Exploring the Posterolateral Corner of the Knee Joint: A Detailed Review of Recent Literature
Abstract
:1. Introduction
2. Epidemiology
3. Anatomy and Biomechanics
- (1)
- Stability against varus stress is primarily provided by the FCL, while other structures contribute to it as a secondary function [18].
- (2)
- Rotational stability is divided into external and internal tibial rotations. Stability against external rotation is provided mostly by the PFL and PT at higher degrees of flexion [19] and by the deep layer of the ITB, the PC, and the AC at lower knee flexion degrees, while resistance to internal rotation is guaranteed by the BFT and superficial layer of the ITB at higher flexion [20].
- (3)
- Stabilizers against tibial translation are divided into anterior and posterior stabilizers, and posterior stability provided by the PLC reduces the strain on the PCL and is guaranteed mostly by the popliteus complex at approximately 90° [21] and by the AC [22,23]. PC seems to stabilize the knee at early degrees of flexion [24], while biceps femoris long-head activation offers resistance against anterior tibial translation before 40° [25], and both layers of the ITB restrain anterior tibial translation throughout the ROM [20].
- (1)
- The FCL has been the focus of many biomechanical studies, and its main function is to resist varus stress at all knee flexion degrees [28]. Furthermore, it is a constantly considered area when reconstructing the PLC using the different techniques discussed in the Section 7 of this paper. In addition to varus stability, the FCL has a secondary function of rotational stability, which is more noticeable at early flexion angles (0–30°) [29]. The FCL exerts multidirectional stabilization over the tibial translation during the stance, and its effects have been thoroughly discussed by Smith et al. [30].
- (2)
- The PT (which generates from the popliteus muscle) was defined as “the fifth ligament of the knee” and is considered as both a static and dynamic stabilizer. The PT has the primary function of stabilizing the PLC, especially against external rotation, which is mostly exploited above 90° [19]. PT also plays a secondary role in limiting posterior tibial translation throughout knee flexion. Even though PT function is prominent above 90°, it plays a crucial role in full extension as it is responsible for unlocking the gait through its muscular contraction to internally rotate the tibia and externally rotate the femur with respect to one another [33].
- (3)
- The PFL primarily stabilizes the functional unit against external tibial rotation, varus angulation, and anterior tibial translation through its synergetic function in conjunction with the FCL and PT. Also, PFL sectioning leads to an increase in the ACL load at all degrees of knee flexion [34,35,36,37].
4. Clinical Examination
4.1. Gait
4.2. Evaluating Instability
4.3. Tests
5. Diagnostics
5.1. Plain Radiographs
5.2. Magnetic Resonance Imaging (MRI)
5.3. Computerized Tomography
5.4. Arthroscopic Diagnosis
6. Conservative Management
7. Surgical Techniques
- (1)
- Non-anatomical Treatment (NAT)
- -
- BFT rerouting to achieve posterolateral stability was first described in 1988 [84]. It started as tenodesis by repositioning the BFT insertion 1 cm anterior to the FCL origin at the femur and was effective in restoring the knee varus and external rotation stability. However, in the same study, overconstraints of the varus and external rotation were reported [78]. NAT then evolved into biceps rerouting, which was used until recently [85,86].
- -
- NAT techniques include ligament avulsion repairs through advancement into a bony bed, and intrasubstance tear repair through sutures made in a “pants over vest” fashion [51]. The combined advancement of the lateral gastrocnemius, posterolateral capsule, and arcuate complex was performed and reported by Hughston in 1985 using a single staple to connect and insert all three structures over the lateral femoral condyle [14].
- (2)
- Anatomic reconstructions (AR)
- -
- The Arciero technique reported the use of a single 7 mm trans-fibular tunnel, and the limbs of the graft are fixed into the femoral sockets through passage from the popliteal hiatus and BFT. This technique is similar to the modified Larson technique; however, it requires passage through different knee layers and has been classified as anatomic, although a single fibular tunnel is made, making it functionally partially anatomic [96].
- -
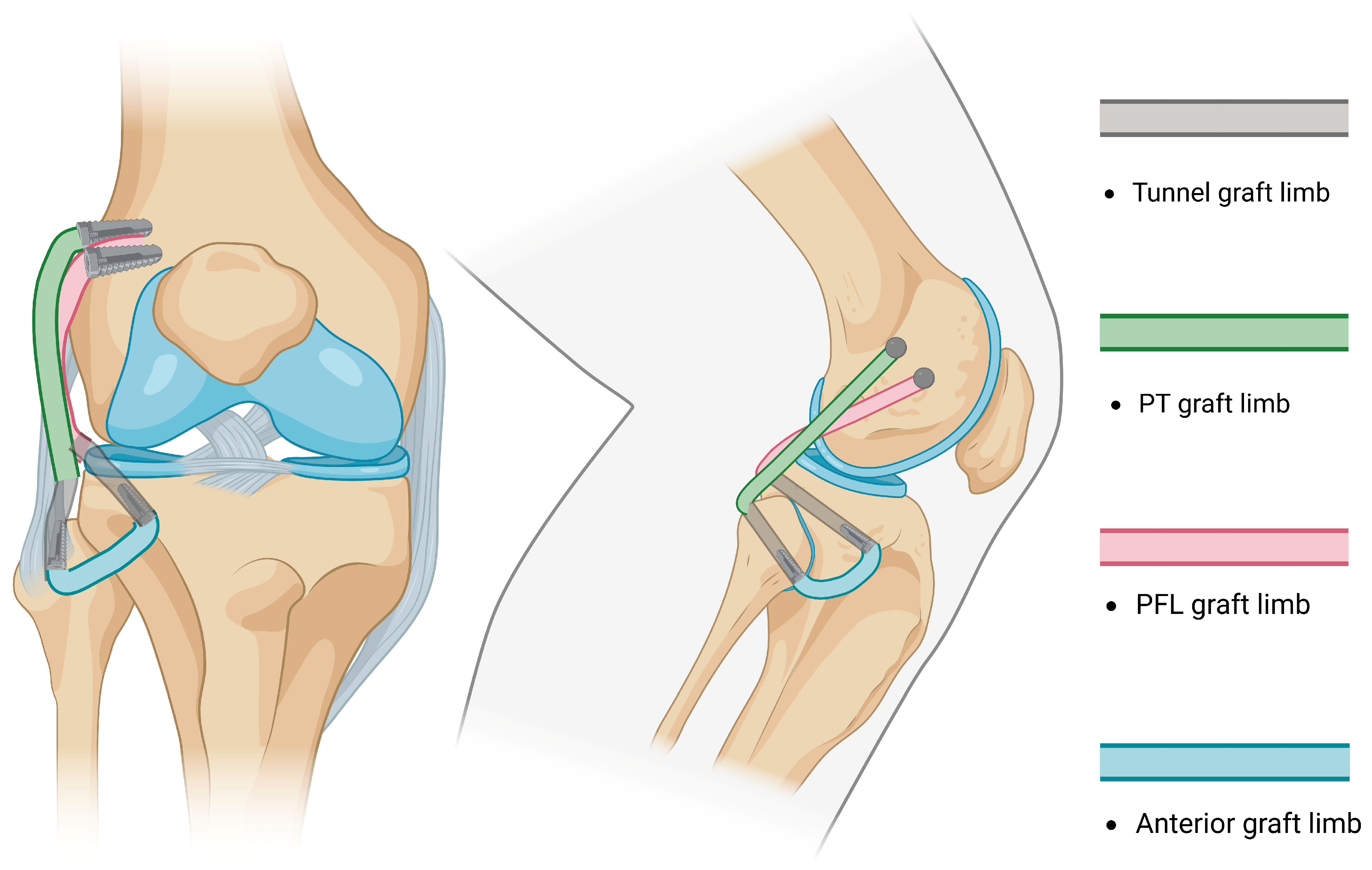
- Subsequently, AR was reported by LaPrade in 2010 [97]. The method consisted of drilling two femoral sockets for fixation and two tunnels (one tibial and one fibular) for graft passage and fixation, thus restoring the PLC by reconstructing the FCL, PT, and PFL (Figure 4). Subsequent studies reported the use of different fixation methods [98].
- -
- (3)
- Arthroscopic reconstructions
- -
- -
- Arthroscopic non-anatomical techniques [105] have been used to treat posterolateral rotatory instability in cases of an intact anatomical but non-functional PLC [106]. Arthroscopic NAT involves the stabilization of the posterolateral joint capsule with the lateral meniscus, yet has been reported to influence meniscal excursion.
- -
- -
- A true all-anatomic, all-arthroscopic technique was first described by Ahn et al., in which the reconstruction of the FCL, PT, and PFL was performed arthroscopically [6], followed by Kolb [109]. The Ahn technique requires the arthroscopic identification of landmarks, expertise in arthroscopic techniques and accessory portals, and extensive knowledge of the posterolateral knee anatomy. Once the landmarks are recognized, the sling reconstruction of the PT is performed, while both ends of the graft (FCL and PFL) are fixed at the fibular tunnel (Figure 6).
| Name | Reconstruction | Advantage | Disadvantage |
|---|---|---|---|
| Albright et al. [83] 1994 |
|
|
|
| Kim et al. [85] 2001 |
|
|
|
| Fanelli-Larson et al. [90] 2002 |
|
|
|
| Arciero [96] 2005 |
|
|
|
| LaPrade et al. [97] 2010 |
|
|
|
| Ahn-Jang et al. [6] 2019 |
|
|
|
| Reference | Technique | FU Duration | Graft | Results |
|---|---|---|---|---|
| Yoon et al. [113] 2011 Level III | Semi-Anatomic reconstruction: Tibiofibular technique with PT versus without PT reconstruction | 24 months | Achilles tendon allograft | Retrospective study on 32 patients: 17 with PT reconstruction, 15 without PT. Varus stress radiographs significantly improved in both groups. No preoperative or postoperative differences between the groups. Popliteal tendon reconstruction had no effect on anatomic reconstruction stability and clinical results. |
| Van Gennip et al. 2020 [114] Level IV | Larson vs. LaPrade (nonanatomic vs. anatomic) | 24 months | - | 11 Larson reconstructions were compared with a different study with LaPrade reconstruction. PROMs improved significantly. Median varus laxity of the injured knee on varus stress radiographs improved significantly, but did not return to the level of the uninjured knee. In comparison with LaPrade reconstruction, no statistically significant differences in clinical outcome were observed. |
| Yeatts et al. 2021 [115] Level IV | Larson vs. LaPrade PLC reconstruction | 12+ months | Allograft | Fibular-based technique (350 knee) and tibiofibular-based technique (593 knees). No statistically significant differences in subjective or objective clinical outcome measurements after fibular-based versus combined TF-based PLC reconstruction were observed. |
| Sharma et al. 2021 [116] Level II | Modified Larson vs. LaPrade (partial anatomic vs. anatomic) | 24 months | Hamstring autografts | Prospective study of 25 patients, 12 LaPrade versus 13 modified Larson reconstructions. Both techniques show good clinical results and restore varus and rotational stability of knee in grade III posterolateral corner injury. No statistical difference between groups. |
| Wiess et al. 2023 [117] Level II | Arthroscopic Arciero vs. Arthroscopic LaPrade (partial-anatomic vs. anatomic) | 12 months | - | Prospective study of 19 patients. Arthroscopic Arciero patients showed significantly higher maximum flexion angles compared with Arthroscopic LaPrade (134.17° ± 3.76° vs. 126.60° ± 4.22°; p = 0.021) at 12 months. Duration of surgery was significantly longer in LaPrade than in Arciero group (121.88 ± 11.63 vs. 165.00 ± 35.65 min; p = 0.003). PROMs showed no significant differences between groups. Complications: Arciero group had a dislocation of femoral PCL button while LaPrade presented arthrofibrosis requiring revision. |
| Khalis et al. 2023 [118] Level IV | Fibular versus Tibiofibular reconstructions | 24+ months | Autologous Gracilis and/or Semitendinosus Achilles/Tibialis posterior allograft | Meta-analysis on 183 patients (90 fibular-based, 93 tibiofibular-based reconstructions). There was no difference between PROMs at 20.3 months. The techniques were equally effective in restoring varus and rotational stability. |
| Fahlbusch et al. (2024) [110] Level II | Open Arciero versus Arthroscopic Arciero (open partial anatomic vs. arthroscopic partial anatomic) | 14.9 ± 7.2 months | Autologous Gracilis (mentioned in Open Arciero) | Prospective study of 26 patients: 12 Open Arciero versus 14 Arthroscopic Arciero. No clinically relevant differences in PROMs were shown in both groups. Arthroscopic reconstruction showed significantly shorter operation time (p = 0.0109). |
| Colatruglio et al. 2024 [119] Level IV | Tibial- versus fibular-based PLCR | 39.6 months | - | Analysis of tibial- and fibular-based posterolateral corner reconstruction suggests no clinical difference. Four studies reported both tibial- and fibular-based PLCR were found to have no significant differences in PROMs. |
| Jackson et al. 2024 [95] Level IV | Anatomic versus non-anatomic techniques | 24 months | Hamstrings, Tibialis posterior allograft Biceps tendon autograft | Systematic review of 230 patients; 80% (n = 8/10) of study cohorts performed anatomic reconstruction technique. Failure rates range from 4.3% to 36%. Subgroup analysis revealed a failure rate of 4.3–24.2% for anatomic reconstruction techniques, and 0–36% failure rate for non-anatomic reconstruction. Arthrofibrosis was the most common complication (range, 0–12.1%) following surgery; 0–8% of patients require revision PLC surgery. |
8. Postoperative Rehabilitation
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.G.; Han, S.B.; Rhim, H.C.; Jeon, O.H.; Jang, K.M. Anatomy of the anterolateral ligament of the knee joint. World J. Clin. Cases 2022, 10, 7215–7223. [Google Scholar] [CrossRef]
- Park, J.G.; Han, S.B.; Lee, C.S.; Jeon, O.H.; Jang, K.M. Anatomy, biomechanics, and reconstruction of the anterolateral ligament of the knee joint. Medicina 2022, 58, 786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhim, H.C.; Jeon, O.H.; Han, S.B.; Bae, J.H.; Suh, D.W.; Jang, K.M. Mesenchymal stem cells for enhancing biological healing after meniscal injuries. World J. Stem Cells 2021, 13, 1005–1029. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, M.C.; Tolstykh, I.; Shakoor, N.; Nguyen, U.S.D.T.; Segal, N.A.; Lewis, C.; Felson, D.T.; Multicenter Osteoarthritis Study Investigators. Symptoms of knee instability as risk factors for Recurrent Falls. Arthritis Care Res. 2016, 68, 1089–1097. [Google Scholar] [CrossRef]
- Hughston, J.C.; Andrews, J.R.; Cross, M.J.; Moschi, A. Classification of knee ligament instabilities. Part II. The lateral compartment. J. Bone Jt. Surg. Am. 1976, 58, 173–179. [Google Scholar] [CrossRef]
- Ahn, J.H.; Wang, J.H.; Lee, S.Y.; Rhyu, I.J.; Suh, D.W.; Jang, K.M. Arthroscopic-assisted anatomical reconstruction of the posterolateral corner of the knee joint. Knee 2019, 26, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Engebretsen, L. Editorial Commentary: Those Who Don’t Know History Are Condemned to Repeat It-What Are the Next Steps to Improve Posterolateral Knee Outcomes? Arthroscopy 2020, 36, 1386–1389. [Google Scholar] [CrossRef]
- Pacheco, R.J.; Ayre, C.A.; Bollen, S.R. Posterolateral corner injuries of the knee: A serious injury commonly missed. J. Bone Jt. Surg. Br. 2011, 93, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, C.I.; Kurtz, C.A.; Sekiya, J.K. Nonsurgical management of lateral side injuries of the knee. Sports Med. Arthrosc. Rev. 2006, 14, 20–22. [Google Scholar] [CrossRef]
- Figueroa, F.; Figueroa, D.; Putnis, S.; Guiloff, R.; Caro, P.; Espregueira-Mendes, J. Posterolateral corner knee injuries: A narrative review. EFORT Open Rev. 2021, 6, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.L.; Poppe, T.; Kim, K.; Barnds, B.; Schroeppel, P.; Mullen, S.; Tarakemeh, A.; Bechtold, M.; Vopat, B.G. Weightbearing protocols after posterolateral corner reconstruction: A systematic review. Orthop. J. Sports Med. 2021, 9, 2325967120988274. [Google Scholar] [CrossRef]
- Frings, J.; Weiß, S.; Kolb, J.; Behrendt, P.; Frosch, K.H.; Krause, M. Arthroscopic anatomy of the posterolateral corner of the knee: Anatomic relations and arthroscopic approaches. Arch. Orthop. Trauma Surg. 2022, 142, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Hughston, J.C.; Jacobson, K.E. Chronic posterolateral rotatory instability of the knee. J. Bone Jt. Surg. Am. 1985, 67, 351–359. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Wentorf, F.A.; Fritts, H.; Gundry, C.; Hightower, C.D. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy 2007, 23, 1341–1347. [Google Scholar] [CrossRef]
- Chen, F.S.M.; Rokito, A.S.M.; Pitman, M.I.M. Acute and chronic posterolateral rotatory instability of the knee. J. Am. Acad. Orthop. Surg. 2000, 8, 97–110. [Google Scholar] [CrossRef]
- Li, E.; Tan, J.; Xu, K.; Pan, Y.; Xu, P. Global burden and socioeconomic impact of knee osteoarthritis: A comprehensive analysis. Front. Med. 2024, 11, 1323091. [Google Scholar] [CrossRef] [PubMed]
- Yaras, R.J.; O’Neill, N.; Mabrouk, A.; Yaish, A.M. Lateral Collateral Ligament Knee Injury. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Wang, B.; Ye, T.; Zhang, B.; Wang, Y.; Zhu, Y.; Luo, C. Relationship of Fracture Morphological Characteristics with Posterolateral Corner Injuries in Hyperextension Varus Tibial Plateau Fractures. J. Bone Jt. Surg. Am. 2024, 106, 2001–2008. [Google Scholar] [CrossRef]
- Kittl, C.; El-Daou, H.; Athwal, K.K.; Gupte, C.M.; Weiler, A.; Williams, A.; Amis, A.A. The Role of the Anterolateral Structures and the ACL in Controlling Laxity of the Intact and ACL-Deficient Knee. Am. J. Sports Med. 2016, 44, 345–354. [Google Scholar] [CrossRef]
- Höher, J.; Harner, C.D.; Vogrin, T.M.; Baek, G.H.; Carlin, G.J.; Woo, S.L. In situ forces in the posterolateral structures of the knee under posterior tibial loading in the intact and posterior cruciate ligament-deficient knee. J. Orthop. Res. 1998, 16, 675–681. [Google Scholar] [CrossRef]
- Thaunat, M.; Pioger, C.; Chatellard, R.; Conteduca, J.; Khaleel, A.; Sonnery-Cottet, B. The arcuate ligament revisited: Role of the posterolateral structures in providing static stability in the knee joint. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Sudasna, S.; Harnsiriwattanagit, K. The ligamentous structures of the posterolateral aspect of the knee. Bull. Hosp. Jt. Dis. Orthop. Inst. 1990, 50, 35–40. [Google Scholar]
- Hsieh, H.H.; Walker, P.S. Stabilizing mechanisms of the loaded and unloaded knee joint. J. Bone Jt. Surg. Am. 1976, 58, 87–93. [Google Scholar] [CrossRef]
- Azmi, N.L.; Ding, Z.; Xu, R.; Bull, A.M.J. Activation of biceps femoris long head reduces tibiofemoral anterior shear force and tibial internal rotation torque in healthy subjects. PLoS ONE 2018, 13, e0190672. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, J.K.; Haemmerle, M.J.; Stabile, K.J.; Vogrin, T.M.; Harner, C.D. Biomechanical analysis of a combined double-bundle posterior cruciate ligament and posterolateral corner reconstruction. Am. J. Sports Med. 2005, 33, 360–369. [Google Scholar] [CrossRef]
- Hodel, S.; Hasler, J.; Fürnstahl, P.; Fucentese, S.F.; Vlachopoulos, L. Elongation patterns of posterolateral corner reconstruction techniques: Results using 3-dimensional weightbearing computed tomography simulation. Orthop. J. Sports Med. 2022, 10, 23259671221090219. [Google Scholar] [CrossRef] [PubMed]
- Grood, E.S.; Stowers, S.F.; Noyes, F.R. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J. Bone Jt. Surg. Am. 1988, 70, 88–97. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Tso, A.; Wentorf, F.A. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am. J. Sports Med. 2004, 32, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.R.; Lenhart, R.L.; Kaiser, J.; Vignos, M.F.; Thelen, D.G. Influence of ligament properties on tibiofemoral mechanics in walking. J. Knee Surg. 2016, 29, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Amis, A.A. Anatomic and biomechanical study of the lateral collateral and popliteofibular ligaments. Am. J. Sports Med. 2001, 29, 466–472. [Google Scholar] [CrossRef]
- Harner, C.D.; Höher, J.; Vogrin, T.M.; Carlin, G.J.; Woo, S.L. The effects of a popliteus muscle load on in situ forces in the posterior cruciate ligament and on knee kinematics. A human cadaveric study. Am. J. Sports Med. 1998, 26, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Mohamad, N.; Yusoff, B.A.H.M. Popliteus tendon injury: A rare cause of acute locked knee. Cureus 2023, 15, e38655. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Resig, S.; Wentorf, F.; Lewis, J.L. The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am. J. Sports Med. 1999, 27, 469–475. [Google Scholar] [CrossRef]
- Gollehon, D.L.; Torzilli, P.A.; Warren, R.F. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J. Bone Jt. Surg. Am. 1987, 69, 233–242. [Google Scholar] [CrossRef]
- Hirokawa, S.; Solomonow, M.; Lu, Y.; Lou, Z.P.; D’Ambrosia, R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am. J. Sports Med. 1992, 20, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Maynard, M.J.; Deng, X.; Wickiewicz, T.L.; Warren, R.F. The popliteofibular ligament. Rediscovery of a key element in posterolateral stability. Am. J. Sports Med. 1996, 24, 311–316. [Google Scholar] [CrossRef]
- Sanchez, A.R.; Sugalski, M.T.; LaPrade, R.F. Anatomy and biomechanics of the lateral side of the knee. Sports Med. Arthrosc. 2006, 14, 2–11. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Wozniczka, J.K.; Stellmaker, M.P.; Wijdicks, C.A. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: The “fifth ligament” of the knee. Am. J. Sports Med. 2010, 38, 543–549. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Wentorf, F. Diagnosis and treatment of posterolateral knee injuries. Clin. Orthop. Relat. Res. 2002, 402, 110–121. [Google Scholar] [CrossRef]
- Goldblatt, J.P.; Richmond, J.C. Anatomy and biomechanics of the knee. Oper. Tech. Sports Med. 2003, 11, 172–186. [Google Scholar] [CrossRef]
- Chevalier, A.; Van Overmeire, A.; Vermue, H.; Pringels, L.; Herregodts, S.; Victor, J.; Loccufier, M. Effect of iliotibial band and gastrocnemius activation on knee kinematics. Knee 2023, 40, 238–244. [Google Scholar] [CrossRef]
- Ko, M.J.; Kang, M.H. The effects of tibial rotation on muscle activity and force of hamstring muscle during isometric knee flexion in healthy women. PNF Mov. 2021, 19, 1–8. [Google Scholar] [CrossRef]
- Azzopardi, C.; Beale, D.; James, S.L.; Botchu, R. Isolated Complete Distal Biceps Femoris Tendon Tears: Case Series and Literature Review. Indian. J. Radiol. Imaging 2021, 31, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Nagura, T.; Dyrby, C.O.; Alexander, E.J.; Andriacchi, T.P. Mechanical loads at the knee joint during deep flexion. J. Orthop. Res. 2002, 20, 881–886. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Ly, T.V.; Wentorf, F.A.; Engebretsen, L. The Posterolateral Attachments of the Knee. Am. J. Sports Med. 2003, 31, 854–860. [Google Scholar] [CrossRef]
- Catalfamo, P.F.; Aguiar, G.; Curi, J.; Braidot, A. Anterior Cruciate Ligament Injury: Compensation during Gait using Hamstring Muscle Activity. Open Biomed. Eng. J. 2010, 4, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wünschel, M.; Leichtle, U.; Obloh, C.; Wülker, N.; Müller, O. The effect of different quadriceps loading patterns on tibiofemoral joint kinematics and patellofemoral contact pressure during simulated partial weight-bearing knee flexion. Knee Surg. Sports Traumatol. Arthrosc. 2011, 19, 1099–1106. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kameyama, M.; Shibata, M.; Shibata, N.; Kato, C.; Kato, T.; Ota, S. The influence of knee varus and valgus on quadriceps muscle activity changes induced by stretching and kneeling. J. Electromyogr. Kinesiol. 2022, 63, 102636. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Xu, D.; Wang, G.; Hendrix, R.W. Muscle strength in knee varus and valgus. Med. Sci. Sports Exerc. 2001, 33, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- DeLee, J.C.; Riley, M.B.; Rockwood, C.A., Jr. Acute posterolateral rotatory instability of the knee. Am. J. Sports Med. 1983, 11, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Madadi-Shad, M.; Jafarnezhadgero, A.; Zago, M.; Granacher, U. Effects of varus knee alignment on gait biomechanics and lower limb muscle activity in boys: A cross sectional study. Gait Posture 2019, 72, 69–75. [Google Scholar] [CrossRef]
- Falworth, M.S.; Allum, R.L. Posterolateral instability of the knee: Its diagnosis and management. Knee 2003, 17, 223–233. [Google Scholar] [CrossRef]
- Miller, M.D.; Bergfeld, J.A.; Fowler, P.J.; Harner, C.D.; Noyes, F.R. The posterior cruciate ligament injured knee: Principles of evaluation and treatment. Instr. Course Lect. 1999, 48, 199–207. [Google Scholar]
- Hughston, J.C.; Norwood, L.A. The posterolateral drawer test and external rotational recurvatum test for posterolateral rotatory instability of the knee. Clin. Orthop. Relat. Res. 1980, 147, 82–87. [Google Scholar] [CrossRef]
- Loomer, R.L. A test for knee posterolateral rotatory instability. Clin. Orthop. Relat. Res. 1991, 264, 235–238. [Google Scholar] [CrossRef]
- Lubowitz, J.H.; Bernardini, B.J.; Reid, J.B. III. Current concepts review: Comprehensive physical examination for instability of the knee. Am. J. Sports Med. 2008, 36, 577–594. [Google Scholar] [CrossRef]
- Kahan, J.B.; Li, D.; Schneble, C.A.; Huang, P.; Bullock, J.; Porrino, J.; Medvecky, M.J. The pathoanatomy of posterolateral corner ligamentous disruption in multiligament knee injuries is predictive of peroneal nerve injury. Am. J. Sports Med. 2020, 48, 3541–3548. [Google Scholar] [CrossRef]
- Shon, O.J.; Park, J.W.; Kim, B.J. Current Concepts of Posterolateral Corner Injuries of the Knee. Knee Surg. Relat. Res. 2017, 29, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Krause, M.; Frosch, K.H. Posterolateral corner of the knee: A systematic literature review of current concepts of arthroscopic reconstruction. Arch. Orthop. Trauma Surg. 2020, 140, 2003–2012. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Heikes, C.; Bakker, A.J.; Jakobsen, R.B. The reproducibility and repeatability of varus stress radiographs in the assessment of isolated fibular collateral ligament and grade-III posterolateral knee injuries. An in vitro biomechanical study. J. Bone Jt. Surg. Am. 2008, 90, 2069–2076. [Google Scholar] [CrossRef]
- Krakowski, P.; Nogalski, A.; Jurkiewicz, A.; Karpiński, R.; Maciejewski, R.; Jonak, J. Comparison of Diagnostic Accuracy of Physical Examination and MRI in the Most Common Knee Injuries. Appl. Sci. 2019, 9, 4102. [Google Scholar] [CrossRef]
- Karpiński, R.; Krakowski, P.; Jonak, J.; Machrowska, A.; Maciejewski, M.; Nogalski, A. Diagnostics of Articular Cartilage Damage Based on Generated Acoustic Signals Using ANN-Part II: Patellofemoral Joint. Sensors 2022, 22, 3765. [Google Scholar] [CrossRef] [PubMed]
- Recondo, J.A.; Salvador, E.; Villanúa, J.A.; Barrera, M.C.; Gervás, C.; Alústiza, J.M. Lateral stabilizing structures of the knee: Functional anatomy and injuries assessed with MR imaging. RadioGraphics 2000, 20, S91–S102. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Gilbert, T.J.; Bollom, T.S.; Wentorf, F.; Chaljub, G. The magnetic resonance imaging appearance of individual structures of the posterolateral knee. A prospective study of normal knees and knees with surgically verified grade III injuries. Am. J. Sports Med. 2000, 28, 191–199. [Google Scholar] [CrossRef]
- Theodorou, D.J.; Theodorou, S.J.; Fithian, D.C.; Paxton, L.; Garelick, D.H.; Resnick, D. Posterolateral complex knee injuries: Magnetic resonance imaging with surgical correlation. Acta Radiol. 2005, 46, 297–305. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.; Shahabpour, M.; Vanderdood, K.; De Ridder, F.; Van Roy, F.; Osteaux, M. Posterolateral supporting structures of the knee: Findings on anatomic dissection, anatomic slices and MR images. Eur. Radiol. 2001, 11, 2170–2177. [Google Scholar] [CrossRef]
- Bencardino, J.T.; Rosenberg, Z.S.; Brown, R.R.; Hassankhani, A.; Lustrin, E.S.; Beltran, J. Traumatic musculotendinous injuries of the knee: Diagnosis with MR imaging. RadioGraphics 2000, 20, S103–S120. [Google Scholar] [CrossRef]
- Vinson, E.N.; Major, N.M.; Helms, C.A. The posterolateral corner of the knee. AJR Am. J. Roentgenol. 2008, 190, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Salonen, D.C.; Hodler, J.; Haghighi, P.; Trudell, D.; Resnick, D. Posterolateral aspect of the knee: Improved MR imaging with a coronal oblique technique. Radiology 1996, 198, 199–204. [Google Scholar] [CrossRef]
- Griffith, J.F.; Antonio, G.E.; Tong, C.W.; Ming, C.K. Cruciate ligament avulsion fractures. Arthroscopy 2004, 20, 803–812. [Google Scholar] [CrossRef]
- Giovannetti de Sanctis, E.; Mesnard, G.; Dejour, D.H. Trochlear dysplasia: When and how to correct. Clin. Sports Med. 2022, 41, 77–88. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, M.; Lei, M.; Lin, F.; Chen, Y.; Chen, J.; Liu, J.; Ye, J. Diagnostic accuracy of ultra-low-dose CT compared to standard-dose CT for identification of non-displaced fractures of the shoulder, knee, ankle, and wrist. Insights Imaging 2023, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Lv, Y.; Hong, L.; Wang, X.; Zhang, J.; Feng, H. Validity of a novel arthroscopic test to diagnose posterolateral rotational instability of the knee joint: The lateral gutter drive-through test. Arthroscopy 2013, 29, 695–700. [Google Scholar] [CrossRef]
- Kannus, P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am. J. Sports Med. 1989, 17, 83–88. [Google Scholar] [CrossRef]
- Sagnard, T.; Picot, B.; Forestier, N. Influence of exercise-induced hamstrings fatigue on proprioceptive reweighting strategies and postural performance in bipedal stance in recreational athletes. Hum. Mov. Sci. 2024, 98, 103298. [Google Scholar] [CrossRef]
- Cavanaugh, J.T.; Saldivar, A.; Marx, R.G. Postoperative Rehabilitation After Posterior Cruciate Ligament Reconstruction and Combined Posterior Cruciate Ligament Reconstruction-Posterior Lateral Corner Surgery. Oper. Tech. Sports Med. 2015, 23, 372–384. [Google Scholar] [CrossRef]
- Wascher, D.C.; Grauer, J.D.; Markoff, K.L. Biceps tendon tenodesis for posterolateral instability of the knee: An in vitro study. Am. J. Sports Med. 1993, 21, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, G.C.; Giannotti, B.F.; Edson, C.J. Arthroscopically assisted combined posterior cruciate ligament/posterior lateral complex reconstruction. Arthroscopy 1996, 12, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.P.; Brown, A.W. Management of chronic posterolateral rotatory instability of the knee: Surgical technique for the posterolateral corner sling procedure. Instr. Course Lect. 1998, 47, 369–378. [Google Scholar]
- Clancy, W.G., Jr.; Shelbourne, K.D.; Zoellner, G.B.; Keene, J.S.; Reider, B.; Rosenberg, T.D. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J. Bone Jt. Surg. Am. 1983, 65, 310–322. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber-Westin, S.D. Surgical restoration to treat chronic deficiency of the posterolateral complex and cruciate ligaments of the knee joint. Am. J. Sports Med. 1996, 24, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.P. Management of chronic posterolateral instability of the knee: Operative technique for the posterolateral corner sling procedure. Iowa Orthop. J. 1994, 14, 94–100. [Google Scholar] [PubMed]
- Clancy, W.G.J. Repair and reconstruction of the posterior cruciate ligament. In Operative Orthopedics; Chapman, M.W., Ed.; JP Lippincott: Philadelphia, PA, USA, 1988; Volume 3, pp. 1651–1655. [Google Scholar]
- Kim, S.J.; Shin, S.J.; Choi, C.H.; Kim, H.C. Reconstruction by biceps tendon rerouting for posterolateral rotatory instability of the knee: Modification of the Clancy technique. Arthroscopy 2001, 17, 664–667. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, T.W.; Kim, S.G.; Kim, H.P.; Chun, Y.M. Clinical comparisons of the anatomical reconstruction and modified biceps rerouting technique for chronic posterolateral instability combined with posterior cruciate ligament reconstruction. J. Bone Jt. Surg. Am. 2011, 93, 809–818. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, Y.B.; Ko, Y.B.; Kim, S.H.; Kwon, H.B.; Yu, D.S.; Jung, Y.B. The necessity of clinical application of tibial reduction for detection of underestimated posterolateral rotatory instability in combined posterior cruciate ligament and posterolateral corner deficient knee. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3062–3069. [Google Scholar] [CrossRef] [PubMed]
- Sidles, J.A.; Larson, R.V.; Garbini, J.L.; Downey, D.J.; Matsen, F.A. Ligament length relationships in the moving knee. J. Orthop. Res. 1988, 6, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://BioRender.com (accessed on 20 February 2025).
- Fanelli, G.C.; Larson, R.V. Practical management of posterolateral instability of the knee. Arthroscopy 2002, 18 (Suppl. S1), 1–8. [Google Scholar] [CrossRef]
- Niki, Y.; Matsumoto, H.; Otani, T.; Enomoto, H.; Toyama, Y.; Suda, Y. A modified Larson’s method of posterolateral corner reconstruction of the knee reproducing the physiological tensioning pattern of the lateral collateral and popliteofibular ligaments. Sports Med. Arthrosc. Rehabil. Ther. Technol. 2012, 4, 21. [Google Scholar] [CrossRef]
- Noyes, F.R.; Barber-Westin, S.D. Posterolateral knee reconstruction with an anatomical bone-patellar tendon-bone reconstruction of the fibular collateral ligament. Am. J. Sports Med. 2007, 35, 259–273. [Google Scholar] [CrossRef]
- Yoon, K.H.; Bae, D.K.; Ha, J.H.; Park, S.W. Anatomic reconstructive surgery for posterolateral instability of the knee. Arthroscopy 2006, 22, 159–165. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, R.F.; Johansen, S.; Wentorf, F.A.; Engebretsen, L.; Esterberg, J.L.; Tso, A. An analysis of an anatomical posterolateral knee reconstruction: An in vitro biomechanical study and development of a surgical technique. Am. J. Sports Med. 2004, 32, 1405–1414. [Google Scholar] [CrossRef]
- Jackson, G.R.; Mameri, E.S.; Condon, J.; DeWald, D.; Batra, A.; Salazar, L.M.; Familiari, F.; Matava, M.; Knapik, D.M.; Verma, N.N.; et al. Non-anatomical reconstruction of chronic posterolateral corner knee injuries show failure rates from 0% to 36% versus 4.3% to 24.2% for anatomic reconstruction techniques: An updated systematic review reflecting the 2019 expert consensus statement. J. ISAKOS 2024, 9, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Arciero, R.A. Anatomic posterolateral corner knee reconstruction. Arthroscopy 2005, 21, 1147. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Johansen, S.; Agel, J.; Risberg, M.A.; Moksnes, H.; Engebretsen, L. Outcomes of an anatomic posterolateral knee reconstruction. J. Bone Jt. Surg. Am. 2010, 92, 16–22. [Google Scholar] [CrossRef]
- Franciozi, C.E.; Albertoni, L.J.B.; Kubota, M.S.; Abdalla, R.J.; Luzo, M.V.M.; Cohen, M.; LaPrade, R.F. A hamstring-based anatomic posterolateral knee reconstruction with autografts improves both radiographic instability and functional outcomes. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 1676–1685.e3. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Choi, D.-H.; Hwang, B.-Y. The influence of posterolateral rotatory instability on ACL reconstruction: Comparison between isolated ACL reconstruction and ACL reconstruction combined with posterolateral corner reconstruction. J. Bone Jt. Surg. Am. 2012, 94, 253–259. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.G.; Lee, I.S.; Han, H.D.; Chung, I.H.; Kim, S.H.; Gorthi, V. Effect of physiological posterolateral rotatory laxity on early results of posterior cruciate ligament reconstruction with posterolateral corner reconstruction. J. Bone Jt. Surg. Am. 2013, 95, 1222–1227. [Google Scholar] [CrossRef]
- Sekiya, J.K.; Kurtz, C.A. Posterolateral corner reconstruction of the knee: Surgical technique utilizing a bifid Achilles tendon allograft and a double femoral tunnel. Arthroscopy 2005, 21, 1400. [Google Scholar] [CrossRef]
- Feng, H.; Hong, L.; Geng, X.S.; Zhang, H.; Wang, X.S.; Zhang, J. Posterolateral sling reconstruction of the popliteus tendon: An all-arthroscopic technique. Arthroscopy 2009, 25, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Frosch, K.H.; Akoto, R.; Heitmann, M.; Enderle, E.; Giannakos, A.; Preiss, A. Arthroscopic reconstruction of the popliteus complex: Accuracy and reproducibility of a new surgical technique. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3114–3120. [Google Scholar] [CrossRef]
- Song, G.Y.; Zhang, H.; Zhang, J.; Li, Y.; Feng, H. Anatomical popliteofibular ligament reconstruction of the knee joints: An all-arthroscopic technique. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2925–2929. [Google Scholar] [CrossRef] [PubMed]
- Hermanowicz, K.; Góralczyk, A.; Malinowski, K.; Jancewicz, P. Arthroscopic posterolateral corner stabilization with popliteus tenodesis. Arthrosc. Tech. 2018, 7, e669–e674. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, Y.; Pascual-Garrido, C.; Kumagae, H.; Sakai, A.; Uchida, S. Arthroscopic Technique for Isolated Posterolateral Rotational Instability of the Knee. Arthrosc. Tech. 2017, 6, e291–e295. [Google Scholar] [CrossRef]
- Hermanowicz, K.; Malinowski, K.; Góralczyk, A.; Guszczyn, T.; LaPrade, R.F. Minimally Invasive, Arthroscopic-Assisted, Anatomic Posterolateral Corner Reconstruction. Arthrosc. Tech. 2019, 8, e251–e257. [Google Scholar] [CrossRef]
- Frings, J.; Kolb, J.P.; Drenck, T.C.; Krause, M.; Alm, L.; Akoto, R.; Frosch, K.H. Anatomic reconstruction of the posterolateral corner: An all-arthroscopic technique. Arthrosc. Tech. 2019, 8, e153–e161. [Google Scholar] [CrossRef]
- Kolb, J.P.; Frings, J.; Krause, M.; Hartel, M.; Frosch, K.H. An all-arthroscopic technique for complex posterolateral corner reconstruction. Arthrosc. Tech. 2019, 8, e999–e1006. [Google Scholar] [CrossRef]
- Fahlbusch, H.; Weiß, S.; Landenberger, J.; von Rehlingen Prinz, F.; Dust, T.; Akoto, R.; Krause, M.; Frosch, K.H. Arthroscopic and open reconstruction of the posterolateral corner of the knee have equally good clinical results: First results of a prospective 12-month follow-up study. Arch. Orthop. Trauma Surg. 2024, 144, 2745–2752. [Google Scholar] [CrossRef]
- Liu, P.; Gong, X.; Zhang, J.; Ao, Y. Anatomic, All-Arthroscopic Reconstruction of Posterolateral Corner of the Knee: A Cadaveric Biomechanical Study. Arthroscopy 2020, 36, 1121–1131. [Google Scholar] [CrossRef]
- Zielinski, K.P.; Wieland, M.D.; Sequeira, S.B.; Gould, H.P.; Dreese, J.C. Posterolateral Corner Reconstruction: A Systematic Review and Meta-analysis of Biomechanical Studies. Am. J. Sports Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Lee, J.H.; Bae, D.K.; Song, S.J.; Chung, K.Y.; Park, Y.W. Comparison of clinical results of anatomic posterolateral corner reconstruction for posterolateral rotatory instability of the knee with or without popliteal tendon reconstruction. Am. J. Sports Med. 2011, 39, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- van Gennip, S.; van der Wal, W.A.; Heesterbeek, P.J.; Wymenga, A.B.; Busch, V.J. Posterolateral corner reconstruction in combined injuries of the knee: Improved stability with Larson’s fibular sling reconstruction and comparison with LaPrade anatomical reconstruction. Knee 2020, 27, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, N.C.; Rao, A.J.; Trofa, D.P.; Hong, I.S.; Moorman, C.T.; Piasecki, D.P.; Fleischli, J.E.; Saltzman, B.M. Comparable subjective and objective clinical outcomes after fibular or combined tibial-fibular-based reconstruction of the posterolateral corner of the knee: A systematic review and meta-analysis. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21. [Google Scholar] [CrossRef]
- Sharma, A.; Saha, P.; Bandyopadhyay, U. Reconstruction of the Posterolateral Corner of the Knee Using LaPrade and Modified Larson Technique: A Prospective Study. Indian J. Orthop. 2021, 56, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Krause, M.; Frosch, K.H. Clinical results after arthroscopic reconstruction of the posterolateral corner of the knee: A prospective randomized trial comparing two different surgical techniques. Arch. Orthop. Trauma Surg. 2023, 143, 967–975. [Google Scholar] [CrossRef]
- Boksh, K.; Ghosh, A.; Narayan, P.; Divall, P.; Aujla, R. Fibular- versus tibiofibular-based reconstruction of the posterolateral corner of the knee: A systematic review and meta-analysis. Am. J. Sports Med. 2023, 51, 3880–3892. [Google Scholar] [CrossRef]
- Colatruglio, M.R.; Lamplot, J.D.; Murphy, J.; Bernholt, D.L. There Is No Difference in Clinical Outcomes of Tibial-Based Versus Fibular-Based Posterolateral Corner Reconstruction: A Systematic Review. Arthroscopy 2024. [Google Scholar] [CrossRef] [PubMed]
- Pache, S.; Sienra, M.; Larroque, D.; Talamás, R.; Aman, Z.S.; Vilensky, E.; LaPrade, R.F. Anatomic Posterolateral Corner Reconstruction Using Semitendinosus and Gracilis Autografts: Surgical Technique. Arthrosc. Tech. 2021, 10, e487–e497. [Google Scholar] [CrossRef]
- Yagiz, G.; Fredianto, M.; Ulfa, M.; Ariani, I.; Agustin, A.D.; Shida, N.; Moore, E.W.G.; Kubis, H.P. A retrospective comparison of the biceps femoris long head muscle structure in athletes with and without hamstring strain injury history. PLoS ONE 2024, 19, e0298146. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.Y.; Jang, E.; Do, J.H.; Kim, H.S.; Song, H.S. Anatomical posterolateral ligament reconstruction of the knee using Achilles tendon allograft. Arthrosc. Orthop. Sports Med. 2014, 1, 12–16. [Google Scholar] [CrossRef]
- Cabarcas, B.; Patel, R.; Mahler, R.; Kumar, N.; Warnick, D. Anatomic Posterolateral Corner Reconstruction with Single Tibialis Allograft and Suspensory Tibial Fixation. Arthrosc. Tech. 2024, 103265. [Google Scholar] [CrossRef]
- Camarda, L.; Condello, V.; Madonna, V.; Cortese, F.; D’Arienzo, M.; Zorzi, C. Results of isolated posterolateral corner reconstruction. J. Orthop. Traumatol. 2010, 11, 73–79. [Google Scholar] [CrossRef]
- Jones, M.; Pinheiro, V.H.; Church, J.S.; Ball, S.V.; Williams, A. Ligament augmentation and reconstruction system (LARS) synthetic grafts are safe and effective for medial collateral ligament and posterolateral corner reconstructions in elite athletes. Knee Surg. Sports Traumatol. Arthrosc. 2025, 33, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Arthrex, Inc. Data on File (APT-04275); Arthrex, Inc.: Naples, FL, USA, 2019. [Google Scholar]
- Noyes, F.R.; Mangine, R.E.; Barber, S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am. J. Sports Med. 1987, 15, 149–160. [Google Scholar] [CrossRef]
- Salter, R.B.; Simmonds, D.F.; Malcolm, B.W.; Rumble, E.J.; MacMichael, D.; Clements, N.D. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J. Bone Jt. Surg. Am. 1980, 62, 1232–1251. [Google Scholar] [CrossRef]
- Arms, S.W.; Pope, M.H.; Johnson, R.J.; Fischer, R.A.; Arvidsson, I.; Eriksson, E. The biomechanics of anterior cruciate ligament rehabilitation and reconstruction. Am. J. Sports Med. 1984, 12, 8–18. [Google Scholar] [CrossRef]
- Guskiewicz, K.M. Regaining balance and postural equilibrium. In Rehabilitation Techniques in Sports Medicine, 3rd ed.; Prentice, W.E., Ed.; WCB/McGraw-Hill: New York, NY, USA, 1999; pp. 107–133. [Google Scholar]
- Flynn, T.W.; Soutas-Little, R.W. Patellofemoral joint compressive forces in forward and backward running. J. Orthop. Sports Phys. Ther. 1995, 21, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ankar, P.; Phansopkar, P. Rehabilitation Strategies Following Posterolateral Corner Repair for Left Knee Dislocation with Multiligament Injury: A Case Report. Cureus 2024, 16, e56863. [Google Scholar] [CrossRef] [PubMed]
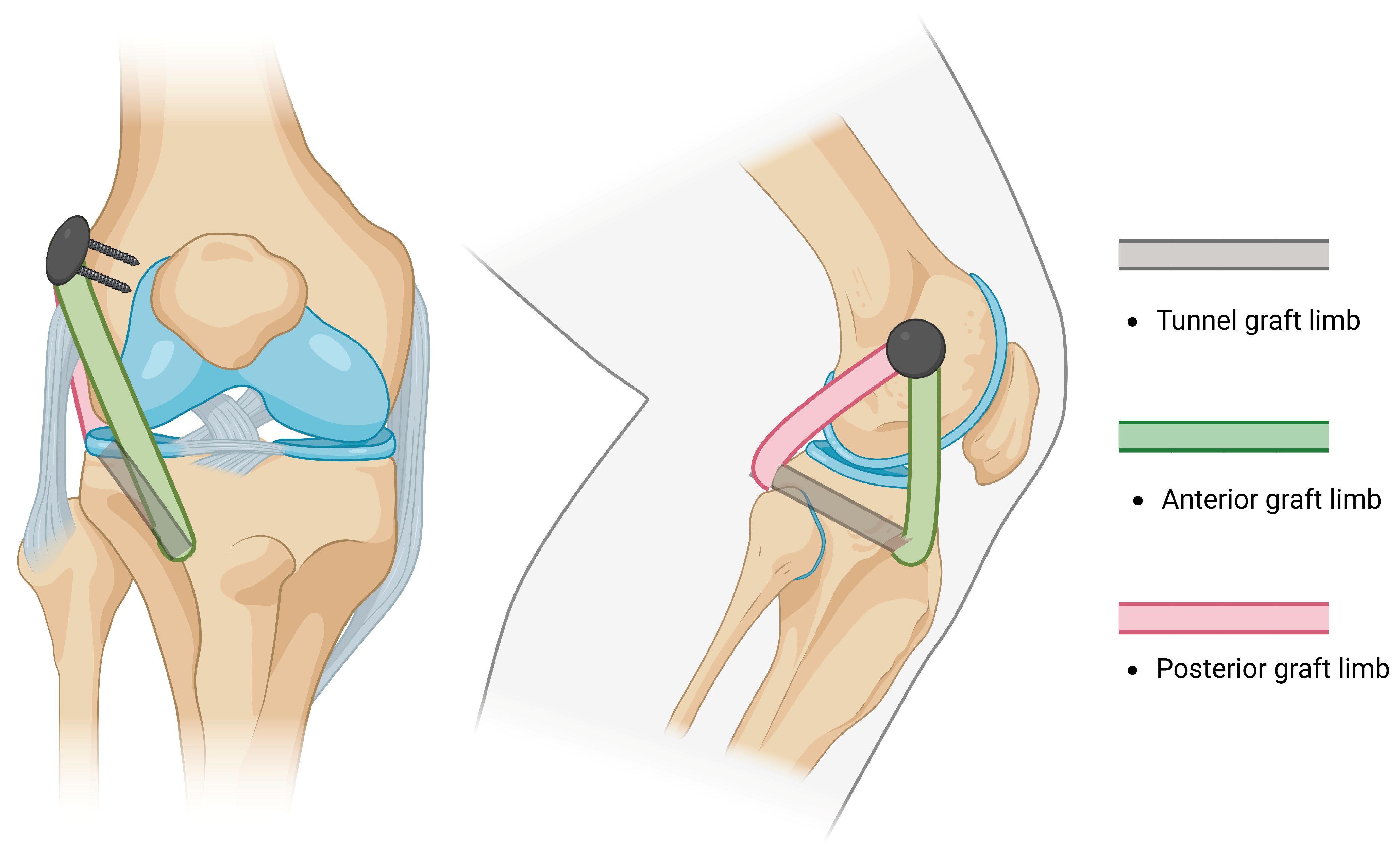
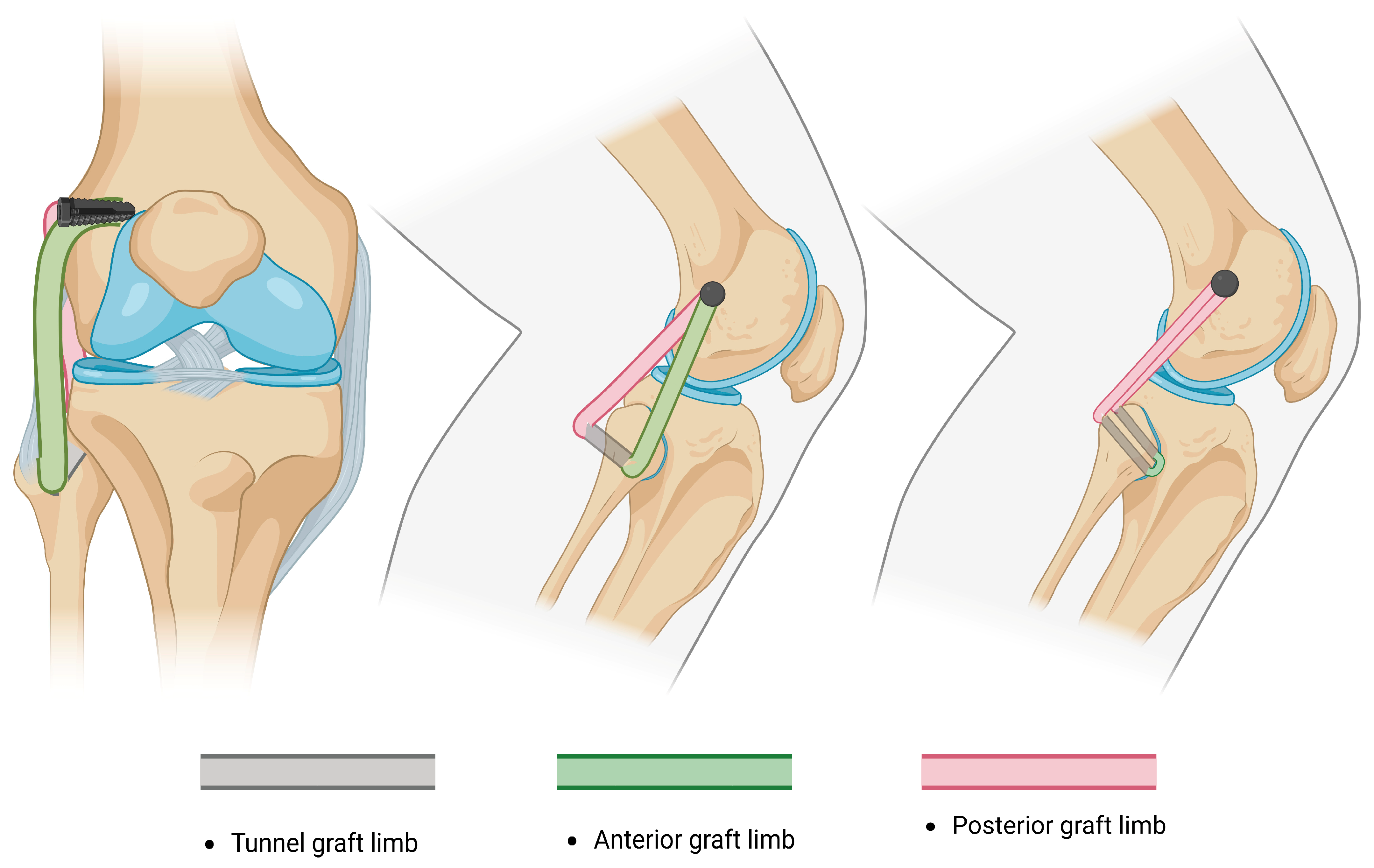
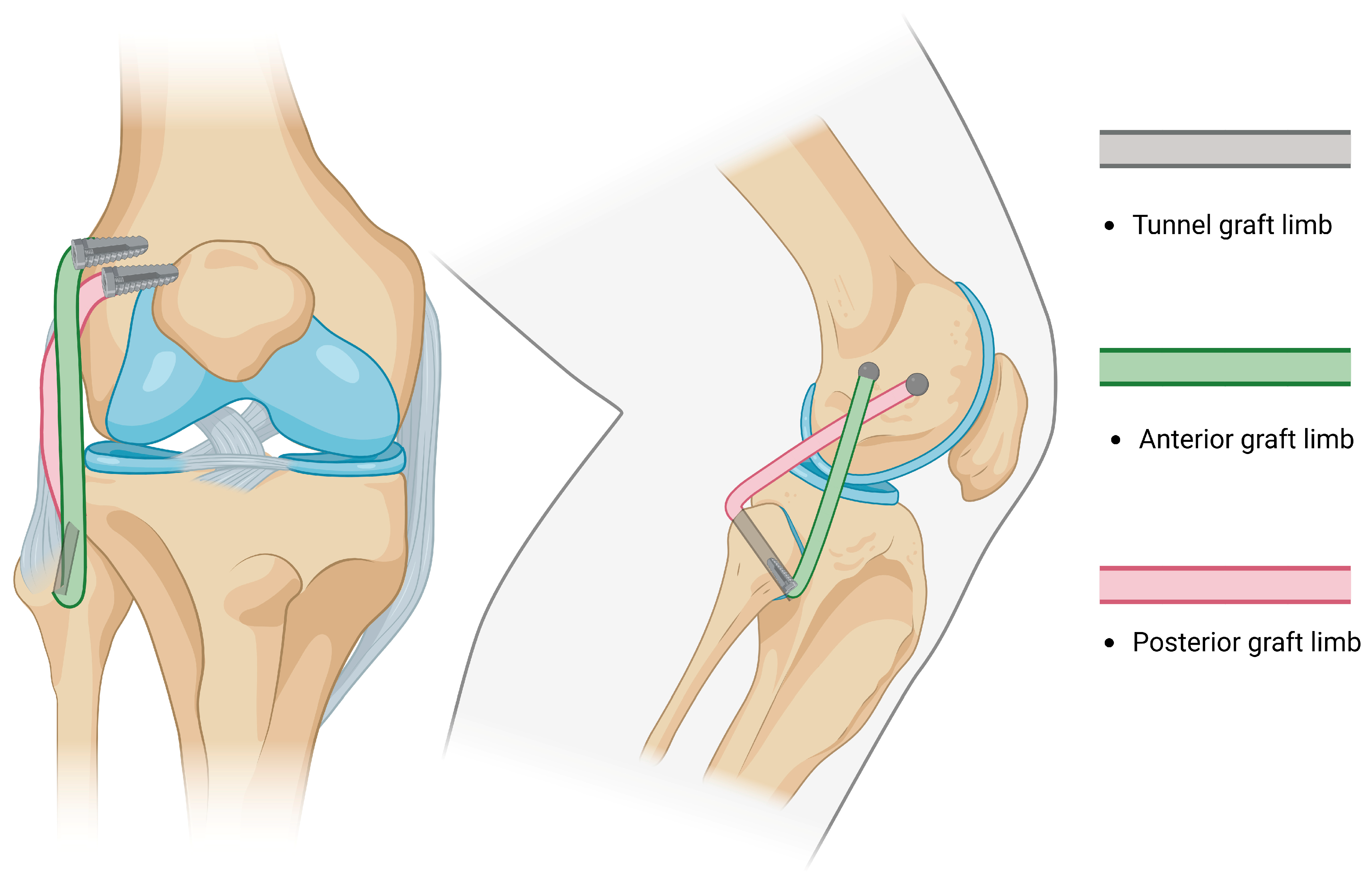
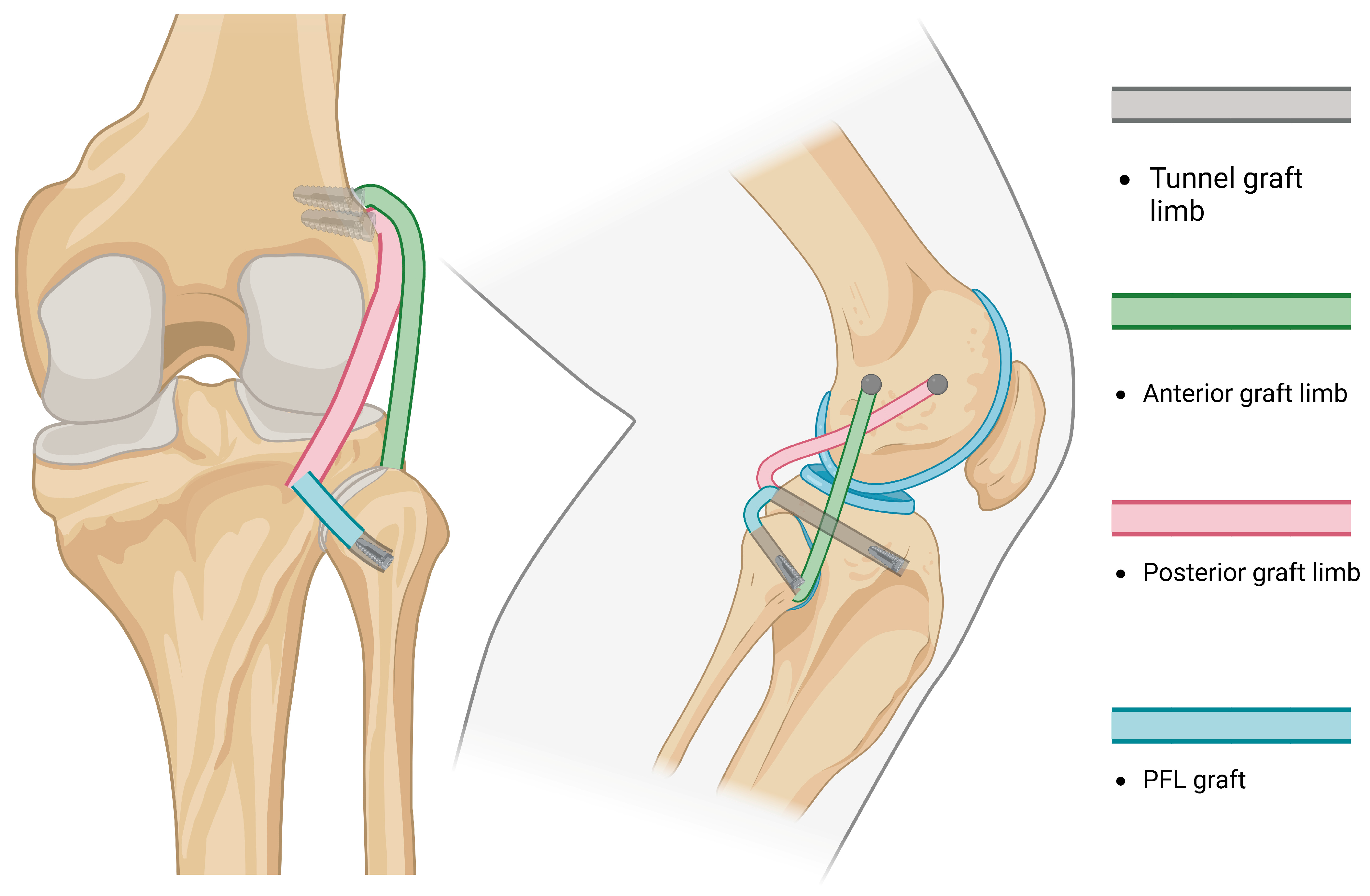


| Structure [References] | Direction of Stabilization | Range of Stabilization | Anatomic Course |
|---|---|---|---|
| Fibular collateral ligament (FCL) [18,28,29,30,38] | Varus | Maximal primary stabilizer at 0° gradually decreasing until 90° | Origin 1.4 mm proximal 3.1 mm posterior to the lateral femoral epicondyle Insertion Anteriorly on the fibular head 28.4 mm distal to the fibular styloid tip |
| External rotation | Maximal function around 0–30° | ||
| Tibial translation | Secondary multidirectional stabilizer of tibial translation | ||
| Popliteus tendon (PT) [19,33,39] PT is essential to unlock flexion by internally rotating the tibia and externally rotating the femur | Varus | Secondary function against varus angulation between 20 and 60° * | Origin around 18.5 mm anteriorly (17–23 mm) from the FCL attachment Insertion Posteromedial proximal tibia |
| Rotation | Primary function against external rotation emphasized between 30 and 90° * Secondary minimal function against internal rotation at all angles * | ||
| Tibial translation | Secondary function against anterior tibial translation between 0 and 30° * | ||
| Popliteofibular ligament (PFL) [34,35,37] | Varus | Small but significant effect between 0 and 30° * | Origin 5–10 mm distal to the lateral femoral epicondyle, expanding from the popliteus muscle-tendon Insertion 20–40 mm below the tibial plateau at the posterolateral fibular head |
| External rotation | Significant effect between 0 and 90°: favours external rotation in full extension, reduces it between 30 and 90° * | ||
| Tibial translation | Variable yet significant effect that seems to depend on coupled directional forces at different degrees of knee flexion * | ||
| Ileotibial band (ITB) [20,40,41,42] Knee extensor between 0–30 and flexor above 40° | Varus | Prominent role against varus angulation during extension, the tract is tightest between 10 and 30° | Origin 20–30 mm from antero-superior iliac crest Insertion 20–40 mm distal to tibial plateau at Gerdy’s tubercle |
| Tibial rotation | Produces 2.4 degrees of external rotation when activated * | ||
| Tibial translation | During ITB activation, there is less anterior tibial translation | ||
| Biceps femoris tendon (BFT) [20,25,43,44] | Varus | Secondary role | Origin Long head: 100–120 mm below PSIS, from ischial tuberosity Short head: Lateral tip of the linea aspera, 120–150 mm from femoral head Insertion 10–15 mm below the fibular head |
| Tibial rotation | Lateral hamstring activity peaks with external rotation *; activation causes external rotation | ||
| Tibial translation | Secondary role of resistance against anterior tibial translation at 90° crucial in extension to lower ACL strain | ||
| Arcuate complex (AC) [22,23] | Varus stress | Secondary function | Considered as a thickening and merge of structures and not a single ligament on its own Origin Posterolateral femoral condyle, 10–20 mm below FCL Insertion Posterior fibular head |
| Rotational stability | Primary function against external tibial rotation | ||
| Tibial translation | Secondary function reducing posterior tibial translation, reduces strain on PCL | ||
| Posterolateral capsule (PC) [24,45] | Varus | Secondary role | Origin Posterolateral femoral condyle and lateral intercondylar notch, connects to LCL and popliteal groove Insertion fibular head 10–15 mm from fibular head tip |
| Rotational stability | Static stabilizer against external tibial rotation | ||
| Posterior tibial translation | Anatomical passive resistance to posterior tibial translation is extension, and increases overall with posterior forces between 30° and 75° | ||
| Lateral gastrocnemius muscle (LGM) [42,46] | Varus | Influences 1.28–1.42° of varus and valgus at different degrees of ROM | Origin 50–70 mm superior to joint line from the lateral femoral condyle Insertion 20–30 mm below the calcaneal tuberosity at the posterior calcaneus Achilles insertion |
| Rotational stability | Significantly limits rotation with variable effect based on knee flexion degrees; −8.0° at 90–100° flexion and +4.81° at 20–30° flexion | ||
| Tibial translation | Significantly limits anterior–posterior lateral drawer effect by 18.64 mm at 90–100° in conjunction with ITB | ||
| Quadriceps muscle (QM) [36,47,48,49,50] Synergetic effect, not a part of PLC | Varus | Resists both varus and valgus stresses through patellar tendon | Origin AIIS, supra-acetabular region of acetabulum (rectus femoris) Greater trochanter, lateral lip of linea aspera (vastus lateralis) Medial linea aspera, medial intermuscular septum (vastus medialis) Anterolateral femoral shaft (vastus intermedius) Insertion Tibial tuberosity as patellar tendon |
| Rotational stability | Limits both internal and external rotation through patellar tendon; however, internal rotation is elicited by vastus medialis obliquus | ||
| Tibial translation | Mostly leads to anterior tibial translation between 0 and 80° and slightly favors posterior tibial translation between 80 and 120°. |
| Prominently Acting Forces | ||
|---|---|---|
| Varus Stability | Rotational Stability | Translational Stability |
| FCL ITB | FCL PT PFL BFT AC LGM | QM PC |
| Graft Type | Advantage | Disadvantage |
|---|---|---|
Autograft
| Economic No host reaction Reduced risk of infection Biologically advantageous No risk of disease transmission BFT can be used for multiple ligamentous reconstructions Semitendinosus strength 1060 N Gracilis strength 838 N | Limited availability Usually available for a single reconstruction Longer surgical time Additional scar Donor site morbidity Fewer reported trials compared with allografts BFT is part of the PLC; its use may weaken the PLC in certain degrees of ROM A hip flexor is implicated which means possible movement limitation and reduced return to sports owing to altered quadriceps/hamstring strength Alteration of the long-head BFT predisposed to injury and delayed return to sports [121] |
| Allograft | Can be used for multiple reconstructions Availability No donor site morbidity | Economic burden Risk of host reaction Higher risk of infection Not available in all countries Limited biological integration |
| Synthetic graft | Can be used for multiple reconstructions Availability No donor site morbidity | Economic burden Rare reaction of host Non-biological, thus bears a high risk of subsequent infection Rejection response/biocompatibility Tissue integration is limited Biomechanical performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Mukh, A.; Lee, S.; Rhim, H.C.; Jang, K.-M. Exploring the Posterolateral Corner of the Knee Joint: A Detailed Review of Recent Literature. J. Clin. Med. 2025, 14, 1549. https://doi.org/10.3390/jcm14051549
Abu-Mukh A, Lee S, Rhim HC, Jang K-M. Exploring the Posterolateral Corner of the Knee Joint: A Detailed Review of Recent Literature. Journal of Clinical Medicine. 2025; 14(5):1549. https://doi.org/10.3390/jcm14051549
Chicago/Turabian StyleAbu-Mukh, Assala, Seungyup Lee, Hye Chang Rhim, and Ki-Mo Jang. 2025. "Exploring the Posterolateral Corner of the Knee Joint: A Detailed Review of Recent Literature" Journal of Clinical Medicine 14, no. 5: 1549. https://doi.org/10.3390/jcm14051549
APA StyleAbu-Mukh, A., Lee, S., Rhim, H. C., & Jang, K.-M. (2025). Exploring the Posterolateral Corner of the Knee Joint: A Detailed Review of Recent Literature. Journal of Clinical Medicine, 14(5), 1549. https://doi.org/10.3390/jcm14051549







