Functional Foods and Nutraceuticals in Irritable Bowel Syndrome
Abstract
1. Introduction
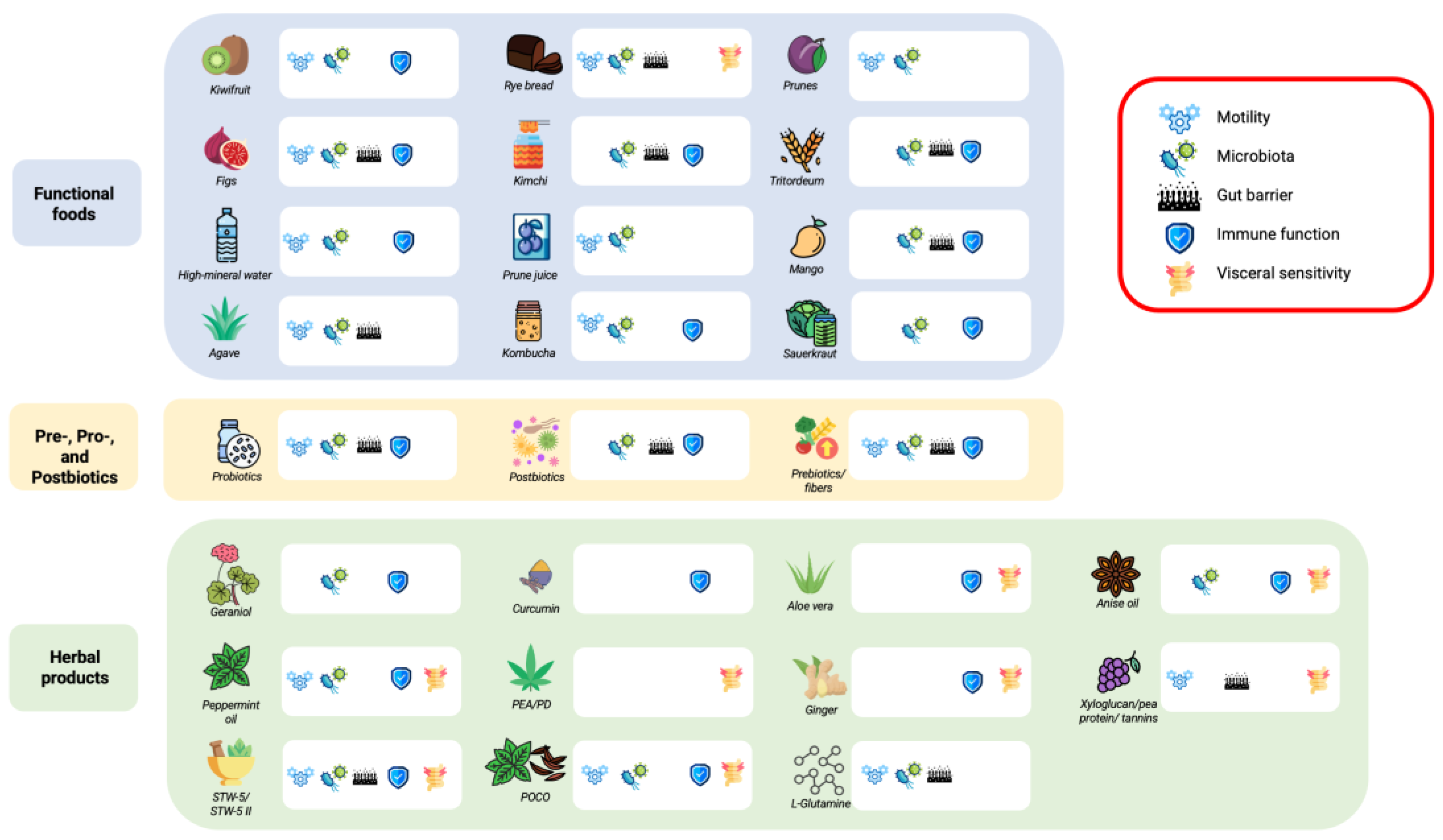
2. Functional Foods
2.1. Fruit
2.1.1. Kiwifruit
2.1.2. Mango, Prune, and Fig
2.2. Cereals
2.2.1. Rye Bread
2.2.2. Tritordeum
2.2.3. Agave
2.2.4. Fermented Foods
2.3. Drinks
High-Mineral Water
2.4. Prune Juice
2.5. Kombucha
3. Herbal Products and Other Nutraceuticals
3.1. Essential Oils
3.2. Curcumin
3.3. Ginger
3.4. Palmitoylethanolamide and Polydatin
3.5. Glutamine
3.6. Other Nutraceuticals
4. Prebiotics, Probiotics, and Postbiotics
4.1. Prebiotics
4.2. Probiotics
4.3. Postbiotics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Simrén, M.; Barbara, G.; Flint, H.J.; Spiegel, B.M.R.; Spiller, R.C.; Vanner, S.; Verdu, E.F.; Whorwell, P.J.; Zoetendal, E.G. Intestinal microbiota in functional bowel disorders: A Rome foundation report. Gut 2013, 62, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Cremon, C.; Staiano, A.; Stanghellini, V.; Borrelli, O.; Strisciuglio, C.; Romano, C.; Mallardo, S.; Scarpato, E.; Marasco, G.; et al. Role of inflammation in pediatric irritable bowel syndrome. Neurogastroenterol. Motil. 2023, 35, e14365. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Gut microbiota signatures and modulation in irritable bowel syndrome. Microbiome Res. Rep. 2022, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Bianco, F.; Stanghellini, V.; Barbara, G. Microbiota modulation in disorders of gut-brain interaction. Dig. Liver Dis. 2024, 56, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Andrews, C.N.; MacQueen, G.; Korownyk, C.; Marsiglio, M.; Graff, L.; Kvern, B.; Lazarescu, A.; Liu, L.; Paterson, W.G.; et al. Canadian Association of Gastroenterology Clinical Practice Guideline for the Management of Irritable Bowel Syndrome (IBS). J. Can. Assoc. Gastroenterol. 2019, 2, 6–29. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Cremon, C.; Bellini, M.; Corsetti, M.; Di Nardo, G.; Falangone, F.; Fuccio, L.; Galeazzi, F.; Iovino, P.; Sarnelli, G.; et al. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroen-terology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig. Liver Dis. 2023, 55, 187–207. [Google Scholar]
- Savarino, E.; Zingone, F.; Barberio, B.; Marasco, G.; Akyuz, F.; Akpinar, H.; Barboi, O.; Bodini, G.; Bor, S.; Chiarioni, G.; et al. Functional bowel disorders with diarrhoea: Clinical guidelines of the United Eu-ropean Gastroenterology and European Society for Neurogastroenterology and Motility. United Eur. Gastroenterol. J. 2022, 10, 556–584. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef]
- Chang, L.; Sultan, S.; Lembo, A.; Verne, G.N.; Smalley, W.; Heidelbaugh, J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Constipation. Gastroenterology 2022, 163, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome With Diarrhea. Gastroenterology 2022, 163, 137–151. [Google Scholar] [CrossRef]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastroin-testinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef]
- Fukudo, S.; Okumura, T.; Inamori, M.; Okuyama, Y.; Kanazawa, M.; Kamiya, T.; Sato, K.; Shiotani, A.; Naito, Y.; Fujikawa, Y.; et al. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J. Gastroenterol. 2021, 56, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Sachdeva, S.; Pratap, N.; Karyampudi, A.; Mustafa, U.; Abraham, P.; Bhatt, C.B.; Chakravartty, K.; Chaudhuri, S.; Goyal, O.; et al. Indian consensus statements on irritable bowel syndrome in adults: A guideline by the Indian Neurogastroenterology and Motility Association and jointly supported by the Indian Society of Gastroenterology. Indian. J. Gastroenterol. 2023, 42, 249–273. [Google Scholar] [CrossRef] [PubMed]
- Bertin, L.; Zanconato, M.; Crepaldi, M.; Marasco, G.; Cremon, C.; Barbara, G.; Barberio, B.; Zingone, F.; Savarino, E.V. The Role of the FODMAP Diet in IBS. Nutrients 2024, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Bellentani, S.; De Bastiani, R.; Tosetti, C.; Cicala, M.; Esposito, G.; Arullani, P.; Annibale, B. A survey of pharmacological and nonpharmacological treatment of functional gastrointestinal disorders. United Eur. Gastroenterol. J. 2013, 1, 385–393. [Google Scholar] [CrossRef]
- Temple, N.J. A rational definition for functional foods: A perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2016, 83, 8. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Talley, N.J.; Spiegel, B.M.R.; Foxx-Orenstein, A.E.; Schiller, L.; Quigley, E.M.M.; Moayyedi, P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: Systematic review and meta-analysis. BMJ 2008, 337, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Entry Details|FAO Terminology Portal|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faoterm/viewentry/en/?entryId=170967 (accessed on 26 February 2025).
- Chang, C.-C.; Lin, Y.-T.; Lu, Y.-T.; Liu, Y.-S.; Liu, J.-F. Kiwifruit improves bowel function in patients with irritable bowel syndrome with constipation. Asia Pac. J. Clin. Nutr. 2010, 19, 451–457. [Google Scholar] [PubMed]
- Dimidi, E.; Staudacher, H.M. Could a kiwifruit a day keep the doctor away? Lancet Gastroenterol. Hepatol. 2020, 5, 648. [Google Scholar] [CrossRef] [PubMed]
- Paturi, G.; Butts, C.A.; Bentley-Hewitt, K.L.; Ansell, J. Influence of green and gold kiwifruit on indices of large bowel function in healthy rats. J. Food Sci. 2014, 79, H1611–H1620. [Google Scholar] [CrossRef]
- Lee, Y.K.; Low, K.Y.; Siah, K.; Drummond, L.M.; Gwee, K.-A. Kiwifruit (Actinidia deliciosa) changes intestinal microbial profile. Microb. Ecol. Health Dis. 2012, 23, 18572. [Google Scholar] [CrossRef]
- Chey, S.W.; Chey, W.D.; Jackson, K.; Eswaran, S. Exploratory Comparative Effectiveness Trial of Green Kiwifruit, Psyllium, or Prunes in US Patients With Chronic Constipation. Am. J. Gastroenterol. 2021, 116, 1304–1312. [Google Scholar] [CrossRef]
- Bayer, S.B.; Heenan, P.; Frampton, C.; Wall, C.L.; Drummond, L.N.; Roy, N.C.; Gearry, R.B. Two Gold Kiwifruit Daily for Effective Treatment of Constipation in Adults—A Randomized Clinical Trial. Nutrients 2022, 14, 4146. [Google Scholar] [CrossRef]
- Gearry, R.; Fukudo, S.; Barbara, G.; Kuhn-Sherlock, B.; Ansell, J.; Blatchford, P.; Eady, S.; Wallace, A.; Butts, C.; Cremon, C.; et al. Consumption of 2 Green Kiwifruits Daily Improves Constipation and Abdominal Comfort—Results of an International Multicenter Randomized Controlled Trial. Am. J. Gastroenterol. 2023, 118, 1058–1068. [Google Scholar] [CrossRef]
- Van Der Schoot, A.; Katsirma, Z.; Whelan, K.; Dimidi, E. Systematic review and meta-analysis: Foods, drinks and diets and their effect on chronic constipation in adults. Aliment. Pharmacol. Ther. 2024, 59, 157–174. [Google Scholar] [CrossRef]
- Kindleysides, S.; Kuhn-Sherlock, B.; Yip, W.; Poppitt, S.D. Encapsulated green kiwifruit extract: A randomised controlled trial investigating alleviation of constipation in otherwise healthy adults. Asia Pac. J. Clin. Nutr. 2015, 24, 421–429. [Google Scholar] [PubMed]
- Ansell, J.; Butts, C.A.; Paturi, G.; Eady, S.L.; Wallace, A.J.; Hedderley, D.; Gearry, R.B. Kiwifruit-derived supplements increase stool frequency in healthy adults: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2015, 35, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Charoensiddhi, S.; Xue, X.; Sun, B.; Liu, Y.; El-Seedi, H.R.; Wang, K. A review on the gastrointestinal protective effects of tropical fruit polyphenols. Crit. Rev. Food Sci. Nutr. 2023, 63, 7197–7223. [Google Scholar] [CrossRef] [PubMed]
- Venancio, V.P.; Kim, H.; Sirven, M.A.; Tekwe, C.D.; Honvoh, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenol-rich Mango (Mangifera indica L.) Ameliorate Functional Constipation Symptoms in Humans beyond Equivalent Amount of Fiber. Mol. Nutr. Food Res. 2018, 62, e1701034. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.K.; Lever, E.; Emery, P.W.; Whelan, K. Nutrient, fibre, sorbitol and chlorogenic acid content of prunes (Prunus domestica): An updated analysis and comparison of different countries of origin and database values. Int. J. Food Sci. Nutr. 2019, 70, 924–931. [Google Scholar] [CrossRef]
- Lever, E.; Scott, S.M.; Louis, P.; Emery, P.W.; Whelan, K. The effect of prunes on stool output, gut transit time and gastrointestinal microbiota: A randomised controlled trial. Clin. Nutr. 2019, 38, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, Y.; Guo, Y.; Jiang, Y.; Wen, L.; Yang, B. New insights of fig (Ficus carica L.) as a potential function food. Trends Food Sci. Technol. 2023, 140, 104146. [Google Scholar] [CrossRef]
- Rtibi, K.; Grami, D.; Wannes, D.; Selmi, S.; Amri, M.; Sebai, H.; Marzouki, L. Ficus carica aqueous extract alleviates delayed gastric emptying and recovers ulcerative colitis-enhanced acute functional gastrointestinal disorders in rats. J. Ethnopharmacol. 2018, 224, 242–249. [Google Scholar] [CrossRef]
- Baek, H.-I.; Ha, K.-C.; Kim, H.-M.; Choi, E.K.; Park, E.O.; Park, B.H.; Yang, H.J.; Kim, M.J.; Kang, H.J.; Chae, S.W. Randomized, double-blind, placebo-controlled trial of Ficus carica paste for the man-agement of functional constipation. Asia Pac. J. Clin. Nutr. 2016, 25, 487–496. [Google Scholar]
- Pourmasoumi, M.; Ghiasvand, R.; Darvishi, L.; Hadi, A.; Bahreini, N.; Keshavarzpour, Z. Comparison and Assessment of Flixweed and Fig Effects on Irritable Bowel Syndrome with Predominant Constipation: A Single-Blind Randomized Clinical Trial. EXPLORE 2019, 15, 198–205. [Google Scholar] [CrossRef]
- Holma, R.; Hongisto, S.-M.; Saxelin, M.; Korpela, R. Constipation Is Relieved More by Rye Bread Than Wheat Bread or Laxatives without Increased Adverse Gastrointestinal Effects. J. Nutr. 2010, 140, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, S.-M.; Paajanen, L.; Saxelin, M.; Korpela, R. A combination of fibre-rich rye bread and yoghurt containing Lactobacillus GG improves bowel function in women with self-reported constipation. Eur. J. Clin. Nutr. 2006, 60, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Pirkola, L.; Laatikainen, R.; Loponen, J.; Hongisto, S.-M.; Hillilä, M.; Nuora, A.; Yang, B.; Linderborg, K.M.; Freese, R. Low-FODMAP vs regular rye bread in irritable bowel syndrome: Randomized SmartPill® study. World J. Gastroenterol. 2018, 24, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Nitride, C.; D’auria, G.; Dente, A.; Landolfi, V.; Picariello, G.; Mamone, G.; Blandino, M.; Romano, R.; Ferranti, P. Tritordeum as an Innovative Alternative to Wheat: A Comparative Digestion Study on Bread. Molecules 2022, 27, 1308. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Gaudioso, G.; Solovyev, P.; Tuohy, K.; Di Cagno, R.; Gobbetti, M.; Fava, F. In vitro faecal fermentation of Tritordeum breads and its effect on the human gut health. Curr. Res. Microb. Sci. 2024, 6, 100214. [Google Scholar] [CrossRef]
- Russo, F.; Riezzo, G.; Orlando, A.; Linsalata, M.; D’Attoma, B.; Prospero, L.; Ignazzi, A.; Giannelli, G. A Comparison of the Low-FODMAPs Diet and a Tritordeum-Based Diet on the Gastro-intestinal Symptom Profile of Patients Suffering from Irritable Bowel Syndrome-Diarrhea Variant (IBS-D): A Randomized Controlled Trial. Nutrients 2022, 14, 1544. [Google Scholar] [CrossRef]
- Riezzo, G.; Prospero, L.; Orlando, A.; Linsalata, M.; D’attoma, B.; Ignazzi, A.; Giannelli, G.; Russo, F. A Tritordeum-Based Diet for Female Patients with Diarrhea-Predominant Irritable Bowel Syndrome: Effects on Abdominal Bloating and Psychological Symptoms. Nutrients 2023, 15, 1361. [Google Scholar] [CrossRef]
- García-Villalba, W.G.; Rodríguez-Herrera, R.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M.; Gallegos-Infante, J.A.; González-Herrera, S.M. Agave fructans: A review of their technological functionality and extraction processes. J. Food Sci. Technol. 2023, 60, 1265–1273. [Google Scholar] [CrossRef]
- García Gamboa, R.; Ortiz Basurto, R.I.; Calderón Santoyo, M.; Bravo Madrigal, J.; Ruiz Álvarez, B.E.; González Avila, M. In vitro evaluation of prebiotic activity, pathogen inhibition and enzymatic metabolism of intestinal bacteria in the presence of fructans extracted from agave: A comparison based on polymerization degree. LWT 2018, 92, 380–387. [Google Scholar] [CrossRef]
- Alvarado-Jasso, G.M.; Camacho-Díaz, B.H.; Arenas Ocampo, M.L.; Jiménez-Ferrer, J.E.; Mora-Escobedo, R.; Osorio-Díaz, P. Prebiotic effects of a mixture of agavins and green banana flour in a mouse model of obesity. J. Funct. Foods 2020, 64, 103685. [Google Scholar] [CrossRef]
- Camacho-Díaz, B.H.; Arenas-Ocampo, M.L.; Osorio-Díaz, P.; Jiménez-Aparicio, A.R.; Alvarado-Jasso, G.M.; Saavedra-Briones, E.V.; Valdovinos-Díaz, M.Á.; Gómez-Reyes, E. The Effects of Agave Fructans in a Functional Food Consumed by Patients with Irritable Bowel Syndrome with Constipation: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2023, 15, 3526. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Park, S.; Ji, Y.; Jung, H.-Y.; Park, H.; Kang, J.; Choi, S.-H.; Shin, H.; Hyun, C.-K.; Kim, K.-T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, K.-Y. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Szaefer, H.; Bartoszek, A.; Baer-Dubowska, W. Modulation of rat hepatic and kidney phase II enzymes by cabbage juices: Comparison with the effects of indole-3-carbinol and phenethyl isothiocyanate. Br. J. Nutr. 2011, 105, 816–826. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Park, E.-S.; Choi, Y.S.; Park, S.J.; Kim, J.H.; Chang, H.K.; Park, K.-Y. Kimchi improves irritable bowel syndrome: Results of a randomized, double-blind placebo-controlled study. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation—A pilot study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Mori, H.; Tack, J.; Suzuki, H. Magnesium Oxide in Constipation. Nutrients 2021, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Constant, F.; Imbert, A.; Hébert, G.; Zourabichvili, O.; Kapel, N. Time to treatment response of a magnesium- and sulphate-rich natural mineral water in functional constipation. Nutrition 2019, 65, 167–172. [Google Scholar] [CrossRef]
- Naumann, J.; Sadaghiani, C.; Alt, F.; Huber, R. Effects of Sulfate-Rich Mineral Water on Functional Constipation: A Double-Blind, Randomized, Placebo-Controlled Study. Complement. Med. Res. 2016, 23, 356–363. [Google Scholar] [CrossRef]
- Morishita, D.; Tomita, T.; Mori, S.; Kimura, T.; Oshima, T.; Fukui, H.; Miwa, H. Senna Versus Magnesium Oxide for the Treatment of Chronic Constipation: A Ran-domized, Placebo-Controlled Trial. Am. J. Gastroenterol. 2021, 116, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Trapani, V.; Petito, V.; Reddel, S.; Pietropaolo, G.; Graziani, C.; Masi, L.; Gasbarrini, A.; Putignani, L.; Scaldaferri, F.; et al. Dietary Magnesium Alleviates Experimental Murine Colitis through Modulation of Gut Microbiota. Nutrients 2021, 13, 4188. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Garcia-Mazcorro, J.F.; Markel, M.; Martino, H.S.; Minamoto, Y.; Steiner, J.M.; Byrne, D.; Suchodolski, J.S.; Mertens-Talcott, S.U. Carbohydrate-Free Peach (Prunus persica) and Plum (Prunus domestica) Juice Affects Fecal Microbial Ecology in an Obese Animal Model. PLoS ONE 2014, 9, e101723. [Google Scholar] [CrossRef]
- Koyama, T.; Nagata, N.; Nishiura, K.; Miura, N.; Kawai, T.; Yamamoto, H. Prune Juice Containing Sorbitol, Pectin, and Polyphenol Ameliorates Subjective Complaints and Hard Feces While Normalizing Stool in Chronic Constipation: A Randomized Pla-cebo-Controlled Trial. Am. J. Gastroenterol. 2022, 117, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Piirainen, L.; Peuhkuri, K.; Bäckström, K.; Korpela, R.; Salminen, S. Prune juice has a mild laxative effect in adults with certain gastrointestinal symptoms. Nutr. Res. 2007, 27, 511–513. [Google Scholar] [CrossRef]
- da Silva Júnior, J.C.; Meireles Mafaldo, Í.; de Lima Brito, I.; Tribuzy de Magalhães Cordeiro, A.M. Kombucha: Formulation, chemical composition, and therapeutic potentialities. Curr. Res. Food Sci. 2022, 5, 360–365. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha Fermentation and Its Antimicrobial Activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, I.; Mannaa, M.; Kim, J.; Wang, S.; Park, I.; Kim, J.; Seo, Y.-S. Effect of Kombucha on gut-microbiota in mouse having non-alcoholic fatty liver disease. Food Sci. Biotechnol. 2019, 28, 261–267. [Google Scholar] [CrossRef]
- Isakov, V.A.; Pilipenko, V.I.; Vlasova, A.V.; Kochetkova, A.A. Evaluation of the Efficacy of Kombucha-Based Drink Enriched with Inulin and Vitamins for the Management of Constipation-Predominant Irritable Bowel Syndrome in Females: A Randomized Pilot Study. Curr. Dev. Nutr. 2023, 7, 102037. [Google Scholar] [CrossRef]
- Jun, H.; Ko, S.-J.; Kim, K.; Kim, J.; Park, J.-W. An Overview of Systematic Reviews of Herbal Medicine for Irritable Bowel Syndrome. Front. Pharmacol. 2022, 13, 894122. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.M.; Hughes, P.A.; Martin, C.M.; Yang, J.; Castro, J.; Isaacs, N.J.; Blackshaw, A.L.; Brierley, S.M. A novel role for TRPM8 in visceral afferent function. Pain 2011, 152, 1459–1468. [Google Scholar] [CrossRef]
- Hawthorn, M.; Ferrante, J.; Luchowski, E.; Rutledge, A.; Wei, X.Y.; Triggle, D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment. Pharmacol. Ther. 1988, 2, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R.; Stöber, M.; Vetter, H. The anti-inflammatory activity of L-menthol compared to mint oil in human monocytes in vitro: A novel perspective for its therapeutic use in inflammatory diseases. Eur. J. Med. Res. 1998, 3, 539–545. [Google Scholar]
- Zsa, Z.; Weerts, R.M.; Masclee, A.A.M.; Witteman, B.J.; Clemens, C.H.; Winkens, B.; Brouwers, J.R.; Frijlink, H.W.; Muris, J.W.; De Wit, N.J.; et al. Efficacy and Safety of Peppermint Oil in a Randomized, Double-Blind Trial of Patients With Irritable Bowel Syndrome. Gastroenterology 2020, 158, 123–136. [Google Scholar] [CrossRef]
- Ingrosso, M.R.; Ianiro, G.; Nee, J.; Lembo, A.J.; Moayyedi, P.; Black, C.J.; Ford, A.C. Systematic review and meta-analysis: Efficacy of peppermint oil in irritable bowel syn-drome. Aliment. Pharmacol. Ther. 2022, 56, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Weerts, Z.Z.R.M.; Essers, B.A.B.; Jonkers, D.M.A.E.; Willems, J.I.A.; Janssen, D.J.P.A.; Witteman, B.J.M.; Clemens, C.H.M.; Westendorp, A.; Masclee, A.A.M.; Keszthelyi, D. A trial-based economic evaluation of peppermint oil for the treatment of irritable bowel syndrome. United Eur. Gastroenterol. J. 2021, 9, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Gwee, K.A.; Lee, Y.Y.; Suzuki, H.; Ghoshal, U.C.; Holtmann, G.; Bai, T.; Barbara, G.; Chen, M.H.; Chua AS, B.; Gibson, P.R.; et al. Asia-Pacific guidelines for managing functional dyspepsia overlapping with other gas-trointestinal symptoms. J. Gastroenterol. Hepatol. 2023, 38, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Botschuijver, S.; Welting, O.; Levin, E.; Maria-Ferreira, D.; Koch, E.; Montijn, R.C.; Seppen, J.; Hakvoort TB, M.; Schuren FH, J.; de Jonge, W.J.; et al. Reversal of visceral hypersensitivity in rat by Menthacarin®, a proprietary com-bination of essential oils from peppermint and caraway, coincides with mycobiome modulation. Neurogastroenterol. Motil. 2018, 30, e13299. [Google Scholar] [CrossRef]
- Thapa, S.; Luna, R.A.; Chumpitazi, B.P.; Oezguen, N.; Abdel-Rahman, S.M.; Garg, U.; Musaad, S.; Versalovic, J.; Kearns, G.L.; Shulman, R.J. Peppermint oil effects on the gut microbiome in children with functional abdominal pain. Clin. Transl. Sci. 2022, 15, 1036–1049. [Google Scholar] [CrossRef]
- Madisch, A.; Frieling, T.; Zimmermann, A.; Hollenz, M.; Labenz, J.; Stracke, B.; Miehlke, S. Menthacarin, a Proprietary Peppermint Oil and Caraway Oil Combination, Improves Multiple Complaints in Patients with Functional Gastrointestinal Disorders: A Systematic Review and Me-ta-Analysis. Dig Dis. 2023, 41, 522–532. [Google Scholar] [CrossRef]
- Allescher, H.-D.; Burgell, R.; Malfertheiner, P.; Mearin, F. Multi-target treatment for irritable bowel syndrome with STW 5: Pharmacological modes of action. J. Gastrointest. Liver Dis. 2020, 29, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Madisch, A.; Holtmann, G.; Plein, K.; Hotz, J. Treatment of irritable bowel syndrome with herbal preparations: Results of a double-blind, randomized, placebo-controlled, multi-centre trial. Aliment. Pharmacol. Ther. 2004, 19, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Noor-Mohammadi, E.; Yuan, T.; Ligon, C.O.; Ammar, R.M.; Rabini, S.; Johnson, A.C.; Meerveld, B.G.-V. Anti-nociceptive effect of STW 5-II in rodent models of stress and post-inflammatory visceral hypersensitivity. Phytomedicine 2024, 135, 156167. [Google Scholar] [CrossRef] [PubMed]
- Koriem, K.M.M. Approach to pharmacological and clinical applications of Anisi aetheroleum. Asian Pac. J. Trop. Biomed. 2015, 5, 60–67. [Google Scholar] [CrossRef]
- Mosaffa-Jahromi, M.; Lankarani, K.B.; Pasalar, M.; Afsharypuor, S.; Tamaddon, A.-M. Efficacy and safety of enteric coated capsules of anise oil to treat irritable bowel syndrome. J. Ethnopharmacol. 2016, 194, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.Q.; Khan, R.; Qamar, W.; Lateef, A.; Rehman, M.U.; Tahir, M.; Ali, F.; Hamiza, O.O.; Hasan, S.K.; Sultana, S. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: Possible role of p38 MAP Kinase and NF-κB. Exp. Mol. Pathol. 2013, 94, 419–429. [Google Scholar] [CrossRef]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology (Reading) 2012, 158, 2870–2877. [Google Scholar] [CrossRef]
- Rizzello, F.; Ricci, C.; Scandella, M.; Cavazza, E.; Giovanardi, E.; Valerii, M.C.; Campieri, M.; Comparone, A.; De Fazio, L.; Candela, M.; et al. Dietary geraniol ameliorates intestinal dysbiosis and relieves symptoms in irritable bowel syndrome patients: A pilot study. BMC Complement. Altern. Med. 2018, 18, 338. [Google Scholar] [CrossRef]
- Ricci, C.; Rizzello, F.; Valerii, M.C.; Spisni, E.; Gionchetti, P.; Turroni, S.; Candela, M.; D’amico, F.; Spigarelli, R.; Bellocchio, I.; et al. Geraniol Treatment for Irritable Bowel Syndrome: A Double-Blind Randomized Clinical Trial. Nutrients 2022, 14, 4208. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Alt, F.; Chong, P.W.; Teng, E.; Uebelhack, R. Evaluation of Benefit and Tolerability of IQP-CL-101 (Xanthofen) in the Symptomatic Improvement of Irritable Bowel Syndrome: A Double-Blinded, Randomised, Placebo-Controlled Clinical Trial. Phytother. Res. 2017, 31, 1056. [Google Scholar] [CrossRef] [PubMed]
- Brinkhaus, B.; Hentschel, C.; Von Keudell, C.; Schindler, G.; Lindner, M.; Stützer, H.; Kohnen, R.; Willich, S.N.; Lehmacher, W.; Hahn, E.G.G. Herbal medicine with curcuma and fumitory in the treatment of irritable bowel syndrome: A randomized, placebo-controlled, double-blind clinical trial. Scand. J. Gastroenterol. 2005, 40, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Scribano, M.; Kohn, A.; Caporaso, N.; Festi, D.; Campanale, M.C.; Di Rienzo, T.; Guarino, M.; Taddia, M.; et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J. Gastrointest. Liver Dis. 2016, 25, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Yeo, W.-S. A Meta-Analysis of the Clinical Use of Curcumin for Irritable Bowel Syndrome (IBS). J. Clin. Med. 2018, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-C.; Zhao, L.-X.; Lou, H.-X. Curcumin alters the pharmacokinetics of warfarin and clopidogrel in Wistar rats but has no effect on anticoagulation or antiplatelet aggregation. Planta Medica 2013, 79, 971–977. [Google Scholar] [CrossRef]
- van Tilburg, M.A.; Palsson, O.S.; Ringel, Y.; Whitehead, W.E. Is ginger effective for the treatment of Irritable Bowel Syndrome? A double blind randomized controlled pilot trial. Complement. Ther. Med. 2014, 22, 17–20. [Google Scholar] [CrossRef]
- Al-Jassim, Z.G. Using brewer’s yeast and ginger in the management of constipation-predominant irritable bowel syndrome: A randomized double-blind placebo-controlled trial. Asian J. Pharm. Clin. Res. 2019, 12, 372–376. [Google Scholar] [CrossRef]
- Piomelli, D.; Sasso, O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014, 17, 164–174. [Google Scholar] [CrossRef]
- Fichna, J.; Wood, J.T.; Papanastasiou, M.; Vadivel, S.K.; Oprocha, P.; Sałaga, M.; Sobczak, M.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; et al. Endocannabinoid and Cannabinoid-Like Fatty Acid Amide Levels Correlate with Pain-Related Symptoms in Patients with IBS-D and IBS-C: A Pilot Study. PLoS ONE 2013, 8, e85073. [Google Scholar] [CrossRef]
- Cremon, C.; Stanghellini, V.; Barbaro, M.R.; Cogliandro, R.F.; Bellacosa, L.; Santos, J.; Vicario, M.; Pigrau, M.; Cotoner, C.A.; Lobo, B.; et al. Randomised clinical trial: The analgesic properties of dietary supplementation with palmitoylethanolamide and polydatin in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2017, 45, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Bernardo, L.; Cremon, C.; Barbara, G.; Felici, E.; Evangelisti, M.; Ferretti, A.; Furio, S.; Piccirillo, M.; Coluzzi, F.; et al. Palmitoylethanolamide and polydatin in pediatric irritable bowel syndrome: A multicentric randomized controlled trial. Nutrition 2024, 122, 112397. [Google Scholar] [CrossRef]
- Zhou, Q.; Souba, W.W.; Croce, C.M.; Verne, G.N. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010, 59, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Costinean, S.; Croce, C.M.; Brasier, A.R.; Merwat, S.; Larson, S.A.; Basra, S.; Verne, G.N. MicroRNA 29 targets nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology 2015, 148, 158–169.e8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Verne, M.L.; Fields, J.Z.; Lefante, J.J.; Basra, S.; Salameh, H.; Verne, G.N. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019, 68, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, B.; Magnusson, M.K.; Isaksson, S.; Larsson, F.; Öhman, L. Effects of Aloe barbadensis Mill. extract (AVH200®) on human blood T cell activity in vitro. J. Ethnopharmacol. 2016, 179, 301–309. [Google Scholar] [CrossRef]
- Langmead, L.; Makins, R.J.; Rampton, D.S. Anti-inflammatory effects of aloe vera gel in human colorectal mucosa in vitro. Aliment. Pharmacol. Ther. 2004, 19, 521–527. [Google Scholar] [CrossRef]
- Størsrud, S.; Pontén, I.; Simrén, M. A pilot study of the effect of aloe barbadensis mill. Extract (AVH200®) in patients with irritable bowel syndrome: A randomized, double-blind, placebo-controlled study. J. Gastrointest. Liver Dis. 2015, 24, 275–280. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Magnusson, M.K.; Böhn, L.; Störsrud, S.; Larsson, F.; Savolainen, O.; Ross, A.; Simrén, M.; Öhman, L. Randomized clinical trial: Effects of Aloe barbadensis Mill. extract on symptoms, fecal microbiota and fecal metabolite profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2020, 32, e13860. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A review of toxicity and adverse clinical effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 77–96. [Google Scholar] [CrossRef]
- Inczefi, O.; Eutamene, H.; Placide, F.; Tondereau, V.; Pallagi, P.; Bagyánszki, M.; Bódi, N.; Gémes, N.; Szebeni, G.; Molnár, T.; et al. Translational evaluation of Gelsectan® effects on gut barrier dysfunction and visceral pain in animal models and irritable bowel syndrome with diarrhoea. United Eur. Gastroenterol. J. 2024, 12, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Burta, O.; Tiuca, N.; Petrisor, D.C.; Lenghel, A.; Santos, J. Efficacy and safety of Gelsectan for diarrhoea-predominant irritable bowel syndrome: A randomised, crossover clinical trial. United Eur. Gastroenterol. J. 2019, 7, 1093–1101. [Google Scholar] [CrossRef]
- de los Rios, C.C.; Falcón, B.S.; Arguelles-Arias, F.; Pérez, E.; Teruel, C.; Geijo, F.; Rey, E. Long-term safety and efficacy study of a medical device containing xy-loglucan, pea protein reticulated with tannins and xylo-oligosaccharides, in patients with diarrhoea-predominant irritable bowel syndrome. Therap Adv. Gastroenterol. 2021, 14, 17562848211020570. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Hutkins, R.; Walter, J.; Gibson, G.R.; Bedu-Ferrari, C.; Scott, K.; Tancredi, D.J.; Wijeyesekera, A.; Sanders, M.E. Classifying compounds as prebiotics—Scientific perspectives and recommendations. Nat. Rev. Gastroenterol. Hepatol. 2024, 22, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Goetze, O.; Fruehauf, H.; Pohl, D.; Giarrè, M.; Rochat, F.; Ornstein, K.; Menne, D.; Fried, M.; Thumshirn, M. Effect of a prebiotic mixture on intestinal comfort and general wellbeing in health. Br. J. Nutr. 2008, 100, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- Silk, D.B.A.; Davis, A.; Vulevic, J.; Tzortzis, G.; Gibson, G.R. Clinical trial: The effects of a trans-galactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2009, 29, 508–518. [Google Scholar] [CrossRef]
- Huaman, J.-W.; Mego, M.; Manichanh, C.; Cañellas, N.; Cañueto, D.; Segurola, H.; Jansana, M.; Malagelada, C.; Accarino, A.; Vulevic, J.; et al. Effects of Prebiotics vs a Diet Low in FODMAPs in Patients With Functional Gut Disorders. Gastroenterology 2018, 155, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Vulevic, J.; Tzortzis, G.; Juric, A.; Gibson, G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS®) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 2018, 30, e13440. [Google Scholar] [CrossRef] [PubMed]
- Guarner, F.; Sanders, M.E.; Szajewska, H.; Cohen, H.; Eliakim, R.; Herrera-deGuise, C.; Karakan, T.; Merenstein, D.; Piscoya, A.; Ramakrishna, B.; et al. World Gastroenterology Organisation Global Guidelines: Probiotics and Prebi-otics. J. Clin. Gastroenterol. 2024, 58, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Drago, L.; De Vecchi, E.; Stronati, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study Basic Study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef]
- Gargari, G.; Mantegazza, G.; Cremon, C.; Taverniti, V.; Valenza, A.; Barbaro, M.R.; Marasco, G.; Duncan, R.; Fiore, W.; Ferrari, R.; et al. Collinsella aerofaciens as a predictive marker of response to probiotic treatment in non-constipated irritable bowel syndrome. Gut Microbes 2024, 16, 2298246. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1–18. [Google Scholar] [CrossRef]
- Goodoory, V.C.; Tuteja, A.K.; Black, C.J.; Ford, A.C. Systematic Review and Meta-analysis: Efficacy of Mesalamine in Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2023, 22, 243–251.e5. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marasco, G.; Cremon, C.; Salvi, D.; Meacci, D.; Dajti, E.; Colecchia, L.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Functional Foods and Nutraceuticals in Irritable Bowel Syndrome. J. Clin. Med. 2025, 14, 1830. https://doi.org/10.3390/jcm14061830
Marasco G, Cremon C, Salvi D, Meacci D, Dajti E, Colecchia L, Barbaro MR, Stanghellini V, Barbara G. Functional Foods and Nutraceuticals in Irritable Bowel Syndrome. Journal of Clinical Medicine. 2025; 14(6):1830. https://doi.org/10.3390/jcm14061830
Chicago/Turabian StyleMarasco, Giovanni, Cesare Cremon, Daniele Salvi, David Meacci, Elton Dajti, Luigi Colecchia, Maria Raffaella Barbaro, Vincenzo Stanghellini, and Giovanni Barbara. 2025. "Functional Foods and Nutraceuticals in Irritable Bowel Syndrome" Journal of Clinical Medicine 14, no. 6: 1830. https://doi.org/10.3390/jcm14061830
APA StyleMarasco, G., Cremon, C., Salvi, D., Meacci, D., Dajti, E., Colecchia, L., Barbaro, M. R., Stanghellini, V., & Barbara, G. (2025). Functional Foods and Nutraceuticals in Irritable Bowel Syndrome. Journal of Clinical Medicine, 14(6), 1830. https://doi.org/10.3390/jcm14061830






