Predicting Major Preoperative Risk Factors for Retears After Arthroscopic Rotator Cuff Repair Using Machine Learning Algorithms
Abstract
1. Introduction
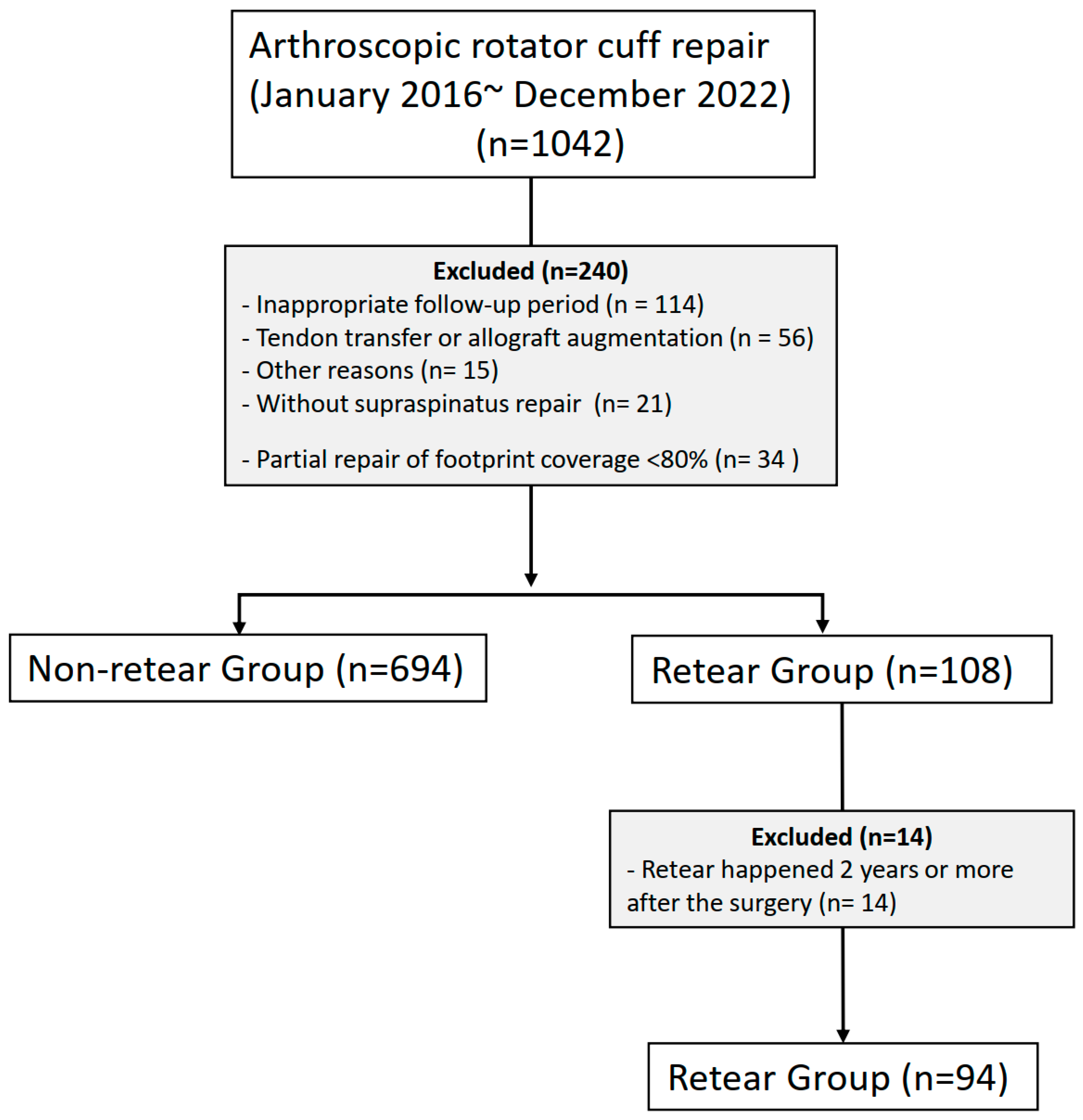
2. Materials and Methods
2.1. Operative Technique and Rehabilitation
2.2. Variable Selection and Clinical Assessment
2.3. Model Development and Feature Importance
2.4. Statistical Analysis
3. Results
3.1. Demographic Features
3.2. Performance of Machine Learning Algorithms
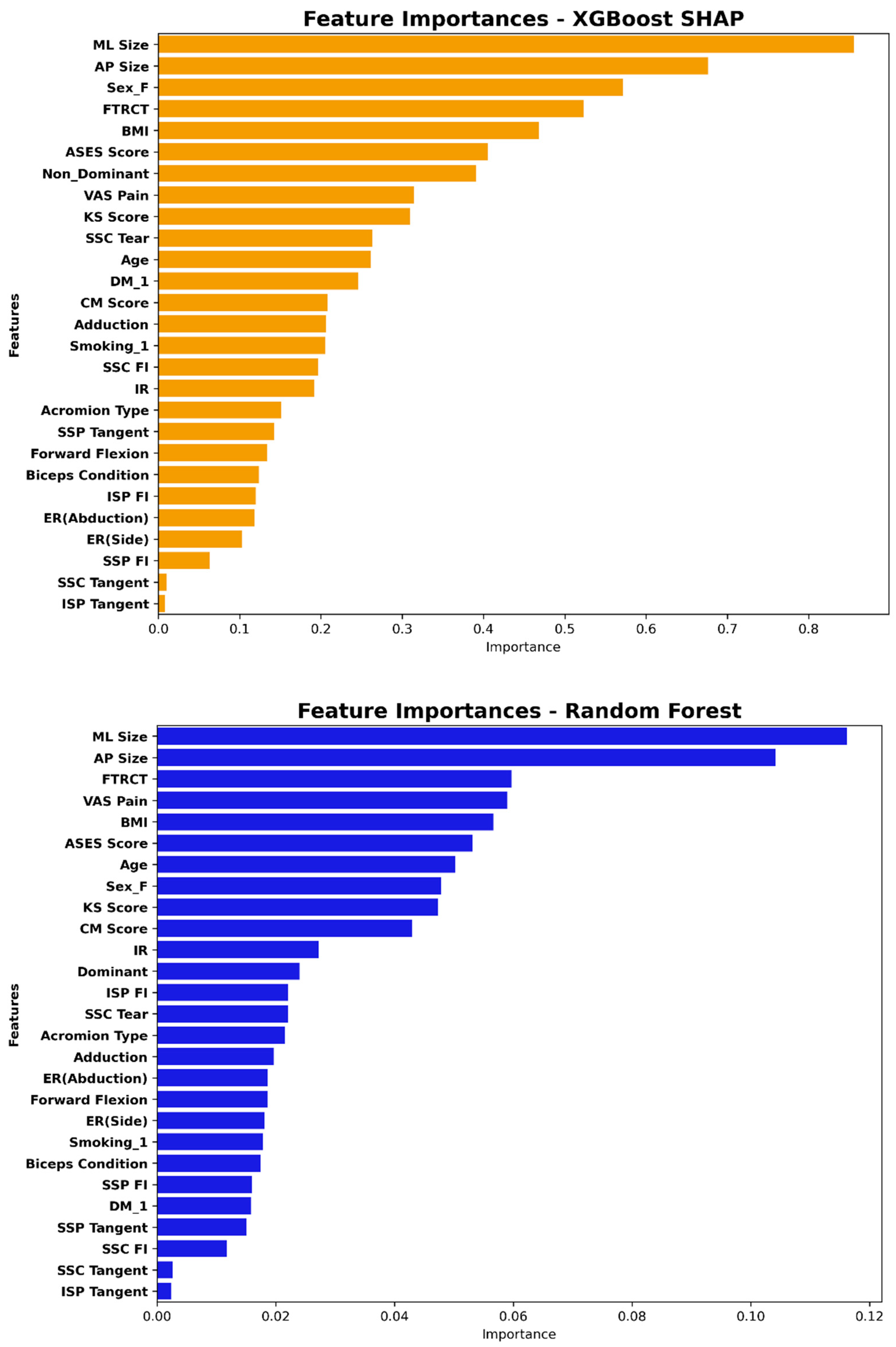
3.3. Feature Importance in the XGBoost and Random Forest Model
3.4. Univariate and Multivariate Logistic Regression Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harada, N.; Gotoh, M.; Ishitani, E.; Kakuma, T.; Yano, Y.; Tatara, D.; Kawakami, J.; Imai, T.; Karasuyama, M.; Kudoh, Y.; et al. Combination of risk factors affecting retear after arthroscopic rotator cuff repair: A decision tree analysis. J. Shoulder Elb. Surg. 2021, 30, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.B.; Jung, D.W.; Suh, K.T. Prediction of the Irreparability of Rotator Cuff Tears. Arthroscopy 2018, 34, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kim, S.H.; Lee, Y.H.; Kim, T.I.; Oh, J.H. The Rotator Cuff Healing Index: A New Scoring System to Predict Rotator Cuff Healing After Surgical Repair. Am. J. Sports Med. 2019, 47, 173–180. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeong, J.Y.; Park, C.D.; Kang, S.G.; Yoo, J.C. Evaluation of the Risk Factors for a Rotator Cuff Retear After Repair Surgery. Am. J. Sports Med. 2017, 45, 1755–1761. [Google Scholar] [CrossRef]
- Lobo-Escolar, L.; Ramazzini-Castro, R.; Codina-Grano, D.; Lobo, E.; Minguell-Monyart, J.; Ardevol, J. Risk factors for symptomatic retears after arthroscopic repair of full-thickness rotator cuff tears. J. Shoulder Elb. Surg. 2021, 30, 27–33. [Google Scholar] [CrossRef]
- Manop, P.; Apivatgaroon, A.; Puntu, W.; Chernchujit, B. Risk Factors for Rotator Cuff Repair Failure and Reliability of the Rotator Cuff Healing Index (RoHI) in Thai Patients: Comparison of the RoHI With a Modified Scoring System. Orthop. J. Sports Med. 2023, 11, 23259671231179449. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, M.; Pan, J.; Liang, G.; Feng, W.; Zeng, L.; Yang, W.; Liu, J. Risk factors affecting rotator cuff retear after arthroscopic repair: A meta-analysis and systematic review. J. Shoulder Elb. Surg. 2021, 30, 2660–2670. [Google Scholar] [CrossRef]
- O’Connor, J.P.; Holden, P.; Gagnier, J.J. Systematic review: Preoperative psychological factors and total hip arthroplasty outcomes. J. Orthop. Surg. Res. 2022, 17, 457. [Google Scholar] [CrossRef] [PubMed]
- Jochimsen, K.N.; Noehren, B.; Mattacola, C.G.; Di Stasi, S.; Duncan, S.T.; Jacobs, C. Preoperative Psychosocial Factors and Short-term Pain and Functional Recovery After Hip Arthroscopy for Femoroacetabular Impingement Syndrome. J. Athl. Train. 2021, 56, 1064–1071. [Google Scholar] [CrossRef]
- Rizvi, S.M.T.; Bishop, M.; Lam, P.H.; Murrell, G.A.C. Factors Predicting Frequency and Severity of Postoperative Pain After Arthroscopic Rotator Cuff Repair Surgery. Am. J. Sports Med. 2021, 49, 146–153. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.K.; Tang, Y.X.; Liu, T.C.; Lu, C.J. Application of SHAP for Explainable Machine Learning on Age-Based Subgrouping Mammography Questionnaire Data for Positive Mammography Prediction and Risk Factor Identification. Healthcare 2023, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Witt, H.; Walker, J.; Ludwig, M.; Geelhoed, B.; Kossack, N.; Schild, M.; Miller, R.; Kirchhof, P. Machine learning-based identification of risk-factor signatures for undiagnosed atrial fibrillation in primary prevention and post-stroke in clinical practice. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 9, 16–23. [Google Scholar] [CrossRef]
- Lu, Y.; Ning, Y.; Li, Y.; Zhu, B.; Zhang, J.; Yang, Y.; Chen, W.; Yan, Z.; Chen, A.; Shen, B.; et al. Risk factor mining and prediction of urine protein progression in chronic kidney disease: A machine learning- based study. BMC Med. Inform. Decis. Mak. 2023, 23, 173. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Mingardi, L.; Zhuo, Y.D. Comparing interpretability and explainability for feature selection. arXiv 2021, arXiv:2105.05328. Available online: https://ui.adsabs.harvard.edu/abs/2021arXiv210505328D (accessed on 1 May 2021).
- Fryer, D.; Strümke, I.; Nguyen, H. Shapley values for feature selection: The good, the bad, and the axioms. IEEE Access 2021, 9, 144352–144360. [Google Scholar] [CrossRef]
- Kursa, M.B.; Rudnicki, W.R. The All Relevant Feature Selection Using Random Forest. arXiv 2011, arXiv:1106.5112. Available online: https://ui.adsabs.harvard.edu/abs/2011arXiv1106.5112K (accessed on 1 June 2011).
- Wang, M.; Sun, M.; Yu, Y.; Li, X.; Ren, Y.; Yin, D. Predictive value of machine learning algorithm of coronary artery calcium score and clinical factors for obstructive coronary artery disease in hypertensive patients. BMC Med. Inform. Decis. Mak. 2023, 23, 244. [Google Scholar] [CrossRef]
- Li, B.; Verma, R.; Beaton, D.; Tamim, H.; Hussain, M.A.; Hoballah, J.J.; Lee, D.S.; Wijeysundera, D.N.; de Mestral, C.; Mamdani, M.; et al. Predicting Major Adverse Cardiovascular Events Following Carotid Endarterectomy Using Machine Learning. J. Am. Heart Assoc. 2023, 12, e030508. [Google Scholar] [CrossRef]
- Iannotti, J.P.; Deutsch, A.; Green, A.; Rudicel, S.; Christensen, J.; Marraffino, S.; Rodeo, S. Time to failure after rotator cuff repair: A prospective imaging study. J. Bone Jt. Surg. Am. 2013, 95, 965–971. [Google Scholar] [CrossRef]
- Miller, B.S.; Downie, B.K.; Kohen, R.B.; Kijek, T.; Lesniak, B.; Jacobson, J.A.; Hughes, R.E.; Carpenter, J.E. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am. J. Sports Med. 2011, 39, 2064–2070. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, H.J.; Park, I.; Sung, G.Y.; Kim, D.J.; Kim, J.H. Arthroscopic In Situ Superior Capsular Reconstruction Using the Long Head of the Biceps Tendon. Arthrosc. Tech. 2018, 7, e97–e103. [Google Scholar] [CrossRef] [PubMed]
- Lafosse, L.; Jost, B.; Reiland, Y.; Audebert, S.; Toussaint, B.; Gobezie, R. Structural integrity and clinical outcomes after arthroscopic repair of isolated subscapularis tears. J. Bone Jt. Surg. Am. 2007, 89, 1184–1193. [Google Scholar] [CrossRef]
- Goutallier, D.; Postel, J.M.; Bernageau, J.; Lavau, L.; Voisin, M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin. Orthop. Relat. Res. 1994, 304, 78–83. [Google Scholar] [CrossRef]
- Smid, P.; Hart, R.; Puskeiler, M. Tangent sign—a reliable predictor of risk for tendon re-rupture in rotator cuff repair. Acta Chir. Orthop. Traumatol. Cech. 2014, 81, 227–232. [Google Scholar] [CrossRef]
- Sugaya, H.; Maeda, K.; Matsuki, K.; Moriishi, J. Functional and structural outcome after arthroscopic full-thickness rotator cuff repair: Single-row versus dual-row fixation. Arthroscopy 2005, 21, 1307–1316. [Google Scholar] [CrossRef]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Fernández, A.; Garcia, S.; Herrera, F.; Chawla, N.V. SMOTE for learning from imbalanced data: Progress and challenges, marking the 15-year anniversary. J. Artif. Intell. Res. 2018, 61, 863–905. [Google Scholar] [CrossRef]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. J. Math. Methods Biosci. 2008, 50, 419–430. [Google Scholar] [CrossRef]
- Marcílio, W.E.; Eler, D.M. From explanations to feature selection: Assessing SHAP values as feature selection mechanism. In Proceedings of the 2020 33rd SIBGRAPI conference on Graphics, Patterns and Images (SIBGRAPI), Porto de Galinhas, Brazil, 7–10 November 2020; IEEE: New York, NY, USA, 2020; pp. 340–347. [Google Scholar]
- Chung, S.W.; Oh, J.H.; Gong, H.S.; Kim, J.Y.; Kim, S.H. Factors affecting rotator cuff healing after arthroscopic repair: Osteoporosis as one of the independent risk factors. Am. J. Sports Med. 2011, 39, 2099–2107. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jung, K.H.; Kim, J.W.; Kim, U.S.; Hwang, D.H. Factors affecting rotator cuff integrity after arthroscopic repair for medium-sized or larger cuff tears: A retrospective cohort study. J. Shoulder Elb. Surg. 2018, 27, 1012–1020. [Google Scholar] [CrossRef]
- Cao, Z.; Li, Q.; Li, Y.; Wu, J. The association of metabolic syndrome with rotator cuff tendinopathy: A two-sample Mendelian randomization study. Diabetol. Metab. Syndr. 2023, 15, 211. [Google Scholar] [CrossRef] [PubMed]
- Gumina, S.; Kim, H.; Jung, Y.; Song, H.S. Rotator cuff degeneration and healing after rotator cuff repair. Clin. Shoulder Elb. 2023, 26, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Luo, M.; Liang, G.; Pan, J.; Han, Y.; Zeng, L.; Yang, W.; Liu, J. What Factors Are Associated with Symptomatic Rotator Cuff Tears: A Meta-analysis. Clin. Orthop. Relat. Res. 2022, 480, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Beam, A.L.; Kohane, I.S. Big data and machine learning in health care. Jama 2018, 319, 1317–1318. [Google Scholar] [CrossRef]
- Rajkomar, A.; Dean, J.; Kohane, I. Machine learning in medicine. New Engl. J. Med. 2019, 380, 1347–1358. [Google Scholar] [CrossRef]

| Non-Retear (n = 694) | Retear (n = 94) | p-Value | |
|---|---|---|---|
| Sex, male–female | 253:441 | 64:30 | 0.424 |
| Age, years | 61.7 ± 8.4 | 64.9 ± 7.4 | 0.001 |
| BMI, | 24.6 ± 3.2 | 24.1 ± 2.9 | 0.115 |
| DM, yes–no | 95:599 | 17:77 | 0.270 |
| Smoking, yes–no | 54:640 | 11:83 | 0.228 |
| Dominant side, yes–no | 488:206 | 72:22 | 0.227 |
| Acromion Type, 1:2:3 | 94:511:89 | 16:61:17 | 0.055 |
| Full-thickness tear, +:− | 442:252 | 84:10 | <0.001 |
| AP tear size, cm | 1.2 ± 0.7 | 1.7 ± 0.9 | <0.001 |
| ML tear size, cm | 1.3 ± 0.9 | 1.9 ± 1.0 | <0.001 |
| Subscapularis tear, I:II:III:IV:V | 440:133:91:21:9 | 55:26:9:3:1 | 0.454 |
| Biceps condition, 0:1:2:3:4 | 341:152:91:101:9 | 36:17:17:24:0 | 0.029 |
| Supraspinatus FI, 0:I:II:III:IV | 137:373:134:37:13 | 10:42:29:12:1 | 0.002 |
| Subscapularis FI, 0:I:II:III:IV | 452:230:12:0:0 | 47:44:2:0:1 | 0.004 |
| Infraspinatus FI, 0:I:II:III:IV | 375:248:63:7:1 | 36:46:11:0:1 | 0.015 |
| Supraspinatus Tangent, 1:0 | 511:183 | 35:59 | 0.070 |
| Subscapularis Tangent, 1:0 | 676:18 | 2:92 | 1.000 |
| Infraspinatus Tangent, 1:0 | 680:14 | 3:91 | 0.493 |
| Forward flexion | 140.9 ± 15.3 | 142.8 ± 12.8 | 0.189 |
| External rotation (side) | 82.3 ± 13.3 | 83.4 ± 12.6 | 0.411 |
| External rotation (abd.) | 84.0 ± 12.7 | 84.8 ± 10.7 | 0.473 |
| Internal rotation | 9.2 ± 3.4 | 9.7 ± 3.2 | 0.157 |
| Adduction | 23.3 ± 4.8 | 24.0 ± 4.4 | 0.179 |
| VAS for pain | 4.2 ± 2.0 | 3.8 ± 2.4 | 0.206 |
| ASES score | 57.8 ± 18.9 | 58.7 ± 22.4 | 0.710 |
| CM score | 69.1 ± 16.5 | 68.8 ± 16.1 | 0.873 |
| KS score | 65.9 ± 15.9 | 64.3 ± 16.1 | 0.368 |
| Model | AUC-ROC | Accuracy | Sensitivity | Specificity |
|---|---|---|---|---|
| RF | 0.653 | 0.576 | 0.737 | 0.554 |
| LR | 0.570 | 0.430 | 0.842 | 0.374 |
| SVM | 0.597 | 0.380 | 0.947 | 0.302 |
| NN | 0.641 | 0.487 | 0.895 | 0.432 |
| XGBoost | 0.676 | 0.506 | 0.842 | 0.460 |
| Variables | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Sex (M) | 0.817 (0.510–1.284) | 0.39 | 0.614 (0.330–1.099) | 0.11 |
| Age | 1.051 (1.023–1.081) | <0.001 | 1.040 (1.006–1.075) | 0.02 |
| BMI | 0.947 (0.879–1.016) | 0.140 | 0.958 (0.883–1.035) | 0.289 |
| DM | 1.392 (0.767–2.405) | 0.254 | 1.240 (0.649–2.274) | 0.5 |
| Smoking | 1.571 (0.753–3.017) | 0.198 | 2.192 (0.886–5.231) | 0.081 |
| Dominant side | 1.382 (0.847–2.335) | 0.209 | 1.318 (0.759–2.361) | 0.339 |
| Acromion Type | ||||
| Type 1 | -[Reference] | -[Reference] | ||
| Type 2 | 0.701 (0.396–1.306) | 0.241 | 0.514 (0.268–1.022) | 0.05 |
| Type 3 | 1.122 (0.533–2.373) | 0.761 | 0.673 (0.287–1.569) | 0.357 |
| FTRCT | 4.789 (2.559–9.985) | <0.001 | 2.106 (0.972–4.898) | 0.069 |
| AP size | 2.345 (1.794–3.081) | <0.001 | 1.476 (0.926–2.348) | 0.1 |
| ML size | 2.122 (1.690–2.678) | <0.001 | 1.580 (1.052–2.392) | 0.029 |
| SSC Lafosse | 1.008 (0.790–1.260) | 0.945 | 0.920 (0.700–1.185) | 0.532 |
| BC | 1.265 (1.060–1.506) | 0.009 | 1.148 (0.938–1.400) | 0.176 |
| SSC FI | 1.787 (1.234–2.579) | 0.002 | 1.258 (0.714–2.210) | 0.425 |
| SSP FI | 1.487 (1.181–1.868) | <0.001 | 0.996 (0.645–1.544) | 0.985 |
| ISP FI | 1.406 (1.060–1.805) | 0.016 | 0.864 (0.508–1.454) | 0.585 |
| SSC Tangent | 0.816 (0.128–2.891) | 0.788 | 0.425 (0.520–2.194) | 0.354 |
| SSP Tangent | 1.656 (1.047–2.588) | 0.028 | 0.683 (0.363–1.257) | 0.227 |
| ISP Tangent | 1.601 (0.364–5.021) | 0.466 | 1.127 (0.193–5.450) | 0.885 |
| Forward flexion | 1.010 (0.994–1.029) | 0.25 | 1.008 (0.974–1.046) | 0.673 |
| ER (side) | 1.007 (0.991–1.028) | 0.431 | 1.008 (0.959–1.054) | 0.748 |
| ER (abduction) | 1.006 (0.989–1.028) | 0.528 | 0.980 (0.946–1.032) | 0.347 |
| IR | 1.048 (0.981–1.128) | 0.177 | 1.022 (0.919–1.142) | 0.701 |
| Adduction | 1.031 (0.984–1.082) | 0.205 | 1.019 (0.955–1.077) | 0.491 |
| VAS pain | 0.925 (0.830–1.028) | 0.152 | 0.849 (0.668–1.080) | 0.181 |
| ASES score | 1.002 (0.991–1.014) | 0.672 | 0.986 (0.956–1.016) | 0.374 |
| CM score | 0.999 (0.986–1.012) | 0.873 | 1.032 (0.998–1.069) | 0.073 |
| KS score | 0.994 (0.981–1.007) | 0.361 | 0.972 (0.967–1.008) | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.-H.; Kim, Y.-S. Predicting Major Preoperative Risk Factors for Retears After Arthroscopic Rotator Cuff Repair Using Machine Learning Algorithms. J. Clin. Med. 2025, 14, 1843. https://doi.org/10.3390/jcm14061843
Cho S-H, Kim Y-S. Predicting Major Preoperative Risk Factors for Retears After Arthroscopic Rotator Cuff Repair Using Machine Learning Algorithms. Journal of Clinical Medicine. 2025; 14(6):1843. https://doi.org/10.3390/jcm14061843
Chicago/Turabian StyleCho, Sung-Hyun, and Yang-Soo Kim. 2025. "Predicting Major Preoperative Risk Factors for Retears After Arthroscopic Rotator Cuff Repair Using Machine Learning Algorithms" Journal of Clinical Medicine 14, no. 6: 1843. https://doi.org/10.3390/jcm14061843
APA StyleCho, S.-H., & Kim, Y.-S. (2025). Predicting Major Preoperative Risk Factors for Retears After Arthroscopic Rotator Cuff Repair Using Machine Learning Algorithms. Journal of Clinical Medicine, 14(6), 1843. https://doi.org/10.3390/jcm14061843







