Variability in Corneal and Epithelial Pachymetry: A Comparison of Optopol Revo 130 and Optovue RTV XR Avanti in Healthy Patients
Abstract
:1. Introduction
2. Materials and Methods
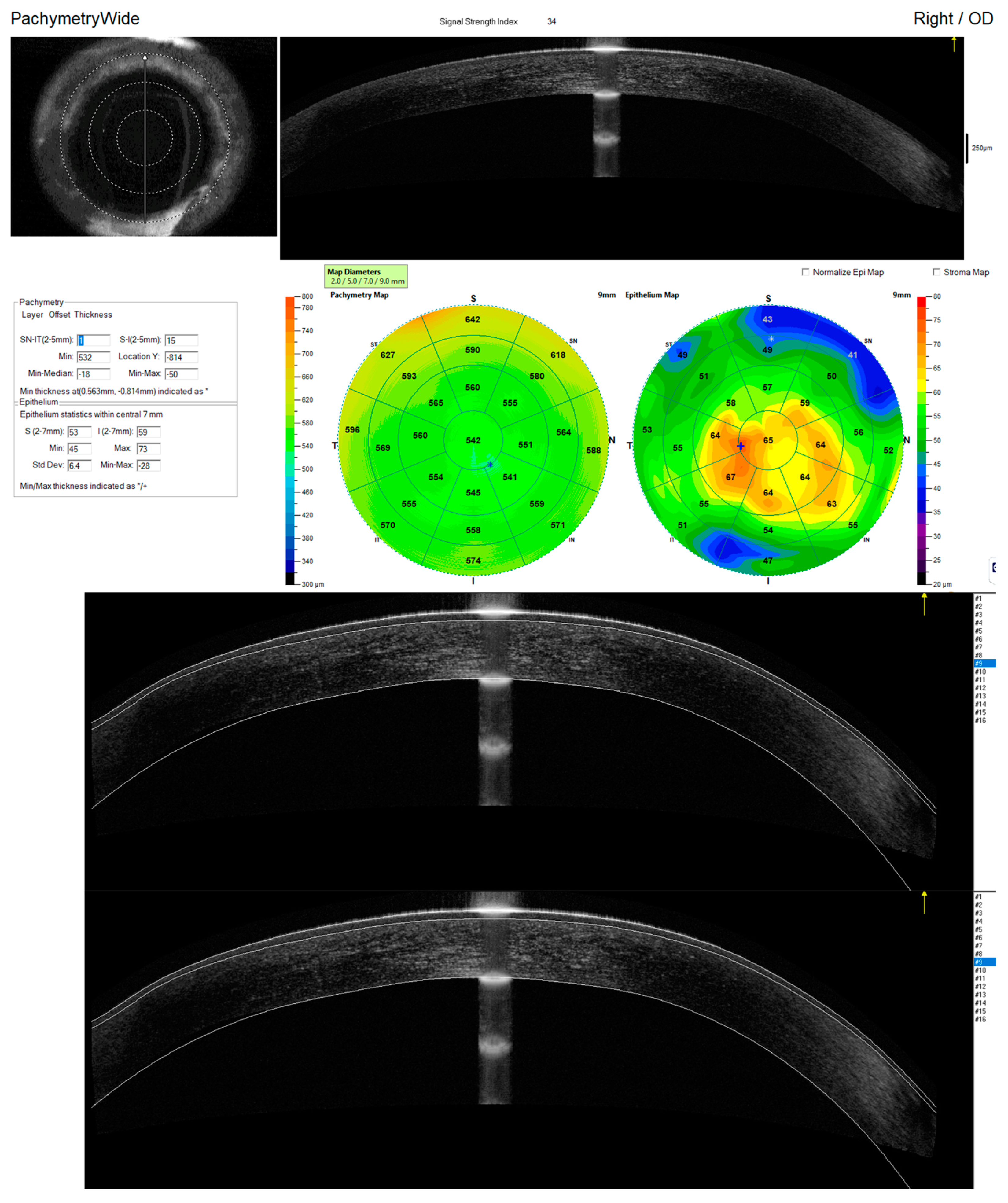
2.1. Study Procedures
2.2. Quality Assessment and Segmentation Correction
2.3. Statistical Analyses
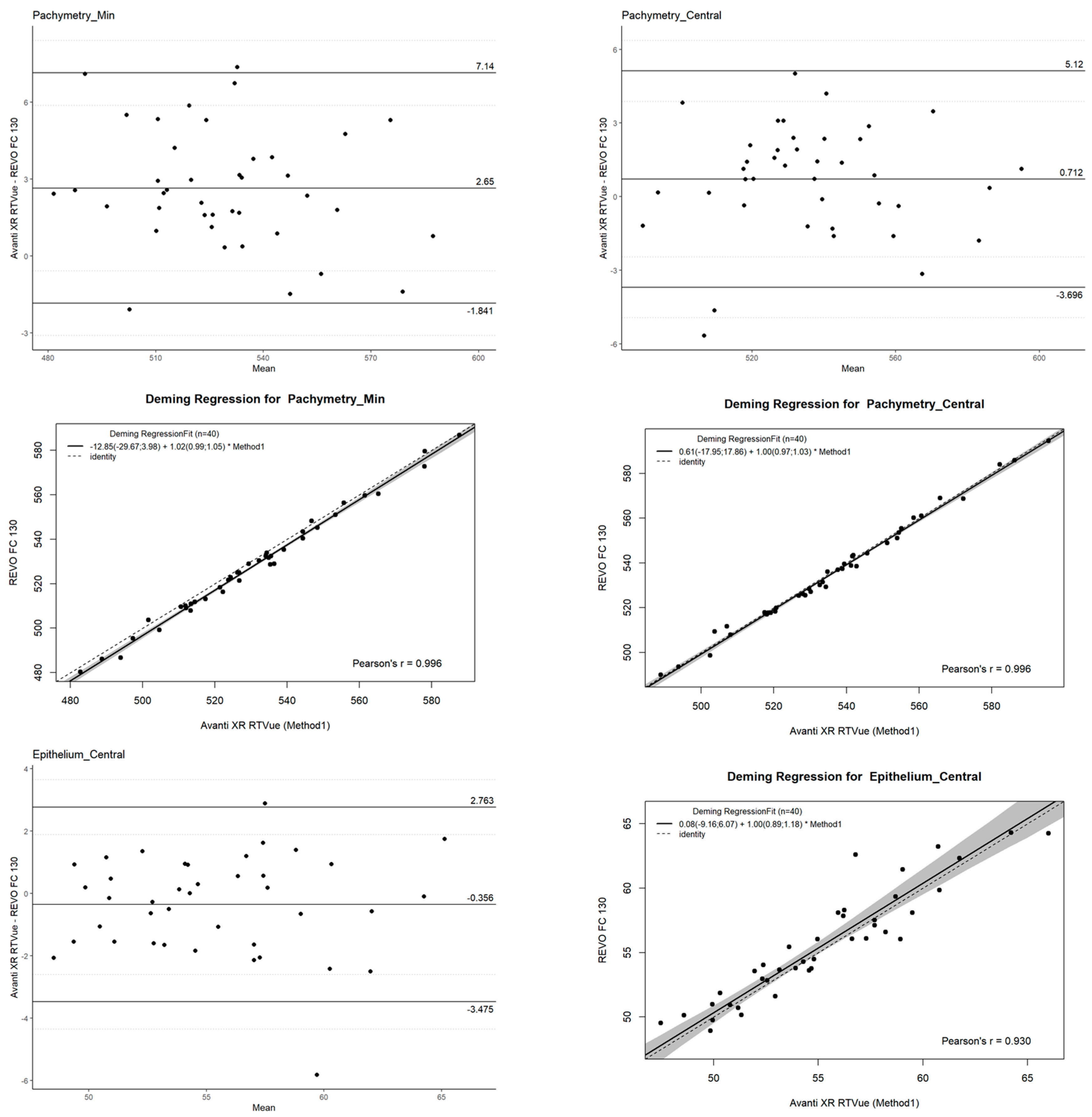
2.4. Agreement
- Mean difference (bias): Representing the systematic difference between the two devices.
- Limits of Agreement (LOAs): Defined as the mean difference ±1.96 standard deviations (SD), providing the range within which 95% of differences between measurements are expected to lie.
- Confidence Intervals (CI): Calculated for the LOAs to provide an estimate of their precision and reliability.
2.5. Precision
- Overall means (Ῡ) with standard deviation (SD): Provided a baseline measure of the central tendency and dispersion of thickness values.
- Within-subject standard deviation (SW): Quantified the variability in measurements for the same patient under identical conditions, reflecting the reliability of repeated scans.
- Test–retest variability (TRT): Calculated as √2 × 1.96 × SW, this metric offered a direct estimate of the range within which repeated measurements could be expected to fall.
- Coefficient of variation (COV): Expressed as a percentage, calculated as (100 × SW)/Ῡ, it indicated relative variability in relation to the mean thickness values.
- Intraclass correlation coefficient (ICC): Provided a statistical measure of the reliability of measurements, with values closer to 1.0 indicating stronger agreement between scans.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khamar, P.; Rao, K.; Wadia, K.; Dalal, R.; Grover, T.; Versaci, F.; Gupta, K. Advanced epithelial mapping for refractive surgery. Indian. J. Ophthalmol. 2020, 68, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Abusamak, M. Corneal Epithelial Mapping Characteristics in Normal Eyes Using Anterior Segment Spectral Domain Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2022, 11, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abtahi, M.A.; Beheshtnejad, A.H.; Latifi, G.; Akbari-Kamrani, M.; Ghafarian, S.; Masoomi, A.; Sonbolastan, S.A.; Jahanbani-Ardakani, H.; Atighechian, M.; Banan, L.; et al. Corneal Epithelial Thickness Mapping: A Major Review. J. Ophthalmol. 2024, 2024, 6674747. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. RA Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 September 2024).
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 10 September 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilke, C.; Wiernik, B. _ggtext: Improved Text Rendering Support for ’ggplot2’_. R Package, Version 0.1.2; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Auguie, B. _gridExtra: Miscellaneous Functions for “Grid” Graphics_. R Package, Version 2.3; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Kassambara, A. _ggpubr: ’ggplot2’ Based Publication Ready Plots_. R package, Version 0.6.0; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Caldwell, A.R. SimplyAgree: An R package and jamovi Module for Simplifying Agreement and Reliability Analyses. J. Open Source Softw. 2022, 7, 4148. [Google Scholar] [CrossRef]
- Potapov, S.; Model, F.; Schuetzenmeister, A.; Manuilova, E.; Dufey, F.; Raymaekers, J. _mcr: Method Comparison Regression_. R Package, Version 1.3.2; R Core Team: Vienna, Austria, 2023. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R Package for Assessment, Comparison and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Giavarina, D. Understanding Bland Altman analysis. Biochem. Med. 2015, 25, 141–151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; Murray, G. Statistical strategies to assess reliability in ophthalmology. Eye 2006, 20, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- CCarroll, R.J.; Ruppert, D. The Use and Misuse of Orthogonal Regression in Linear Errors-in-Variables Models. Am. Stat. 1996, 50, 1–6. [Google Scholar] [CrossRef]
- Payne, R.B. Deming’s Regression Analysis in Method Comparison Studies. Ann. Clin. Biochem. 1985, 22, 430. [Google Scholar] [CrossRef]
- Ge, L.; Yuan, Y.; Shen, M.; Tao, A.; Wang, J.; Lu, F. The role of axial resolution of optical coherence tomography on the measurement of corneal and epithelial thicknesses. Invest. Ophthalmol. Vis. Sci. 2013, 54, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Tao, A.; Wang, J.; Chen, Q.; Shen, M.; Lu, F.; Dubovy, S.R.; Shousha, M.A. Topographic thickness of Bowman’s layer determined by ultra-high resolution spectral domain-optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3901–3907. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, T.; Balakrishna, N. Intrasession repeatability of pachymetry measurements with RTVue XR 100 optical coherence tomography in normal cornea. Saudi J. Ophthalmol. 2017, 2, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Vienola, K.V.; Braaf, B.; Sheehy, C.K.; Yang, Q.; Tiruveedhula, P.; Arathorn, D.W.; de Boer, J.F.; Roorda, A. Real-time eye motion compensation for OCT imaging with tracking SLO. Biomed. Opt. Express. 2012, 3, 2950–2963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shekhar, R.; Huang, D. Corneal pachymetry mapping with high-speed optical coherence tomography. Ophthalmology 2006, 113, 792–799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Read, S.A.; Collins, M.J.; Carney, L.G.; Franklin, R.J. The topography of the central and peripheral cornea. Invest. Ophthalmol. Vis. Sci. 2006, 47, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Bergmanson, J.P.G.; Burns, A.R.; Walker, M.K. Central versus peripheral thickness in the human cornea explained. Cont. Lens Anterior Eye. 2024, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- King-Smith, P.E.; Fink, B.A.; Fogt, N.; Nichols, K.K.; Hill, R.M.; Wilson, G.S. The thickness of the human precorneal tear film: Evidence from reflection spectra. Invest. Ophthalmol. Vis. Sci. 2000, 41, 3348–3359. [Google Scholar] [PubMed]
- Averich, V.V.; Bubnova, I.A. Izmenenie prekorneal’noi sleznoi plenki pri razlichnykh stadiyakh keratokonusa [Changes in the precorneal tear film at various stages of keratoconus]. Vestn. Oftalmol. 2024, 140, 43–49. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xu, Z.; Wang, H.; Wang, Y.; Wei, L.; Ma, H.; Zhao, J.; Lu, F.; Hu, L. Comparison of the repeatability and reproducibility of corneal thickness mapping using optical coherence tomography according to tear film break-up time. BMC Ophthalmol. 2024, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Temstet, C.; Sandali, O.; Bouheraoua, N.; Hamiche, T.; Galan, A.; El Sanharawi, M.; Basli, E.; Laroche, L.; Borderie, V. Corneal epithelial thickness mapping using Fourier-domain optical coherence tomography for detection of form fruste keratoconus. J Cataract. Refract. Sur. 2015, 41, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Lozano García, I.; Romero Caballero, M.D.; Sellés Navarro, I. High resolution anterior segment optical coherence tomography for differential diagnosis between corneo-conjunctival intraepithelial neoplasia and pterygium. Arch. Soc. Esp. Oftalmol. (Engl Ed). 2020, 95, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Yusufoğlu, E.; Güngör Kobat, S.; Keser, S. Evaluation of central corneal epithelial thickness with anterior segment OCT in patients with type 2 diabetes mellitus. Int. Ophthalmol. 2023, 43, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Babić Leko, M.; Pleić, N.; Lešin, M.; Gunjača, I.; Torlak, V.; Škunca Herman, J.; Vatavuk, Z.; Punda, A.; Polašek, O.; Hayward, C.; et al. Association between Thyroid Function and Ocular Parameters. Biology 2022, 11, 1847. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Archer, T.J.; Vida, R.S. Epithelial thickness mapping for corneal refractive surgery. Curr. Opin. Ophthalmol. 2022, 33, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Reinstein, D.Z.; Nitter, T.; Archer, T.J.; McAlinden, C.; Chen, X.; Bertelsen, G.; Utheim, T.P.; Stojanovic, A. Heidelberg Anterion Swept-Source OCT Corneal Epithelial Thickness Mapping: Repeatability and Agreement with Optovue Avanti. J. Refract. Surg. 2022, 38, 6. [Google Scholar] [CrossRef] [PubMed]
- Tatar-Mathes, P.; Hansen, R.E.; Choi, S.; Marques, M.J.; Israelsen, N.M.; Podoleanu, A. Temperature Dependence of a Depth-Encoded System for Polarization-Sensitive Optical Coherence Tomography using a PM Fiber. arXiv 2023, arXiv:2309.00833. [Google Scholar]
| Mean Measurement | Mean Measurement | Mean Measurement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avanti Mean | Avanti SD | REVO Mean | REVO SD | Avanti Mean | Avanti SD | REVO Mean | REVO SD | Avanti Mean | Avanti SD | REVO Mean | REVO SD | |||
| 2 mm Epi_2 Central | 55.2 | 4.26 | 55.55 | 4.28 | 2 mm Pachy_2 Central | 536.56 | 24.19 | 535.85 | 24.13 | 2 mm Stroma_2 Central | 481.36 | 23.72 | 480.29 | 23.56 |
| 2–5 mm Epi_I_2_5 | 56.43 | 4.35 | 55.99 | 4.2 | 2–5 mm Pachy_I_2_5 | 556.54 | 24.38 | 548.89 | 25.09 | 2–5 mm Stroma_I_2_5 | 500.11 | 23.88 | 492.9 | 24.42 |
| 2–5 mm Epi_IN_2_5 | 55.87 | 3.96 | 55.69 | 4.08 | 2–5 mm Pachy_IN_2_5 | 563.43 | 23.55 | 557.42 | 24.67 | 2–5 mm Stroma_IN_2_5 | 507.56 | 23.39 | 501.72 | 24.14 |
| 2–5 mm Epi_IT_2_5 | 55.67 | 4.54 | 55.3 | 4.41 | 2–5 mm Pachy_IT_2_5 | 547.15 | 24.89 | 539.15 | 25.23 | 2–5 mm Stroma_IT_2_5 | 491.48 | 24.26 | 483.85 | 24.37 |
| 2–5 mm Epi_S_2_5 | 54.02 | 3.47 | 53.96 | 3.61 | 2–5 mm Pachy_N_2_5 | 567.9 | 24.01 | 563.87 | 24.52 | 2–5 mm Stroma_N_2_5 | 512.92 | 23.65 | 508.92 | 24.12 |
| 2–5 mm Epi_SN_2_5 | 54.54 | 3.62 | 54.43 | 3.58 | 2–5 mm Pachy_S_2_5 | 570.9 | 27.15 | 566.36 | 25.83 | 2–5 mm Stroma_S_2_5 | 516.88 | 26.56 | 512.4 | 25.22 |
| 2–5 mm Epi_ST_2_5 | 53.87 | 3.66 | 53.99 | 3.72 | 2–5 mm Pachy_SN_2_5 | 572.9 | 25.78 | 569.59 | 25.06 | 2–5 mm Stroma_SN_2_5 | 518.36 | 25.29 | 515.16 | 24.72 |
| 2–5 mm Epi_T_2_5 | 54.67 | 4.06 | 54.28 | 4.05 | 2–5 mm Pachy_ST_2_5 | 557.11 | 27.3 | 551.5 | 25.82 | 2–5 mm Stroma_ST_2_5 | 503.24 | 26.76 | 497.51 | 25.06 |
| 2–5 mmEpi_N_2_5 | 54.98 | 3.73 | 54.96 | 3.98 | 2–5 mm Pachy_T_2_5 | 545.37 | 25.96 | 538.02 | 25.27 | 2–5 mm Stroma_T_2_5 | 490.69 | 25.45 | 483.74 | 24.49 |
| 5–7 mm Epi_I_5_7 | 56.44 | 3.67 | 55.93 | 3.15 | 5–7 mm Pachy_I_5_7 | 592.19 | 26.14 | 586.41 | 26.86 | 5–7 mm Stroma_I_5_7 | 535.75 | 25.63 | 530.48 | 26.23 |
| 5–7 mm Epi_IN_5_7 | 55.58 | 3.13 | 55.74 | 2.96 | 5–7 mm Pachy_IN_5_7 | 600.8 | 26.07 | 598.77 | 26.43 | 5–7 mm Stroma_IN_5_7 | 545.21 | 25.78 | 543.03 | 25.95 |
| 5–7 mm Epi_IT_5_7 | 56.15 | 3.88 | 55.45 | 3.55 | 5–7 mm Pachy_IT_5_7 | 575.17 | 26.47 | 568.25 | 26.39 | 5–7 mm Stroma_IT_5_7 | 519.01 | 25.86 | 512.8 | 25.75 |
| 5–7 mm Epi_N_5_7 | 55.14 | 3.45 | 55.6 | 3.23 | 5–7 mm Pachy_N_5_7 | 605.71 | 25.63 | 606.3 | 25.9 | 5–7 mm Stroma_N_5_7 | 550.57 | 25.43 | 550.69 | 25.44 |
| 5–7 mm Epi_S_5_7 | 51.82 | 3.32 | 52.15 | 2.64 | 5–7 mm Pachy_S_5_7 | 615.73 | 29.77 | 614.67 | 27.68 | 5–7 mm Stroma_S_5_7 | 563.91 | 29.65 | 562.52 | 27.64 |
| 5–7 mm Epi_SN_5_7 | 54.05 | 3.25 | 54.31 | 2.94 | 5–7 mm Pachy_SN_5_7 | 615.9 | 27.4 | 617.31 | 26.31 | 5–7 mm Stroma_SN_5_7 | 561.86 | 27.45 | 563 | 26.28 |
| 5–7 mm Epi_ST_5_7 | 52.47 | 3.2 | 53.05 | 2.54 | 5–7 mm Pachy_ST_5_7 | 591.68 | 30.16 | 588.45 | 26.7 | 5–7 mm Stroma_ST_5_7 | 539.21 | 29.83 | 535.4 | 26.68 |
| 5–7 mm Epi_T_5_7 | 54.05 | 3.34 | 54.01 | 3.12 | 5–7 mm Pachy_T_5_7 | 568.76 | 27.6 | 562.59 | 25.79 | 5–7 mm Stroma_T_5_7 | 514.72 | 27.31 | 508.58 | 25.44 |
| Epi_Median | 53.13 | 3.6 | 54.69 | 3.09 | Pachy_Median | 554.92 | 24.55 | 578.96 | 25.4 | Stroma_Median | 519.16 | 24.88 | 524.23 | 25.08 |
| Epi_Min | 48.85 | 2.93 | 48.15 | 2.42 | Pachy_Min | 530.18 | 24.09 | 527.53 | 24.55 | Stroma_Min | 474.96 | 23.69 | 472.47 | 23.79 |
| Parameter Name | Repeatability Operator 1 | Repeatability Operator 2 | Reproducibility | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SW | TRT | COV% | ICC | Mean | SD | SW | TRT | COV% | ICC | Mean_Rep | SD_Rep | SW_Rep | TRT_Rep | COV_Rep | ICC_Rep | |
| Pachymetry | ||||||||||||||||||
| Pachymetry_Min | 531.14 | 31.97 | 1.84 | 5.1 | 0.35 | 0.9968 | 531.01 | 31.88 | 1.25 | 3.47 | 0.24 | 0.9985 | 531.08 | 31.85 | 1.92 | 5.32 | 0.36 | 0.9976 |
| Pachymetry_Median | 581.15 | 31.85 | 2.39 | 6.63 | 0.41 | 0.9945 | 580.77 | 31.88 | 1.9 | 5.27 | 0.33 | 0.9965 | 580.96 | 31.8 | 2.52 | 7 | 0.43 | 0.9955 |
| Pachymetry_Central | 539.45 | 31.99 | 1.8 | 4.98 | 0.33 | 0.9969 | 539.3 | 31.77 | 1.55 | 4.31 | 0.29 | 0.9977 | 539.38 | 31.81 | 1.87 | 5.17 | 0.35 | 0.9973 |
| Pachymetry 2–5 mm | ||||||||||||||||||
| Pachymetry_S_2–5 mm | 570.2 | 33.88 | 3.2 | 8.88 | 0.56 | 0.9913 | 569.68 | 33.55 | 3.2 | 8.86 | 0.56 | 0.9911 | 569.94 | 33.64 | 3.69 | 10.24 | 0.65 | 0.9912 |
| Pachymetry_SN_2–5 mm | 573.92 | 33.26 | 3.69 | 10.24 | 0.64 | 0.9878 | 573.62 | 32.7 | 3.92 | 10.85 | 0.68 | 0.986 | 573.77 | 32.91 | 4.24 | 11.75 | 0.74 | 0.9869 |
| Pachymetry_N_2–5 mm | 567.73 | 32.14 | 3.3 | 9.15 | 0.58 | 0.9897 | 567.76 | 31.9 | 3.58 | 9.93 | 0.63 | 0.9877 | 567.74 | 31.95 | 3.87 | 10.72 | 0.68 | 0.9887 |
| Pachymetry_IN_2–5 mm | 560.87 | 31.65 | 2.6 | 7.2 | 0.46 | 0.9935 | 561.31 | 31.51 | 2.96 | 8.22 | 0.53 | 0.9914 | 561.09 | 31.51 | 3.1 | 8.6 | 0.55 | 0.9924 |
| Pachymetry_I_2–5 mm | 552.4 | 31.53 | 2.19 | 6.06 | 0.4 | 0.9953 | 552.65 | 31.49 | 1.99 | 5.53 | 0.36 | 0.9961 | 552.52 | 31.44 | 2.42 | 6.71 | 0.44 | 0.9957 |
| Pachymetry_IT_2–5 mm | 542.86 | 31.6 | 2.42 | 6.7 | 0.45 | 0.9943 | 542.67 | 31.56 | 1.69 | 4.7 | 0.31 | 0.9972 | 542.76 | 31.51 | 2.53 | 7 | 0.47 | 0.9958 |
| Pachymetry_T_2–5 mm | 541.58 | 32.42 | 2.41 | 6.67 | 0.44 | 0.9947 | 541.02 | 32.71 | 1.77 | 4.92 | 0.33 | 0.9971 | 541.3 | 32.5 | 2.53 | 7.01 | 0.47 | 0.9959 |
| Pachymetry_ST_2–5 mm | 555.08 | 33.6 | 2.74 | 7.6 | 0.49 | 0.9935 | 554.42 | 33.73 | 2.09 | 5.79 | 0.38 | 0.9962 | 554.75 | 33.59 | 2.86 | 7.94 | 0.52 | 0.9949 |
| Pachymetry 5–7 mm | ||||||||||||||||||
| Pachymetry_S_5–7 mm | 616.33 | 35.17 | 4.36 | 12.09 | 0.71 | 0.9852 | 615.72 | 33.98 | 4.48 | 12.42 | 0.73 | 0.9833 | 616.02 | 34.49 | 5.48 | 15.19 | 0.89 | 0.9837 |
| Pachymetry_SN_5–7 mm | 619.12 | 34.12 | 4.48 | 12.41 | 0.72 | 0.9838 | 620.87 | 34.26 | 5.41 | 15 | 0.87 | 0.9757 | 620.01 | 34.13 | 6.08 | 16.84 | 0.98 | 0.9792 |
| Pachymetry_N_5–7 mm | 608.87 | 33.73 | 4.33 | 12.01 | 0.71 | 0.984 | 609.05 | 33.16 | 5.23 | 14.5 | 0.86 | 0.9759 | 608.96 | 33.37 | 5.49 | 15.22 | 0.9 | 0.98 |
| Pachymetry_IN_5–7 mm | 600.19 | 33.34 | 3.56 | 9.87 | 0.59 | 0.989 | 600.84 | 32.77 | 4.4 | 12.19 | 0.73 | 0.9827 | 600.52 | 32.98 | 4.55 | 12.62 | 0.76 | 0.9859 |
| Pachymetry_I_5–7 mm | 587.91 | 32.43 | 2.83 | 7.85 | 0.48 | 0.9926 | 587.64 | 32.47 | 2.98 | 8.27 | 0.51 | 0.9919 | 587.78 | 32.38 | 3.36 | 9.3 | 0.57 | 0.9923 |
| Pachymetry_IT_5–7 mm | 570.73 | 31.13 | 3.45 | 9.56 | 0.6 | 0.988 | 570.78 | 31.72 | 3.54 | 9.82 | 0.62 | 0.9877 | 570.76 | 31.36 | 4.2 | 11.64 | 0.74 | 0.9879 |
| Pachymetry_T_5–7 mm | 565.94 | 32.37 | 3.67 | 10.17 | 0.65 | 0.9874 | 565.44 | 32.94 | 3.51 | 9.73 | 0.62 | 0.9888 | 565.69 | 32.59 | 4.17 | 11.55 | 0.74 | 0.9881 |
| Pachymetry_ST_5–7 mm | 590.2 | 33.09 | 3.74 | 10.36 | 0.63 | 0.9879 | 590.05 | 34.16 | 3.41 | 9.44 | 0.58 | 0.9901 | 590.13 | 33.56 | 4.17 | 11.57 | 0.71 | 0.989 |
| Epithelium | ||||||||||||||||||
| Epithelium_Central | 55.83 | 4.28 | 1.18 | 3.26 | 2.11 | 0.9247 | 55.84 | 4.24 | 0.98 | 2.72 | 1.76 | 0.9455 | 55.83 | 4.25 | 1.29 | 3.56 | 2.3 | 0.9348 |
| Epithelium 2–5 mm | ||||||||||||||||||
| Epithelium_S_2–5 mm | 54.15 | 4.12 | 1.21 | 3.37 | 2.24 | 0.9156 | 54.15 | 3.98 | 1.18 | 3.28 | 2.18 | 0.9127 | 54.15 | 4.04 | 1.5 | 4.16 | 2.77 | 0.9141 |
| Epithelium_SN_2–5 mm | 55.05 | 4.11 | 1.3 | 3.59 | 2.35 | 0.9024 | 54.87 | 3.91 | 1.09 | 3.03 | 1.99 | 0.9221 | 54.96 | 4.01 | 1.47 | 4.08 | 2.68 | 0.9119 |
| Epithelium_N_2–5 mm | 55.45 | 4.19 | 1.32 | 3.65 | 2.37 | 0.9026 | 55.28 | 4.13 | 1.03 | 2.87 | 1.87 | 0.9368 | 55.37 | 4.15 | 1.4 | 3.88 | 2.53 | 0.9192 |
| Epithelium_IN_2–5 mm | 56.14 | 4.45 | 1.3 | 3.6 | 2.31 | 0.9174 | 56.12 | 4.37 | 1.23 | 3.41 | 2.19 | 0.9206 | 56.13 | 4.4 | 1.52 | 4.21 | 2.7 | 0.919 |
| Epithelium_I_2–5 mm | 56.34 | 4.62 | 1.31 | 3.62 | 2.32 | 0.9233 | 56.34 | 4.4 | 1.12 | 3.09 | 1.98 | 0.9376 | 56.34 | 4.5 | 1.47 | 4.06 | 2.6 | 0.93 |
| Epithelium_IT_2–5 mm | 55.61 | 4.63 | 1.23 | 3.41 | 2.21 | 0.932 | 55.59 | 4.43 | 1.02 | 2.83 | 1.84 | 0.9484 | 55.6 | 4.52 | 1.42 | 3.94 | 2.56 | 0.9398 |
| Epithelium_T_2–5 mm | 54.33 | 4.17 | 1.14 | 3.15 | 2.09 | 0.9285 | 54.25 | 4.1 | 0.99 | 2.75 | 1.83 | 0.9429 | 54.29 | 4.13 | 1.44 | 3.98 | 2.64 | 0.9355 |
| Epithelium_ST_2–5 mm | 53.93 | 4.09 | 1.2 | 3.33 | 2.23 | 0.917 | 53.85 | 3.95 | 1.09 | 3.01 | 2.02 | 0.9251 | 53.89 | 4.02 | 1.52 | 4.2 | 2.81 | 0.9208 |
| Epithelium 5–7 mm | ||||||||||||||||||
| Epithelium_S_5–7 mm | 52.47 | 3.65 | 1.15 | 3.18 | 2.18 | 0.9047 | 52.9 | 3.28 | 1.09 | 3.03 | 2.07 | 0.8871 | 52.69 | 3.47 | 1.37 | 3.8 | 2.6 | 0.894 |
| Epithelium_SN_5–7 mm | 54.42 | 3.45 | 1.06 | 2.93 | 1.94 | 0.9082 | 54.64 | 3.1 | 0.87 | 2.41 | 1.59 | 0.924 | 54.53 | 3.27 | 1.12 | 3.1 | 2.05 | 0.9149 |
| Epithelium_N_5–7 mm | 55.48 | 3.27 | 0.87 | 2.4 | 1.56 | 0.9325 | 55.28 | 3.24 | 0.77 | 2.13 | 1.39 | 0.9454 | 55.38 | 3.25 | 0.99 | 2.75 | 1.79 | 0.9389 |
| Epithelium_IN_5–7 mm | 55.7 | 3.4 | 0.97 | 2.69 | 1.75 | 0.9216 | 55.57 | 3.28 | 0.76 | 2.1 | 1.36 | 0.9486 | 55.63 | 3.33 | 0.98 | 2.71 | 1.76 | 0.9347 |
| Epithelium_I_5–7 mm | 55.83 | 3.71 | 1.05 | 2.91 | 1.88 | 0.9229 | 55.73 | 3.31 | 0.63 | 1.75 | 1.14 | 0.965 | 55.78 | 3.51 | 0.97 | 2.68 | 1.74 | 0.9416 |
| Epithelium_IT_5–7 mm | 55.48 | 3.39 | 0.78 | 2.16 | 1.41 | 0.9493 | 55.29 | 3.47 | 0.75 | 2.09 | 1.36 | 0.9546 | 55.39 | 3.42 | 0.86 | 2.39 | 1.56 | 0.952 |
| Epithelium_T_5–7 mm | 53.87 | 3.21 | 0.64 | 1.77 | 1.18 | 0.9619 | 53.69 | 3.4 | 0.8 | 2.22 | 1.49 | 0.9461 | 53.78 | 3.3 | 0.93 | 2.59 | 1.74 | 0.9537 |
| Epithelium_ST_5–7 mm | 53.11 | 3.59 | 0.78 | 2.15 | 1.46 | 0.9529 | 53.18 | 3.51 | 0.9 | 2.5 | 1.69 | 0.9321 | 53.14 | 3.54 | 0.98 | 2.71 | 1.84 | 0.9419 |
| Stroma | ||||||||||||||||||
| Stroma_Min | 475.78 | 30.83 | 1.18 | 3.28 | 0.25 | 0.9986 | 475.55 | 30.81 | 0.57 | 1.57 | 0.12 | 0.9997 | 475.66 | 30.75 | 1.2 | 3.32 | 0.25 | 0.9991 |
| Stroma_Median | 526.33 | 31.53 | 2.1 | 5.82 | 0.4 | 0.9957 | 526.07 | 31.6 | 1.75 | 4.84 | 0.33 | 0.997 | 526.2 | 31.5 | 2.27 | 6.3 | 0.43 | 0.9964 |
| Stroma_Central | 483.63 | 30.89 | 1.34 | 3.73 | 0.28 | 0.9982 | 483.46 | 30.73 | 1.07 | 2.97 | 0.22 | 0.9988 | 483.54 | 30.74 | 1.41 | 3.91 | 0.29 | 0.9985 |
| Stroma 2–5 mm | ||||||||||||||||||
| Stroma_S_2–5 mm | 516.06 | 33.03 | 3 | 8.31 | 0.58 | 0.9919 | 515.53 | 32.7 | 2.86 | 7.93 | 0.56 | 0.9925 | 515.79 | 32.79 | 3.34 | 9.27 | 0.65 | 0.9922 |
| Stroma_SN_2–5 mm | 518.87 | 32.43 | 3.5 | 9.7 | 0.67 | 0.9885 | 518.75 | 31.99 | 3.66 | 10.13 | 0.7 | 0.9873 | 518.81 | 32.13 | 3.83 | 10.63 | 0.74 | 0.9879 |
| Stroma_N_2–5 mm | 512.27 | 31.65 | 3.19 | 8.84 | 0.62 | 0.9902 | 512.48 | 31.37 | 3.35 | 9.29 | 0.65 | 0.9889 | 512.38 | 31.44 | 3.51 | 9.73 | 0.69 | 0.9896 |
| Stroma_IN_2–5 mm | 504.73 | 31.38 | 2.3 | 6.38 | 0.46 | 0.9948 | 505.19 | 31.22 | 2.63 | 7.3 | 0.52 | 0.9931 | 504.96 | 31.23 | 2.74 | 7.59 | 0.54 | 0.994 |
| Stroma_I_2–5 mm | 496.06 | 31.24 | 1.61 | 4.45 | 0.32 | 0.9974 | 496.3 | 31.17 | 1.73 | 4.81 | 0.35 | 0.997 | 496.18 | 31.14 | 1.99 | 5.52 | 0.4 | 0.9972 |
| Stroma_IT_2–5 mm | 487.25 | 31.02 | 1.78 | 4.94 | 0.37 | 0.9968 | 487.08 | 30.84 | 1.47 | 4.07 | 0.3 | 0.9978 | 487.16 | 30.86 | 2.11 | 5.84 | 0.43 | 0.9973 |
| Stroma_T_2–5 mm | 487.25 | 31.47 | 1.98 | 5.5 | 0.41 | 0.9962 | 486.77 | 31.67 | 1.68 | 4.65 | 0.34 | 0.9973 | 487.01 | 31.5 | 2.16 | 5.98 | 0.44 | 0.9967 |
| Stroma_ST_2–5 mm | 501.15 | 32.56 | 2.41 | 6.68 | 0.48 | 0.9947 | 500.57 | 32.67 | 1.95 | 5.42 | 0.39 | 0.9965 | 500.86 | 32.54 | 2.46 | 6.81 | 0.49 | 0.9956 |
| Stroma 5–7 mm | ||||||||||||||||||
| Stroma_S_5–7 mm | 563.86 | 34.49 | 4.14 | 11.49 | 0.74 | 0.9862 | 562.81 | 33.37 | 4.36 | 12.07 | 0.77 | 0.9838 | 563.33 | 33.85 | 5.24 | 14.52 | 0.93 | 0.9845 |
| Stroma_SN_5–7 mm | 564.7 | 33.66 | 4.34 | 12.04 | 0.77 | 0.9843 | 566.23 | 34.04 | 5.13 | 14.21 | 0.91 | 0.9779 | 565.48 | 33.78 | 5.78 | 16.02 | 1.02 | 0.9806 |
| Stroma_N_5–7 mm | 553.38 | 33.78 | 4.33 | 11.99 | 0.78 | 0.9841 | 553.77 | 33.27 | 5.05 | 14.01 | 0.91 | 0.9776 | 553.58 | 33.45 | 5.3 | 14.7 | 0.96 | 0.9809 |
| Stroma_IN_5–7 mm | 544.5 | 33.42 | 3.47 | 9.61 | 0.64 | 0.9896 | 545.27 | 32.88 | 4.31 | 11.94 | 0.79 | 0.9835 | 544.89 | 33.08 | 4.46 | 12.36 | 0.82 | 0.9866 |
| Stroma_I_5–7 mm | 532.09 | 32.48 | 2.48 | 6.89 | 0.47 | 0.9943 | 531.91 | 32.44 | 2.88 | 7.99 | 0.54 | 0.9924 | 532 | 32.39 | 3.21 | 8.9 | 0.6 | 0.9934 |
| Stroma_IT_5–7 mm | 515.25 | 31.18 | 3.29 | 9.12 | 0.64 | 0.9891 | 515.48 | 31.59 | 3.5 | 9.71 | 0.68 | 0.9879 | 515.36 | 31.32 | 4.09 | 11.33 | 0.79 | 0.9885 |
| Stroma_T_5–7 mm | 512.07 | 31.79 | 3.53 | 9.79 | 0.69 | 0.9879 | 511.75 | 32.38 | 3.57 | 9.89 | 0.7 | 0.988 | 511.91 | 32.01 | 4.02 | 11.13 | 0.78 | 0.988 |
| Stroma_ST_5–7 mm | 537.1 | 32.38 | 3.63 | 10.07 | 0.68 | 0.9881 | 536.87 | 33.36 | 3.39 | 9.4 | 0.63 | 0.9897 | 536.98 | 32.8 | 4.08 | 11.3 | 0.76 | 0.9889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wylęgała, E.; Kryszan, K.; Rogacz, K.; Bujała, K.; Dugiełło, B.; Woźniak, P.; Wylęgała, A. Variability in Corneal and Epithelial Pachymetry: A Comparison of Optopol Revo 130 and Optovue RTV XR Avanti in Healthy Patients. J. Clin. Med. 2025, 14, 1295. https://doi.org/10.3390/jcm14041295
Wylęgała E, Kryszan K, Rogacz K, Bujała K, Dugiełło B, Woźniak P, Wylęgała A. Variability in Corneal and Epithelial Pachymetry: A Comparison of Optopol Revo 130 and Optovue RTV XR Avanti in Healthy Patients. Journal of Clinical Medicine. 2025; 14(4):1295. https://doi.org/10.3390/jcm14041295
Chicago/Turabian StyleWylęgała, Edward, Katarzyna Kryszan, Kamila Rogacz, Katarzyna Bujała, Bogdan Dugiełło, Przemysław Woźniak, and Adam Wylęgała. 2025. "Variability in Corneal and Epithelial Pachymetry: A Comparison of Optopol Revo 130 and Optovue RTV XR Avanti in Healthy Patients" Journal of Clinical Medicine 14, no. 4: 1295. https://doi.org/10.3390/jcm14041295
APA StyleWylęgała, E., Kryszan, K., Rogacz, K., Bujała, K., Dugiełło, B., Woźniak, P., & Wylęgała, A. (2025). Variability in Corneal and Epithelial Pachymetry: A Comparison of Optopol Revo 130 and Optovue RTV XR Avanti in Healthy Patients. Journal of Clinical Medicine, 14(4), 1295. https://doi.org/10.3390/jcm14041295






