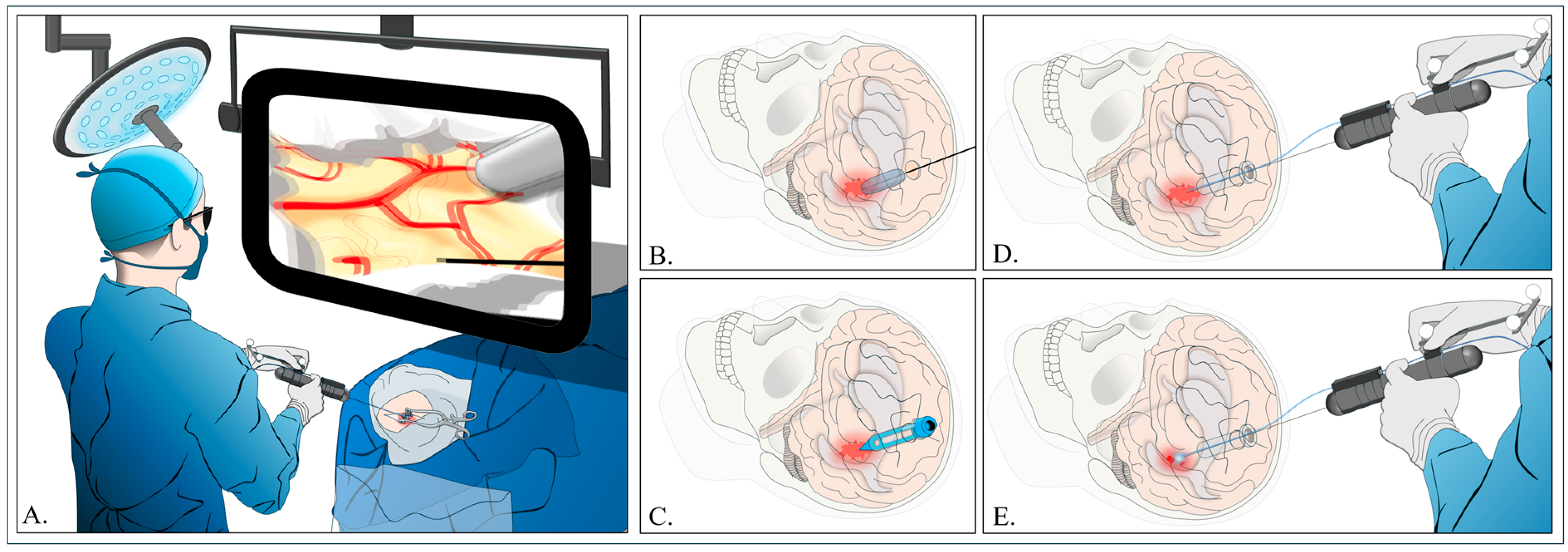
4.4. Stereotactic Endoscopic ICH Evacuation Under Blood Water Aspiration (SCUBA)
4.4.1. Trajectory
4.4.2. Hematoma Removal
4.4.3. Implications and Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef]
- Bankole, N.D.A.; Adjiou, D.K.F.d.P.; Moune, M.Y.; Hemama, M.; El Fatemi, N.; El Maaqili, M.R. Spontaneous intraparenchymal hemorrhage in young adults: Cross-sectional study. Interdiscip. Neurosurg. 2023, 33, 101762. [Google Scholar] [CrossRef]
- Feigin, V.L.; Lawes, C.M.M.; Bennett, D.A.; Anderson, C.S. Stroke epidemiology: A review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003, 2, 43–53. [Google Scholar] [CrossRef] [PubMed]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pinho, J.; Costa, A.S.; Araújo, J.M.; Amorim, J.M.; Ferreira, C. Intracerebral hemorrhage outcome: A comprehensive update. J. Neurol. Sci. 2019, 398, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, R.; Murros, K.; Rissanen, A.; Avikainen, S. Long term survival after primary intracerebral haemorrhage: A retrospective population based study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1534–1538. [Google Scholar] [CrossRef]
- Alkhiri, A.; Alamri, A.; Almaghrabi, A.A.; Alghamdi, B.A.; Alharbi, A.; Alturki, F.; Alhazzani, A.A.; Al-ajlan, F.S. Abstract WMP73: Minimally Invasive Surgery for Intracerebral Hemorrhage: Meta-Analysis of High Quality RCTs. Stroke 2024, 55, AWMP73. [Google Scholar] [CrossRef]
- Mendelow, A.D.; Gregson, B.A.; Fernandes, H.M.; Murray, G.D.; Teasdale, G.M.; Hope, D.T.; Karimi, A.; Shaw, M.D.M.; Barer, D.H.; STICH Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): A randomised trial. Lancet 2005, 365, 387–397. [Google Scholar] [CrossRef]
- Mendelow, A.D.; Gregson, B.A.; Rowan, E.N.; Murray, G.D.; Gholkar, A.; Mitchell, P.M.; STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 2013, 382, 397–408. [Google Scholar] [CrossRef]
- Xu, H.-Z.; Guo, J.; Wang, C.; Liu, X.; Song, Z.-Q.; Chen, R.-F.; Qiu, B.; Wang, Q.; Huang, Y. A Novel Stereotactic Aspiration Technique for Intracerebral Hemorrhage. World Neurosurg. 2023, 170, e28–e36. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, S. Analysis of the Therapeutic Effect and Prognostic Factors of 126 Patients with Hypertensive Cerebral Hemorrhage Treated by Soft-Channel Minimally Invasive Puncture and Drainage. Front. Surg. 2022, 9, 885580. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ascanio, L.C.; Smith, C.; Odland, I.; Murtaza-Ali, M.; Vasan, V.; Downes, M.; Schuldt, B.R.; Lin, A.; Dullea, J.; et al. Early and effective intracerebral hemorrhage evacuation is associated with a lower 1-year residual cavity volume and better functional outcomes. J. NeuroInterv. Surg. 2023, 16, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.J.; Carpenter, A.B.; Kellner, C.; Sigounas, D.; Godage, I.; Sengupta, S.; Oluigbo, C.; Cleary, K.; Chen, Y. Minimally Invasive Intracerebral Hemorrhage Evacuation: A review. Ann. Biomed. Eng. 2022, 50, 365–386. [Google Scholar] [CrossRef]
- Ibrahim, A.; Arifianto, M.R.; Al Fauzi, A. Minimally Invasive Neuroendoscopic Surgery for Spontaneous Intracerebral Hemorrhage: A Review of the Rationale and Associated Complications. Acta Neurochir. Suppl. 2023, 130, 103–108. [Google Scholar] [CrossRef]
- Pradilla, G.; Ratcliff, J.J.; Hall, A.J.; Saville, B.R.; Allen, J.W.; Paulon, G.; McGlothlin, A.; Lewis, R.J.; Fitzgerald, M.; Caveney, A.F.; et al. Trial of Early Minimally Invasive Removal of Intracerebral Hemorrhage. N. Engl. J. Med. 2024, 390, 1277–1289. [Google Scholar] [CrossRef]
- Morris, N.A.; Simard, J.M.; Chaturvedi, S. Surgical Management for Primary Intracerebral Hemorrhage. Neurology 2024, 103, e209714. [Google Scholar] [CrossRef]
- Seiffge, D.J.; Fandler-Höfler, S.; Du, Y.; Goeldlin, M.B.; Jolink, W.M.T.; Klijn, C.J.M.; Werring, D.J. Intracerebral haemorrhage—Mechanisms, diagnosis and prospects for treatment and prevention. Nat. Rev. Neurol. 2024, 20, 708–723. [Google Scholar] [CrossRef]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral haemorrhage. Lancet 2009, 373, 1632–1644. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Huster, G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993, 24, 987–993. [Google Scholar] [CrossRef] [PubMed]
- He, G.-N.; Guo, H.-Z.; Han, X.; Wang, E.-F.; Zhang, Y.-Q. Comparison of CT black hole sign and other CT features in predicting hematoma expansion in patients with ICH. J. Neurol. 2018, 265, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Hillal, A.; Ullberg, T.; Ramgren, B.; Wassélius, J. Computed tomography in acute intracerebral hemorrhage: Neuroimaging predictors of hematoma expansion and outcome. Insights Imaging 2022, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, S.; Elia, A.; Del Maestro, M.; Morotti, A.; Elbabaa, S.K.; Cavallini, A.; Galzio, R. Indication, Timing, and Surgical Treatment of Spontaneous Intracerebral Hemorrhage: Systematic Review and Proposal of a Management Algorithm. World Neurosurg. 2019, 124, e769–e778. [Google Scholar] [CrossRef]
- Sondag, L.; Schreuder, F.H.B.M.; Boogaarts, H.D.; Rovers, M.M.; Vandertop, W.P.; Dammers, R.; Klijn, C.J.M.; Dutch ICH Surgery Trial Study Group, Part of the CONTRAST Consortium. Neurosurgical Intervention for Supratentorial Intracerebral Hemorrhage. Ann. Neurol. 2020, 88, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gregson, B.A.; Broderick, J.P.; Auer, L.M.; Batjer, H.; Chen, X.-C.; Juvela, S.; Morgenstern, L.B.; Pantazis, G.C.; Teernstra, O.P.; Wang, W.-Z.; et al. Individual patient data subgroup meta-analysis of surgery for Spontaneous Supratentorial Intracerebral Haemorrhage. Stroke 2012, 43, 1496–1504. [Google Scholar] [CrossRef] [PubMed]
- Griepp, D.W.; Miller, A.; Moawad, S.; Rahme, R. Minimally Invasive Microsurgical Technique for Evacuation of Deep Intracerebral Hematomas. World Neurosurg. 2021, 149, 103. [Google Scholar] [CrossRef]
- Katta, N.; Estrada, A.D.; McErloy, A.B.; Milner, T.E. Fiber-laser platform for precision brain surgery. Biomed. Opt. Express 2022, 13, 1985–1994. [Google Scholar] [CrossRef]
- Awad, I.A.; Polster, S.P.; Carrión-Penagos, J.; Thompson, R.E.; Cao, Y.; Stadnik, A.; Money, P.L.; Fam, M.D.; Koskimäki, J.; Girard, R.; et al. Surgical Performance Determines Functional Outcome Benefit in the Minimally Invasive Surgery Plus Recombinant Tissue Plasminogen Activator for Intracerebral Hemorrhage Evacuation (MISTIE) Procedure. Neurosurgery 2019, 84, 1157–1168. [Google Scholar] [CrossRef]
- Hanley, D.F.; Thompson, R.E.; Rosenblum, M.; Yenokyan, G.; Lane, K.; McBee, N.; Mayo, S.W.; Bistran-Hall, A.J.; Gandhi, D.; Mould, W.A.; et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019, 393, 1021–1032. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, X.; Ding, Y.; Zhang, L. Assessing the Clinical Efficacy of Stereotactic Puncture in Combination with Postoperative Rehabilitation Training for Hypertensive Cerebral Hemorrhage. Altern. Ther. Health Med. 2023, 30, 122–127. [Google Scholar] [PubMed]
- Hou, X.; Li, D.; Yao, Y.; Zeng, L.; Li, C. Clinical application of 3DSlicer and Sina in minimally invasive puncture drainage of elderly patients with spontaneous intracerebral hemorrhage under local anesthesia. J. Stroke Cerebrovasc. Dis. 2023, 32, 107192. [Google Scholar] [CrossRef]
- Ratcliff, J.J.; Hall, A.J.; Porto, E.; Saville, B.R.; Lewis, R.J.; Allen, J.W.; Frankel, M.; Wright, D.W.; Barrow, D.L.; Pradilla, G. Early Minimally Invasive Removal of Intracerebral Hemorrhage (ENRICH): Study protocol for a multi-centered two-arm randomized adaptive trial. Front. Neurol. 2023, 14, 1126958. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, T.; Wang, P.; Zhang, S.; Yin, Y.; Chen, L.; Duan, H.; Wu, N.; Feng, H.; Hu, R. Efficacy and safety of NeuroEndoscopic Surgery for IntraCerebral Hemorrhage: A randomized, controlled, open-label, blinded endpoint trial (NESICH). Int. J. Stroke 2024, 19, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Lin, X.; Wang, C.; Zhou, K.; Wei, Y.; Tian, X. Endoscopic surgery versus craniotomy in the treatment of spontaneous intracerebral hematoma: A systematic review and meta-analysis. Chin. Neurosurg. J. 2022, 8, 36. [Google Scholar] [CrossRef]
- Ali, M.; Zhang, X.; Ascanio, L.C.; Troiani, Z.; Smith, C.; Dangayach, N.S.; Liang, J.W.; Selim, M.; Mocco, J.; Kellner, C.P. Long-term functional independence after minimally invasive endoscopic intracerebral hemorrhage evacuation. J. Neurosurg. 2023, 138, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Peng, Y.; Li, J.; Liu, C.; Tao, L. Long-term outcomes and cost-effectiveness evaluation of robot-assisted stereotactic hematoma drainage for spontaneous intracerebral hemorrhage. Front. Neurol. 2023, 14, 1291634. [Google Scholar] [CrossRef]
- Elguindy, M.M.; Haddad, A.F.; Lu, A.; Savastano, L.E. Minimally Invasive Endoscopic Evacuation of Cerebellar Intracerebral Hemorrhage: An Illustrative Case Report. Stroke 2024, 55, e144–e147. [Google Scholar] [CrossRef] [PubMed]
- Noiphithak, R.; Yindeedej, V.; Ratanavinitkul, W.; Duangprasert, G.; Nimmannitya, P.; Yodwisithsak, P. Treatment outcomes between endoscopic surgery and conventional craniotomy for spontaneous supratentorial intracerebral hemorrhage: A randomized controlled trial. Neurosurg. Rev. 2023, 46, 136. [Google Scholar] [CrossRef]
- Shapiro, S.D.; Alkayyali, M.; Reynolds, A.; Reilly, K.; Selim, M.; Dangayach, N.; Mocco, J.; Kellner, C.P.; Liang, J.W. Stereotactic IntraCerebral Underwater Blood Aspiration (SCUBA) Improves Survival Following Intracerebral Hemorrhage as Compared with Predicted Mortality. World Neurosurg. 2022, 161, e289–e294. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, H.; Li, D.; Liu, L.; Yang, J.; Wang, W. An effective treatment for cerebral hemorrhage: Minimally invasive craniopuncture combined with urokinase infusion therapy. Neurol. Res. 2010, 32, 371–377. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Zhang, J.; Luo, M.; Wang, Q.; Zhao, Y.; Gan, Z.; Xu, B.; Chen, X.; MISICH Study Team. Minimally invasive surgeries for spontaneous hypertensive intracerebral hemorrhage (MISICH): A multicenter randomized controlled trial. BMC Med. 2024, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Lu, Y.; Wu, D.; Tang, Y.; Dong, Q. Minimally Invasive Surgery in Patients with Intracerebral Hemorrhage: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2021, 12, 789757. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, J.; Li, Q.; Ren, G.; Yao, G.; Liu, M.; Dong, Q.; Guo, J.; Li, L.; Guo, J.; et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: A meta-analysis of randomized controlled trials. Stroke 2012, 43, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Liu, L.; Han, X.; Tao, Y.; Tang, Y.; Hua, W.; Xue, J.; Dong, Q. A prospective controlled study: Minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol. 2011, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Rost, N.S.; Smith, E.E.; Chang, Y.; Snider, R.W.; Chanderraj, R.; Schwab, K.; FitzMaurice, E.; Wendell, L.; Goldstein, J.N.; Greenberg, S.M.; et al. Prediction of functional outcome in patients with primary intracerebral hemorrhage: The FUNC score. Stroke 2008, 39, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C.; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef] [PubMed]
- He, X.-W.; Chen, M.; Du, C.-N.; Zhao, K.; Yang, M.-F.; Ma, Q.-F. A novel model for predicting the outcome of intracerebral hemorrhage: Based on 1186 Patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104867. [Google Scholar] [CrossRef]
- Ohwaki, K.; Yano, E.; Nagashima, H.; Hirata, M.; Nakagomi, T.; Tamura, A. Surgery for patients with severe supratentorial intracerebral hemorrhage. Neurocrit. Care 2006, 5, 15–20. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, J.-P.; Song, Y.-L.; Tan, K.; Wang, Y.; Li, T.; Guo, P.; Li, X.; Wang, Y.; Zhao, Q.-H. Stereotactic aspiration versus craniotomy for primary intracerebral hemorrhage: A meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e107614. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-Y.; Chen, C.-C.; Chang, C.-S.; Lee, W.-Y.; Tso, M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: Comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg. Neurol. 2006, 65, 547–555, discussion 555–556. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, D.; Zhou, L.; Wang, R.; Wang, D.; Wang, S.; Yuan, G.; Kang, S.; Zhao, Y.; Ji, N.; et al. The efficacy of three different approaches in treatment of hypertensive intracerebral hemorrhage: A multi-center single-blind study of 2464 patients. Zhonghua Yi Xue Za Zhi 2005, 85, 2238–2242. [Google Scholar] [PubMed]
- Sondag, L.; Schreuder, F.H.B.M.; Pegge, S.A.H.; Coutinho, J.M.; Dippel, D.W.J.; Janssen, P.M.; Vandertop, W.P.; Boogaarts, H.D.; Dammers, R.; Klijn, C.J.M.; et al. Safety and technical efficacy of early minimally invasive endoscopy-guided surgery for intracerebral haemorrhage: The Dutch Intracerebral haemorrhage Surgery Trial pilot study. Acta Neurochir. 2023, 165, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Kleinig, T.J.; Abou-Hamden, A.; Laidlaw, J.; Churilov, L.; Kellner, C.P.; Wu, T.; Mocco, J.; Lau, H.; Adamides, A.; Kavar, B.; et al. Early minimally invasive intracerebral hemorrhage evacuation: A phase 2a feasibility, safety, and promise of surgical efficacy study. J. NeuroInterv. Surg. 2023, 16, 555–558. [Google Scholar] [CrossRef]
- Videla, C.G.; Plou, P.; Chicue, L.V.; Yampolsky, C.; Ajler, P.M.; Ciarrocchi, N.M. Minimally Invasive Drainage of Intracerebral Hemorrhage. A South American Experience with the MISTIE Procedure. World Neurosurg. 2022, 168, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Ali, M.; Smith, C.; Jankowitz, B.; Hom, D.; Mocco, J.; Kellner, C.P. Initial Experience with the NICO Myriad Device for Minimally Invasive Endoscopic Evacuation of Intracerebral Hemorrhage. Oper. Neurosurg. 2022, 23, 194–199. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, Z.; Zhang, S.; Chen, S.; Wu, X.; Shi, J.; Liu, N.; Pan, C.; Tang, Y.; Meng, C.; et al. Evolving Therapeutic Landscape of Intracerebral Hemorrhage: Emerging Cutting-Edge Advancements in Surgical Robots, Regenerative Medicine, and Neurorehabilitation Techniques. Transl. Stroke Res. 2024. [Google Scholar] [CrossRef]
- Bako, A.T.; Potter, T.; Pan, A.P.; Tannous, J.; Britz, G.; Ziai, W.C.; Awad, I.; Hanley, D.; Vahidy, F.S. Minimally Invasive Surgery with Thrombolysis for Intracerebral Hemorrhage Evacuation: Bayesian Reanalysis of a Randomized Controlled Trial. Neurology 2023, 101, e1614–e1622. [Google Scholar] [CrossRef]
- Kellner, C.P.; Chartrain, A.G.; Nistal, D.A.; Scaggiante, J.; Hom, D.; Ghatan, S.; Bederson, J.B.; Mocco, J. The Stereotactic Intracerebral Hemorrhage Underwater Blood Aspiration (SCUBA) technique for minimally invasive endoscopic intracerebral hemorrhage evacuation. J. NeuroInterv. Surg. 2018, 10, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Hallenberger, T.J.; Guzman, R.; Soleman, J. Minimally invasive image-guided endoscopic evacuation of intracerebral haemorrhage: How I Do it. Acta Neurochir. 2023, 165, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Scullen, T.; Tucci, M.; Delashaw, J.; Khan, P.; Dumont, A.; Wang, A. Minimally Invasive Hematoma Evacuation Using the MindsEye Expandable Tubular Retractor: A Technical Note. World Neurosurg. 2023, 176, 162–167. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, J.; Zhou, D.; Liu, D. The Binding Technique for Endoscopic Spontaneous Intracerebral Hemorrhage Evacuation. World Neurosurg. 2022, 161, 64–70. [Google Scholar] [CrossRef]

| Trials | Selection Criteria for MIS TRIAL | Sample | Age Mean (Range or SD) | NIHSS at RCT Time | Timing from Ictus (hours) | ICH Volume (Mean, SD or Range) mL | mRs | Rebleeding | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| G. Pradilla et al. (2024) [15] ENRICH | ICH V: 30–80 mL, IVH < 50% Onset < 24 h Age: 18–80 Years GCS: 5–14, NIHSS score > 5 mRs ≤ 1 before ICH | MIS (150) vs. MM (150) for lobar and basal ganglia hemorrhage | 64 (56–72) | 16 (11–22) | MIS: 16.75 (10.70–21.25) MM: NA | 54 (39–72) | mRs (0–3) at 180 days: MIS (50.3%) vs. MM (41%), OR = 0.658 (95% CI: 0.433–0.957) | MIS (3.3%) MM (NA) | At 180 days: MIS (20%) vs. MM (23%) |
| Trials | Selection Criteria for MIS TRIAL | Sample | Age Mean (Range or SD) | NIHSS at RCT Time | Timing from Ictus (hours) | ICH Volume (Mean, SD or Range) mL | mRs | Rebleeding | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Noiphithak et al. (2023) [38] | Age: 18 to 80 years ICH ≥ 30 mL GCS: 5 to 14 NIHSS > 6 mRs ≤ 1 before ICH Onset < 24 h No-obstructive hydrocephalus IVH < 50% | MIS(95) vs. CC(93) for lobar and basal ganglia hemorrhage | 51 (18) | 18 (8) | MIS: 6.8 (2) vs. CC: 6.6 (2.5) | MIS: 50.1 (33) vs. CC: 49.3 (28.9) | mRs (0–3) at 180 days: MIS (48.4%) vs. CC (35.5%) (adjusted RD 17.3, 95% CI [4.6–30.0], p = 0.01). | MIS (3.2%) vs. CC (5.4%) p = 0.5 | At 180 days: MIS (22.1%) vs. CC (21.5%) |
| Xu X et al. 2024 [41] MISICH | ICH V: ≥25 mL, Onset < 24 h Age: 18–80 Years GCS: ≥5, mRs ≤ 1 before ICH | MIS (239) vs. CC (236) for lobar and basal ganglia hemorrhage | 56.7 (11.3) | NA | From ictus < 24 | MIS: 49.1 (SD 20.3) vs. CC: 49.9 (17.6) | mRs (0–2) at 180 days: MIS (33.3%) vs. CC (22.2%), p = 0.017 | MIS (3.8%) vs. CC (5.1%) p = 0.89 | At 180 days: MIS (13.7%) vs. CC (13.2%), p = 0.60 |
| Wang L et al. 2024 [33] NESICH | ICH V: ≥25 mL, Onset < 24 h Age: 18–80 Years GCS: ≥5, mRs ≤ 1 before ICH | Estimation of 560 patients to be enrolled in ongoing trial for lobar and basal ganglia hemorrhage | NA | NA | NA | NA | NA | NA | NA |
| Trials | Selection Criteria for MIS TRIAL | Sample | Age Mean (Range or SD) | NIHSS at RCT Time | Timing from Ictus (Hours) to Surgery | ICH Volume (Mean, SD or Range) mL | ADL or mRs | Rebleeding | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Sun et al. (2010) [40] | ICH V: 30–80 mL Age: 40–75 years From onset ≤ 72 h | MIS (159) vs. CC (145) for basal ganglia hemorrhage | 56.9 (8.9) | NA | From ictus ≤ 72 | MIS: 52.3 (14.5) vs. CC: 51.7 (14.7) | mRs (0–3) at 14 days: MIS (13.6%) vs. CC (18.8%) (p = 0.69) Favorable outcome with ADL at 90 days (Barthel Index > 95): MIS (20.6%) vs. CC (11.1%) (p < 0.05) | MIS (8.8%) vs. CC (21.4%) (p = 0.002) | At 90 days: MIS (14.5%) vs. CC (25%) (p = 0.02) |
| Zhou H et al. 2011 [44] MISPT | Age: 40–75 years ICH: 30–100 mL Onset < 24 h GCS: ≥5 | MIS (90) vs. CC (78) for basal ganglia hemorrhage vs. conventional craniotomy | 57.6 (11.2) | NA | From ictus < 24 | ICH: 30–100 mL for both MIS and CC | MIS: mRs (2.2 ± 0.3) vs. CC (3.9 ± 0.4), p = 0.042 | MIS (10%) vs. CC (15.4%), p = 0.29 | MIS (18.9%) vs. CC (24.4%), p = 0.38 |
| Hanley et al. (2019) [29] MISTIE III | ICH V: ≥30 mL, Age: ≥18 years, GCS: ≤14, NIHSS score ≥ 6 mRs ≤ 1 before ICH | MIS (250) vs. MM (249) for lobar and basal ganglia hemorrhage | 62 (52–70) | 19 (15–23) | 35.1 (23.4–52.8) | MIS: 45.8 (35.4–59.6) vs. MM: 45.3 (35.4–57.2), | mRs (0–3) at 365 days: 45% (MIS) vs. 41% (MM) Adjust risk difference 4% (95% CI: 4–12), p = 0.33 | MIS (2.4%) vs. MM (1.2%) p = 0.32 | At 180 days: MIS (15.3%) vs. MM (22.7%) HR = 0.67, 95% CI (0.45–0.98), p = 0.03 |
| Xu X et al. 2024 [41] MISICH | ICH V: ≥25 mL, Onset < 24 h Age: 18–80 Years GCS: ≥5, mRs ≤ 1 before ICH | MIS (246) vs. CC (236) for lobar and basal ganglia hemorrhage | 56.7 (11.3) | NA | From ictus < 24 | MIS: 48.5 (14.9) vs. CC: 49.9 (17.6) | mRs (0–2) at 180 days: MIS (32.7%) vs. CC (22.2%), p = 0.017 | MIS (6.1%) vs. CC (5.1%) p = 0.89 | At 180 days: MIS (16.4%) vs. CC (13.2%), p = 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bankole, N.D.A.; Kuntz, C.; Planty-Bonjour, A.; Beaufort, Q.; Gaberel, T.; Cordonnier, C.; Pasi, M.; Schlunk, F.; Nawabi, J.; Zemmoura, I.; et al. Minimally Invasive Surgery for Spontaneous Intracerebral Hemorrhage: A Review. J. Clin. Med. 2025, 14, 1155. https://doi.org/10.3390/jcm14041155
Bankole NDA, Kuntz C, Planty-Bonjour A, Beaufort Q, Gaberel T, Cordonnier C, Pasi M, Schlunk F, Nawabi J, Zemmoura I, et al. Minimally Invasive Surgery for Spontaneous Intracerebral Hemorrhage: A Review. Journal of Clinical Medicine. 2025; 14(4):1155. https://doi.org/10.3390/jcm14041155
Chicago/Turabian StyleBankole, Nourou Dine Adeniran, Cyrille Kuntz, Alexia Planty-Bonjour, Quentin Beaufort, Thomas Gaberel, Charlotte Cordonnier, Marco Pasi, Frieder Schlunk, Jawed Nawabi, Ilyess Zemmoura, and et al. 2025. "Minimally Invasive Surgery for Spontaneous Intracerebral Hemorrhage: A Review" Journal of Clinical Medicine 14, no. 4: 1155. https://doi.org/10.3390/jcm14041155
APA StyleBankole, N. D. A., Kuntz, C., Planty-Bonjour, A., Beaufort, Q., Gaberel, T., Cordonnier, C., Pasi, M., Schlunk, F., Nawabi, J., Zemmoura, I., & Boulouis, G. (2025). Minimally Invasive Surgery for Spontaneous Intracerebral Hemorrhage: A Review. Journal of Clinical Medicine, 14(4), 1155. https://doi.org/10.3390/jcm14041155






