Association Between Plasma Granzyme B Levels, Organ Failure, and 28-Day Mortality Prediction in Patients with Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Population
2.2. Definitions
2.3. Data Collection
2.4. Blood Sample Collection and Measurement of Plasma Granzyme B
2.5. Statistical Analysis
3. Results
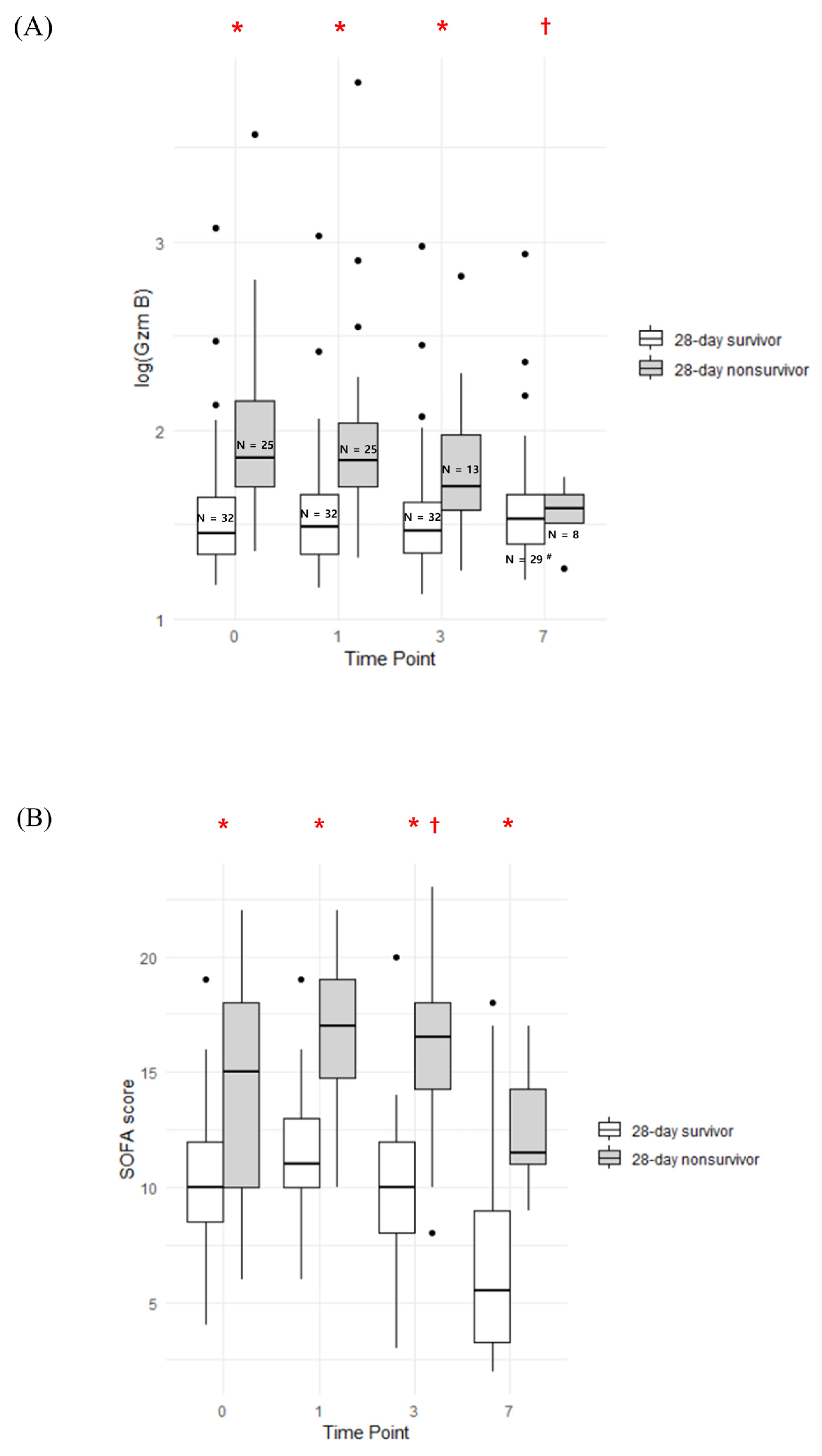
3.1. Serial Trend of Plasma Granzyme B and SOFA Score
3.2. Correlation Analysis Between Plasma Granzyme B and Various Cytokines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Coelho, L.; Dal-Pizzol, F.; Ferrer, R.; Huttner, A.; Morris, A.C.; Nobre, V.; Ramirez, P.; Rouze, A.; Salluh, J.; et al. How to use biomarkers of infection or sepsis at the bedside: Guide to clinicians. Intensive Care Med. 2023, 49, 142–153. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, G.; Xie, J. Immune dysregulation in sepsis: Experiences, lessons and perspectives. Cell Death Discov. 2023, 9, 465. [Google Scholar] [CrossRef]
- Voskoboinik, I.; Whisstock, J.C.; Trapani, J.A. Trapani. Perforin and granzymes: Function, dysfunction and human pathology. Nat. Rev. Immunol. 2015, 15, 388–400. [Google Scholar] [CrossRef]
- Hay, Z.L.Z.; Slansky, J.E. Granzymes: The molecular executors of immune-mediated cytotoxicity. Int. J. Mol. Sci. 2022, 23, 1833. [Google Scholar] [CrossRef]
- Velotti, F.; Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Granzyme B in inflammatory diseases: Apoptosis, inflammation, extracellular matrix remodeling, epithelial-to-mesenchymal transition and fibrosis. Front. Immunol. 2020, 11, 587581. [Google Scholar] [CrossRef]
- Turner, C.T.; Lim, D.; Granville, D.J. Granzyme B in skin inflammation and disease. Matrix Biol. 2019, 75, 126–140. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Ortiz-López, R.; González-Rivero, A.F.; Gómez-Bernal, F.; González-Mesa, A.; Jiménez, A.; Pérez-Cejas, A. High mortality rate of septic patients with high blood granzyme B concentrations. Diagn. Microbiol. Infect. Dis. 2022, 103, 115694. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Sadaka, F.; EthmaneAbouElMaali, C.; Cytron, M.A.; Fowler, K.; Javaux, V.M.; O’brien, J. Predicting mortality of patients with sepsis: A comparison of APACHE II and APACHE III scoring systems. J. Clin. Med. Res. 2017, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.A.; Lange, T.; Saugel, B.; Petzoldt, M.; Fuhrmann, V.; Metschke, M.; Kluge, S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med. 2016, 42, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Srisangthong, P.; Wongsa, A.; Kittiworawitkul, P.; Wattanathum, A. Early IL-6 response in sepsis is correlated with mortality and severity score. Crit. Care 2013, 17 (Suppl. S2), P34. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Nedeva, C.; Menassa, J.; Puthalakath, H. Sepsis: Inflammation is a necessary evil. Front. Cell Dev. Biol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Wensink, A.C.; Hack, C.E.; Bovenschen, N. Granzymes regulate proinflammatory cytokine responses. J. Immunol. 2015, 194, 491–497. [Google Scholar] [CrossRef]
- Garzón-Tituaña, M.; Arias, M.A.; Sierra-Monzón, J.L.; Morte-Romea, E.; Santiago, L.; Ramirez-Labrada, A.; Martinez-Lostao, L.; Paño-Pardo, J.R.; Galvez, E.M.; Pardo, J. The multifaceted function of granzymes in sepsis: Some facts and a lot to discover. Front. Immunol. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Hiebert, P.R.; Granville, D.J. Granzyme B in injury, inflammation, and repair. Trends Mol. Med. 2012, 18, 732–741. [Google Scholar] [CrossRef]
- Napoli, A.M.; Fast, L.D.; Gardiner, F.; Nevola, M.; Machan, J.T. Increased granzyme levels in cytotoxic T lymphocytes are associated with disease severity in emergency department patients with severe sepsis. Shock 2012, 37, 257–262. [Google Scholar] [CrossRef]
- Lauw, F.N.; Simpson, A.J.H.; Hack, C.E.; Prins, J.M.; Wolbink, A.M.; van Deventer, S.J.H.; Chaowagul, W.; White, N.J.; van der Poll, T. Soluble granzymes are released during human endotoxemia and in patients with severe infection due to gram-negative bacteria. J. Infect. Dis. 2000, 182, 206–213. [Google Scholar] [CrossRef]
- Afonina, I.S.; Tynan, G.A.; Logue, S.E.; Cullen, S.P.; Bots, M.; Lüthi, A.U.; Reeves, E.P.; McElvaney, N.G.; Medema, J.P.; Lavelle, E.C.; et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol. Cell 2011, 44, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Buzza, M.S.; Dyson, J.M.; Choi, H.; Gardiner, E.E.; Andrews, R.K.; Kaiserman, D.; Mitchell, C.A.; Berndt, M.C.; Dong, J.-F.; Bird, P.I. Antihemostatic activity of human granzyme B mediated by cleavage of von Willebrand factor. J. Biol. Chem. 2008, 283, 22498–22504. [Google Scholar] [CrossRef] [PubMed]
- Freishtat, R.J.; Natale, J.; Benton, A.S.; Cohen, J.; Sharron, M.; Wiles, A.A.; Ngor, W.-M.; Mojgani, B.; Bradbury, M.; Degnan, A.; et al. Sepsis alters the megakaryocyte–platelet transcriptional axis resulting in granzyme B–mediated lymphotoxicity. Am. J. Respir. Crit. Care Med. 2009, 179, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sharron, M.; Hoptay, C.E.; Wiles, A.A.; Garvin, L.M.; Geha, M.; Benton, A.S.; Nagaraju, K.; Freishtat, R.J. Platelets induce apoptosis during sepsis in a contact-dependent manner that is inhibited by GPIIb/IIIa blockade. PLoS ONE 2012, 7, e41549. [Google Scholar] [CrossRef]
- Hendel, A.; Hsu, I.; Granville, D.J. Granzyme B releases vascular endothelial growth factor from extracellular matrix and induces vascular permeability. Lab. Investig. 2014, 4, 716–725. [Google Scholar] [CrossRef]
- Casserly, B.; Phillips, G.S.; Schorr, C.; Dellinger, R.P.; Townsend, S.R.; Osborn, T.M.; Reinhart, K.; Selvakumar, N.; Levy, M.M. Lactate measurements in sepsis-induced tissue hypoperfusion: Results from the Surviving Sepsis Campaign database. Crit. Care Med. 2015, 43, 567–573. [Google Scholar] [CrossRef]
- Hermsen, C.C.; Konijnenberg, Y.; Mulder, L.; Loé, C.; VAN Deuren, M.; VAN DER Meer, J.W.M.; VAN Mierlo, G.J.; Eling, W.M.C.; Hack, C.E.; Sauerwein, R.W. Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin. Exp. Immunol. 2003, 132, 467–472. [Google Scholar] [CrossRef]
- Otani, N.; Nakajima, K.; Ishikawa, K.; Ichiki, K.; Ueda, T.; Takesue, Y.; Yamamoto, T.; Tanimura, S.; Shima, M.; Okuno, T. Changes in cell-mediated immunity (ifn-γ and granzyme b) following influenza vaccination. Viruses 2021, 13, 1137. [Google Scholar] [CrossRef]
- Coomes, S.M.; Kannan, Y.; Pelly, V.S.; Entwistle, L.J.; Guidi, R.; Perez-Lloret, J.; Nikolov, N.; Müller, W.; Wilson, M.S. CD4+ Th2 cells are directly regulated by IL-10 during allergic airway inflammation. Mucosal Immunol. 2017, 10, 150–161. [Google Scholar] [CrossRef]
- Kienzle, N.; Buttigieg, K.; Groves, P.; Kawula, T.; Kelso, A. A clonal culture system demonstrates that IL-4 induces a subpopulation of noncytolytic T cells with low CD8, perforin, and granzyme expression. J. Immunol. 2002, 168, 1672–1681. [Google Scholar] [CrossRef]
- Li, X.-Y.; Liu, M.; Fu, Y.-J.; Jiang, Y.-J.; Zhang, Z.-N. Alterations in levels of cytokine following treatment to predict outcome of sepsis: A meta-analysis. Cytokine 2023, 161, 156056. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Aschenbrenner, A.C.; Bauer, M.; Bock, C.; Calandra, T.; Gat-Viks, I.; Kyriazopoulou, E.; Lupse, M.; Monneret, G.; Pickkers, P.; et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat. Immunol. 2024, 25, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.K.; Pickkers, P.; van der Poll, T. Sepsis-induced immunosuppression. Annu. Rev. Physiol. 2022, 84, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D., 2nd; Krupnick, A.S.; et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- Dolin, H.H.; Papadimos, T.J.; Stepkowski, S.; Chen, X.; Pan, Z.K. A novel combination of biomarkers to herald the onset of sepsis prior to the manifestation of symptoms. Shock 2018, 49, 364–370. [Google Scholar] [CrossRef]


| 28-Day Survivors (N = 32) | 28-Day Nonsurvivors (N = 25) | p-Value | |
|---|---|---|---|
| Age | 69.0 [63.0; 79.5] | 69.0 [65.0; 81.0] | 0.693 |
| Sex, male, n (%) | 20 (62.5%) | 16 (64.0%) | 1.000 |
| Charlson comorbidity index | 4.0 [2.0; 5.5] | 6.0 [4.0; 7.0] | 0.004 |
| Immunocompromised or autoimmune disease, n (%) | |||
| Malignancy | 6 (18.8%) | 12 (48.0%) | 0.038 |
| Solid organ transplantation | 5 (15.6%) | 2 (8.0%) | 0.643 |
| Autoimmune disease | 1 (3.2%) | 0 (0.0%) | 1.000 |
| Infection focus, n (%) | 0.283 | ||
| -Pulmonary | 13 (50.0%) | 14 (63.6%) | |
| -Urogenital | 7 (26.9%) | 1 (4.5%) | |
| -GI tract | 2 (7.7%) | 2 (9.1%) | |
| -Skin, Soft tissue, and Bone | 0 (0.0%) | 2 (9.1%) | |
| -Cardiac | 1 (3.8%) | 1 (4.5%) | |
| -Brain/CSF | 0 (0.0%) | 1 (4.5%) | |
| -Miscellaneous | 1 (3.8%) | 0 (0.0%) | |
| -Multiple | 2 (7.7%) | 1 (4.5%) | |
| Bacteremia, n (%) | 12 (38.7%) | 12 (50.0%) | 0.573 |
| Bacteremia pathogen, n (%) | 0.587 | ||
| -Gram-negative | 8 (66.7%) | 7 (58.3%) | |
| -Gram-positive | 4 (33.3%) | 4 (33.3%) | |
| -Fungal | 0 (0.0%) | 1 (8.3%) | |
| Shock, n (%) | 28 (87.5%) | 22 (88.0%) | 1.000 |
| APACHE II score | 23.5 [19.0; 30.5] | 34.0 [27.0; 39.0] | 0.007 |
| Total SOFA score | 10.0 [8.5; 12.0] | 15.0 [10.0; 18.0] | 0.001 |
| Use of vasopressor | 31 (96.9%) | 25 (100.0%) | 1.000 |
| Number of different vasopressors | 0.016 | ||
| 0 | 1 (3.1%) | 0 (0.0%) | |
| 1 | 21 (65.6%) | 6 (24.0%) | |
| 2 | 9 (28.1%) | 17 (68.0%) | |
| 3 | 1 (3.1%) | 1 (4.0%) | |
| 4 | 0 (0.0%) | 1 (4.0%) | |
| Peak norepinephrine infusion rate (mcg/kg/min) * | 0.3 ± 0.3 | 0.8 ± 0.6 | 0.001 |
| Acute kidney injury | 25 (78.1%) | 25 (100.0%) | 0.037 |
| CRRT | 13 (40.6%) | 22 (88.0%) | 0.001 |
| Mechanical ventilation | 20 (62.5%) | 22 (88.0%) | 0.062 |
| ECMO | 3 (9.4%) | 0 (0.0%) | 0.329 |
| Laboratory tests | |||
| White blood cell count (103/uL) | 13.3 [11.3; 17.8] | 11.0 [7.1; 23.9] | 0.539 |
| Procalcitonin (ng/mL) | 5.5 [0.7; 19.4] | 11.5 [2.0; 19.7] | 0.333 |
| C-reactive protein (mg/L) | 189.4 [99.2; 311.1] | 140.2 [49.2; 201.5] | 0.053 |
| Lactate, venous (mmol/L) | 1.8 [0.9; 2.4] | 9.2 [1.8; 10.6] | <0.001 |
| Granzyme B (pg/mL) | 28.2 [21.8; 46.8] | 71.0 [50.5; 143.5] | <0.001 |
| Interleukin-6 (pg/mL) | 353.1 [89.6; 1605.0] | 1282.2 [88.5; 12,950.0] | 0.186 |
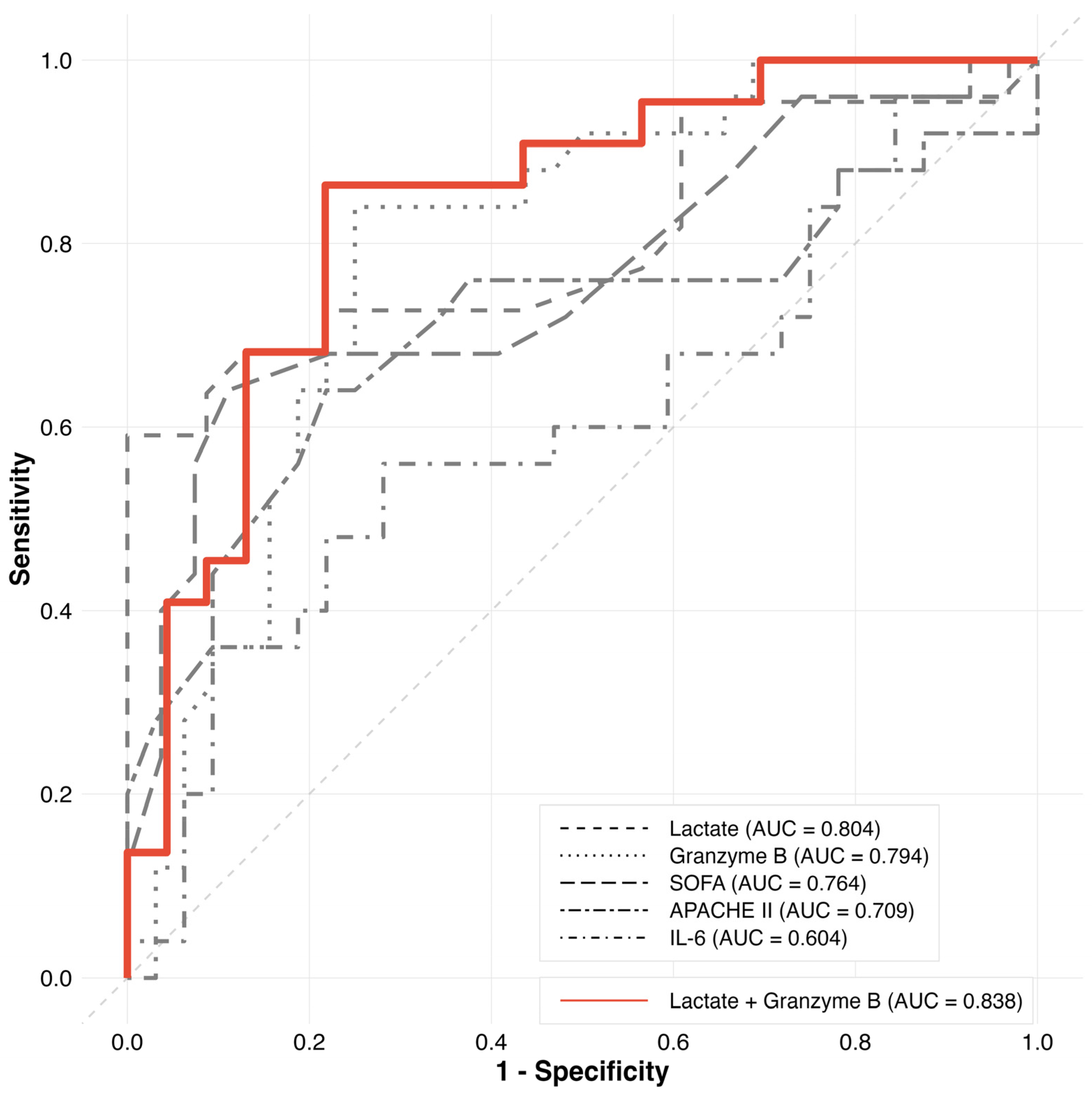
| Variable | AUC | SE | p-Value | 95% CI | Cutoff | Sensitivity | Specificity | AUC Difference | p-Value (DeLong Test) |
|---|---|---|---|---|---|---|---|---|---|
| Granzyme B | 0.794 | 0.060 | <0.001 | 0.676–0.913 | 41.75 | 0.840 | 0.750 | Reference | |
| Lactate | 0.804 | 0.070 | <0.001 | 0.668–0.941 | 6.7 | 0.591 | 1.000 | −0.010 | 0.778 |
| SOFA | 0.764 | 0.069 | <0.001 | 0.629–0.898 | 13.5 | 0.640 | 0.889 | 0.030 | 0.956 |
| APACHE II | 0.709 | 0.076 | 0.006 | 0.560–0.857 | 31.5 | 0.640 | 0.781 | 0.085 | 0.372 |
| IL-6 | 0.604 | 0.079 | 0.910 | 0.449–0.758 | 1048.1 | 0.560 | 0.719 | 0.190 | 0.009 |
| Lactate + Granzyme B | 0.838 | 0.061 | <0.001 | 0.718–0.958 | 43.7 | 0.864 | 0.783 | −0.044 | 0.252 |
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age | 1 | 0.97–1.03 | 0.979 | 0.98 | 0.96–1.01 | 0.275 |
| Male (vs. Female) | 1.13 | 0.50–2.55 | 0.776 | |||
| Charlson comorbidity index | 1.26 | 1.08–1.47 | 0.003 | 1.18 | 1.02–1.36 | 0.031 |
| Malignancy | 2.6 | 1.18–5.73 | 0.018 | |||
| APACHE II score | 1.07 | 1.02–1.12 | 0.003 | |||
| Total SOFA score | 1.21 | 1.09–1.34 | <0.001 | 1.08 | 0.96–1.22 | 0.209 |
| C-reactive protein (mg/L) | 1 | 0.99–1.00 | 0.055 | |||
| Lactate, venous (mmol/L) | 1.21 | 1.12–1.31 | <0.001 | |||
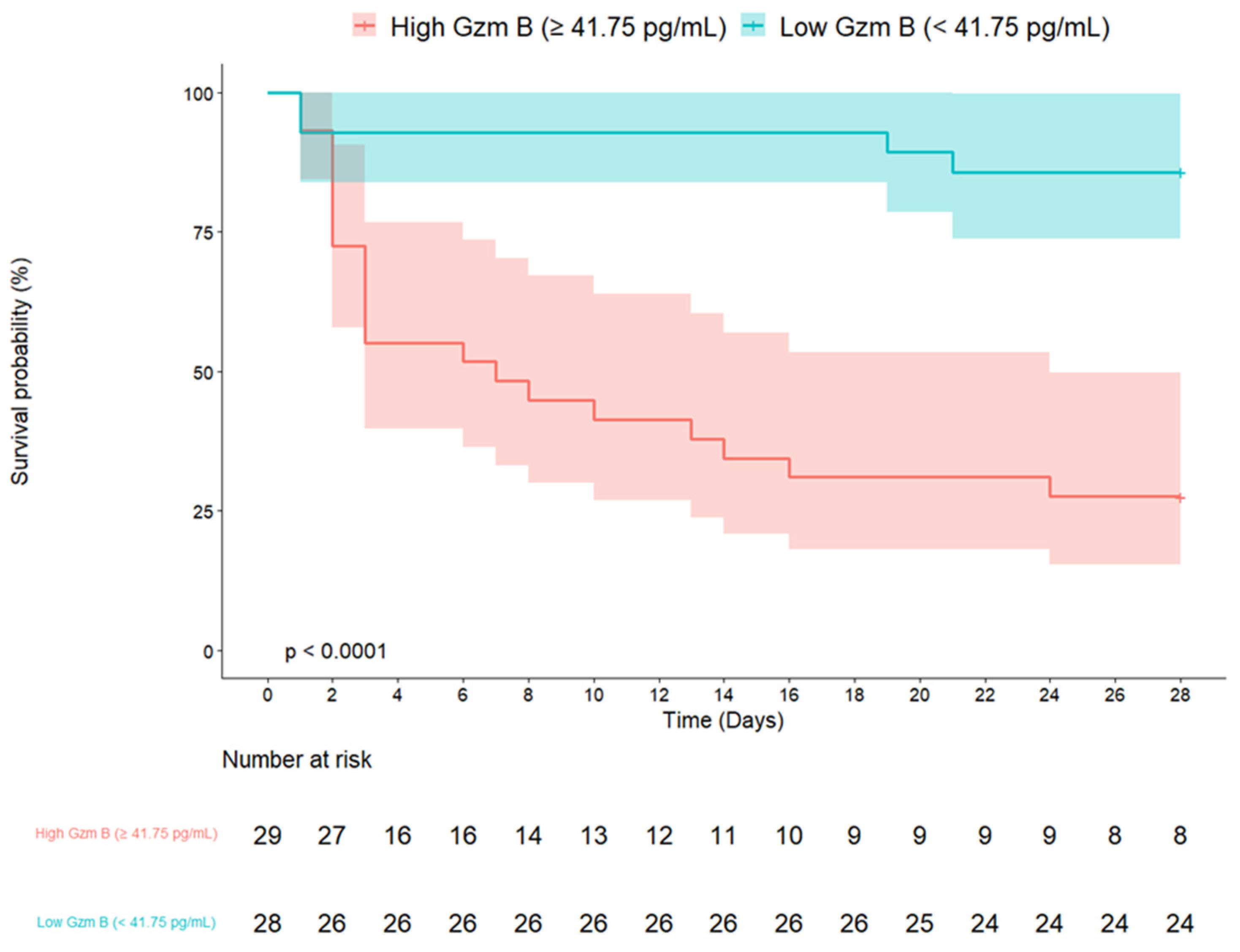
| Granzyme B ≥ 41.75 pg/mL | 8.16 | 2.77–24.02 | <0.001 | 4.47 | 1.29–15.44 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ki, M.S.; Shin, J.H.; Sung, M.D.; Chang, S.; Leem, A.Y.; Lee, S.H.; Park, M.S.; Kim, Y.S.; Chung, K.S. Association Between Plasma Granzyme B Levels, Organ Failure, and 28-Day Mortality Prediction in Patients with Sepsis. J. Clin. Med. 2025, 14, 1461. https://doi.org/10.3390/jcm14051461
Ki MS, Shin JH, Sung MD, Chang S, Leem AY, Lee SH, Park MS, Kim YS, Chung KS. Association Between Plasma Granzyme B Levels, Organ Failure, and 28-Day Mortality Prediction in Patients with Sepsis. Journal of Clinical Medicine. 2025; 14(5):1461. https://doi.org/10.3390/jcm14051461
Chicago/Turabian StyleKi, Min Seo, Ju Hye Shin, Min Dong Sung, Shihwan Chang, Ah Young Leem, Su Hwan Lee, Moo Suk Park, Young Sam Kim, and Kyung Soo Chung. 2025. "Association Between Plasma Granzyme B Levels, Organ Failure, and 28-Day Mortality Prediction in Patients with Sepsis" Journal of Clinical Medicine 14, no. 5: 1461. https://doi.org/10.3390/jcm14051461
APA StyleKi, M. S., Shin, J. H., Sung, M. D., Chang, S., Leem, A. Y., Lee, S. H., Park, M. S., Kim, Y. S., & Chung, K. S. (2025). Association Between Plasma Granzyme B Levels, Organ Failure, and 28-Day Mortality Prediction in Patients with Sepsis. Journal of Clinical Medicine, 14(5), 1461. https://doi.org/10.3390/jcm14051461






