Transcatheter Aortic Valve Implantation and Replacement: The Latest Advances and Prospects
Abstract
1. Introduction
2. Discussion
2.1. Early Development and Initial Indications
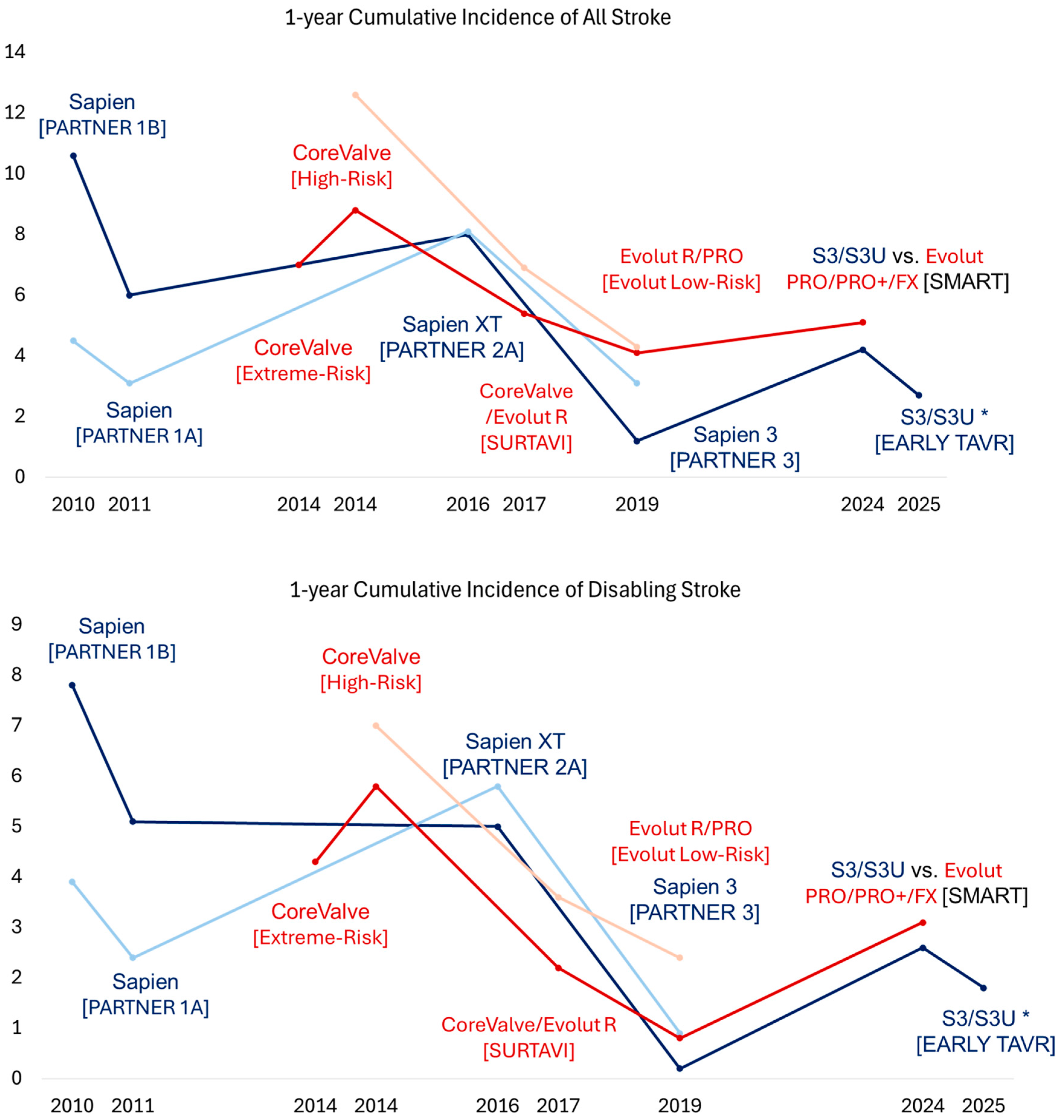
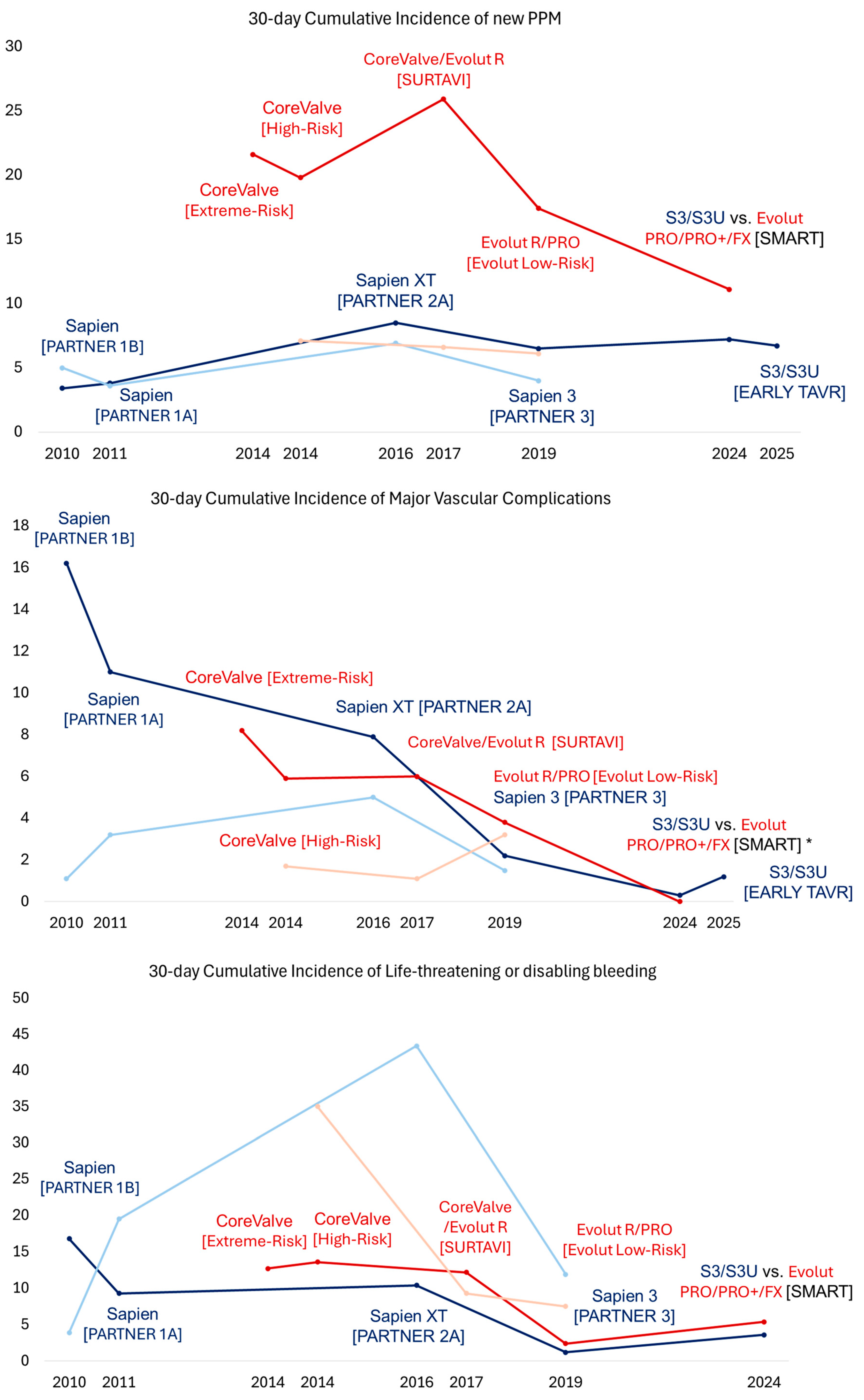
TAVR for Symptomatic Severe AS with High and Prohibitive Surgical Risk
2.2. Contemporary Trials and Expanding Indications
2.2.1. TAVR for Symptomatic Severe AS Across All Risk Categories
2.2.2. TAVR for Symptomatic Severe AS with Bicuspid Aortic Valve or Small Aortic Annulus
2.2.3. TAVR for Asymptomatic Severe or Moderate AS
2.3. Advances in Valve Design
2.3.1. Edwards SAPIEN Series and RESILIA Technology
2.3.2. Medtronic CoreValve Evolut Series
2.3.3. Abbott Portico Navitor Systems
2.3.4. Boston Scientific ACURATE Neo2 and LOTUS Series
2.3.5. JenaValve
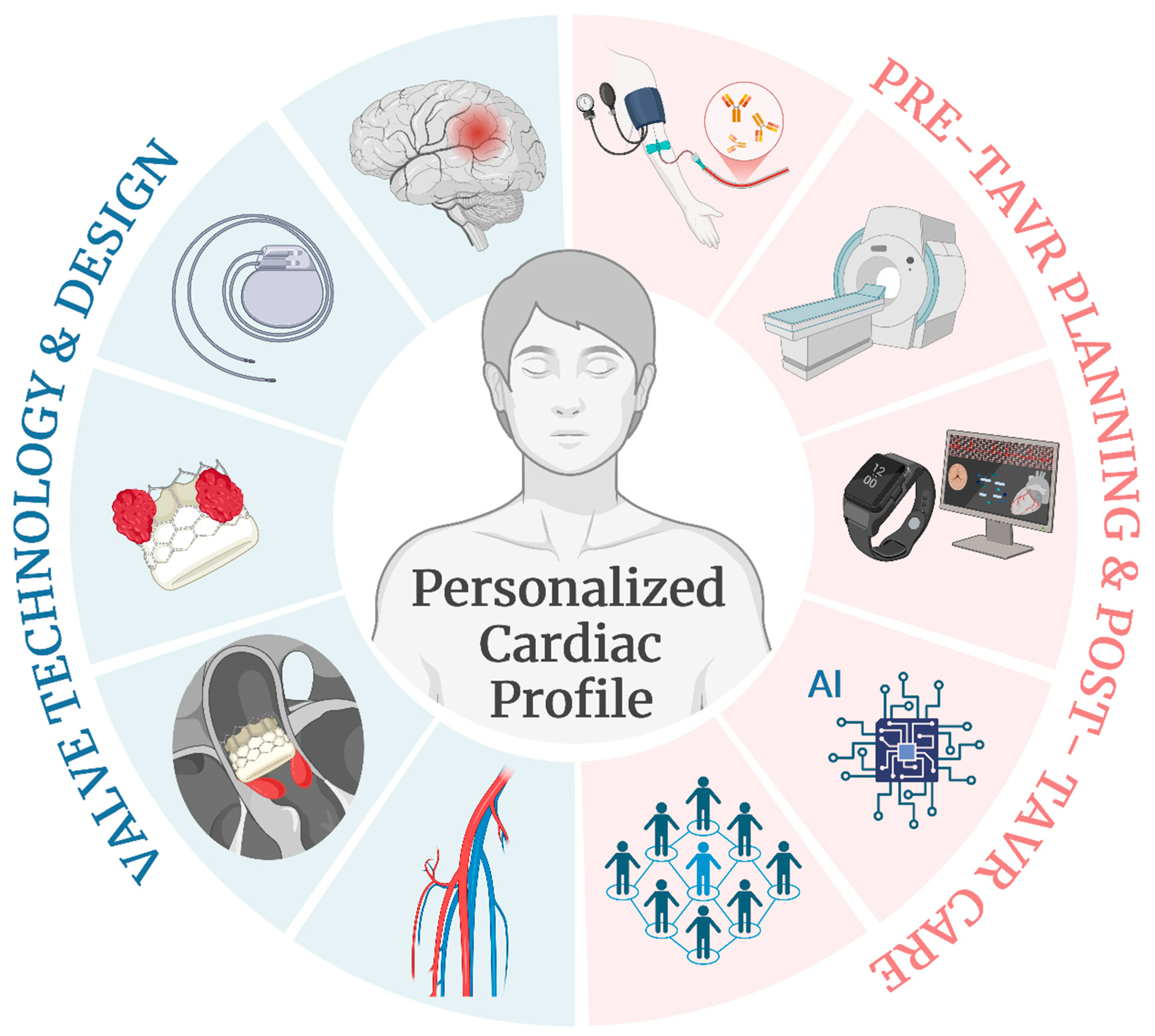
2.4. Personalized Profiles and Multidisciplinary Care
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| PVL | Paravalvular leak |
| SAVR | Surgical aortic valve replacement |
| TAVR | Transcatheter aortic valve replacement |
| CMR | Cardiac magnetic resonance |
| PET | Polyethylene terephthalate |
| FDA | Food and Drug Administration |
References
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease. J. Am. Coll. Cardiol. 2021, 77, e25–e197. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Reardon, M.J.; Mieghem, N.M.V.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Elmariah, S.; Spitzer, E.; Pibarot, P.; Nazif, T.M.; Bax, J.J.; Hahn, R.T.; Popma, A.; Ben-Yehuda, O.; Kallel, F.; et al. Transcatheter Aortic Valve Replacement in Patients With Systolic Heart Failure and Moderate Aortic Stenosis: TAVR UNLOAD. J. Am. Coll. Cardiol. 2024, 85, 878–890. [Google Scholar] [CrossRef]
- Généreux, P.; Schwartz, A.; Oldemeyer, J.B.; Pibarot, P.; Cohen, D.J.; Blanke, P.; Lindman, B.R.; Babaliaros, V.; Fearon, W.F.; Daniels, D.V.; et al. Transcatheter Aortic-Valve Replacement for Asymptomatic Severe Aortic Stenosis. N. Engl. J. Med. 2024, 392, 217–227. [Google Scholar] [CrossRef]
- Loganath, K.; Craig, N.J.; Everett, R.J.; Bing, R.; Tsampasian, V.; Molek, P.; Botezatu, S.; Aslam, S.; Lewis, S.; Graham, C.; et al. Early Intervention in Patients With Asymptomatic Severe Aortic Stenosis and Myocardial Fibrosis: The EVOLVED Randomized Clinical Trial. JAMA 2025, 333, 213–221. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Tron, C.; Bauer, F.; Agatiello, C.; Nercolini, D.; Tapiero, S.; Litzler, P.Y.; Bessou, J.P.; Babaliaros, V. Treatment of calcific aortic stenosis with the percutaneous heart valve: Mid-term follow-up from the initial feasibility studies: The French experience. J. Am. Coll. Cardiol. 2006, 47, 1214–1223. [Google Scholar] [CrossRef]
- Webb, J.G.; Pasupati, S.; Humphries, K.; Thompson, C.; Altwegg, L.; Moss, R.; Sinhal, A.; Carere, R.G.; Munt, B.; Ricci, D.; et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007, 116, 755–763. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Leon, M.B.; Makkar, R.R.; Tuzcu, E.M.; Svensson, L.G.; Kodali, S.; Webb, J.G.; Mack, M.J.; Douglas, P.S.; Thourani, V.H.; et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2485–2491. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet 2015, 385, 2477–2484. [Google Scholar] [CrossRef]
- Popma, J.J.; Adams, D.H.; Reardon, M.J.; Yakubov, S.J.; Kleiman, N.S.; Heimansohn, D.; Hermiller, J., Jr.; Hughes, G.C.; Harrison, J.K.; Coselli, J.; et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J. Am. Coll. Cardiol. 2014, 63, 1972–1981. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B., Jr.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; De Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary. J. Am. Coll. Cardiol. 2014, 63, 2438–2488. [Google Scholar] [CrossRef]
- Gilard, M.; Eltchaninoff, H.; Iung, B.; Donzeau-Gouge, P.; Chevreul, K.; Fajadet, J.; Leprince, P.; Leguerrier, A.; Lievre, M.; Prat, A.; et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N. Engl. J. Med. 2012, 366, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Moat, N.E.; Ludman, P.; de Belder, M.A.; Bridgewater, B.; Cunningham, A.D.; Young, C.P.; Thomas, M.; Kovac, J.; Spyt, T.; MacCarthy, P.A.; et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: The U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J. Am. Coll. Cardiol. 2011, 58, 2130–2138. [Google Scholar] [CrossRef]
- Onorati, F.; D’Errigo, P.; Barbanti, M.; Rosato, S.; Covello, R.D.; Maraschini, A.; Ranucci, M.; Santoro, G.; Tamburino, C.; Grossi, C.; et al. Different impact of sex on baseline characteristics and major periprocedural outcomes of transcatheter and surgical aortic valve interventions: Results of the multicenter Italian OBSERVANT Registry. J. Thorac. Cardiovasc. Surg. 2014, 147, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef]
- Oikonomou, G.; Apostolos, A.; Drakopoulou, M.; Simopoulou, C.; Karmpalioti, M.; Toskas, P.; Stathogiannis, K.; Xanthopoulou, M.; Ktenopoulos, N.; Latsios, G.; et al. Long-Term Outcomes of Aortic Stenosis Patients with Different Flow/Gradient Patterns Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2024, 13, 1200. [Google Scholar] [CrossRef]
- Karra, N.; Sharon, A.; Massalha, E.; Fefer, P.; Maor, E.; Guetta, V.; Ben-Zekry, S.; Kuperstein, R.; Matetzky, S.; Beigel, R.; et al. Temporal Trends in Patient Characteristics and Clinical Outcomes of TAVR: Over a Decade of Practice. J. Clin. Med. 2024, 13, 5027. [Google Scholar] [CrossRef]
- Arnold, S.V.; Zhang, Y.; Baron, S.J.; McAndrew, T.C.; Alu, M.C.; Kodali, S.K.; Kapadia, S.; Thourani, V.H.; Miller, D.C.; Mack, M.J.; et al. Impact of Short-Term Complications on Mortality and Quality of Life After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 362–369. [Google Scholar] [CrossRef]
- Makkar, R.R.; Thourani, V.H.; Mack, M.J.; Kodali, S.K.; Kapadia, S.; Webb, J.G.; Yoon, S.-H.; Trento, A.; Svensson, L.G.; Herrmann, H.C.; et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, E.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. Self-expanding Transcatheter vs Surgical Aortic Valve Replacement in Intermediate-Risk Patients: 5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Pibarot, P.; Hahn, R.T.; Genereux, P.; Kodali, S.K.; Kapadia, S.R.; Cohen, D.J.; Pocock, S.J.; et al. Transcatheter Aortic-Valve Replacement in Low-Risk Patients at Five Years. N. Engl. J. Med. 2023, 389, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Cannata, S.; Gandolfo, C.; Ribichini, F.L.; van Mieghem, N.; Buccheri, S.; Barbanti, M.; Berti, S.; Teles, R.C.; Bartorelli, A.L.; Musumeci, G.; et al. One-year outcomes after transcatheter aortic valve implantation with the latest-generation SAPIEN balloon-expandable valve: The S3U registry. EuroIntervention 2023, 18, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Forrest, J.K.; Deeb, G.M.; Yakubov, S.J.; Gada, H.; Mumtaz, M.A.; Ramlawi, B.; Bajwa, T.; Teirstein, P.S.; Tchétché, D.; Huang, J.; et al. 4-Year Outcomes of Patients With Aortic Stenosis in the Evolut Low Risk Trial. J. Am. Coll. Cardiol. 2023, 82, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Talanas, G.; Laconi, A.; Kereiakes, D.J.; Merella, P.; Reardon, M.J.; Spano, A.; Petretto, G.; Lauriola, F.; Casula, M.; Micheluzzi, V.; et al. Long-Term Outcomes of Transcatheter vs Surgical Aortic Valve Replacement: Meta-analysis of Randomized Trials. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102143. [Google Scholar] [CrossRef]
- Grubb, K.J.; Tom, S.K.; Xie, J.; Kalra, K.; Camaj, A. Transcatheter Aortic Valve Replacement Versus Surgical Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis-We Need a Well-Designed Randomized Control Trial. J. Clin. Med. 2024, 13, 6565. [Google Scholar] [CrossRef]
- Leone, P.P.; Regazzoli, D.; Pagnesi, M.; Sanz-Sanchez, J.; Chiarito, M.; Cannata, F.; Van Mieghem, N.M.; Barbanti, M.; Tamburino, C.; Teles, R.; et al. Predictors and Clinical Impact of Prosthesis-Patient Mismatch After Self-Expandable TAVR in Small Annuli. JACC Cardiovasc. Interv. 2021, 14, 1218–1228. [Google Scholar] [CrossRef]
- Abdelghani, M.; Mankerious, N.; Allali, A.; Landt, M.; Kaur, J.; Sulimov, D.S.; Merten, C.; Sachse, S.; Mehilli, J.; Neumann, F.J.; et al. Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement With Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights From the CHOICE Trial and the CHOICE-Extend Registry. JACC Cardiovasc. Interv. 2018, 11, 2507–2518. [Google Scholar] [CrossRef]
- Kamioka, N.; Arita, T.; Hanyu, M.; Hayashi, M.; Watanabe, S.; Miura, S.; Isotani, A.; Arai, Y.; Kakumoto, S.; Ando, K.; et al. Valve Hemodynamics and Clinical Outcomes After Transcatheter Aortic Valve Replacement for a Small Aortic Annulus. Int. Heart J. 2019, 60, 86–92. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Mehran, R.; Blackman, D.J.; Bailey, S.; Möllmann, H.; Abdel-Wahab, M.; Ben Ali, W.; Mahoney, P.D.; Ruge, H.; Wood, D.A.; et al. Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus. N. Engl. J. Med. 2024, 390, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Tchétché, D.; Mehran, R.; Blackman, D.J.; Khalil, R.F.; Möllmann, H.; Abdel-Wahab, M.; Ben Ali, W.; Mahoney, P.D.; Ruge, H.; Bleiziffer, S.; et al. Transcatheter Aortic Valve Implantation by Valve Type in Women With Small Annuli: Results From the SMART Randomized Clinical Trial. JAMA Cardiol. 2024, 9, 1106–1114. [Google Scholar] [CrossRef]
- Kang, D.-H.; Park, S.-J.; Lee, S.-A.; Lee, S.; Kim, D.-H.; Kim, H.-K.; Yun, S.-C.; Hong, G.-R.; Song, J.-M.; Chung, C.-H.; et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Banovic, M.; Putnik, S.; Penicka, M.; Doros, G.; Deja, M.A.; Kockova, R.; Kotrc, M.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: The AVATAR Trial. Circulation 2022, 145, 648–658. [Google Scholar] [CrossRef]
- Richardson, C.; Gilbert, T.; Aslam, S.; Brookes, C.L.; Singh, A.; Newby, D.E.; Dweck, M.R.; Stewart, R.A.H.; Myles, P.S.; Briffa, T.; et al. Rationale and design of the early valve replacement in severe asymptomatic aortic stenosis trial. Am. Heart J. 2024, 275, 119–127. [Google Scholar] [CrossRef]
- Singh, S.K.; Kachel, M.; Castillero, E.; Xue, Y.; Kalfa, D.; Ferrari, G.; George, I. Polymeric prosthetic heart valves: A review of current technologies and future directions. Front. Cardiovasc. Med. 2023, 10, 1137827. [Google Scholar] [CrossRef]
- Kapadia, S.R.; Makkar, R.; Leon, M.; Abdel-Wahab, M.; Waggoner, T.; Massberg, S.; Rottbauer, W.; Horr, S.; Sondergaard, L.; Karha, J.; et al. Cerebral Embolic Protection during Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2022, 387, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Gupta, A.; Waggoner, T.E.; Horr, S.; Karha, J.; Satler, L.; Stoler, R.C.; Alvarez, J.; Sakhuja, R.; MacDonald, L.; et al. Cerebral Embolic Protection by Geographic Region: A Post Hoc Analysis of the PROTECTED TAVR Randomized Clinical Trial. JAMA Cardiol. 2025, 10, 17–24. [Google Scholar] [CrossRef]
- Lansky, A.J.; Schofer, J.; Tchetche, D.; Stella, P.; Pietras, C.G.; Parise, H.; Abrams, K.; Forrest, J.K.; Cleman, M.; Reinöhl, J.; et al. A prospective randomized evaluation of the TriGuard™ HDH embolic DEFLECTion device during transcatheter aortic valve implantation: Results from the DEFLECT III trial. Eur. Heart J. 2015, 36, 2070–2078. [Google Scholar] [CrossRef]
- Nazif, T.M.; Moses, J.; Sharma, R.; Dhoble, A.; Rovin, J.; Brown, D.; Horwitz, P.; Makkar, R.; Stoler, R.; Forrest, J.; et al. Randomized Evaluation of TriGuard 3 Cerebral Embolic Protection After Transcatheter Aortic Valve Replacement: REFLECT II. JACC Cardiovasc Interv. 2021, 14, 515–527. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; van Gils, L.; Ahmad, H.; van Kesteren, F.; van der Werf, H.W.; Brueren, G.; Storm, M.; Lenzen, M.; Daemen, J.; van den Heuvel, A.F.; et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: The randomised MISTRAL-C trial. EuroIntervention 2016, 12, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.R.; Kodali, S.; Makkar, R.; Mehran, R.; Lazar, R.M.; Zivadinov, R.; Dwyer, M.G.; Jilaihawi, H.; Virmani, R.; Anwaruddin, S.; et al. Protection Against Cerebral Embolism During Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017, 69, 367–377. [Google Scholar] [CrossRef]
- Kaur, A.; Dhaliwal, A.S.; Sohal, S.; Gwon, Y.; Gupta, S.; Bhatia, K.; Dominguez, A.C.; Basman, C.; Tamis-Holland, J. Role of Cerebral Embolic Protection Devices in Patients Undergoing Transcatheter Aortic Valve Replacement: An Updated Meta-Analysis. J. Am. Heart Assoc. 2024, 13, e030587. [Google Scholar] [CrossRef] [PubMed]
- Beaver, T.; Bavaria, J.E.; Griffith, B.; Svensson, L.G.; Pibarot, P.; Borger, M.A.; Sharaf, O.M.; Heimansohn, D.A.; Thourani, V.H.; Blackstone, E.H.; et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. J. Thorac Cardiovasc Surg. 2024, 168, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Sondergaard, L.; Walton, A.S.; Worthley, S.G.; Smith, D.; Chehab, B.; Manoharan, G.; Yong, G.; Bedogni, F.; Bates, N.; Reardon, M.J. Thirty-day and one-year outcomes of the Navitor transcatheter heart valve in patients with aortic stenosis: The prospective, multicentre, global PORTICO NG Study. EuroIntervention 2023, 19, 248–255. [Google Scholar] [CrossRef]
- Vahl, T.P.; Thourani, V.H.; Makkar, R.R.; Hamid, N.; Khalique, O.K.; Daniels, D.; McCabe, J.M.; Satler, L.; Russo, M.; Cheng, W.; et al. Transcatheter aortic valve implantation in patients with high-risk symptomatic native aortic regurgitation (ALIGN-AR): A prospective, multicentre, single-arm study. Lancet 2024, 403, 1451–1459. [Google Scholar] [CrossRef]
- Hecht, S.; Giuliani, C.; Nuche, J.; Farjat Pasos, J.I.; Bernard, J.; Tastet, L.; Abu-Alhayja’a, R.; Beaudoin, J.; Côté, N.; DeLarochellière, R.; et al. Multimarker Approach to Improve Risk Stratification of Patients Undergoing Transcatheter Aortic Valve Implantation. JACC Adv. 2024, 3, 100761. [Google Scholar] [CrossRef]
- Dahou, A.; Awasthi, V.; Bkhache, M.; Djellal, M.; Yang, X.; Wang, H.; Bouchareb, R. Sex-Related Differences in the Pathophysiology, Cardiac Imaging, and Clinical Outcomes of Aortic Stenosis: A Narrative Review. J. Clin. Med. 2024, 13, 6359. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Rabbat, M.G. Artificial Intelligence in Transcatheter Aortic Valve Replacement: Its Current Role and Ongoing Challenges. Diagnostics 2024, 14, 261. [Google Scholar] [CrossRef]
- Sun, S.; Yeh, L.; Imanzadeh, A.; Kooraki, S.; Kheradvar, A.; Bedayat, A. The Current Landscape of Artificial Intelligence in Imaging for Transcatheter Aortic Valve Replacement. Curr. Radiol. Rep. 2024, 12, 113–120. [Google Scholar] [CrossRef]
- Ocagli, H.; Lorenzoni, G.; Lanera, C.; Schiavo, A.; D’Angelo, L.; Liberti, A.D.; Besola, L.; Cibin, G.; Martinato, M.; Azzolina, D.; et al. Monitoring Patients Reported Outcomes after Valve Replacement Using Wearable Devices: Insights on Feasibility and Capability Study: Feasibility Results. Int. J. Environ. Res. Public. Health 2021, 18, 7171. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, J.; Guo, Y.; Dai, H.; Xu, J.; Wang, L.; Hu, P.; Lin, X.; Li, C.; Zhou, D.; et al. Wearable Smartwatch Facilitated Remote Health Management for Patients Undergoing Transcatheter Aortic Valve Replacement. J. Am. Heart Assoc. 2022, 11, e023219. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Dai, H.; Guo, Y.; Xu, J.; Wang, L.; Jiang, J.; Lin, X.; Li, C.; Zhou, D.; Li, H.; et al. Smartwatch-Detected Arrhythmias in Patients After Transcatheter Aortic Valve Replacement (TAVR): Analysis of the SMART TAVR Trial. J. Med. Internet Res. 2024, 26, e41843. [Google Scholar] [CrossRef]
- Tran, J.H.; Itagaki, S.; Zeng, Q.; Leon, M.B.; O’Gara, P.T.; Mack, M.J.; Gillinov, A.M.; El-Hamamsy, I.; Tang, G.H.L.; Mikami, T.; et al. Transcatheter or Surgical Replacement for Failed Bioprosthetic Aortic Valves. JAMA Cardiol. 2024, 9, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, D.; Yousef, S.; Kliner, D.; Brown, J.A.; Serna-Gallegos, D.; Toma, C.; Makani, A.; West, D.; Wang, Y.; Thoma, F.W.; et al. Outcomes of Valve-in-Valve Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2024, 215, 1–7. [Google Scholar] [CrossRef]
- Khan, J.M.; Greenbaum, A.B.; Babaliaros, V.C.; Rogers, T.; Eng, M.H.; Paone, G.; Leshnower, B.G.; Reisman, M.; Satler, L.; Waksman, R.; et al. The BASILICA Trial. JACC Cardiovasc. Interv. 2019, 12, 1240–1252. [Google Scholar] [CrossRef]
- Hatoum, H.; Maureira, P.; Lilly, S.; Dasi, L.P. Impact of Leaflet Laceration on Transcatheter Aortic Valve-in-Valve Washout: BASILICA to Solve Neosinus and Sinus Stasis. JACC Cardiovasc. Interv. 2019, 12, 1229–1237. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brankovic, M.; Sharma, A. Transcatheter Aortic Valve Implantation and Replacement: The Latest Advances and Prospects. J. Clin. Med. 2025, 14, 1844. https://doi.org/10.3390/jcm14061844
Brankovic M, Sharma A. Transcatheter Aortic Valve Implantation and Replacement: The Latest Advances and Prospects. Journal of Clinical Medicine. 2025; 14(6):1844. https://doi.org/10.3390/jcm14061844
Chicago/Turabian StyleBrankovic, Milos, and Abhishek Sharma. 2025. "Transcatheter Aortic Valve Implantation and Replacement: The Latest Advances and Prospects" Journal of Clinical Medicine 14, no. 6: 1844. https://doi.org/10.3390/jcm14061844
APA StyleBrankovic, M., & Sharma, A. (2025). Transcatheter Aortic Valve Implantation and Replacement: The Latest Advances and Prospects. Journal of Clinical Medicine, 14(6), 1844. https://doi.org/10.3390/jcm14061844





