Clinical and Neuroradiological Manifestations of Cerebral Amyloid Angiopathy: A Closer Look into the Natural History of a Frequent Disease
Abstract
1. Introduction
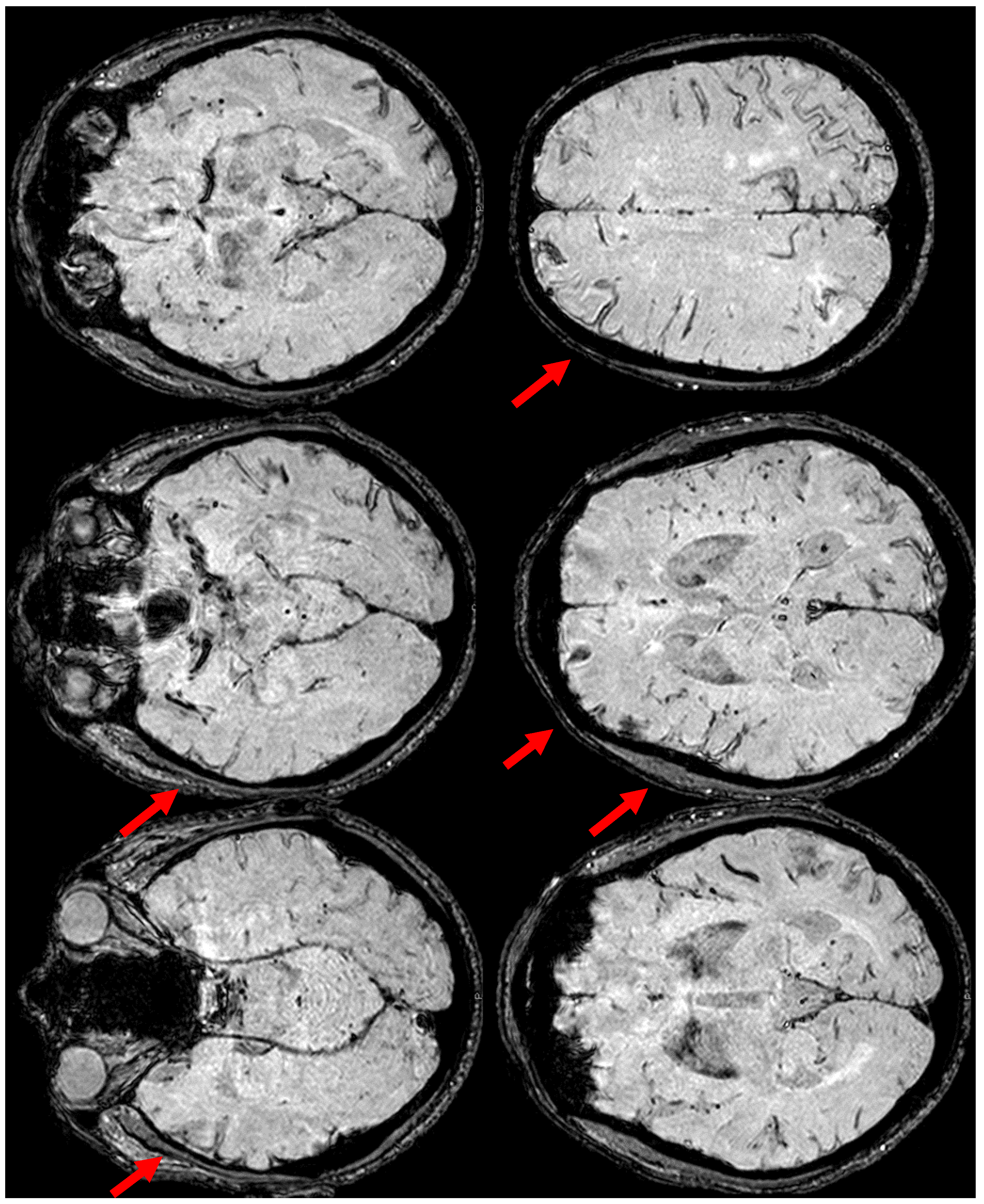
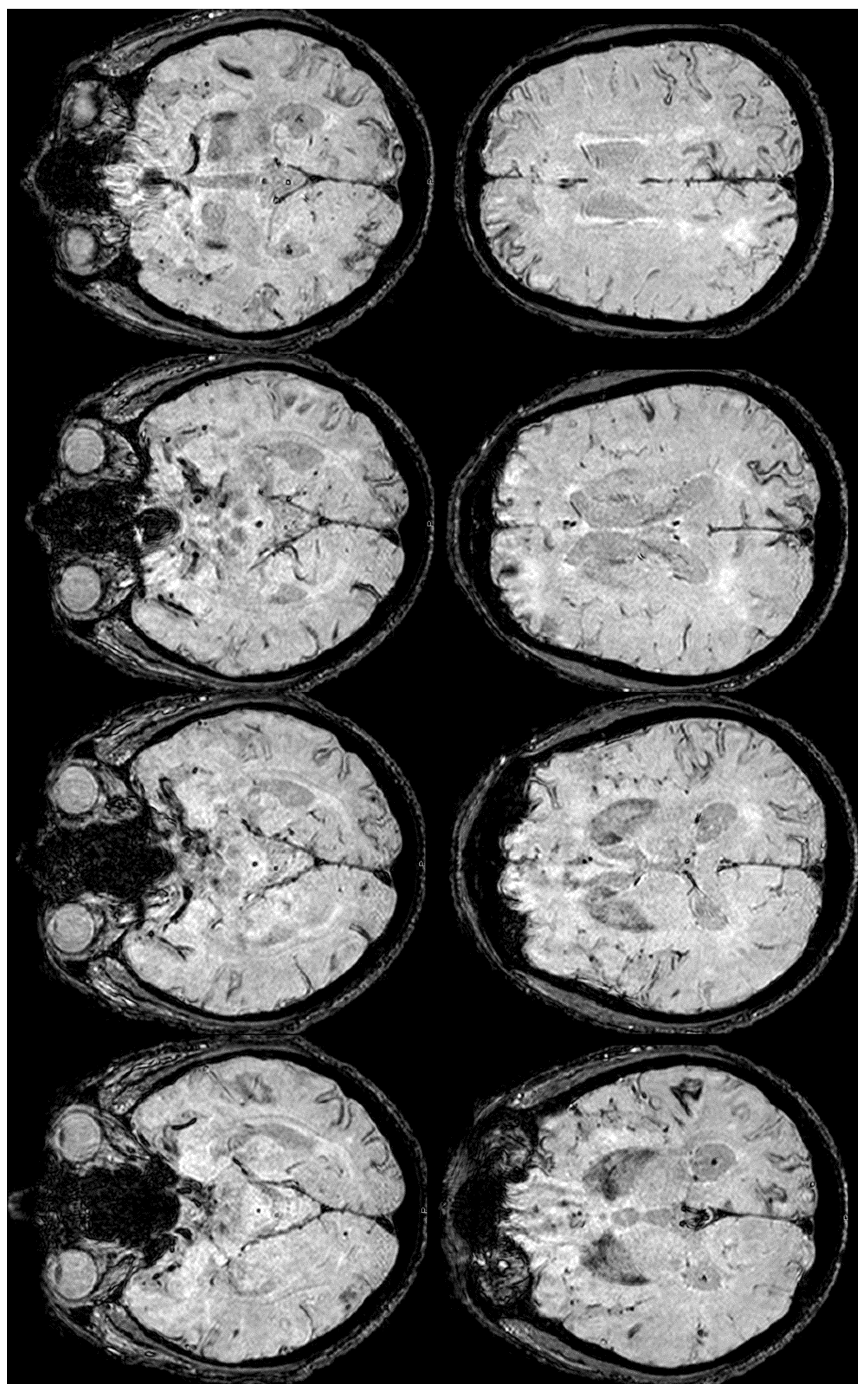
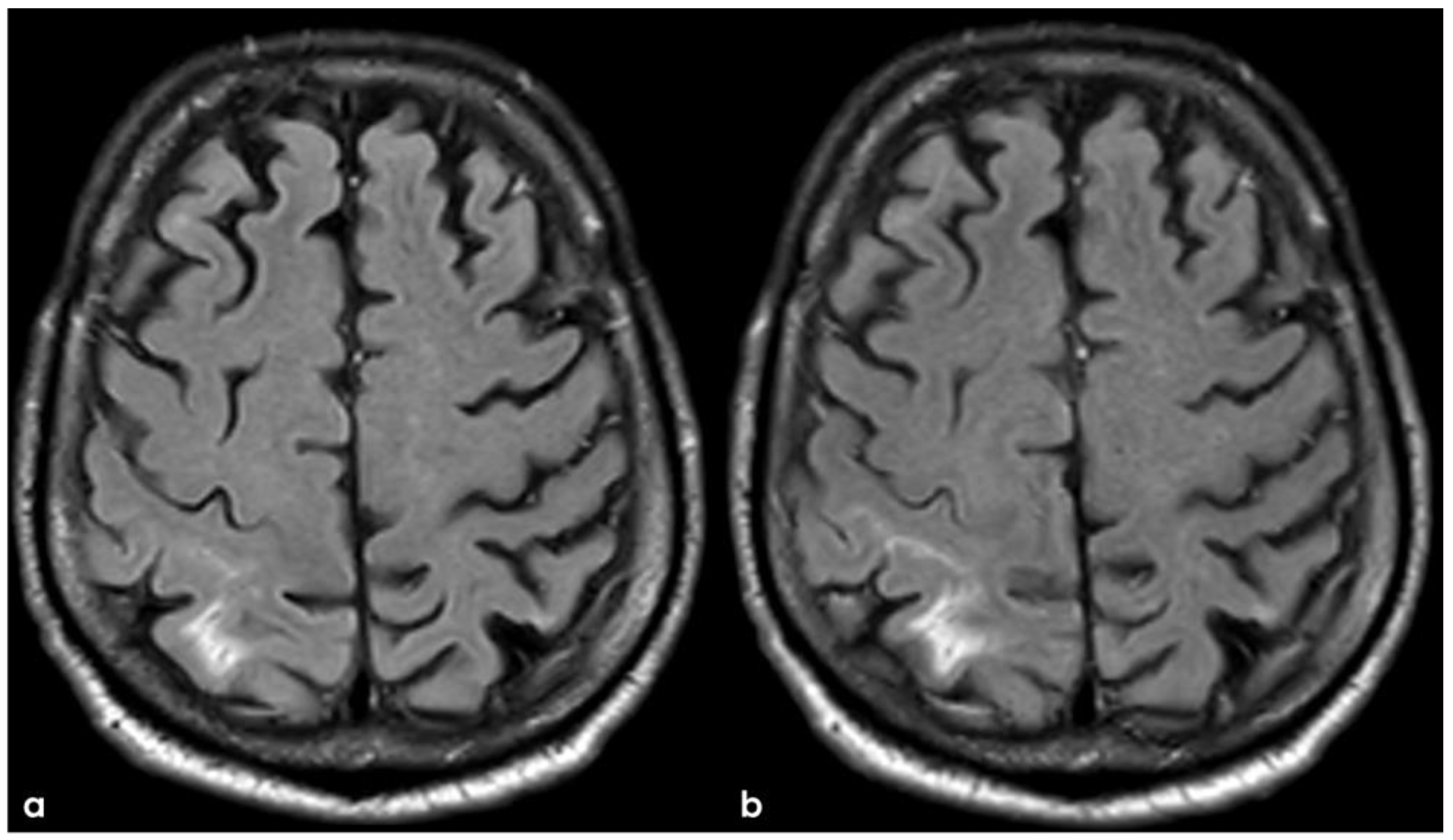
2. December 2013: Sulcal SAH and TFNEs
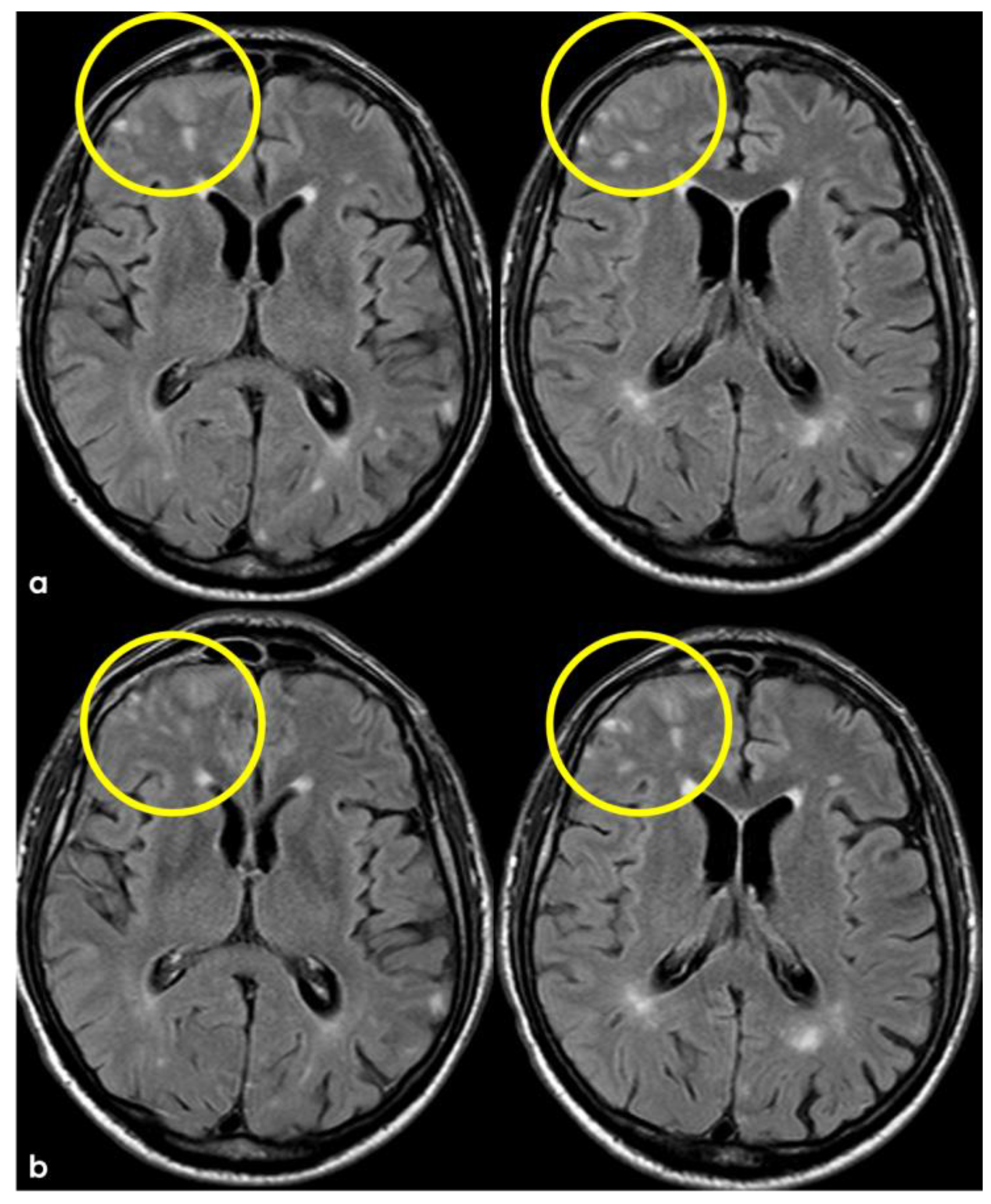
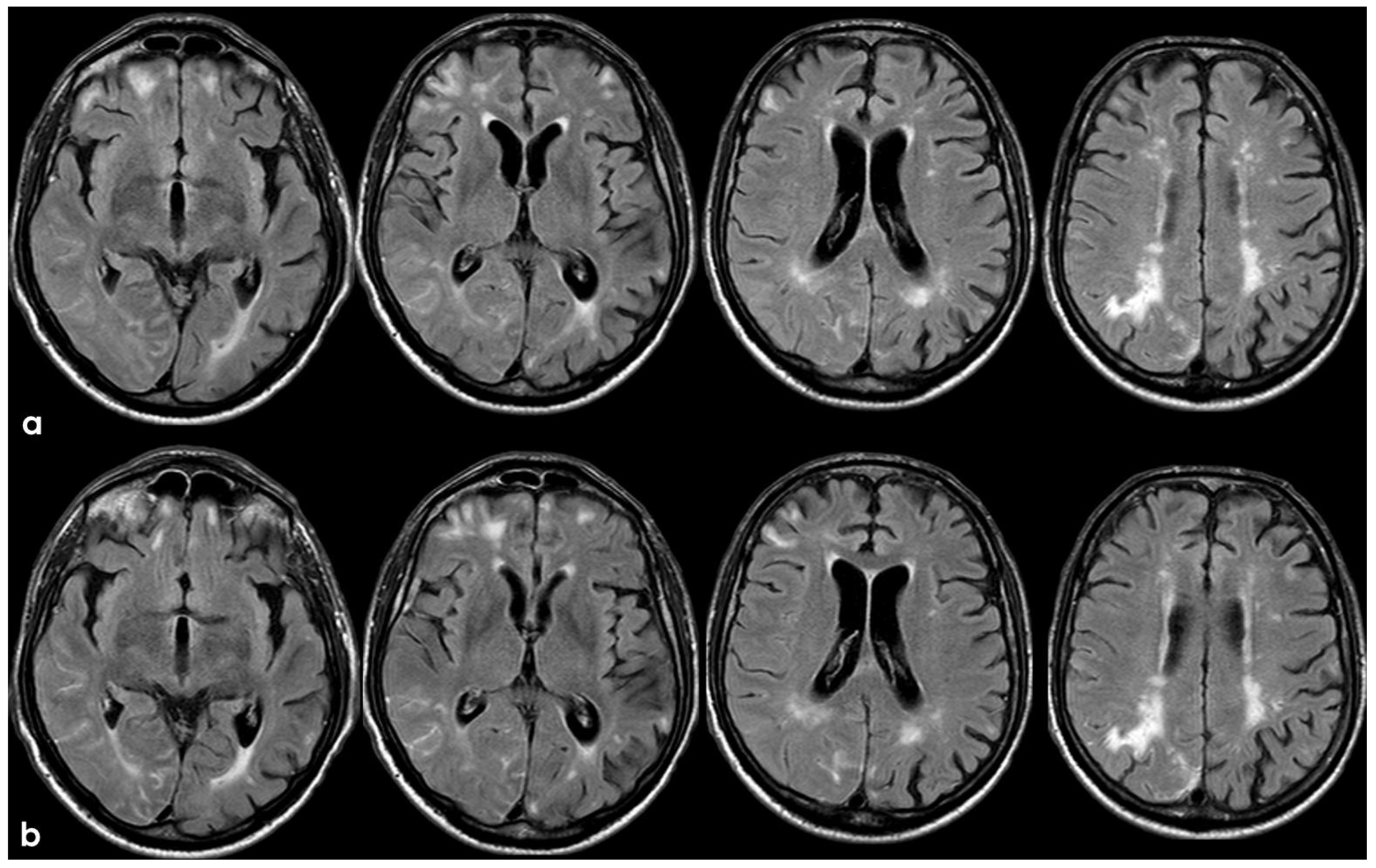
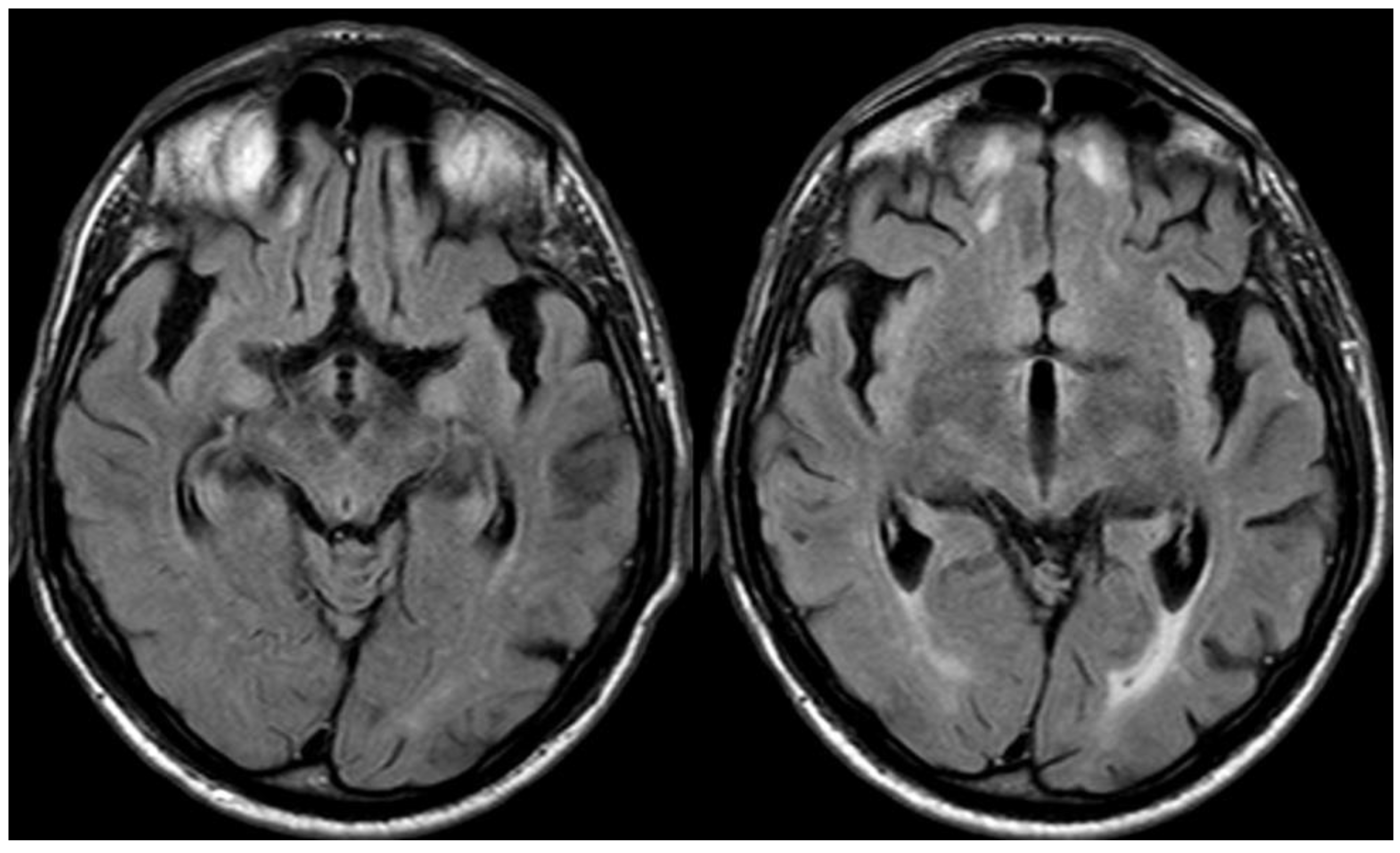
3. May 2014: CAA-Related Inflammation
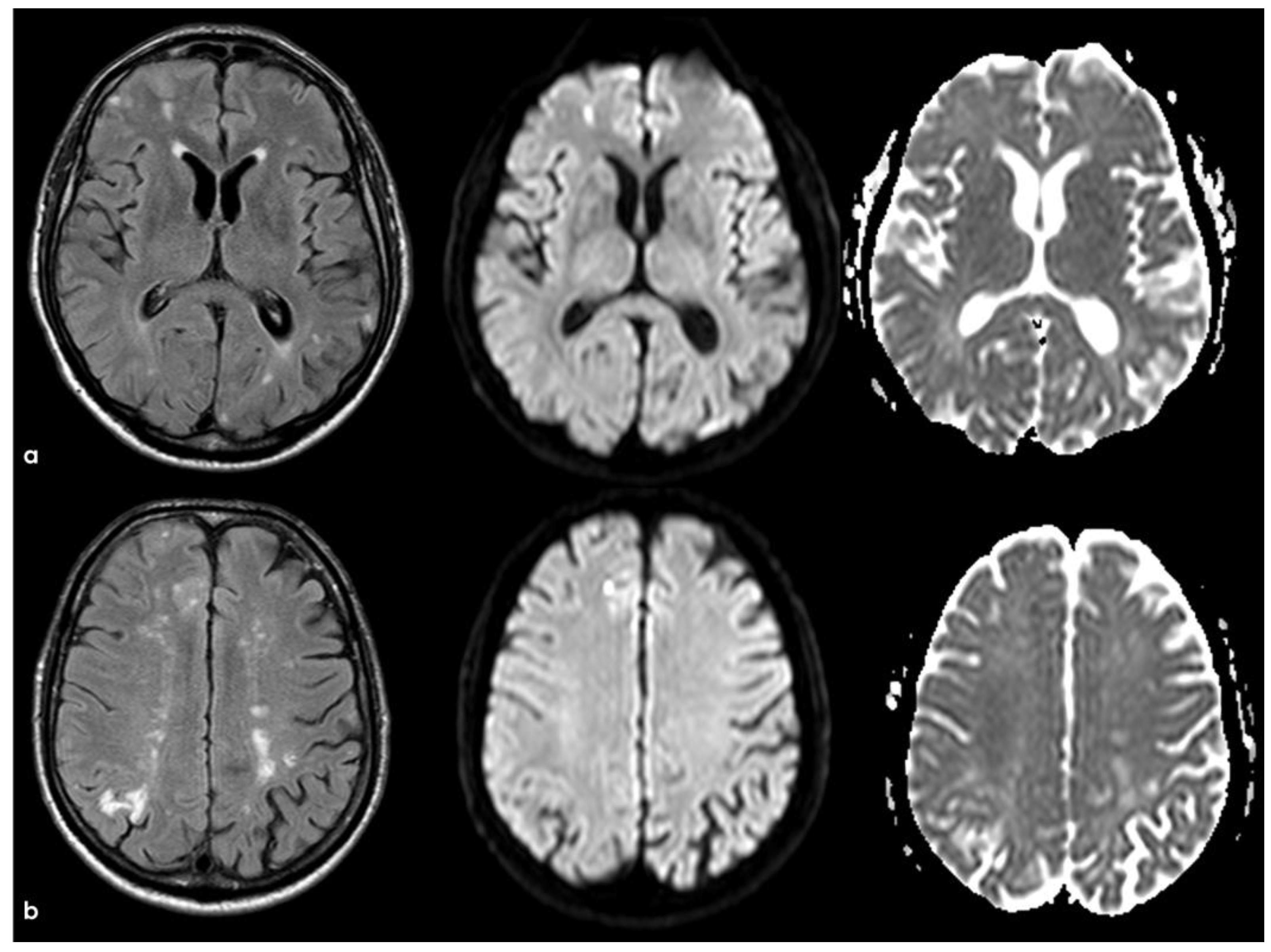
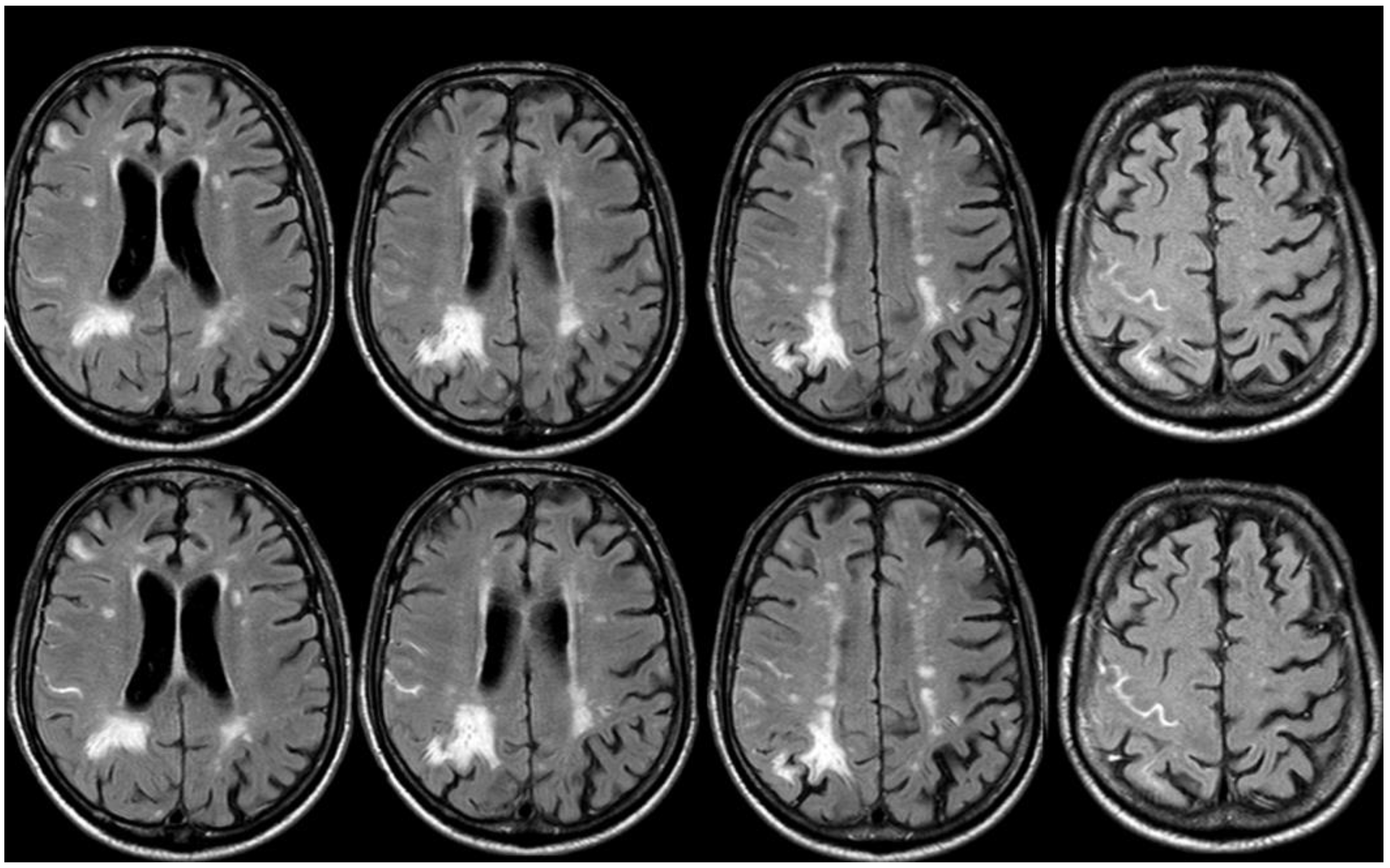
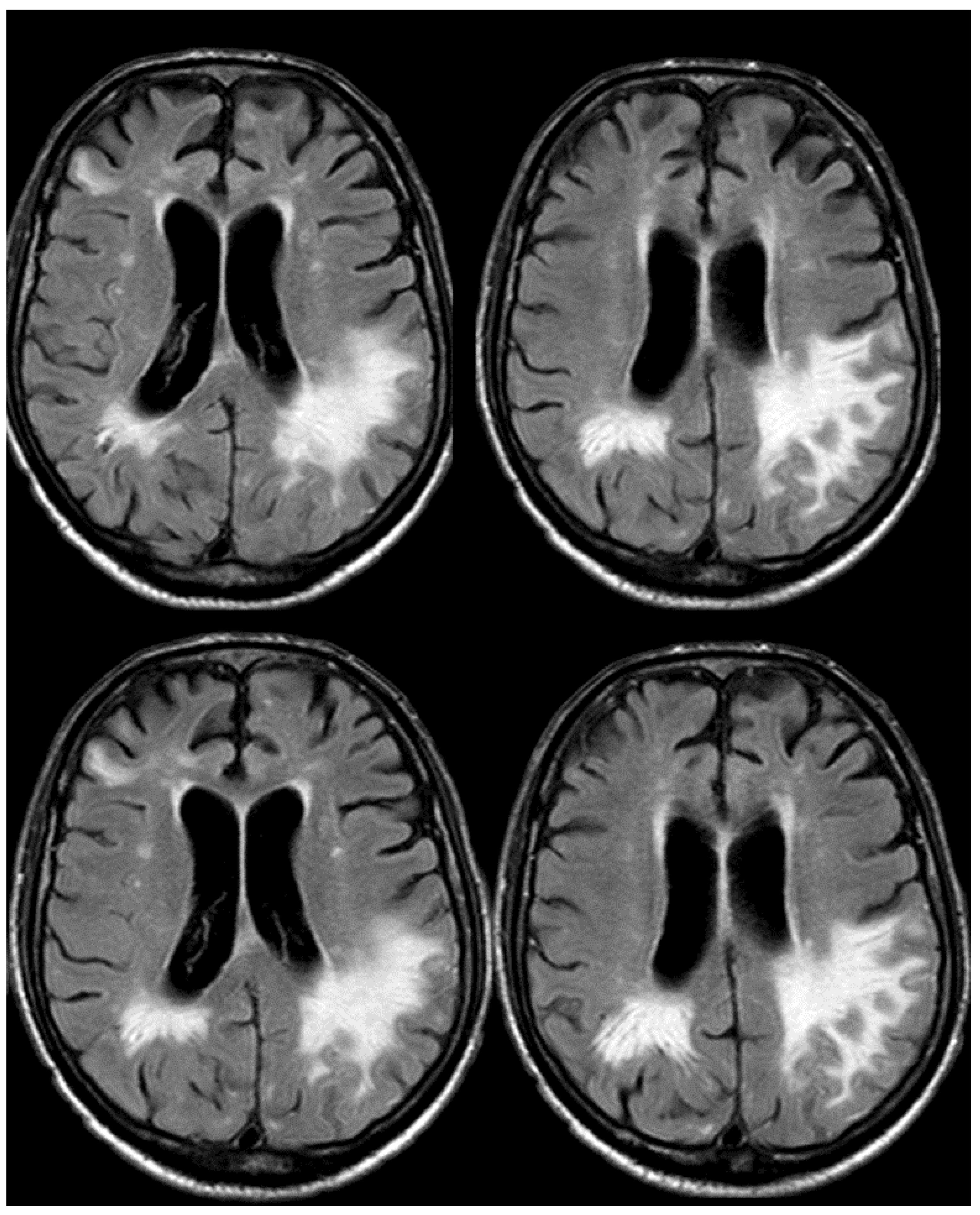
4. December 2015: Ischemic Stroke
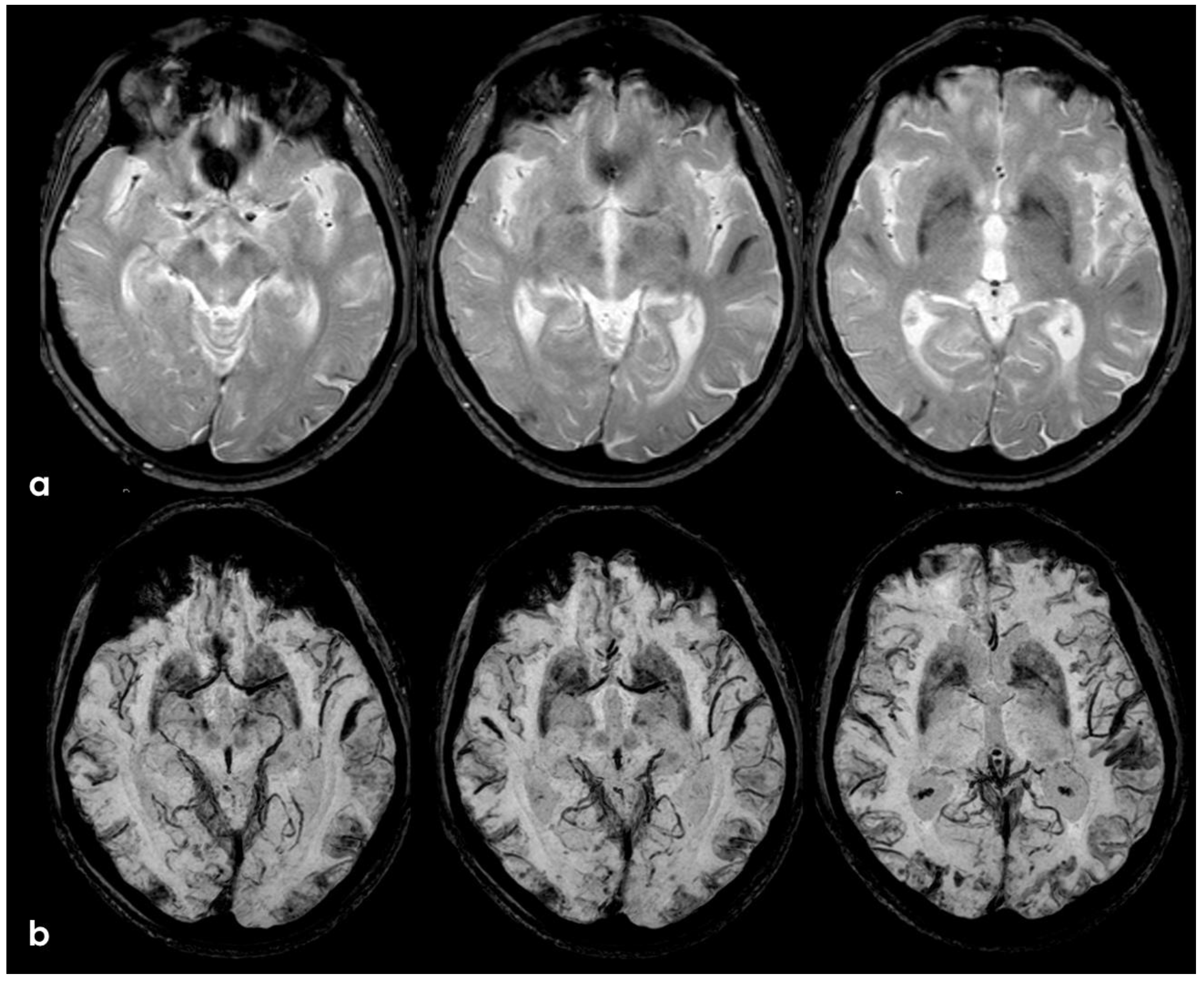
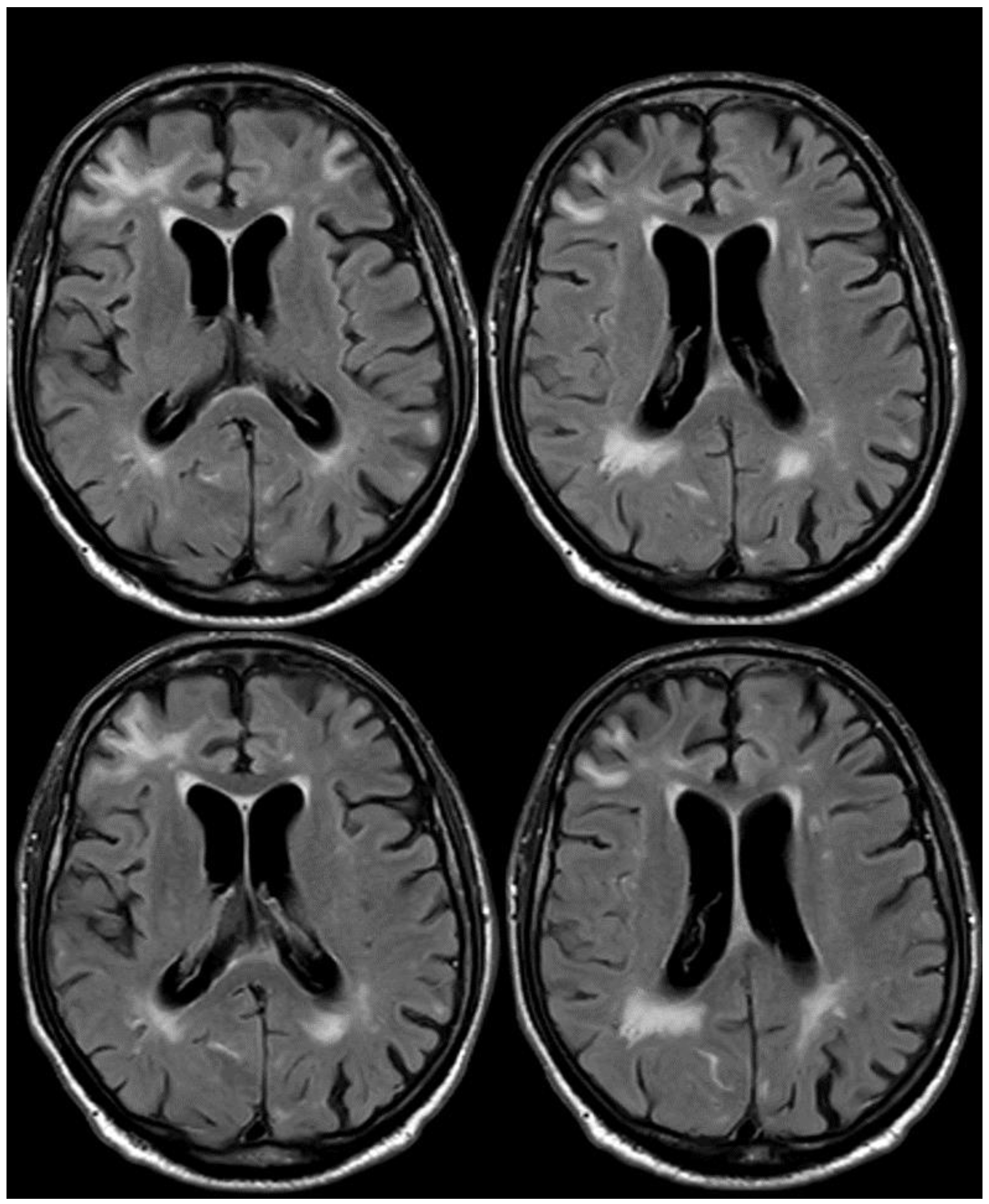
5. February 2017: First Recurrent CAA-Related Inflammation
6. December 2017: Recurrent SAH
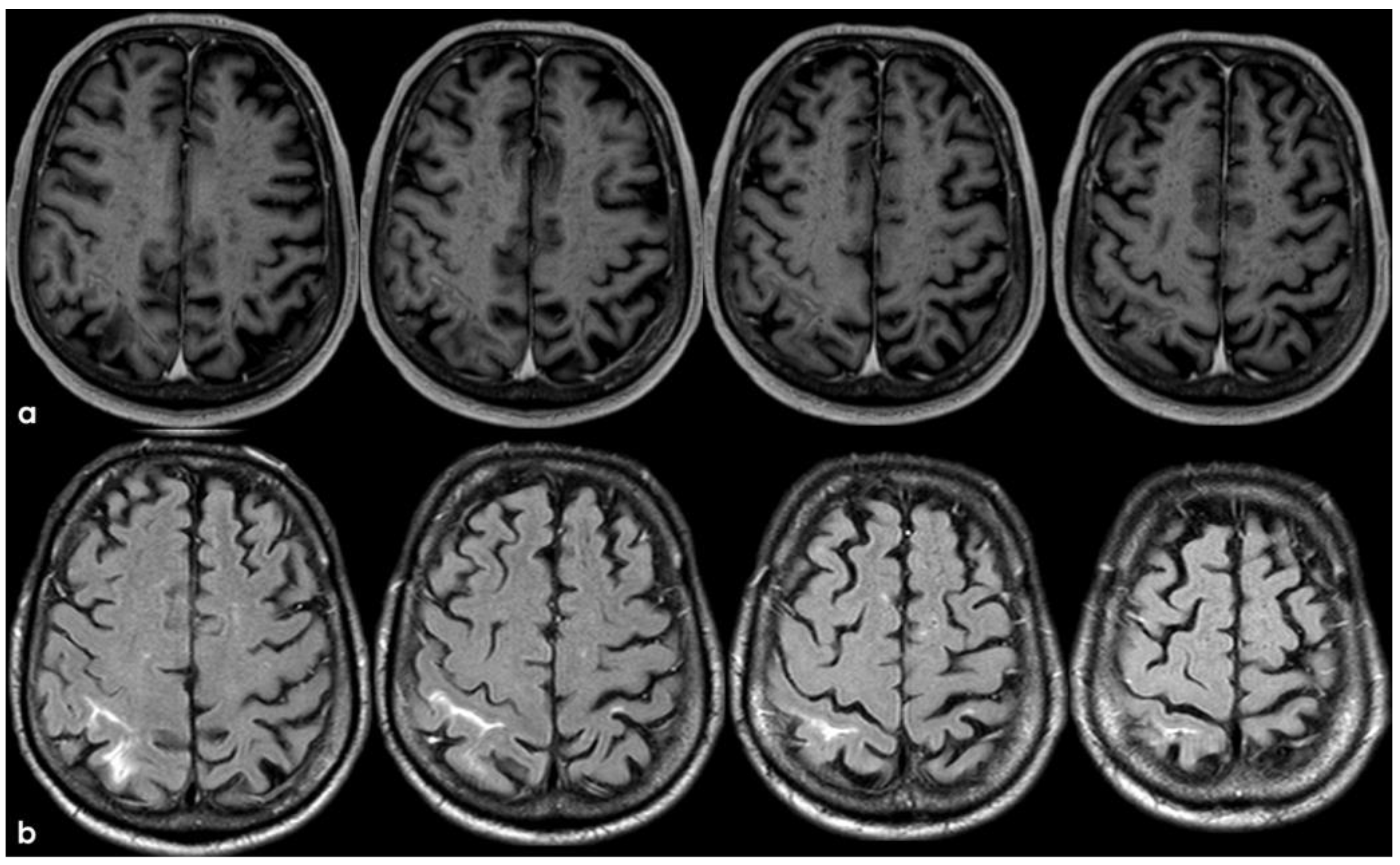
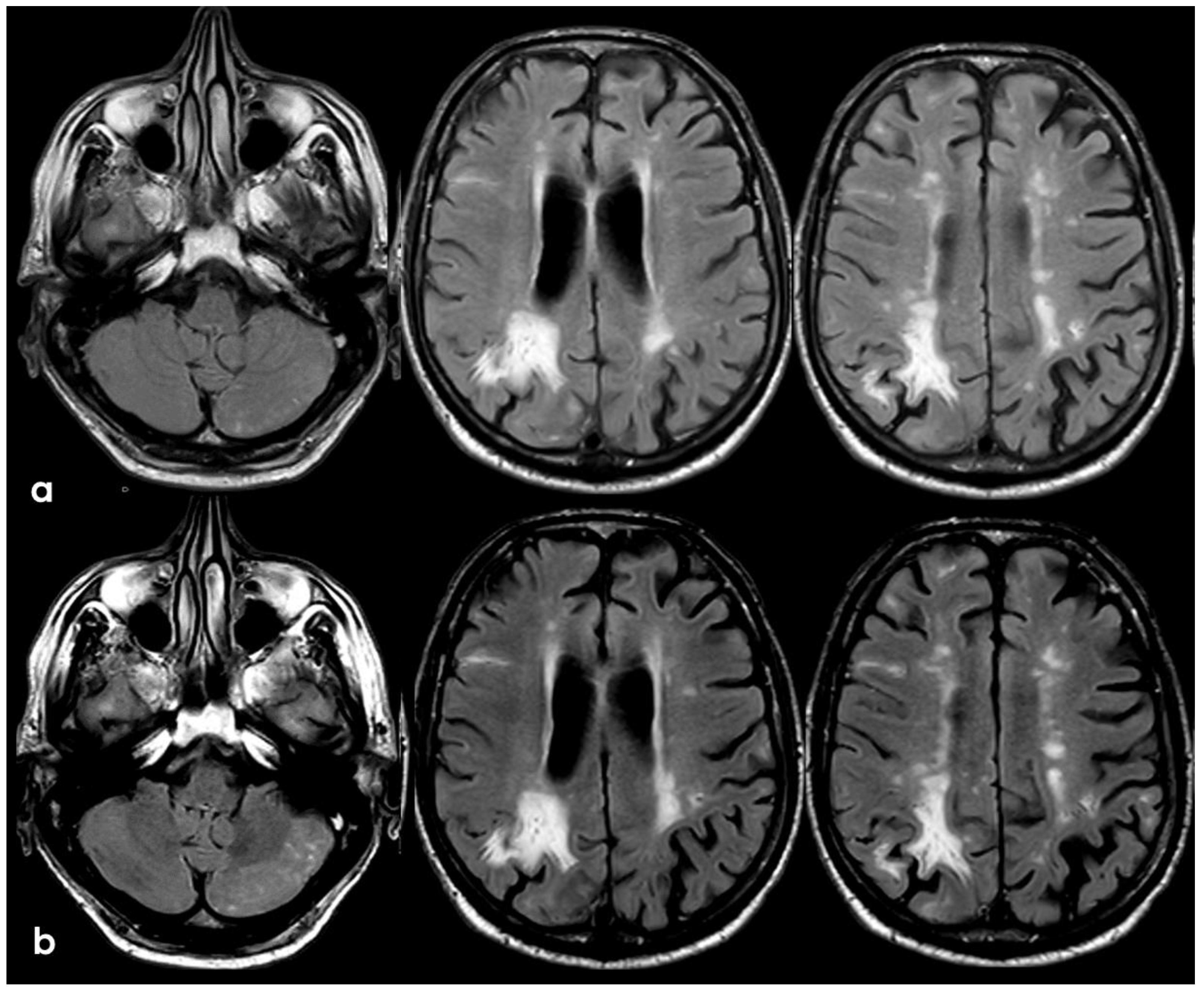
7. April 2018: Second Recurrent CAA-Related Inflammation
8. April 2018: Third Recurrent CAA-Related Inflammation
9. Additional Information and Discussion: How This Case Helps to Better Understand CAA
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jokinen, H.; Koikkalainen, J.; Laakso, H.M.; Melkas, S.; Nieminen, T.; Brander, A.; Korvenoja, A.; Rueckert, D.; Barkhof, F.; Scheltens, P.; et al. Global Burden of Small Vessel Disease-Related Brain Changes on MRI Predicts Cognitive and Functional Decline. Stroke 2020, 51, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Jansma, A.; de Bresser, J.; Schoones, J.W.; van Heemst, D.; Akintola, A.A. Sporadic cerebral small vessel disease and cognitive decline in healthy older adults: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2024, 44, 660–679. [Google Scholar] [CrossRef] [PubMed]
- Seiffge, D.J.; Wilson, D.; Ambler, G.; Banerjee, G.; Hostettler, I.C.; Houlden, H.; Shakeshaft, C.; Cohen, H.; Yousry, T.A.; Salman, R.A.-S.; et al. Small vessel disease burden and intracerebral haemorrhage in patients taking oral anticoagulants. J. Neurol. Neurosurg. Psychiatry 2021, 92, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef]
- Mahammedi, A.; Wang, L.; Williamson, B.; Khatri, P.; Kissela, B.; Sawyer, R.; Shatz, R.; Khandwala, V.; Vagal, A. Small Vessel Disease, a Marker of Brain Health: What the Radiologist Needs to Know. AJNR Am. J. Neuroradiol. 2022, 43, 650–660. [Google Scholar] [CrossRef]
- Duering, M.; Biessels, G.J.; Brodtmann, A.; Chen, C.; Cordonnier, C.; de Leeuw, F.-E.; Debette, S.; Frayne, R.; Jouvent, E.; Rost, N.S.; et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 2023, 22, 602–618, Erratum in Lancet Neurol. 2023, 22, e10. [Google Scholar] [CrossRef]
- Puy, L.; Pasi, M.; Rodrigues, M.; van Veluw, S.J.; Tsivgoulis, G.; Shoamanesh, A.; Cordonnier, C. Cerebral microbleeds: From depiction to interpretation. J. Neurol. Neurosurg. Psychiatry 2021, 92, 598–607. [Google Scholar] [CrossRef]
- Pasi, M.; Charidimou, A.; Boulouis, G.; Auriel, E.; Ayres, A.; Schwab, K.M.; Goldstein, J.N.; Rosand, J.; Viswanathan, A.; Pantoni, L.; et al. Mixed-location cerebral hemorrhage/microbleeds: Underlying microangiopathy and recurrence risk. Neurology 2018, 90, e119–e126. [Google Scholar] [CrossRef]
- Charidimou, A.; Linn, J.; Vernooij, M.W.; Opherk, C.; Akoudad, S.; Baron, J.-C.; Greenberg, S.M.; Jäger, H.R.; Werring, D.J. Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015, 138 Pt 8, 2126–2139. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bacskai, B.J.; Hernandez-Guillamon, M.; Pruzin, J.; Sperling, R.; van Veluw, S.J. Cerebral amyloid angiopathy and Alzheimer disease—One peptide, two pathways. Nat. Rev. Neurol. 2020, 16, 30–42. [Google Scholar] [CrossRef]
- Piazza, F.; Winblad, B. Amyloid-Related Imaging Abnormalities (ARIA) in Immunotherapy Trials for Alzheimer’s Disease: Need for Prognostic Biomarkers? J. Alzheimers Dis. 2016, 52, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, D.A. Antibody-Mediated Clearance of Brain Amyloid-β: Mechanisms of Action, Effects of Natural and Monoclonal Anti-Aβ Antibodies, and Downstream Effects. J. Alzheimers Dis. Rep. 2023, 7, 873–899. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Jack, C.R., Jr.; Black, S.E.; Frosch, M.P.; Greenberg, S.M.; Hyman, B.T.; Scheltens, P.; Carrillo, M.C.; Thies, W.; Bednar, M.M.; et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: Recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011, 7, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, V.; Brahmbhatt, P.; Desai, A.; Vibhute, P.; Joseph-Mathurin, N.; Bathla, G. Amyloid-related Imaging Abnormalities in Alzheimer Disease Treated with Anti-Amyloid-β Therapy. Radiographics 2023, 43, e230009. [Google Scholar] [CrossRef]
- Barakos, J.; Purcell, D.; Suhy, J.; Chalkias, S.; Burkett, P.; Grassi, C.M.; Castrillo-Viguera, C.; Rubino, I.; Vijverberg, E. Detection and Management of Amyloid-Related Imaging Abnormalities in Patients with Alzheimer’s Disease Treated with Anti-Amyloid Beta Therapy. J. Prev. Alzheimers Dis. 2022, 9, 211–220. [Google Scholar] [CrossRef]
- Roytman, M.; Mashriqi, F.; Al-Tawil, K.; Schulz, P.E.; Zaharchuk, G.; Benzinger, T.L.S.; Franceschi, A.M. Amyloid-Related Imaging Abnormalities: An Update. AJR Am. J. Roentgenol. 2023, 220, 562–574. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Suh, C.H.; Shim, W.H.; Lim, J.-S.; Lee, J.-H.; Kim, S.J. Incidence of Amyloid-Related Imaging Abnormalities in Patients With Alzheimer Disease Treated With Anti-β-Amyloid Immunotherapy: A Meta-analysis. Neurology 2022, 99, e2092–e2101. [Google Scholar] [CrossRef]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- DiFrancesco, J.C.; Longoni, M.; Piazza, F. Anti-Aβ Autoantibodies in Amyloid Related Imaging Abnormalities (ARIA): Candidate Biomarker for Immunotherapy in Alzheimer’s Disease and Cerebral Amyloid Angiopathy. Front. Neurol. 2015, 6, 207. [Google Scholar] [CrossRef]
- Piazza, F.; Caminiti, S.P.; Zedde, M.; Presotto, L.; DiFrancesco, J.C.; Pascarella, R.; Giossi, A.; Sessa, M.; Poli, L.; Basso, G.; et al. Association of Microglial Activation With Spontaneous ARIA-E and CSF Levels of Anti-Aβ Autoantibodies. Neurology 2022, 99, e1265–e1277. [Google Scholar] [CrossRef]
- Piazza, F.; Greenberg, S.M.; Savoiardo, M.; Gardinetti, M.; Chiapparini, L.; Raicher, I.; Nitrini, R.; Sakaguchi, H.; Brioschi, M.; Billo, G.; et al. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: Implications for amyloid-modifying therapies. Ann. Neurol. 2013, 73, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Zedde, M.; Pascarella, R.; Piazza, F. CAA-ri and ARIA: Two Faces of the Same Coin? AJNR Am. J. Neuroradiol. 2023, 44, E13–E14. [Google Scholar] [CrossRef] [PubMed]
- Antolini, L.; DiFrancesco, J.C.; Zedde, M.; Basso, G.; Arighi, A.; Shima, A.; Cagnin, A.; Caulo, M.; Carare, R.O.; Charidimou, A.; et al. Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study. Neurology 2021, 97, e1809–e1822. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Frosch, M.P.; Baron, J.-C.; Pasi, M.; Albucher, J.F.; Banerjee, G.; Barbato, C.; Bonneville, F.; Brandner, S.; et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022, 21, 714–725. [Google Scholar] [CrossRef]
- Cogswell, P.; Barakos, J.; Barkhof, F.; Benzinger, T.; Jack, C.; Poussaint, T.; Raji, C.; Ramanan, V.; Whitlow, C. Amyloid-Related Imaging Abnormalities with Emerging Alzheimer Disease Therapeutics: Detection and Reporting Recommendations for Clinical Practice. AJNR Am. J. Neuroradiol. 2022, 43, E19–E35. [Google Scholar] [CrossRef] [PubMed]
- Auriel, E.; Charidimou, A.; Gurol, M.E.; Ni, J.; Van Etten, E.S.; Martinez-Ramirez, S.; Boulouis, G.; Piazza, F.; DiFrancesco, J.C.; Frosch, M.P.; et al. Validation of Clinicoradiological Criteria for the Diagnosis of Cerebral Amyloid Angiopathy-Related Inflammation. JAMA Neurol. 2016, 73, 197–202. [Google Scholar] [CrossRef]
- Zedde, M.; Grisendi, I.; Assenza, F.; Napoli, M.; Moratti, C.; Pavone, C.; Bonacini, L.; Di Cecco, G.; D’aniello, S.; Pezzella, F.R.; et al. Spontaneous Non-Aneurysmal Convexity Subarachnoid Hemorrhage: A Scoping Review of Different Etiologies beyond Cerebral Amyloid Angiopathy. J. Clin. Med. 2024, 13, 4382. [Google Scholar] [CrossRef]
- Khurram, A.; Kleinig, T.; Leyden, J. Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke 2014, 45, 1151–1153. [Google Scholar] [CrossRef]
- Kumar, S.; Goddeau, R.P.; Selim, M.H.; Thomas, A.; Schlaug, G.; Alhazzani, A.; Searls, D.E.; Caplan, L.R. Atraumatic convexal subarachnoid hemorrhage: Clinical presentation, imaging patterns, and etiologies. Neurology 2010, 74, 893–899. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Vonsattel, J.; Stakes, J.W.; Gruber, M.; Finklestein, S.P. The clinical spectrum of cerebral amyloid angiopathy: Presentations without lobar hemorrhage. Neurology 1993, 43, 2073–2079. [Google Scholar] [CrossRef]
- Switzer, A.R.; Charidimou, A.; McCarter, S.; Vemuri, P.; Nguyen, A.T.; Przybelski, S.A.; Lesnick, T.G.; Rabinstein, A.A.; Brown, R.D.; Knopman, D.S.; et al. Boston Criteria v2.0 for Cerebral Amyloid Angiopathy Without Hemorrhage: An MRI-Neuropathologic Validation Study. Neurology 2024, 102, e209386. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, I.C.; Wilson, D.; Fiebelkorn, C.A.; Aum, D.; Ameriso, S.F.; Eberbach, F.; Beitzke, M.; Kleinig, T.; Phan, T.; Marchina, S.; et al. Risk of intracranial haemorrhage and ischaemic stroke after convexity subarachnoid haemorrhage in cerebral amyloid angiopathy: International individual patient data pooled analysis. J. Neurol. 2022, 269, 1427–1438. [Google Scholar] [CrossRef]
- Regenhardt, R.W.; Thon, J.M.; Das, A.S.; Thon, O.R.; Charidimou, A.; Viswanathan, A.; Gurol, M.E.; Chwalisz, B.K.; Frosch, M.P.; Cho, T.A.; et al. Association between immunosuppressive treatment and outcomes of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2020, 77, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Charidimou, A.; Ayata, C.; Werring, D.J.; Greenberg, S.M. Cerebral Amyloid Angiopathy-Related Transient Focal Neurologic Episodes. Neurology 2021, 97, 231–238. [Google Scholar] [CrossRef]
- Ni, J.; Auriel, E.; Jindal, J.; Ayres, A.; Schwab, K.M.; Martinez-Ramirez, S.; Gurol, E.M.; Greenberg, S.M.; Viswanathan, A. The characteristics of superficial siderosis and convexity subarachnoid hemorrhage and clinical relevance in suspected cerebral amyloid angiopathy. Cerebrovasc. Dis. 2015, 39, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.; Salloway, S.; Brooks, D.J.; Tampieri, D.; Barakos, J.; Fox, N.C.; Raskind, M.; Sabbagh, M.; Honig, L.S.; Porsteinsson, A.P.; et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: A retrospective analysis. Lancet Neurol. 2012, 11, 241–249. [Google Scholar] [CrossRef]
- Filippi, M.; Cecchetti, G.; Spinelli, E.G.; Vezzulli, P.; Falini, A.; Agosta, F. Amyloid-Related Imaging Abnormalities and β-Amyloid-Targeting Antibodies: A Systematic Review. JAMA Neurol. 2022, 79, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Werring, D.J.; Sperling, R. Inflammatory cerebral amyloid angiopathy and amyloid-modifying therapies: Variations on the same ARIA? Ann. Neurol. 2013, 73, 439–441. [Google Scholar] [CrossRef]
- Cogswell, P.M.; Andrews, T.J.; Barakos, J.A.; Barkhof, F.; Bash, S.; Benayoun, M.D.; Chiang, G.C.; Franceschi, A.M.; Jack, C.R.; Pillai, J.J.; et al. Alzheimer’s Disease Anti-Amyloid Immunotherapies: Imaging Recommendations and Practice Considerations for ARIA Monitoring. AJNR Am. J. Neuroradiol. 2024, 46, 24–32. [Google Scholar] [CrossRef]
- Sin, M.-K.; Zamrini, E.; Ahmed, A.; Nho, K.; Hajjar, I. Anti-Amyloid Therapy, AD, and ARIA: Untangling the Role of CAA. J. Clin. Med. 2023, 12, 6792. [Google Scholar] [CrossRef]
- Available online: https://sites.google.com/site/icabinternationalnetwork/home (accessed on 1 December 2024).
- Reijmer, Y.D.; van Veluw, S.J.; Greenberg, S.M. Ischemic brain injury in cerebral amyloid angiopathy. J. Cereb. Blood Flow Metab. 2016, 36, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.E.; Schneider, J.A.; Wardlaw, J.M.; Greenberg, S.M. Cerebral microinfarcts: The invisible lesions. Lancet Neurol. 2012, 11, 272–282. [Google Scholar] [CrossRef]
- Brundel, M.; de Bresser, J.; van Dillen, J.J.; Kappelle, L.J.; Biessels, G.J. Cerebral microinfarcts: A systematic review of neuropathological studies. J. Cereb. Blood Flow Metab. 2012, 32, 425–436. [Google Scholar] [CrossRef]
- Okamoto, Y.; Yamamoto, T.; Kalaria, R.N.; Senzaki, H.; Maki, T.; Hase, Y.; Kitamura, A.; Washida, K.; Yamada, M.; Ito, H.; et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012, 123, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Launer, L.J.; Petrovitch, H.; Ross, G.W.; Markesbery, W.; White, L.R. AD brain pathology: Vascular origins? Results from the HAAS autopsy study. Neurobiol. Aging 2008, 29, 1587–1590. [Google Scholar] [CrossRef]
- Gregoire, S.M.; Charidimou, A.; Gadapa, N.; Dolan, E.; Antoun, N.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.-C.; Jäger, H.R.; et al. Acute ischaemic brain lesions in intracerebral haemorrhage: Multicentre cross-sectional magnetic resonance imaging study. Brain 2011, 134, 2376–2386. [Google Scholar] [CrossRef]
- Kimberly, W.T.; Gilson, A.; Rost, N.S.; Rosand, J.; Viswanathan, A.; Smith, E.E.; Greenberg, S.M. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology 2009, 72, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Auriel, E.; Gurol, M.E.; Ayres, A.; Dumas, A.P.; Schwab, K.M.; Vashkevich, A.; Martinez-Ramirez, S.; Rosand, J.; Viswanathan, A.; Greenberg, S.M. Characteristic distributions of intracerebral hemorrhage-associated diffusion-weighted lesions. Neurology 2012, 79, 2335–2341. [Google Scholar] [CrossRef]
- Auriel, E.; Edlow, B.L.; Reijmer, Y.D.; Fotiadis, P.; Ramirez-Martinez, S.; Ni, J.; Reed, A.K.; Vashkevich, A.; Schwab, K.; Rosand, J.; et al. Microinfarct disruption of white matter structure: A longitudinal diffusion tensor analysis. Neurology 2014, 83, 182–188. [Google Scholar] [CrossRef]
- Westover, M.B.; Bianchi, M.T.; Yang, C.; Schneider, J.A.; Greenberg, S.M. Estimating cerebral microinfarct burden from autopsy samples. Neurology 2013, 80, 1365–1369. [Google Scholar] [CrossRef]
- Sonnen, J.A.; Larson, E.B.; Crane, P.K.; Haneuse, S.; Li, G.; Schellenberg, G.D.; Craft, S.; Leverenz, J.B.; Montine, T.J. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann. Neurol. 2007, 62, 406–413. [Google Scholar] [CrossRef]
- Launer, L.J.; Hughes, T.M.; White, L.R. Microinfarcts brain atrophy, and cognitive function: The Honolulu Asia Aging Study Autopsy Study. Ann. Neurol. 2011, 70, 774–780. [Google Scholar] [CrossRef]
- Sinka, L.; Kövari, E.; Gold, G.; Hof, P.R.; Herrmann, F.R.; Bouras, C.; Giannakopoulos, P. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest old. J. Neuropathol. Exp. Neurol. 2010, 69, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- van Veluw, S.J.; Zwanenburg, J.J.; Engelen-Lee, J.; Spliet, W.G.; Hendrikse, J.; Luijten, P.R.; Biessels, G.J. In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J. Cereb. Blood Flow Metab. 2013, 33, 322–329. [Google Scholar] [CrossRef]
- van Veluw, S.J.; Zwanenburg, J.J.; Rozemuller, A.J.; Luijten, P.R.; Spliet, W.G.; Biessels, G.J. The spectrum of MR detectable cortical microinfarcts: A classification study with 7-tesla postmortem MRI and histopathology. J. Cereb. Blood Flow Metab. 2015, 35, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Van Rooden, S.; Doan, N.T.; Versluis, M.J.; Goos, J.D.; Webb, A.G.; Oleksik, A.M.; van der Flier, W.M.; Scheltens, P.; Barkhof, F.; Weverling–Rynsburger, A.W.; et al. 7T T2(*)-weighted magnetic resonance imaging reveals cortical phase differences between early- and late-onset Alzheimer’s disease. Neurobiol. Aging 2015, 36, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Salat, D.; Chen, J.; van der Kouwe, A.; Greve, D.; Fischl, B.; Rosas, H. Hippocampal degeneration is associated with temporal and limbic gray matter/white matter tissue contrast in Alzheimer’s disease. Neuroimage 2011, 54, 1795–1802. [Google Scholar] [CrossRef]
- Charidimou, A.; Peeters, A.P.; Jäger, R.; Fox, Z.; Vandermeeren, Y.; Laloux, P.; Baron, J.-C.; Werring, D.J. Cortical superficial siderosis and intracerebral hemorrhage risk in cerebral amyloid angiopathy. Neurology 2013, 81, 1666–1673. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G.; Roongpiboonsopit, D.; Auriel, E.; Pasi, M.; Haley, K.; van Etten, E.S.; Martinez-Ramirez, S.; Ayres, A.; Vashkevich, A.; et al. Cortical superficial siderosis multifocality in cerebral amyloid angiopathy: A prospective study. Neurology 2017, 89, 2128–2135. [Google Scholar] [CrossRef]
- Moulin, S.; Casolla, B.; Kuchcinski, G.; Boulouis, G.; Rossi, C.; Henon, H.; Leys, D.; Cordonnier, C. Cortical superficial siderosis: A prospective observational cohort study. Neurology 2018, 91, e132–e138. [Google Scholar] [CrossRef]
- Wollenweber, F.A.; Opherk, C.; Zedde, M.; Catak, C.; Malik, R.; Duering, M.; Konieczny, M.J.; Pascarella, R.; Samões, R.; Correia, M.; et al. Prognostic relevance of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 2019, 92, e792–e801. [Google Scholar] [CrossRef]
- Knudsen, K.A.; Rosand, J.; Karluk, D.; Greenberg, S.M. Clinical diagnosis of cerebral amyloid angiopathy: Validation of the Boston criteria. Neurology 2001, 56, 537–539. [Google Scholar] [CrossRef]
- Charidimou, A.; Boulouis, G. Clinical Diagnosis of Probable Cerebral Amyloid Angiopathy: Diagnostic Accuracy Meta-Analysis of the Boston Criteria. Stroke 2022, 53, 3679–3687. [Google Scholar] [CrossRef]
- Charidimou, A.; Jäger, R.H.; Fox, Z.; Peeters, A.; Vandermeeren, Y.; Laloux, P.; Baron, J.-C.; Werring, D.J. Prevalence and mechanisms of cortical superficial siderosis in cerebral amyloid angiopathy. Neurology 2013, 81, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Fandler-Höfler, S.; Gattringer, T.; Enzinger, C.; Werring, D.J. Comparison of Boston Criteria v2.0/v1.5 for Cerebral Amyloid Angiopathy to Predict Recurrent Intracerebral Hemorrhage. Stroke 2023, 54, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Raposo, N.; Périole, C.; Planton, M. In-vivo diagnosis of cerebral amyloid angiopathy: An updated review. Curr. Opin. Neurol. 2024, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Roongpiboonsopit, D.; Charidimou, A.; William, C.M.; Lauer, A.; Falcone, G.J.; Martinez-Ramirez, S.; Biffi, A.; Ayres, A.; Vashkevich, A.; Awosika, O.O.; et al. Cortical superficial siderosis predicts early recurrent lobar hemorrhage. Neurology 2016, 87, 1863–1870. [Google Scholar] [CrossRef]
- Pichler, M.; Vemuri, P.; Rabinstein, A.A.; Aakre, J.; Flemming, K.D.; Brown, R.D., Jr.; Kumar, N.; Kantarci, K.; Kremers, W.; Mielke, M.M.; et al. Prevalence and natural history of superficial siderosis: A population-based study. Stroke 2017, 48, 3210–3214. [Google Scholar] [CrossRef]
- Mandybur, T.I. Cerebral amyloid angiopathy: The vascular pathology and complications. J. Neuropathol. Exp. Neurol. 1986, 45, 79–90. [Google Scholar] [CrossRef]
- Chung, D.Y.; Oka, F.; Ayata, C. Spreading depolarizations: A therapeutic target against delayed cerebral ischemia after subarachnoid hemorrhage. J. Clin. Neurophysiol. 2016, 33, 196–202. [Google Scholar] [CrossRef]
- Charidimou, A. Cerebral Amyloid Angiopathy-Related Inflammation Spectrum Disorders: Introduction of a Novel Concept and Diagnostic Criteria. Ann. Neurol. 2025, 97, 470–474. [Google Scholar] [CrossRef]
- Sellimi, A.; Panteleienko, L.; Mallon, D.; Fandler-Höfler, S.; Oliver, R.; Harvey, V.; Zandi, M.S.; Banerjee, G.; Werring, D.J. Inflammation in Cerebral Amyloid Angiopathy-Related Transient Focal Neurological Episodes. Ann. Neurol. 2025, 97, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Charidimou, A.; Boulouis, G.; Xiong, L.; Pasi, M.; Roongpiboonsopit, D.; Ayres, A.; Schwab, K.M.; Rosand, J.; Gurol, M.E.; Viswanathan, A.; et al. Cortical Superficial Siderosis Evolution. Stroke 2019, 50, 954–962. [Google Scholar] [CrossRef]
- Ringman, J.M.; Sachs, M.C.; Zhou, Y.; Monsell, S.E.; Saver, J.L.; Vinters, H.V. Clinical predictors of severe cerebral amyloid angiopathy and influence of APOE genotype in persons with pathologically verified Alzheimer disease. JAMA Neurol. 2014, 71, 878–883. [Google Scholar] [CrossRef]
- Sanchez-Caro, J.M.; de Lorenzo Martínez de Ubago, I.; de Celis Ruiz, E.; Arribas, A.B.; Calviere, L.; Raposo, N.; Blancart, R.G.; Fuentes, B.; Diez-Tejedor, E.; Rodriguez-Pardo, J. Transient Focal Neurological Events in Cerebral Amyloid Angiopathy and the Long-term Risk of Intracerebral Hemorrhage and Death: A Systematic Review and Meta-analysis. JAMA Neurol. 2022, 79, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Catak, C.; Zedde, M.; Malik, R.; Janowitz, D.; Soric, V.; Seegerer, A.; Krebs, A.; Düring, M.; Opherk, C.; Linn, J.; et al. Decreased CSF Levels of ß-Amyloid in Patients With Cortical Superficial Siderosis. Front. Neurol. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.V.; Singhal, S.; Rowe, C.C.; Kempster, P.; Bower, S.; Phan, T.G. Convexity Subarachnoid Hemorrhage with PiB Positive Pet Scans: Clinical Features and Prognosis. J. Neuroimaging 2015, 25, 420–429. [Google Scholar] [CrossRef]
- Tsai, H.H.; Pasi, M.; Liu, C.J.; Tsai, Y.C.; Yen, R.F.; Chen, Y.F.; Jeng, J.S.; Tsai, L.K.; Charidimou, A.; Baron, J.C. Differentiating Cerebral Amyloid Angiopathy From Alzheimer’s Disease Using Dual Amyloid and Tau Positron Emission Tomography. J. Stroke 2025, 27, 65–74. [Google Scholar] [CrossRef]
- Zedde, M.; Napoli, M.; Moratti, C.; Pezzella, F.R.; Seiffge, D.J.; Tsivgoulis, G.; Caputi, L.; Salvarani, C.; Toni, D.; Valzania, F.; et al. The Hemorrhagic Side of Primary Angiitis of the Central Nervous System (PACNS). Biomedicines 2024, 12, 459. [Google Scholar] [CrossRef]
- Pascarella, R.; Antonenko, K.; Boulouis, G.; De Boysson, H.; Giannini, C.; Heldner, M.R.; Kargiotis, O.; Nguyen, T.N.; Rice, C.M.; Salvarani, C.; et al. European Stroke Organisation (ESO) guidelines on Primary Angiitis of the Central Nervous System (PACNS). Eur. Stroke J. 2023, 8, 842–879. [Google Scholar] [CrossRef]
- Scolding, N.J.; Joseph, F.; Kirby, P.A.; Mazanti, I.; Gray, F.; Mikol, J.; Ellison, D.; Hilton, D.A.; Williams, T.L.; MacKenzie, J.M.; et al. Abeta-related angiitis: Primary angiitis of the central nervous system associated with cerebral amyloid angiopathy. Brain 2005, 128 Pt 3, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Grangeon, L.; Boulouis, G.; Capron, J.; Bala, F.; Renard, D.; Raposo, N.; Ozkul-Wermester, O.; Triquenot-Bagan, A.; Ayrignac, X.; Wallon, D.; et al. Cerebral Amyloid Angiopathy-Related Inflammation and Biopsy-Positive Primary Angiitis of the CNS: A Comparative Study. Neurology 2024, 103, e209548. [Google Scholar] [CrossRef] [PubMed]
- Panteleienko, L.; Banerjee, G.; Mallon, D.H.; Harvey, V.; Oliver, R.; Hotton, G.; Knight, W.; Datta, S.; Zandi, M.S.; Jäger, H.R.; et al. Sulcal Hyperintensity as an Early Imaging Finding in Cerebral Amyloid Angiopathy-Related Inflammation. Neurology 2024, 103, e210084. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Tozer, D.J.; Markus, H.S. Cerebral Microbleeds and Their Association With Inflammation and Blood-Brain Barrier Leakage in Small Vessel Disease. Stroke 2025, 56, 427–436. [Google Scholar] [CrossRef]
- Ahn, S.J.; Anrather, J.; Nishimura, N.; Schaffer, C.B. Diverse Inflammatory Response After Cerebral Microbleeds Includes Coordinated Microglial Migration and Proliferation. Stroke 2018, 49, 1719–1726. [Google Scholar] [CrossRef]
- Rosidi, N.L.; Zhou, J.; Pattanaik, S.; Wang, P.; Jin, W.; Brophy, M.; Olbricht, W.L.; Nishimura, N.; Schaffer, C.B. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS ONE 2011, 6, e26612. [Google Scholar] [CrossRef]
- Sumbria, R.K.; Grigoryan, M.M.; Vasilevko, V.; Krasieva, T.B.; Scadeng, M.; Dvornikova, A.K.; Paganini-Hill, A.; Kim, R.; Cribbs, D.H.; Fisher, M.J. A murine model of inflammation-induced cerebral microbleeds. J. Neuroinflammation 2016, 13, 218. [Google Scholar] [CrossRef]
- Koemans, E.A.; Chhatwal, J.P.; van Veluw, S.J.; van Etten, E.S.; van Osch, M.J.P.; van Walderveen, M.A.A.; Sohrabi, H.R.; Kozberg, M.G.; Shirzadi, Z.; Terwindt, G.M.; et al. Progression of cerebral amyloid angiopathy: A pathophysiological framework. Lancet Neurol. 2023, 22, 632–642. [Google Scholar] [CrossRef]
- Kozberg, M.G.; Yi, I.; Freeze, W.M.; Auger, C.A.; Scherlek, A.A.; Greenberg, S.M.; van Veluw, S.J. Blood-brain barrier leakage and perivascular inflammation in cerebral amyloid angiopathy. Brain Commun. 2022, 4, fcac245. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bax, F.; van Veluw, S.J. Amyloid-related imaging abnormalities: Manifestations, metrics and mechanisms. Nat. Rev. Neurol. 2025. [Google Scholar] [CrossRef]
- Makkinejad, N.; Zanon Zotin, M.C.; van den Brink, H.; Auger, C.A.; Vom Eigen, K.A.; Iglesias, J.E.; Greenberg, S.M.; Perosa, V.; van Veluw, S.J. Neuropathological Correlates of White Matter Hyperintensities in Cerebral Amyloid Angiopathy. J. Am. Heart Assoc. 2024, 13, e035744. [Google Scholar] [CrossRef] [PubMed]
- van Veluw, S.J.; Benveniste, H.; Bakker, E.N.T.P.; Carare, R.O.; Greenberg, S.M.; Iliff, J.J.; Lorthois, S.; Van Nostrand, W.E.; Petzold, G.C.; Shih, A.Y.; et al. Is CAA a perivascular brain clearance disease? A discussion of the evidence to date and outlook for future studies. Cell. Mol. Life Sci. 2024, 81, 239. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.A.; Perosa, V.; Greenberg, S.M.; van Veluw, S.J.; Kozberg, M.G. Cortical superficial siderosis is associated with reactive astrogliosis in cerebral amyloid angiopathy. J. Neuroinflammation 2023, 20, 195. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, S.N.; DeLong, J.H.; Kozberg, M.G.; Mazur-Hart, D.J.; van Veluw, S.J.; Alkayed, N.J.; Sansing, L.H. Role of Inflammatory Processes in Hemorrhagic Stroke. Stroke 2023, 54, 605–619. [Google Scholar] [CrossRef]
- Charidimou, A.; Perosa, V.; Frosch, M.P.; Scherlek, A.A.; Greenberg, S.M.; van Veluw, S.J. Neuropathological correlates of cortical superficial siderosis in cerebral amyloid angiopathy. Brain 2020, 143, 3343–3351. [Google Scholar] [CrossRef]
- Koemans, E.A.; van Walderveen, M.A.A.; Voigt, S.; Rasing, I.; van Harten, T.W.; JA van Os, H.; van der Weerd, N.; Terwindt, G.M.; van Osch, M.J.P.; van Veluw, S.J.; et al. Subarachnoid CSF hyperintensities at 7 tesla FLAIR MRI: A novel marker in cerebral amyloid angiopathy. Neuroimage Clin. 2023, 38, 103386. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Piazza, F.; Pascarella, R. Clinical and Neuroradiological Manifestations of Cerebral Amyloid Angiopathy: A Closer Look into the Natural History of a Frequent Disease. J. Clin. Med. 2025, 14, 1697. https://doi.org/10.3390/jcm14051697
Zedde M, Piazza F, Pascarella R. Clinical and Neuroradiological Manifestations of Cerebral Amyloid Angiopathy: A Closer Look into the Natural History of a Frequent Disease. Journal of Clinical Medicine. 2025; 14(5):1697. https://doi.org/10.3390/jcm14051697
Chicago/Turabian StyleZedde, Marialuisa, Fabrizio Piazza, and Rosario Pascarella. 2025. "Clinical and Neuroradiological Manifestations of Cerebral Amyloid Angiopathy: A Closer Look into the Natural History of a Frequent Disease" Journal of Clinical Medicine 14, no. 5: 1697. https://doi.org/10.3390/jcm14051697
APA StyleZedde, M., Piazza, F., & Pascarella, R. (2025). Clinical and Neuroradiological Manifestations of Cerebral Amyloid Angiopathy: A Closer Look into the Natural History of a Frequent Disease. Journal of Clinical Medicine, 14(5), 1697. https://doi.org/10.3390/jcm14051697






