A New Histology-Based Prognostic Index for Acute Myeloid Leukemia: Preliminary Results for the “AML Urayasu Classification”
Abstract
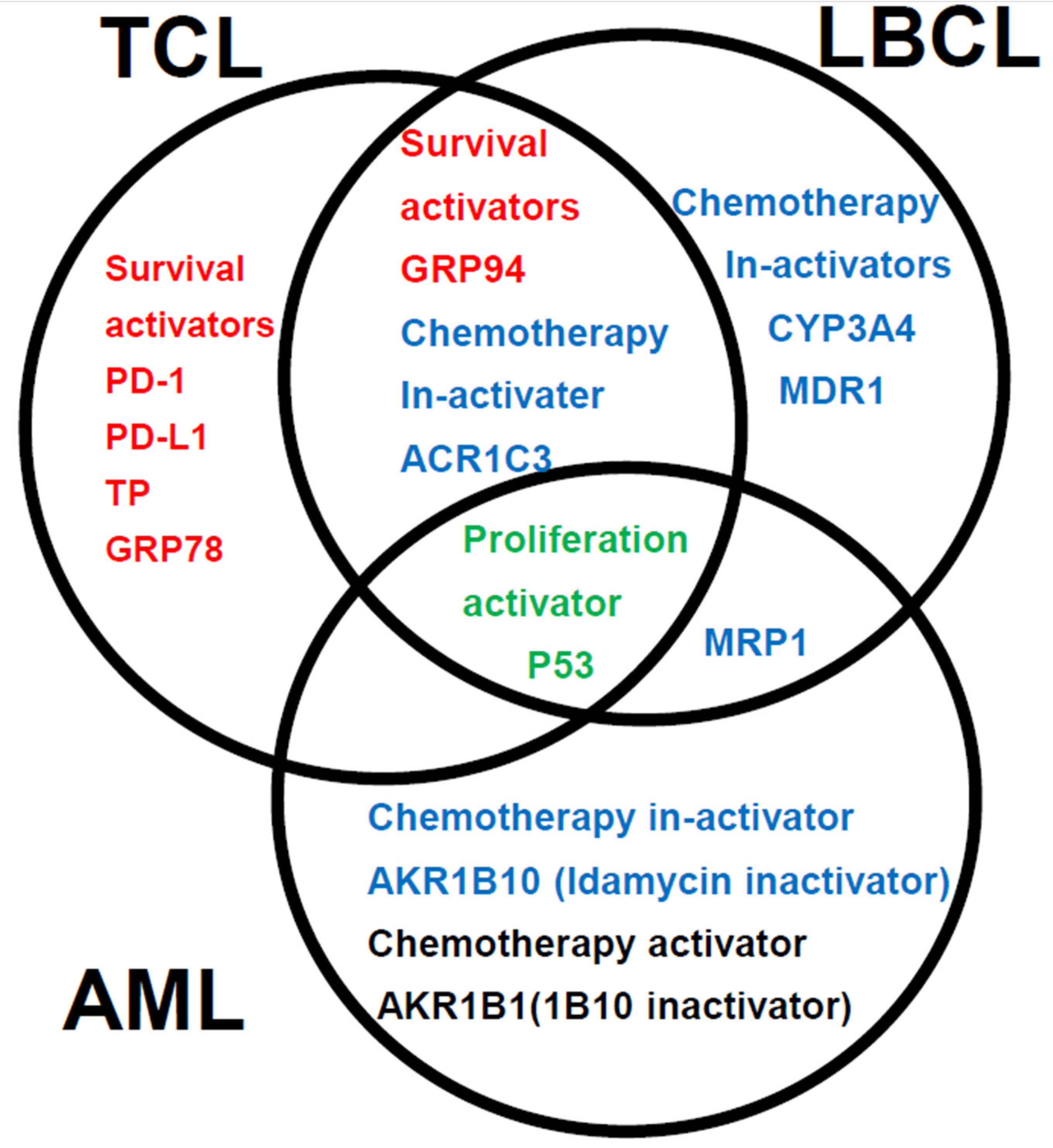
1. Introduction
2. Materials and Methods
2.1. Patients and Sample Collection
2.2. Immunohistochemistry
2.3. Statistical Analysis
3. Results
3.1. Kaplan–Meier Survival Curves and Comparisons Among Groups (Log Rank Test)
3.1.1. Overall Survival of AML Patients with and Without Expression of a Prognostic Factor
3.1.2. Overall Survival of AML Patients with and Without Expression of the Two Prognostic Factors
3.2. AML Urayasu Classification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| IHC | Immunohistochemical staining |
| MRP1 | Multidrug resistance-associated protein 1 |
| AKR1B10 | Aldo-keto reductase family 1 member B10 |
| AKR1B1 | Aldo-keto reductase family 1 member B1 |
| AKR1C3 | Aldo-keto reductase family 1 member C3 |
| OS | Overall survival |
| ELN | European Leukemia Net |
| LBCL | Large B-cell lymphoma |
| TCL | Aggressive T-cell lymphoma |
| GRP94 | Glucose-regulated protein 94 |
| GRP78 | Glucose-regulated protein 78 |
| B-ALL | B-cell acute lymphoblastic leukemia |
| TGFβ1 | Transforming growth factor β1 |
| TNFα1 | Tumor necrosis factor α1 |
| TNFR | Tumor necrosis factor receptor |
| PD-1 | Programmed cell death-1 |
| PD-L1 | Programmed cell death–ligand 1 |
| ENT1 | Equilibrative nucleoside transporter 1 |
| MDR1 | Multidrug resistance 1 |
| MRP1 | Multidrug resistance-associated protein 1 |
| CYP3A4 | Cytochrome P450 3A4 |
| FLT3 | fms related receptor tyrosine kinase 3 |
| TKI | Tyrosin kinase inhibitor |
| CYP2B6 | Cytochrome P450 2B6 |
| AKR1C3 | Aldo-keto reductase family 1 member C3 |
| AKR1B10 | Aldo-keto reductase family 1 member B10 |
| AKR1B1 | Aldo-keto reductase family 1 member B1 |
| TP | Thymidine phosphorylase |
| GST | Glutathione sulfate transferase |
| APL | Acute promyeloid leukemia |
| CBF | Core binding factor |
| CR | Complete remission |
| PD | Progressive disease |
| M | Months |
| NS | Not significant |
| NR | Not reached |
| MCL1 | Myeloid cell leukemia sequence 1 |
| Y | Years |
| ER | Endoplasmic reticulum |
| BCL2 | B-cell/CLL lymphoma 2 |
| Del | Deletion |
| MTX | Methotrexate |
| C | Cyclophosphamide |
| OH | Oncovin hydroxyl doxorubicin; |
| HCCFA | 7-hydroxy-2-(4-methoxyphenylimino)-2H-chromene-3-carboxylic acid benzylamide |
| NSAIDs | N-phenyl-anthranilic acid, diclofenac, and glycyrrhetinic acid |
References
- Bin Emran, T.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Takizawa, H.; Mitsumori, T.; Iizuka-Honma, H.; Araki, Y.; Fujishiro, M.; Tomita, S.; Kishikawa, S.; Hashizume, A.; Sawada, T.; et al. Possible New Histological Prognostic Index for Large B-Cell Lymphoma. J. Clin. Med. 2023, 12, 6324. [Google Scholar] [CrossRef] [PubMed]
- Nitta, H.; Takizawa, H.; Mitsumori, T.; Iizuka-Honma, H.; Ochiai, T.; Furuya, C.; Araki, Y.; Fujishiro, M.; Tomita, S.; Hashizume, A.; et al. A New Histology-Based Prognostic Index for Aggressive T-Cell lymphoma: Preliminary Results of the “TCL Urayasu Classification”. J. Clin. Med. 2024, 13, 3870. [Google Scholar] [CrossRef] [PubMed]
- Metzeler, K.H.; Herold, T.; Rothenberg-Thurley, M.; Amler, S.; Sauerland, M.C.; Görlich, D.; Schneider, S.; Konstandin, N.P.; Dufour, A.; Bräundl, K.; et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 2016, 128, 686–698. [Google Scholar] [CrossRef]
- Koschade, S.E.; Stratmann, J.A.; Finkelmeier, F.; Wagner, S.; Chromik, J.; Steffen, B.; Serve, H.; Brandts, C.H.; Ballo, O. Relapse surveillance of acute myeloid leukemia patients in first remission after consolidation chemotherapy: Diagnostic value of regular bone marrow aspirations. Ann. Hematol. 2022, 101, 1703–1710. [Google Scholar] [CrossRef]
- Duan, X.; Iwanowycz, S.; Ngoi, S.; Hill, M.; Zhao, Q.; Liu, B. Molecular Chaperone GRP94/GP96in Cancers: Oncogenesis and Therapeutic Target. Front. Oncol. 2021, 11, 629846. [Google Scholar] [CrossRef]
- Lee, A.S. Glucose-regulated proteins in cancer: Molecular mechanisms and therapeutic potential. Nat. Rev. Cancer 2014, 14, 263–276. [Google Scholar] [CrossRef]
- Akinyemi, A.O.; Simpson, K.E.; Oyelere, S.F.; Nur, M.; Ngule, C.M.; Owoyemi, B.C.D.; Ayarick, V.A.; Oyelami, F.F.; Obaleye, O.; Esoe, D.-P.; et al. Unveiling the dark side of glucose-regulated protein 78 (GRP78) in cancers and other human pathology: A systematic review. Mol. Med. 2023, 29, 112. [Google Scholar] [CrossRef]
- Angeles-Floriano, T.; Rivera-Torruco, G.; García-Maldonado, P.; Juárez, E.; Gonzalez, Y.; Parra-Ortega, I.; Vilchis-Ordoñez, A.; Lopez-Martinez, B.; Arriaga-Pizano, L.; Orozco-Ruíz, D.; et al. Cell surface expression of GRP78 and CXCR4 is associated with childhood high-risk acute lymphoblastic leukemia at diagnostics. Sci. Rep. 2022, 12, 2322. [Google Scholar] [CrossRef]
- Hebbar, N.; Epperly, R.; Vaidya, A.; Thanekar, U.; Moore, S.E.; Umeda, M.; Ma, J.; Patil, S.L.; Langfitt, D.; Huang, S.; et al. CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat. Commun. 2022, 13, 587. [Google Scholar] [CrossRef]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Timmins, M.A.; Ringshausen, I. Transforming Growth Factor-Beta Orchestrates Tumour and Bystander Cells in B-Cell Non-Hodgkin Lymphoma. Cancers 2022, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Takizawa, H.; Araki, Y.; Fujishiro, M.; Tomita, S.; Kishikawa, S.; Hashizume, A.; Mitsumori, T.; Nitta, H.; Iizuka-Honma, H.; Sawada, T.; et al. Role of TGF-beta1 and TNF-alpha1 produced by neoplastic cells in the pathogenesis of fibrosis in patients with hematologic neoplasms. J. Clin. Exp. Hematop. 2023, 63, 83–89. [Google Scholar] [CrossRef]
- Prada, J.P.; Wangorsch, G.; Kucka, K.; Lang, I.; Dandekar, T.; Wajant, H. A systems-biology model of the tumor necrosis factor (TNF) interactions with TNF receptor 1 and 2. Bioinformatics 2021, 37, 669–676. [Google Scholar] [CrossRef]
- Vinante, F.; Rigo, A.; Tecchio, C.; Morosato, L.; Nadali, G.; Ricetti, M.M.; Krampera, M.; Zanolin, E.; Locatelli, F.; Gallati, H.; et al. Serum levels of p55 and p75 soluble TNF receptors in adult acute leukaemia at diagnosis: Correlation with clinical and biological features and outcome. Br. J. Haematol. 1998, 102, 1025–1034. [Google Scholar] [CrossRef]
- Cisterne, A.; Baraz, R.; Khan, N.I.; Welschinger, R.; Basnett, J.; Fung, C.; Rizos, H.; Bradstock, K.F.; Bendall, L.J. Silencer of death domains controls cell death through tumour necrosis factor-receptor 1 and caspase-10 in acute lymphoblastic leukemia. PLoS ONE 2014, 9, e103383. [Google Scholar] [CrossRef]
- Cao, H.; Wu, T.; Zhou, X.; Xie, S.; Sun, H.; Sun, Y.; Li, Y. Progress of research on PD-1/PD-L1 in leukemia. Front. Immunol. 2023, 14, 1265299. [Google Scholar] [CrossRef]
- Geduk, A.; Atesoglu, E.B.; Mehtap, O.; Demirsoy, E.T.; Menguc, M.U.; Tarkun, P.; Hacihanefioglu, A.; Balcı, S. Programmed Cell Death Ligand 1 Expression Level and Prognostic Significance in Acute Myeloid Leukemia. Indian J. Hematol. Blood Transfus. 2022, 38, 464–472. [Google Scholar] [CrossRef]
- Mortensen, J.B.; Monrad, I.; Enemark, M.B.; Ludvigsen, M.; Kamper, P.; Bjerre, M.; D’Amore, F. Soluble programmed cell death protein 1 (sPD-1) and the soluble programmed cell death ligands 1 and 2 (sPD-L1 and sPD-L2) in lymphoid malignancies. Eur. J. Haematol. 2021, 107, 81–91. [Google Scholar] [CrossRef]
- Candelaria, M.; Corrales-Alfaro, C.; Gutiérrez-Hernández, O.; Díaz-Chavez, J.; Labardini-Méndez, J.; Vidal-Millán, S.; Herrera, L.A. Expression Levels of Human Equilibrative Nucleoside Transporter 1 and Deoxycytidine Kinase Enzyme as Prognostic Factors in Patients with Acute Myeloid Leukemia Treated with Cytarabine. Chemotherapy 2016, 61, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Júnior, L.S.d.S.; Soares, V.d.L.; da Silva, A.S.J.; Gil, E.A.; Araújo, M.d.G.P.d.; Gonçalves, C.A.M.; Paiva, A.d.S.; de Oliveira, T.M.M.; Oliveira, G.H.d.M.; e Silva, D.G.K.C.; et al. P-glycoprotein and multidrug resistance-associated protein-1 expression in acute myeloid leukemia: Biological and prognosis implications. Int. J. Lab. Hematol. 2020, 42, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Kunadt, D.; Dransfeld, C.; Dill, C.; Schmiedgen, M.; Kramer, M.; Altmann, H.; Röllig, C.; Bornhäuser, M.; Mahlknecht, U.; Schaich, M.; et al. Multidrug-related protein 1 (MRP1) polymorphisms rs129081, rs212090, and rs212091 predict survival in normal karyotype acute myeloid leukemia. Ann. Hematol. 2020, 99, 2173–2180. [Google Scholar] [CrossRef]
- Copsel, S.; Bruzzone, A.; May, M.; Beyrath, J.; Wargon, V.; Cany, J.; Russel, F.G.; Shayo, C.; Davio, C. Multidrug resistance protein 4/ATP binding cassette transporter 4: A new potential therapeutic target for acute myeloid leukemia. Oncotarget 2014, 5, 9308–9321. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Hernandez, D.; Alonso, S.; Gao, M.; Su, M.; Ghiaur, G.; Levis, M.J.; Jones, R.J. Role of CYP3A4 in bone marrow microenvironment-mediated protection of FLT3/ITD AML from tyrosine kinase inhibitors. Blood Adv. 2019, 3, 908–916. [Google Scholar] [CrossRef]
- Xiong, X.; Yu, D.; Gao, Q.; Zhang, Y.; Yin, Q.; Chen, X.; Xiao, H.; Tong, R. Association between CYP2B6 c.516G >T variant and acute leukaemia. A protocol for systematic review and meta-analysis. Medicine 2021, 100, e26740. [Google Scholar] [CrossRef]
- Verma, K.; Zang, T.; Penning, T.M.; Trippier, P.C. Potent and Highly Selective Aldo-Keto Reductase 1C3 (AKR1C3) Inhibitors Act as Chemotherapeutic Potentiators in Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. J. Med. Chem. 2019, 62, 3590–3616. [Google Scholar] [CrossRef]
- Laffin, B.; Petrash, J.M. Expression of the aldo-ketoreductases AKR1B1 and AKR1B10inhumancancers. Front. Pharmacol. 2012, 3, 104. [Google Scholar] [CrossRef]
- Penning, T.M.; Jonnalagadda, S.; Trippier, P.C.; Rižner, T.L. Aldo-Keto Reductases and Cancer Drug Resistance. Pharmacol. Rev. 2021, 73, 1150–1171. [Google Scholar] [CrossRef]
- Büküm, N.; Novotná, E.; Morell, A.; Želazková, J.; Laštovičková, L.; Čermáková, L.; Portillo, R.; Solich, P.; Wsól, V. Inhibition of AKR1B10-mediated metabolism of daunorubicin as a novel off-target effect for the Bcr-Abl tyrosine kinase inhibitor dasatinib. Biochem. Pharmacol. 2021, 192, 114710. [Google Scholar] [CrossRef]
- Nie, X.; Clifford, P.M.; Bhat, R.; Heintzelman, R.; Abraham, M.; Hou, J.S. Thymidine phosphorylase expression in B-cell lymphomas and its significance: A new prognostic marker? Anal. Quant. Cytopathol. Histpathol. 2013, 35, 301–305. [Google Scholar] [PubMed]
- Anensen, N.; Oyan, A.M.; Bourdon, J.-C.; Kalland, K.H.; Bruserud, O.; Gjertsen, B.T. A distinct p53 protein isoform signature reflects the onset of induction chemotherapy for acute myeloid leukemia. Clin. Cancer Res. 2006, 12, 3985–3992. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Cheng, A.; Guirales, F.; Yeh, W.; Tirado, C.A. c-MYC Amplification in AML. J. Assoc. Genet. Technol. 2021, 47, 202–212. [Google Scholar]
- Davies, S.M.; Robison, L.L.; Buckley, J.D.; Tjoa, T.; Woods, W.G.; Radloff, G.A.; Ross, J.A.; Perentesis, J.P. Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia. J. Clin. Oncol. 2001, 19, 1279–1287. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Otsuki, A.; Kumondai, M.; Kobayashi, D.; Kikuchi, M.; Ueki, Y.; Sato, Y.; Hayashi, N.; Yagi, A.; Onishi, Y.; Onodera, K.; et al. Plasma Venetoclax Concentrations in Patients with Acute Myeloid Leukemia Treated with CYP3A4 Inhibitors. Yakugaku Zasshi 2024, 144, 775–779. [Google Scholar] [CrossRef]
- Wei, A.H.; Roberts, A.W.; Spencer, A.; Rosenberg, A.S.; Siegel, D.; Walter, R.B.; Caenepeel, S.; Hughes, P.; McIver, Z.; Mezzi, K.; et al. Targeting MCL-1 in hematologic malignancies: Rationale and progress. Blood Rev. 2020, 44, 100672. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Endo, S.; Xia, S.; Suyama, M.; Morikawa, Y.; Oguri, H.; Hu, D.; Ao, Y.; Takahara, S.; Horino, Y.; Hayakawa, Y.; et al. Synthesis of Potent and Selective Inhibitors of Aldo-Keto Reductase 1B10 and Their Efficacy against Proliferation, Metastasis, and Cisplatin Resistance of Lung Cancer Cells. J. Med. Chem. 2017, 60, 8441–8455. [Google Scholar] [CrossRef]
- Kikuya, M.; Furuichi, K.; Hirao, T.; Endo, S.; Toyooka, N.; Ito, K.; Aoki, S. Aldo-keto reductase inhibitors increase the anticancer effects of tyrosine kinase inhibitors in chronic myelogenous leukemia. J. Pharmacol. Sci. 2021, 147, 1–8. [Google Scholar] [CrossRef]
- Endo, S.; Matsunaga, T.; Soda, M.; Tajima, K.; Zhao, H.-T.; El-Kabbani, O.; Hara, A. Selective inhibition of the tumor marker AKR1B10 by antiinflammatory N-phenylanthranilic acids and glycyrrhetic acid. Biol. Pharm. Bull. 2010, 33, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Kamil, S.; Kouser, S.; Naeem, N.; Farroqui, W.; Sharafat, S.; Khan, F.A.; Haider, G.; Kamil, N.; Ali, R.; Abbass, F.F.; et al. Unveiling the Significance of MRP 1 in Achieving Complete Remission Following Induction Therapy in Acute Myeloid Leukemia. Curr. Mol. Med. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Kuss, B.J.; Deeley, R.G.; Cole, S.P.C.; Willman, C.L.; Kopecky, K.J.; Wolman, S.R.; Eyre, H.J.; Callen, D.F. The biological significance of the multidrug resistance gene MRP in inversion 16 leukemias. Leuk. Lymphoma 1996, 20, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Xu, D.; Zhang, T.; Hu, W.; Feng, Z. Gain-of-function mutant p53 in cancer progression and therapy. J. Mol. Cell. Biol. 2020, 12, 674–687. [Google Scholar] [CrossRef]
- Hanssen, K.M.; Haber, M.; Fletcher, J.I. Targeting multidrug resistance-associated protein 1 (MRP1)-expressing cancers: Beyond pharmacological inhibition. Drug Resist. Updat. 2021, 59, 100795. [Google Scholar] [CrossRef]
- Tivnan, A.; Zakaria, Z.; O’Leary, C.; Kögel, D.; Pokorny, J.L.; Sarkaria, J.N.; Prehn, J.H.M.; Kögel, D. Inhibition of multidrug resistance protein 1 (MRP1) improves chemotherapy drug response in primary and recurrent glioblastoma multiforme. Front. Neurosci. 2015, 9, 218. [Google Scholar] [CrossRef]

| Items | Count |
|---|---|
| Age > 60 years (%) | 13 (37%) |
| Male (%) | 20 (57%) |
| Chromosome | |
| CBF t(8;21) (q22;q22) n = 7, inv(16) (p12q22) n = 1 | 8 (23%) |
| Normal | 10 (29%) |
| Complex chromosome | 8 (23%) |
| Chromosome 7 deletion | 9 (26%) |
| European Leukemia Net | |
| Favorable | 12 (34%) |
| Intermediate | 13 (37%) |
| Adverse | 10 (29%) |
| Induction chemotherapy | |
| Idarubicin + cytarabine | 35 (100%) |
| Outcome | |
| CR | 27 (77%) |
| Non-relapse | 18 (51%) |
| Relapse | 9 (26%) |
| PD | 8 (23%) |
| Allogeneic transplantation | 10 (29%) |
| Category | Factors (♯ Significant Difference:) | n | Median OS (Months) | Years (Y) Survival Rate | p Value | Figure |
|---|---|---|---|---|---|---|
| Total | AML | 35 | NR | 5Y 73% | 1A | |
| ELN | ELN Favorable group | 12 | NR | 5Y 93% | NS | |
| ELN Intermediate group | 13 | NR | 5Y76% | NS | ||
| ELN Adverse group | 10 | NR | 5Y72% | NS | 1B | |
| Chromosome abnormality | Deletion chromosome 7 | 9 | NR | 5Y78% | NS | |
| Complex chromosome | 8 | NR | 5Y73% | NS | ||
| 12 | 74 | 5Y62% | NS | |||
| ER stress proteins | GRP94 | 33 | NR | 5Y73% | NS | |
| TGFβ1 | 27 | 63 | 5Y90% | NS | ||
| GRP78 | 30 | NR | 5Y90% | NS | ||
| TNFα1 | 20 | NR | 5Y82% | NS | ||
| OH metabolic enzyme | AKR1C3 | 16 | NR | 5Y68% | NS | |
| AKR1B1 | 4 | NR | 5Y72% | NS | 1F | |
| AKR1B10 (♯) | 17 | NR | 5Y63% | * p < 0.05 | 1E | |
| C metabolic enzyme | CYP2B6 | 0 | ||||
| CHOP metabolic enzyme | CYP3A4 | 5 | NR | 3Y73% | ||
| OH efflux pump | MDR1 | 5 | NR | 3Y72% | ||
| MRP1 (♯) | 1 | 12 | 1Y0% | * p < 0.01 | 1D | |
| MTX efflux pump | MRP4 | 0 | NS | |||
| Immune checkpoint | PD-1 | 0 | NS | |||
| PD-L1 | 1 | NR | 5Y100% | NS | ||
| PD-L2 | 20 | 74 | 5Y74% | NS | ||
| Others | TP | 4 | 74 | 5Y100% | NS | |
| p53 (♯) | 3 | 14 | 2Y0% | * p < 0.01 | 1C | |
| GST | 28 | NR | 5Y88% | NS | ||
| MYC | 28 | 74 | 5Y82% | NS | ||
| ENT-1 | 31 | NR | 5Y88% | NS | ||
| Fibrosis (Silver stain positive) | 11 | NR | 5Y74% | p > 0.05 | ||
| BCL2 | 28 | 74 | 5Y84% | p > 0.05 | ||
| MCL1 | 16 | NR | 5Y88% | p > 0.05 | ||
| Significant combination | ||||||
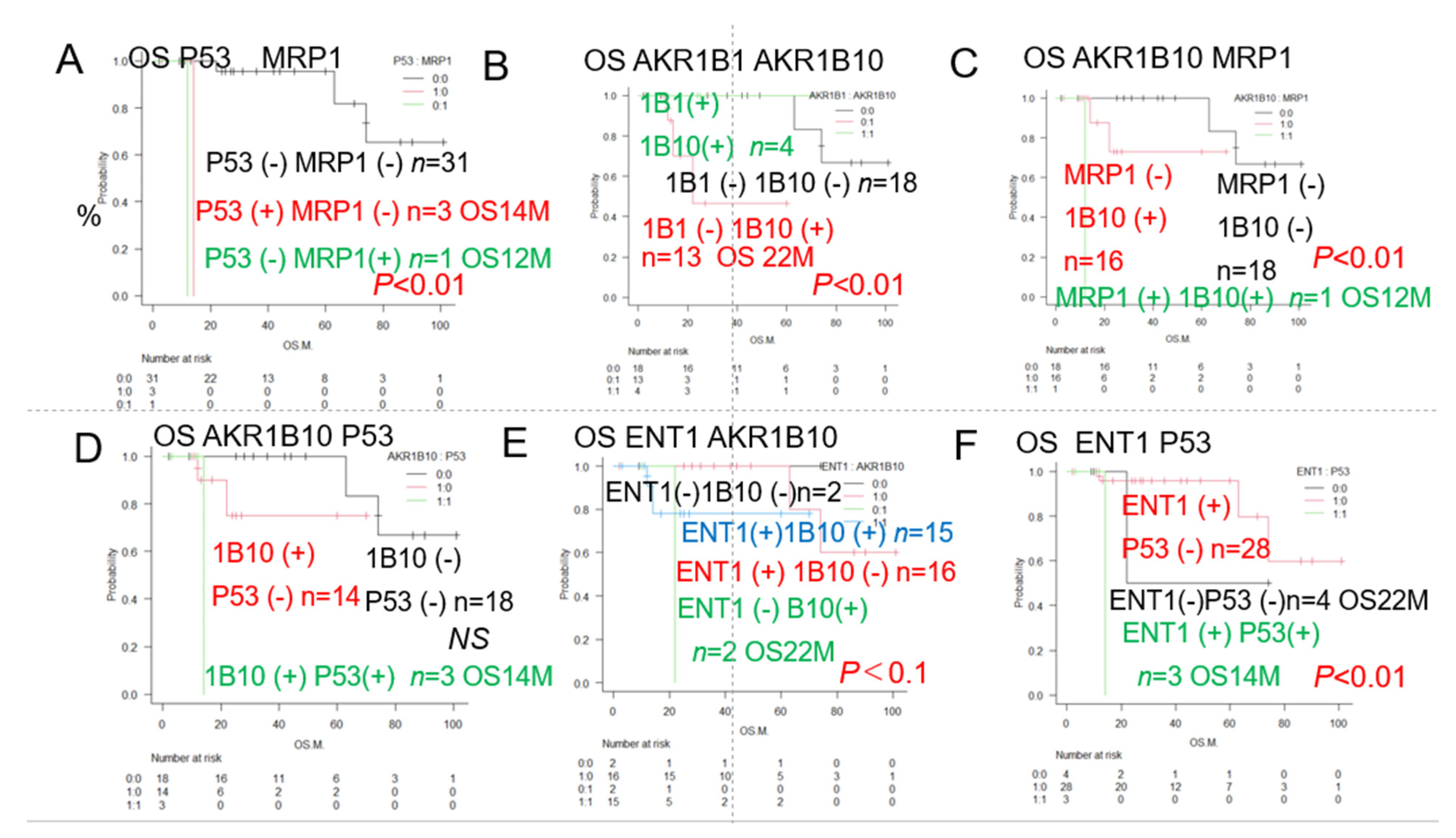
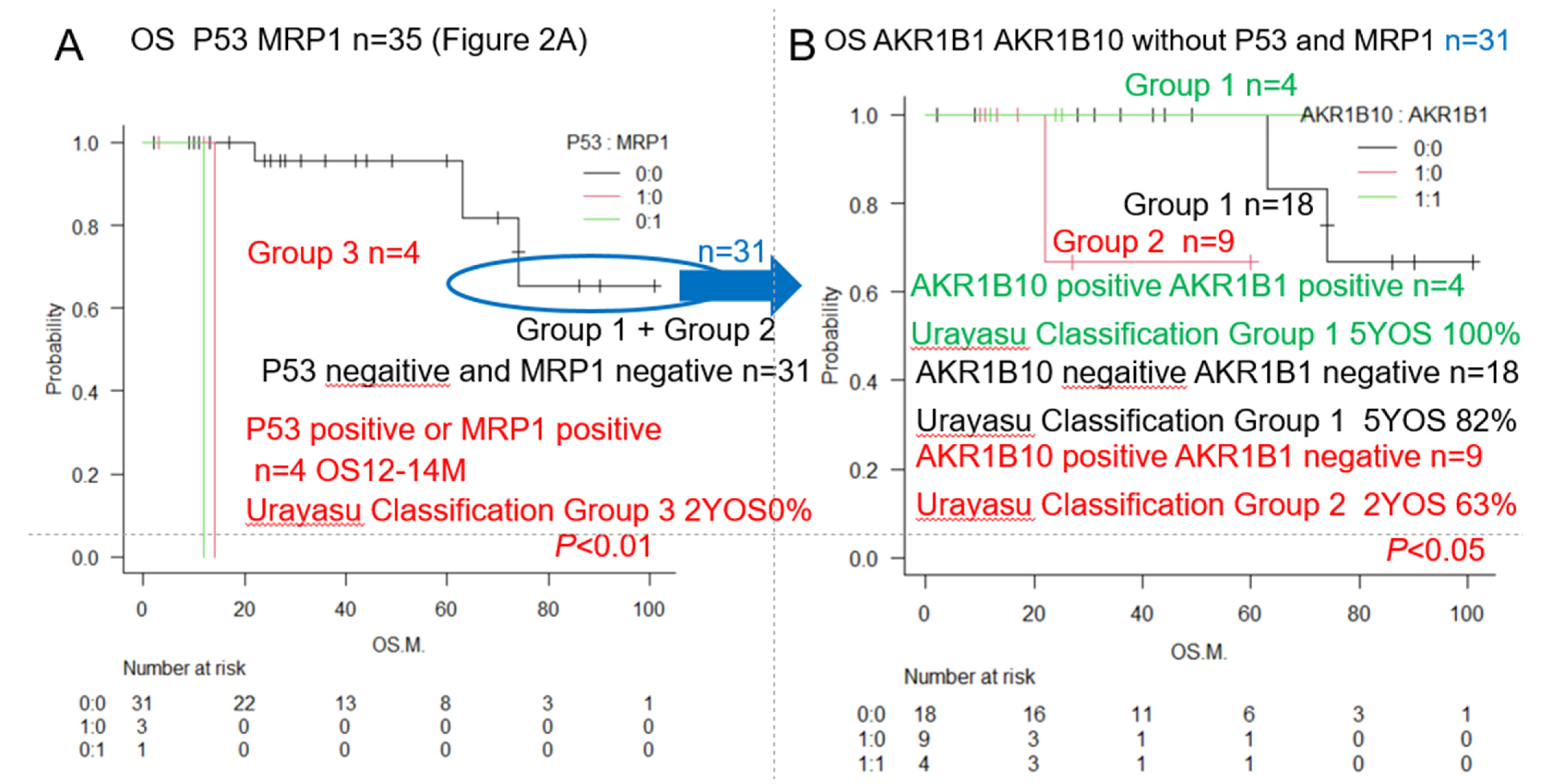
| Urayasu classification G3 | P53(+) or MRP1(+) (♯) | 4 | 13 | 2Y0% | ** p < 0.01 | 2A, 3, 5 |
| Urayasu classification G2 | P53(-) MRP1(-) AKR1B10(+) 1B1(-) (♯) | 9 | NR | 2Y63% | * p < 0.05 | 3, 5 |
| Urayasu classification G1 | P53(-) MRP1(-) AKR1B10(-) 1B1(-) (♯) | 22 | NR | 5Y82% | * p < 0.05 | 3, 5 |
| Urayasu classification G1 | P53(-) MRP1(-) AKR1B10(+) 1B1(+) (♯) | 3 | NR | 5Y100% | * p < 0.05 | 3, 5 |
| P53□ENT1 (♯) P53(+) ENT1(+)□ | 3 | 14 | 2Y 0% | ** p < 0.01 | 2F | |
| MRP1 ENT1 (♯) MRP1(+) ENT1(+) | 1 | 12 | 2Y 0% | ** p < 0.01 | ||
| AKR1B10, AKR1B1 (♯) 1B10(+) 1B1(-) | 13 | 22 | 5Y 44% | ** p < 0.01 | 2B | |
| MRP1, AKR1B10□(♯) MRP1(+) 1B10(+) | 1 | 12 | 2Y 0% | ** p < 0.01 | 2C | |
| P53, BCL2 (♯) P53(+) BCL2(+) | 3 | 14 | 2Y 0% | * p < 0.05 | ||
| P53, MCL1 (♯) P53(+) MCL1(+) | 3 | 14 | 2Y0% | ** p < 0.01 | ||
| P53, PD-L1 (♯) P53(+) PD-L1(-) | 2 | 14 | 2Y0% | ** p < 0.01 | ||
| P53, PD-L2 (♯) P53(+) PD-L2 (+) | 3 | 14 | 2Y0% | * p < 0.05 | ||
| P53, CYP3A4 (♯) P53(+) CYP3A4(+) | 1 | NR | NR | ** p < 0.01 | ||
| P53, GRP78 (♯) P53(+) GRP78(+) | 3 | 14 | 2Y0% | ** p < 0.01 | ||
| P53, GRP94 (♯) P53,(+) GRP94(+) | 3 | 14 | 2Y0% | ** p < 0.01 | ||
| P53, AKR1C3 (♯) P53(+) AKR1C3(+) | 2 | 14 | 2Y0% | ** p < 0.01 | ||
| P53, TGF beta1 (♯) P53(+) TGF beta1(+) | 3 | 14 | 2Y0% | * p < 0.05 | ||
| P53, MYC (♯) P53(+) MYC(+) | 3 | 14 | 2Y0% | * p < 0.05 | ||
| P53, GST (♯) P53(+) GST(+) | 1 | NR | NR | * p < 0.05 | ||
| Combinations with a tendancy towards association with the OS | ||||||
| Del 7, AKRB10(♯) Del 7(+) 1B10(+) | 6 | 14 | 2Y 0% | NS | ||
| BCL2, MCL1 | 23 | NS | ||||
| AKR1B10, P53 | 35 | NS | ||||
| ENT1, AKR1B10 | NS | 2E | ||||
| P53, AKR1B10 P53(+) AKR1B10(+) | 3 | 14 | 2Y 0% | NS | 2D |
| Classification | Group 1 (Favorable) | Group 2 (Intermediate) | Group 3 (Adverse) | Figure |
|---|---|---|---|---|
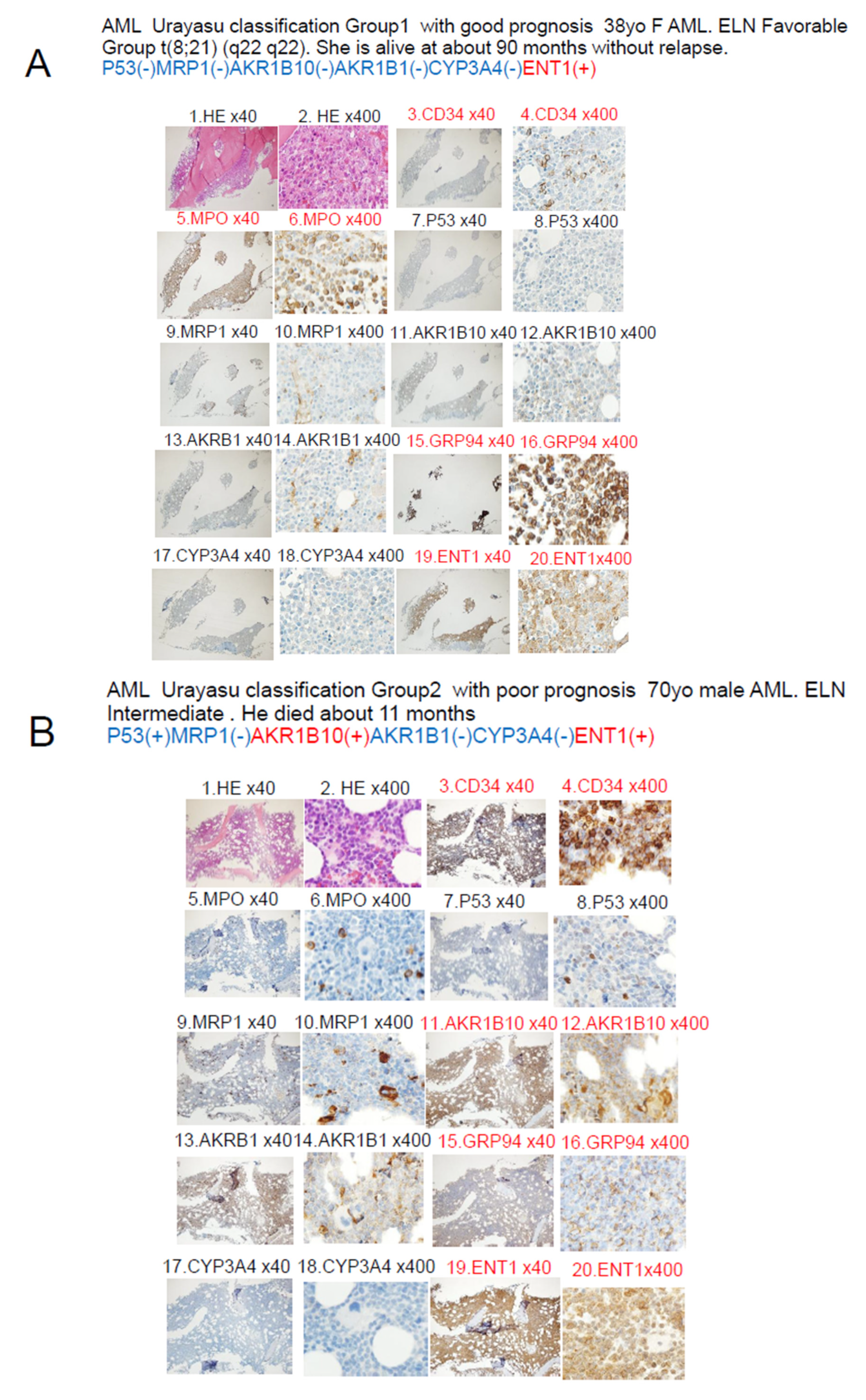
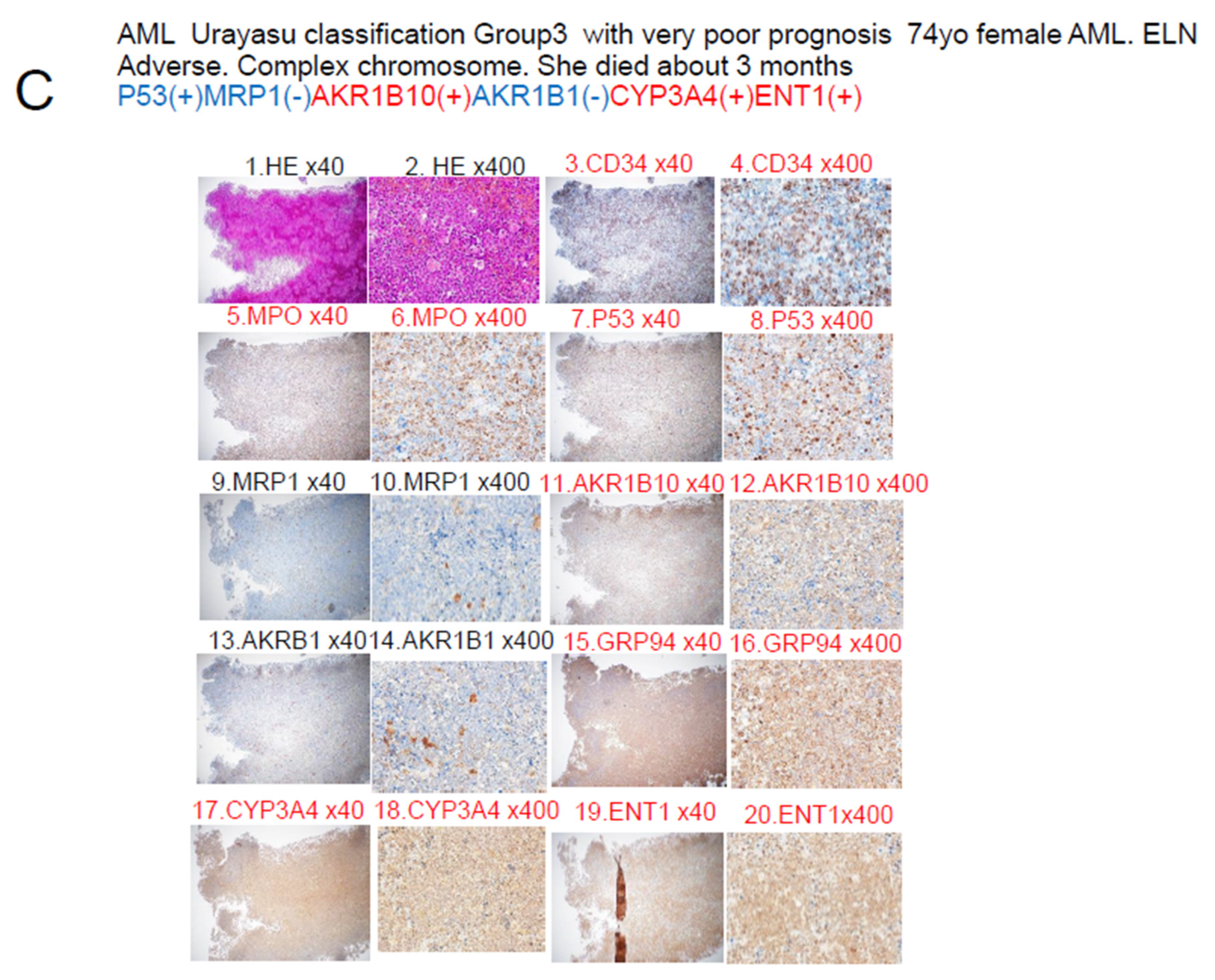
| AML Urayasu | P53(-)MRP(-)AKR1B10(+) | P53(-)MRP(-)AKR1B10(+) | P53(+) or MRP1(+) | 1C–F |
| Classification | AKR1B1(+) or | AKR1B1(-) | □ | 2AB, 3AB |
| □ | P53(-)MRP(-)AKR1B10(-) | □ | □ | □ |
| □ | AKR1B1(-) | □ | □ | □ |
| □ | Cases n = 22 (63%) | Cases n = 9 (26%) | Cases n = 4 (11%) | □ |
| □ | OS 5 Y 82%-100% | OS 2 Y 63% | OS 2 Y 0% | □ |
| □ | Median OS NR | Median OS NR | Median OS 12–14 M | □ |
| ELN AML risk | Cases n = 562 (37%) | Cases n = 355 (23%) | Cases n = 616 (40%) | 1B |
| Classification | OS 5 Y 50% | OS 5 Y 20% | OS 5 Y 8% | □ |
| □ | Median OS 4 Y | Median OS 15 M | Median OS 10 M | □ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsumori, T.; Nitta, H.; Takizawa, H.; Iizuka-Honma, H.; Furuya, C.; Fujishiro, M.; Tomita, S.; Hashizume, A.; Sawada, T.; Miyake, K.; et al. A New Histology-Based Prognostic Index for Acute Myeloid Leukemia: Preliminary Results for the “AML Urayasu Classification”. J. Clin. Med. 2025, 14, 1989. https://doi.org/10.3390/jcm14061989
Mitsumori T, Nitta H, Takizawa H, Iizuka-Honma H, Furuya C, Fujishiro M, Tomita S, Hashizume A, Sawada T, Miyake K, et al. A New Histology-Based Prognostic Index for Acute Myeloid Leukemia: Preliminary Results for the “AML Urayasu Classification”. Journal of Clinical Medicine. 2025; 14(6):1989. https://doi.org/10.3390/jcm14061989
Chicago/Turabian StyleMitsumori, Toru, Hideaki Nitta, Haruko Takizawa, Hiroko Iizuka-Honma, Chiho Furuya, Maki Fujishiro, Shigeki Tomita, Akane Hashizume, Tomohiro Sawada, Kazunori Miyake, and et al. 2025. "A New Histology-Based Prognostic Index for Acute Myeloid Leukemia: Preliminary Results for the “AML Urayasu Classification”" Journal of Clinical Medicine 14, no. 6: 1989. https://doi.org/10.3390/jcm14061989
APA StyleMitsumori, T., Nitta, H., Takizawa, H., Iizuka-Honma, H., Furuya, C., Fujishiro, M., Tomita, S., Hashizume, A., Sawada, T., Miyake, K., Okubo, M., Sekiguchi, Y., Ando, M., & Noguchi, M. (2025). A New Histology-Based Prognostic Index for Acute Myeloid Leukemia: Preliminary Results for the “AML Urayasu Classification”. Journal of Clinical Medicine, 14(6), 1989. https://doi.org/10.3390/jcm14061989








