Role of OCT in Assessing Vasa Vasorum in Chronic Coronary Syndrome: Insights from Long-Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
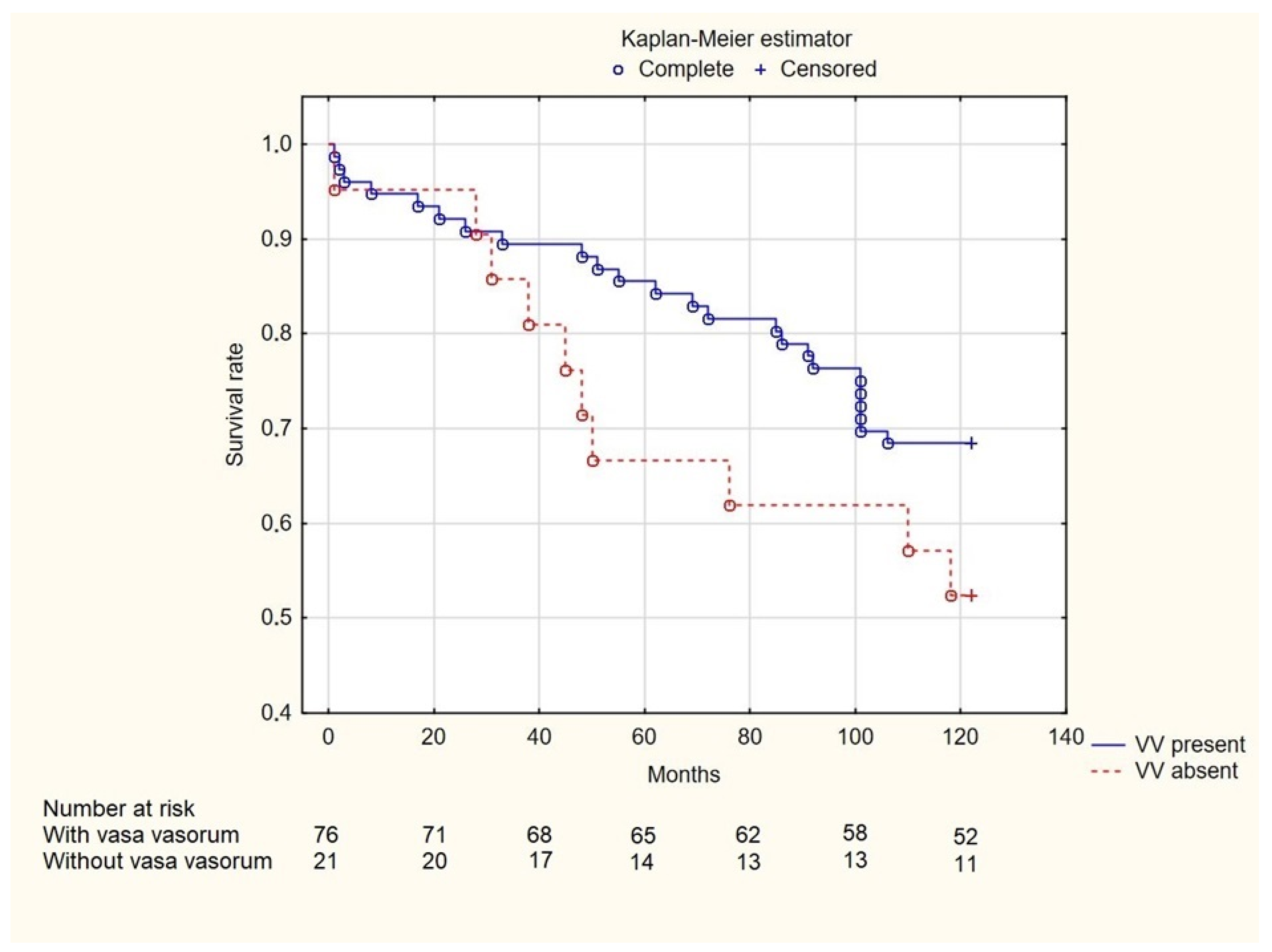
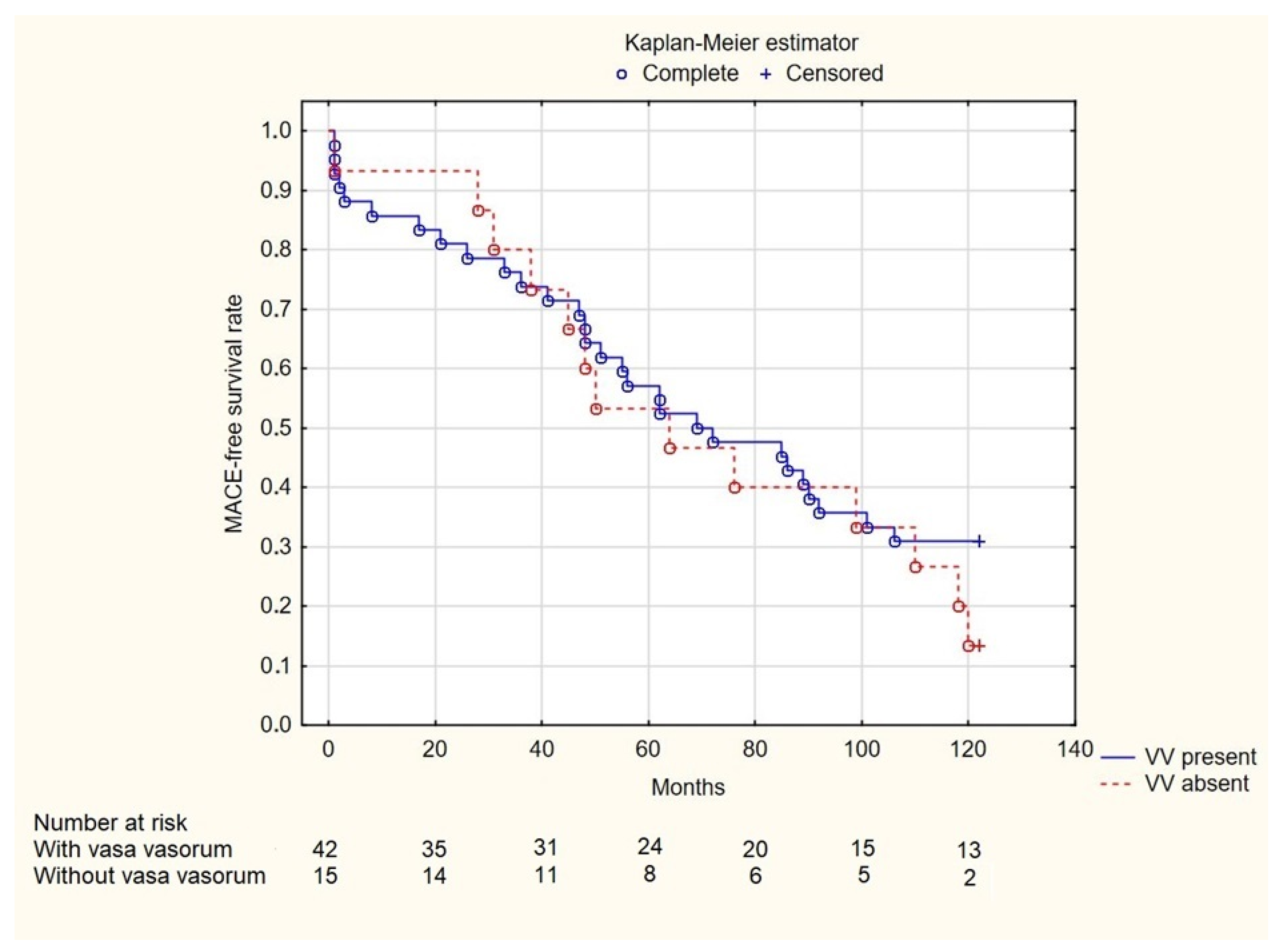
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, Z.A.; Galougahi, K.K.; Mintz, G.S.; Maehara, A.; Shlofmitz, R.A.; Mattesini, A. Intracoronary optical coherence tomography: State of the art and future directions. EuroIntervention 2021, 17, E105–E123. [Google Scholar] [CrossRef] [PubMed]
- Baruś, P.; Modrzewski, J.; Gumiężna, K.; Dunaj, P.; Głód, M.; Bednarek, A.; Wańha, W.; Roleder, T.; Kochman, J.; Tomaniak, M. Comparative Appraisal of Intravascular Ultrasound and Optical Coherence Tomography in Invasive Coronary Imaging: 2022 Update. J. Clin. Med. 2022, 11, 4055. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, H.G.; Costa, M.A.; Guagliumi, G.; Rollins, A.M.; Simon, D.I. Intracoronary Optical Coherence Tomography: A Comprehensive Review: Clinical and Research Applications. JACC Cardiovasc. Interv. 2009, 2, 1035. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.A.; Landmesser, U.; Maehara, A.; Matsumura, M.; Shlofmitz, R.A.; Guagliumi, G.; Price, M.J.; Hill, J.M.; Akasaka, T.; Prati, F.; et al. Optical Coherence Tomography-Guided versus Angiography-Guided PCI. N. Engl. J. Med. 2023, 389, 1466–1476. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Moreno, P.R.; Purushothaman, K.R.; Sirol, M.; Levy, A.P.; Fuster, V. Neovascularization in human atherosclerosis. Circulation 2006, 113, 2245–2252. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Gold, H.K.; Burke, A.P.; Fowler, D.R.; Kruth, H.S.; Weber, D.K.; Farb, A.; Guerrero, L.J.; Hayase, M.; Kutys, R.; et al. Intraplaque hemorrhage and progression of coronary atheroma. N. Engl. J. Med. 2003, 349, 2316–2325. [Google Scholar] [CrossRef]
- Campbell, K.A.; Lipinski, M.J.; Doran, A.C.; Skaflen, M.D.; Fuster, V.; McNamara, C.A. Lymphocytes and the adventitial immune response in atherosclerosis. Circ. Res. 2012, 110, 889–900. [Google Scholar] [CrossRef]
- Mulligan-Kehoe, M.J.; Simons, M. Vasa vasorum in normal and diseased arteries. Circulation 2014, 129, 2557–2566. [Google Scholar] [CrossRef]
- Taruya, A.; Tanaka, A.; Nishiguchi, T.; Matsuo, Y.; Ozaki, Y.; Kashiwagi, M.; Shiono, Y.; Orii, M.; Yamano, T.; Ino, Y.; et al. Vasa Vasorum Restructuring in Human Atherosclerotic Plaque Vulnerability: A Clinical Optical Coherence Tomography Study. J. Am. Coll. Cardiol. 2015, 65, 2469–2477. [Google Scholar] [CrossRef]
- Choi, B.J.; Matsuo, Y.; Aoki, T.; Kwon, T.G.; Prasad, A.; Gulati, R.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2473–2477. [Google Scholar] [CrossRef] [PubMed]
- Vorpahl, M.; Nakano, M.; Virmani, R. Small black holes in optical frequency domain imaging matches intravascular neoangiogenesis formation in histology. Eur. Heart J. 2010, 31, 1889. [Google Scholar] [CrossRef] [PubMed]
- Baruś, P.; Piasecki, A.; Gumiężna, K.; Bednarek, A.; Dunaj, P.; Głód, M.; Sadowski, K.; Ochijewicz, D.; Rdzanek, A.; Pietrasik, A.; et al. Multimodality OCT, IVUS and FFR evaluation of coronary intermediate grade lesions in women vs. men. Front. Cardiovasc. Med. 2023, 10, 1021023. [Google Scholar] [CrossRef] [PubMed]
- Tomaniak, M.; Ochijewicz, D.; Kołtowski, Ł.; Rdzanek, A.; Pietrasik, A.; Jąkała, J.; Slezak, M.; Malinowski, K.P.; Zaleska, M.; Maksym, J.; et al. OCT-Derived Plaque Morphology and FFR-Determined Hemodynamic Relevance in Intermediate Coronary Stenoses. J. Clin. Med. 2021, 10, 2379. [Google Scholar] [CrossRef] [PubMed]
- Baruś, P.; Hunia, J.; Kaczorowski, R.; Bednarek, A.; Ochijewicz, D.; Gumiężna, K.; Kołtowski, Ł.; Kochman, J.; Grabowski, M.; Tomaniak, M. Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate Coronary Artery Lesions. Med. Sci. Monit. 2024, 30, e943956-1. [Google Scholar] [CrossRef]
- Kennedy, M.W.; Fabris, E.; Ijsselmuiden, A.J.; Nef, H.; Reith, S.; Escaned, J.; Alfonso, F.; van Royen, N.; Wojakowski, W.; Witkowski, A.; et al. Combined optical coherence tomography morphologic and fractional flow reserve hemodynamic assessment of non- culprit lesions to better predict adverse event outcomes in diabetes mellitus patients: COMBINE (OCT-FFR) prospective study. Rationale and design. Cardiovasc. Diabetol. 2016, 15, 144. [Google Scholar] [CrossRef]
- Prati, F.; Guagliumi, G.; Mintz, G.S.; Costa, M.; Regar, E.; Akasaka, T.; Barlis, P.; Tearney, G.J.; Jang, I.K.; Arbustini, E.; et al. Expert review document part 2: Methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur. Heart J. 2012, 33, 2513–2520. [Google Scholar] [CrossRef]
- Prati, F.; Regar, E.; Mintz, G.S.; Arbustini, E.; Di Mario, C.; Jang, I.K.; Akasaka, T.; Costa, M.; Guagliumi, G.; Grube, E.; et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: Physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 2010, 31, 401–415. [Google Scholar] [CrossRef]
- Räber, L.; Mintz, G.S.; Koskinas, K.C.; Johnson, T.W.; Holm, N.R.; Onuma, Y.; Radu, M.D.; Joner, M.; Yu, B.; Jia, H.; et al. Clinical use of intracoronary imaging. Part 1: Guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention 2018, 14, 656–677. [Google Scholar] [CrossRef]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Kini, A.S.; Vengrenyuk, Y.; Yoshimura, T.; Matsumura, M.; Pena, J.; Baber, U.; Moreno, P.; Mehran, R.; Maehara, A.; Sharma, S.; et al. Fibrous Cap Thickness by Optical Coherence Tomography In Vivo. J. Am. Coll. Cardiol. 2017, 69, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Koizumi, M.; Okubo, R.; Yabe, T.; Watanabe, I.; Saito, D.; Toda, M.; Ikeda, T. Comparison of Coronary Intimal Plaques by Optical Coherence Tomography in Arteries with Versus Without Internal Running Vasa Vasorum. Am. J. Cardiol. 2017, 119, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Rodriguez-Porcel, M.; Matsuo, Y.; Cassar, A.; Kwon, T.G.; Franchi, F.; Gulati, R.; Kushwaha, S.S.; Lennon, R.J.; Lerman, L.O.; et al. Evaluation of coronary adventitial vasa vasorum using 3D optical coherence tomography—Animal and human studies. Atherosclerosis 2015, 239, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Papazoglou, A.S.; Karagiannidis, E.; Moysidis, D.V.; Sofidis, G.; Bompoti, A.; Stalikas, N.; Panteris, E.; Arvanitidis, C.; Herrmann, M.D.; Michaelson, J.S.; et al. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hell. J. Cardiol. 2021, 62, 399–407. [Google Scholar] [CrossRef]
- Altabas, V.; Biloš, L.S.K. The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. Int. J. Mol. Sci. 2022, 23, 2663. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, M.; Hu, M.; Zhang, Y.; Jiang, W.; Tang, W.; Ouyang, Y.; Jiang, L.; Mi, Y.; Chen, Z.; et al. Emerging applications of single-cell profiling in precision medicine of atherosclerosis. J. Transl. Med. 2024, 22, 97. [Google Scholar] [CrossRef]
- Cesaro, A.; Acerbo, V.; Indolfi, C.; Filardi, P.P.; Calabrò, P. The clinical relevance of the reversal of coronary atherosclerotic plaque. Eur. J. Intern. Med. 2024, 129, 16–24. [Google Scholar] [CrossRef]
- Alfonso, F.; De La Torre Hernández, J.M. Vasa vasorum and coronary artery disease progression: Optical coherence tomography findings. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 280–282. [Google Scholar] [CrossRef]
- Bhindi, R.; Guan, M.; Zhao, Y.; Humphries, K.H.; Mancini, G.B.J. Coronary atheroma regression and adverse cardiac events: A systematic review and meta-regression analysis. Atherosclerosis 2019, 284, 194–201. [Google Scholar] [CrossRef]
- Jackson, R.; Al-Hussaini, A.; Joseph, S.; van Soest, G.; Wood, A.; Macaya, F.; Gonzalo, N.; Cade, J.; Caixeta, A.; Hlinomaz, O.; et al. Spontaneous Coronary Artery Dissection: Pathophysiological Insights from Optical Coherence Tomography. JACC Cardiovasc. Imaging 2019, 12, 2475–2488. [Google Scholar] [CrossRef]
- Kwon, T.G.; Gulati, R.; Matsuzawa, Y.; Aoki, T.; Guddeti, R.R.; Herrmann, J.; Lennon, R.J.; Ritman, E.L.; Lerman, L.O.; Lerman, A. Proliferation of Coronary Adventitial Vasa Vasorum in Patients with Spontaneous Coronary Artery Dissection. JACC Cardiovasc. Imaging 2016, 9, 891–892. [Google Scholar] [CrossRef]
- Tsujita, K.; Kaikita, K.; Araki, S.; Yamada, T.; Nagamatsu, S.; Yamanaga, K.; Sakamoto, K.; Kojima, S.; Hokimoto, S.; Ogawa, H. In Vivo optical coherence tomography visualization of intraplaque neovascularization at the site of coronary vasospasm: A case report. BMC Cardiovasc. Disord. 2016, 16, 235. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, K.; Kubo, T.; Akasaka, T. Evaluation of coronary plaques and atherosclerosis using optical coherence tomography. Expert. Rev. Cardiovasc. Ther. 2021, 19, 379–386. [Google Scholar] [CrossRef]
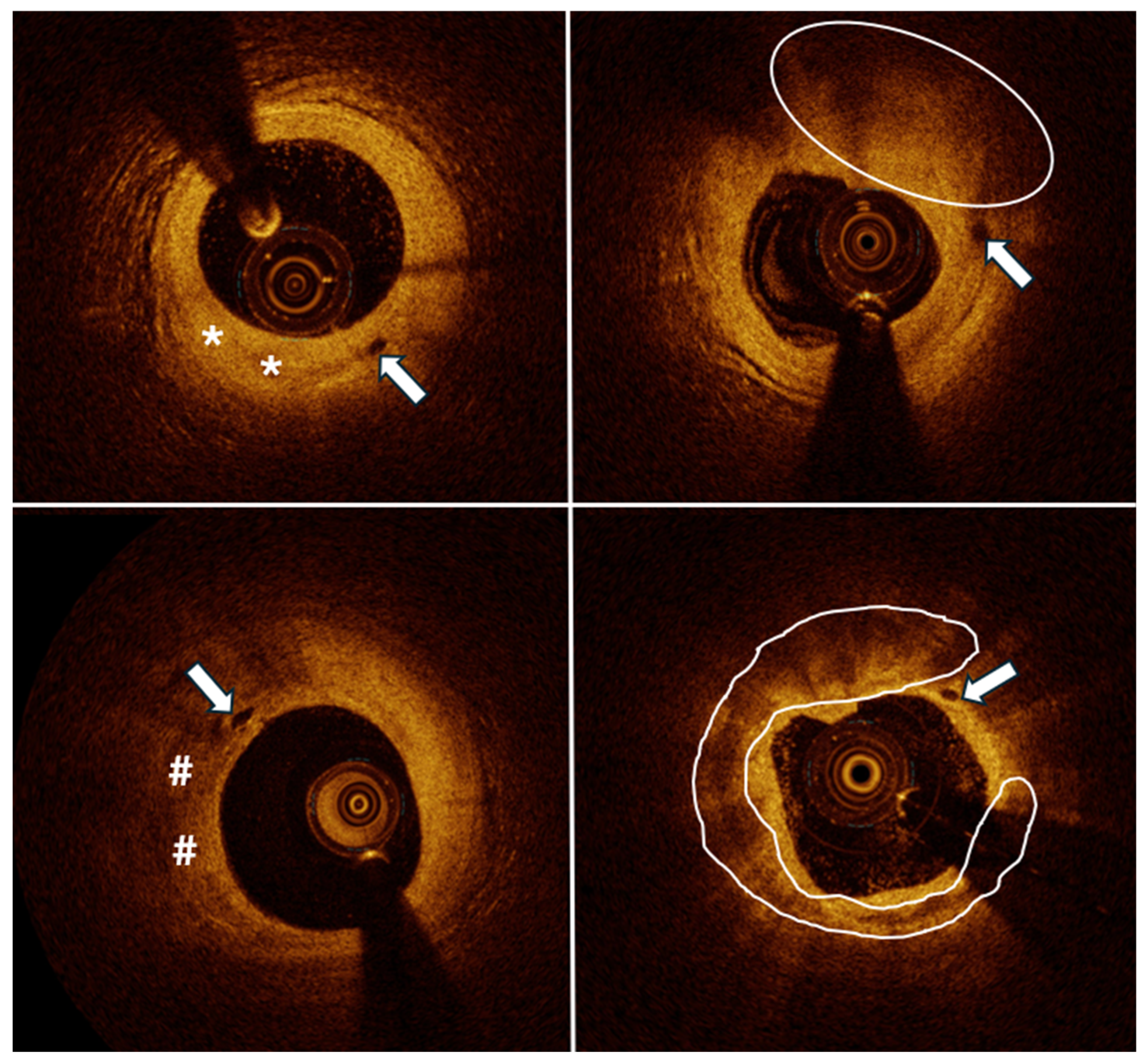
| Feature | Present Coronary Vasa Vasorum (n = 76) | Absence of Coronary Vasa Vasorum (n = 21) | p-Value |
|---|---|---|---|
| Age (years) | 64.79 ± 9.18 | 64.57 ± 9.68 | 0.924 |
| Male | 68 (89.5%) | 12 (57.1%) | 0.024 |
| Diabetes mellitus | 25 (32.9%) | 8 (38.1%) | 0.853 |
| Hypertension | 61 (80.3%) | 20 (95.2%) | 0.181 |
| Dyslipidemia | 50 (65.8%) | 11 (52.4%) | 0.384 |
| Chronic kidney disease | 10 (13.2%) | 1 (4.8%) | 0.447 |
| Serum creatinine (mg/dL) | 1.03 ± 0.24 | 0.87 ± 0.22 | 0.009 |
| eGFR | 75.2 ± 21 | 86.1 ± 21.3 | 0.043 |
| Chronic heart failure | 12 (15.8%) | 4 (19.1%) | 0.744 |
| Previous PCI | 55 (72.4%) | 16 (76.2%) | 0.943 |
| Previous CABG | 3 (4.0%) | 1 (4.1%) | 1.000 |
| Previous MI | 23 (88.5%) | 7 (87.5%) | 0.984 |
| TIA/stroke | 1 (1.3%) | 1 (5.0%) | 0.375 |
| Current smoker | 12 (16.0%) | 4 (19.1%) | 0.746 |
| Feature | Present Coronary Vasa Vasorum (n = 84) | Absence of Coronary Vasa Vasorum (n = 30) | p-Value |
|---|---|---|---|
| LM | 3 (3.6%) | 0 (0.0%) | 0.565 |
| LAD | 50 (59.5%) | 18 (60.0%) | 0.864 |
| Cx | 7 (8.3%) | 5 16.7%) | 0.352 |
| RCA | 19 (22.6%) | 6 (20.0%) | 0.968 |
| Lesion length in CAG (mm) | 14.9 ± 8.3 | 14.7 ± 6.5 | 0.760 |
| Diameter stenosis in CAG (%) | 57.6 ± 13.8 | 60.7 ± 15.3 | 0.311 |
| Mean lumen area (mm2) | 3.88 ± 1.76 | 4.01 ± 2.00 | 0.731 |
| Mean lumen diameter (mm) | 2.26 ± 0.38 | 2.57 ± 0.57 | 0.026 |
| Calcified plaque | 29 (34.9%) | 13 (43.3%) | 0.552 |
| Fibrous plaque | 28 (33.7%) | 10 (33.3%) | 0.853 |
| Lipid-rich plaque | 13 (15.7%) | 1 (3.3%) | 0.108 |
| Mixed plaque | 13 (15.7%) | 6 (20.0%) | 0.795 |
| TCFA | 14 (16.9%) | 5 (16.7%) | 0.795 |
| Mean FCT (mm) | 0.14 ± 0.05 | 0.16 ± 0.06 | 0.309 |
| Minimal FCT (mm) | 0.13 ± 0.05 | 0.16 ± 0.06 | 0.131 |
| Mean angle of the calcium (°) | 115.1 ± 63.8 | 109.7 ± 63.2 | 0.670 |
| Maximal angle of the calcium (°) | 132.8 ± 77.8 | 120.8 ± 80.5 | 0.383 |
| Mean cap thickness over calcium (mm) | 0.11 ± 0.07 | 0.12 ± 0.08 | 0.630 |
| Cap thickness over calcium at the distal part of the lesion (mm) | 0.07 ± 0.06 | 0.18 ± 0.09 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baruś, P.; Bartkiewicz, K.; Pęczek, P.; Libera, A.; Dunaj, P.; Jonik, S.; Kochman, J.; Grabowski, M.; Tomaniak, M. Role of OCT in Assessing Vasa Vasorum in Chronic Coronary Syndrome: Insights from Long-Term Follow-Up. J. Clin. Med. 2025, 14, 1560. https://doi.org/10.3390/jcm14051560
Baruś P, Bartkiewicz K, Pęczek P, Libera A, Dunaj P, Jonik S, Kochman J, Grabowski M, Tomaniak M. Role of OCT in Assessing Vasa Vasorum in Chronic Coronary Syndrome: Insights from Long-Term Follow-Up. Journal of Clinical Medicine. 2025; 14(5):1560. https://doi.org/10.3390/jcm14051560
Chicago/Turabian StyleBaruś, Piotr, Karolina Bartkiewicz, Piotr Pęczek, Anna Libera, Piotr Dunaj, Szymon Jonik, Janusz Kochman, Marcin Grabowski, and Mariusz Tomaniak. 2025. "Role of OCT in Assessing Vasa Vasorum in Chronic Coronary Syndrome: Insights from Long-Term Follow-Up" Journal of Clinical Medicine 14, no. 5: 1560. https://doi.org/10.3390/jcm14051560
APA StyleBaruś, P., Bartkiewicz, K., Pęczek, P., Libera, A., Dunaj, P., Jonik, S., Kochman, J., Grabowski, M., & Tomaniak, M. (2025). Role of OCT in Assessing Vasa Vasorum in Chronic Coronary Syndrome: Insights from Long-Term Follow-Up. Journal of Clinical Medicine, 14(5), 1560. https://doi.org/10.3390/jcm14051560






