Advancements in Regenerative Therapies for Orthopedics: A Comprehensive Review of Platelet-Rich Plasma, Mesenchymal Stem Cells, Peptide Therapies, and Biomimetic Applications
Abstract
:1. Introduction
2. Methods
2.1. Clinical Study Selection
2.2. Risk of Bias Assessment
2.3. Clinical Study Data
2.4. Statistical Analysis
3. Results
3.1. Platelet-Rich Plasma (PRP)
3.2. Mesenchymal Stem Cell Therapies
3.3. Peptide Therapies
3.4. Biomimetic Applications
3.5. Comparative Analysis of Regenerative Therapies
3.6. Comparative Statistical Analysis
4. Discussion
4.1. Platelet-Rich Plasma (PRP)
4.2. Mesenchymal Stem Cell (MSC) Therapies
4.3. Peptide-Based Therapies
4.4. Biomimetic Applications
4.5. Limitations and Challenges
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Choi, Y.S.; Kim, T.W.; Chang, M.J.; Kang, S.-B.; Chang, C.B. Enhanced Recovery After Surgery for Major Orthopedic Surgery: A Narrative Review. Knee Surg. Relat. Res. 2022, 34, 8. [Google Scholar] [CrossRef]
- Islam, M.T.; Bulut, D.; Sharabidze, Z. Regenerative Medicine in Orthopaedic Surgery: Pioneering Advances and Their Applications. Innovations 2025, 9, 82–94. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Chen, H.; Li, X.; Zhang, J. Platelet-Rich Plasma in Orthopedics: Bridging Innovation and Clinical Applications for Bone Repair. J. Orthop. Surg. 2024, 32, 10225536231224952. [Google Scholar] [CrossRef]
- Liebig, B.E.; Lipsky, B.A.; Maher, A.B.; Wu, C.; Millikan, K.W. The Platelet-Rich Plasma and Mesenchymal Stem Cell Milieu: A Review of Therapeutic Effects on Bone Healing. J. Orthop. Res. 2020, 38, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, Y.; Wang, W.; Li, L.; Chen, J. A Biomimetic Platelet-Rich Plasma-Based Interpenetrating Network Printable Hydrogel for Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 887454. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.K.; Meena, S. Emerging trends in PRP and MSC-based therapies: A review of clinical efficacy and limitations. Int. J. Musculoskelet. Med. 2024, 12, 85–98. [Google Scholar]
- Hosoyama, K.; Lazurko, C.; Muñoz, M.; McTiernan, C.D.; Alarcon, E.I. Peptide-based functional biomaterials for soft-tissue repair. Front. Bioeng. Biotechnol. 2019, 7, 205. [Google Scholar] [CrossRef]
- Gupta, A.; Potty, A.G.; Maffulli, N. Regenerative biologics for musculoskeletal injuries. Front. Pain. Res. 2024, 5, 1400548. [Google Scholar] [CrossRef]
- Hsu, W.K.; Mishra, A.; Rodeo, S.R.; Fu, F.; Terry, M.A.; Randelli, P.; Canale, S.T.; Kelly, F.B. Platelet-rich plasma in orthopaedic applications: Evidence-based recommendations for treatment. J. Am. Acad. Orthop. Surg. 2013, 21, 739–748. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-Rich Plasma (PRP): What Is PRP and What Is Not PRP? Implant. Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef]
- Gill, S.S.; Cenci, G.; Falcinelli, S.; Marzano, F.; Carriero, B.; Filippi, N.; Pace, V. Platelet-rich plasma and anterior cruciate ligament repair: A new frontier, or a short-term adjunct. World J. Orthop. 2025, 16, 100693. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-rich plasma: New performance understandings and therapeutic considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Q.; Xu, Y.; He, H. Platelet-rich plasma treatment for talar cartilage repair: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jones, I.A.; Park, C.; Vangsness, C.T. The efficacy of platelet-rich plasma on tendon and ligament healing: A systematic review and meta-analysis with bias assessment. Am. J. Sports Med. 2018, 46, 2020–2032. [Google Scholar] [CrossRef]
- Kwong, C.A.; Woodmass, J.M.; Gusnowski, E.M.; Bois, A.J.; Leblanc, J.; More, K.D.; Lo, I.K.Y. Platelet-Rich Plasma in Patients With Partial-Thickness Rotator Cuff Tears or Tendinopathy Leads to Significantly Improved Short-Term Pain Relief and Function Compared With Corticosteroid Injection: A Double-Blind Randomized Controlled Trial. Arthroscopy 2021, 37, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.T.; Lim Fat, D.; Moran, C.J.; Mullett, H. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: A meta-analysis of randomized controlled trials. Am. J. Sports Med. 2019, 47, 753–761. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zampogna, B.; Parisi, F.R.; Ferrini, A.; Zampoli, A.; Papalia, G.F.; Shanmugasundaram, S.; Papalia, R. Safety and efficacy of autologous adipose-derived stem cells for knee osteoarthritis in the elderly population: A systematic review. J. Clin. Orthop. Trauma. 2024, 59, 102804. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498. [Google Scholar] [CrossRef]
- King, N.M.P.; Perrin, J. Ethical issues in stem cell research and therapy. Stem Cell Res. Ther. 2014, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, V.; Ghorbani, M.; Pooshang Bagheri, K.; Shokrgozar, M.A. Prevention of biofilm formation on orthopedic implants by melittin thin layer on chitosan/bioactive glass/vancomycin coatings. J. Mater. Sci. Mater. Med. 2021, 32, 75. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Park, Y.; Hahm, K.-S. The role of antimicrobial peptides in preventing multidrug-resistant bacterial infections and biofilm formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef]
- Costa, B.; Martínez-de-Tejada, G.; Gomes, P.A.C.; Martins, M.C.L.; Costa, F. Antimicrobial peptides in the battle against orthopedic implant-related infections: A review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Cushman, C.J.; Ibrahim, A.F.; Smith, A.D.; Hernandez, E.J.; MacKay, B.; Zumwalt, M. Local and systemic peptide therapies for soft tissue regeneration: A narrative review. Yale J. Biol. Med. 2024, 97, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2014, 42, 323–337. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Engineering cartilage tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Autologous protein solution injections for the treatment of knee osteoarthritis: 3-year results. Am. J. Sports Med. 2020, 48, 2703–2710. [Google Scholar] [CrossRef]
- Kon, E.; Engebretsen, L.; Verdonk, P.; Nehrer, S.; Filardo, G. Clinical outcomes of knee osteoarthritis treated with an autologous protein solution injection: A 1-year pilot double-blinded randomized controlled trial. Am. J. Sports Med. 2018, 46, 171–180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Rodriguez, J.E.; Williams, D.R.; Bürkner, P.-C. Heterogeneous Heterogeneity by Default: Testing Categorical Moderators in Mixed-Effects Meta-Analysis. Br. J. Math. Stat. Psychol. 2023, 76, 402–433. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.R.; Barendregt, J.J.; Khan, S.; Thalib, L.; Williams, G.M. Advances in the Meta-Analysis of Heterogeneous Clinical Trials II: The Quality Effects Model. Contemp. Clin. Trials 2015, 45, 123–129. [Google Scholar] [CrossRef]
- Paget, L.D.A.; Reurink, G.; de Vos, R.J.; Weir, A.; Moen, M.H.; Bierma-Zeinstra, S.M.A.; Stufkens, S.A.S.; Kerkhoffs, G.M.M.J.; Tol, J.L.; For the PRIMA Study Group. Effect of Platelet-Rich Plasma Injections vs Placebo on Ankle Symptoms and Function in Patients With Ankle Osteoarthritis: A Randomized Clinical Trial. JAMA 2021, 326, 1–12. [Google Scholar] [CrossRef]
- Wongjarupong, A.; Pairuchvej, S.; Laohapornsvan, P.; Kotheeranurak, V.; Jitpakdee, K.; Yeekian, C.; Chanplakorn, P. Platelet-Rich Plasma Epidural Injection: An Emerging Strategy in Lumbar Disc Herniation—A Randomized Controlled Trial. BMC Musculoskelet. Disord. 2023, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kearney, R.S.; Ji, C.; Warwick, J.; Parsons, N.; Brown, J.; Harrison, P.; Young, J.; Costa, M.L.; ATM Trial Collaborators. Effect of Platelet-Rich Plasma Injection vs Sham Injection on Tendon Dysfunction in Patients With Chronic Midportion Achilles Tendinopathy: A Randomized Clinical Trial. JAMA 2021, 326, 137–144. [Google Scholar] [CrossRef]
- Keene, D.J.; Alsousou, J.; Harrison, P.; O’Connor, H.M.; Wagland, S.; Dutton, S.J.; Hulley, P.; Lamb, S.E.; Willett, K. Platelet-rich plasma injection for acute Achilles tendon rupture: Two-year follow-up of the PATH-2 randomized, placebo-controlled, superiority trial. Bone Jt. J. 2022, 104-B, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.; Prabhu, R.M.; Jogani, A.; Mohanty, S.S.; Panchal, S.; Dakhode, S. Ultrasound-guided platelet-rich plasma injection versus steroid injection for lateral elbow tendinopathy: A prospective triple-blinded study with midterm follow-up. Clin. Orthop. Surg. 2023, 15, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Munde, S.L.; Jha, V.; Malik, J.S. Effectiveness of Platelet-Rich Plasma in the Treatment of Moderate Knee Osteoarthritis. Ann. Int. Med. Den. Res. 2017, 3, OR42–OR47. [Google Scholar] [CrossRef]
- Keene, D.J.; Alsousou, J.; Harrison, P.; Hulley, P.; Wagland, S.; Parsons, S.R.; Thompson, J.Y.; O’Connor, H.M.; Schlüssel, M.M.; Dutton, S.J.; et al. Platelet-rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo-controlled, superiority trial. BMJ 2019, 367, l6132. [Google Scholar] [CrossRef]
- Mohammadivahedi, F.; Sadeghifar, A.; Farsinejad, A.; Jambarsang, S.; Mirhosseini, H. Comparative efficacy of platelet-rich plasma (PRP) injection versus PRP combined with vitamin C injection for partial-thickness rotator cuff tears: A randomized controlled trial. J. Orthop. Surg. Res. 2024, 19, 426. [Google Scholar] [CrossRef]
- Rossi, L.A.; Gorodischer, T.D.; Camino, P.; Brandariz, R.N.; Tanoira, I.; Piuzzi, N.S.; Ranalletta, M. Leukocyte-poor platelet-rich plasma as an adjuvant to arthroscopic rotator cuff repair reduces the retear rate but does not improve functional outcomes: A double-blind randomized controlled trial. Am. J. Sports Med. 2024, 52, 1403–1410. [Google Scholar] [CrossRef]
- Reurink, G.; Goudswaard, G.J.; Moen, M.H.; Weir, A.; Verhaar JA, N.; Bierma-Zeinstra SM, A.; Maas, M.; Tol, J.L.; For the Dutch HIT-Study Investigators. Rationale, secondary outcome scores and 1-year follow-up of a randomised trial of platelet-rich plasma injections in acute hamstring muscle injury: The Dutch Hamstring Injection Therapy study. Br. J. Sports Med. 2015, 49, 1206–1212. [Google Scholar] [CrossRef]
- Yoshioka, T.; Arai, N.; Sugaya, H.; Taniguchi, Y.; Kanamori, A.; Gosho, M.; Okuno, K.; Kikuchi, N.; Hyodo, K.; Aoto, K.; et al. The Effectiveness of Leukocyte-Poor Platelet-Rich Plasma Injections for Symptomatic Mild to Moderate Osteoarthritis of the Knee with Joint Effusion or Bone Marrow Lesions in a Japanese Population: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Am. J. Sports Med. 2024, 52, 2493–2502. [Google Scholar] [CrossRef]
- Pitsilos, C.; Karachrysafi, S.; Fragou, A.; Gigis, I.; Papadopoulos, P.; Chalidis, B. The biological effect of platelet-rich plasma on rotator cuff tears: A prospective randomized in vivo study. Int. J. Mol. Sci. 2024, 25, 7957. [Google Scholar] [CrossRef]
- Xu, R.-D.; Li, J.-H.; Zhang, H.; Liang, H.-R.; Duan, S.-Y.; Sun, M.; Wen, H.; Zhou, X.-T.; Liu, H.-F.; Cai, Z.-C. The combined application of pulsed electromagnetic fields and platelet-rich plasma in the treatment of early-stage knee osteoarthritis: A randomized clinical trial. Medicine 2024, 103, e39369. [Google Scholar] [CrossRef] [PubMed]
- Hewavithana, P.B.; Wettasinghe, M.C.; Hettiarachchi, G.; Ratnayaka, M.; Suraweera, H.; Wickramasinghe, N.D.; Kumarasiri, P.V.R. Effectiveness of single intra-bursal injection of platelet-rich plasma against corticosteroid under ultrasonography guidance for shoulder impingement syndrome: A randomized clinical trial. Skelet. Radiol. 2024, 53, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Chen, H.; Qiao, Y.; Wu, C.; Cho, E.; Wu, X.; Li, Z.; Wu, J.; Lu, S.; Xie, G.; et al. Intra-articular platelet-rich plasma injection after anterior cruciate ligament reconstruction: A randomized clinical trial. JAMA Netw. Open 2024, 7, e2410134. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Lee, C.-L.; Chen, Y.-J.; Tien, Y.-C.; Lin, S.-Y.; Chen, C.-H.; Chou, P.P.-H.; Huang, H.-T. Comparing the efficacy of intra-articular single platelet-rich plasma (PRP) versus novel crosslinked hyaluronic acid for early-stage knee osteoarthritis: A prospective, double-blind, randomized controlled trial. Medicina 2022, 58, 1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yeo, S.M.; Noh, S.J.; Ha, C.-W.; Lee, B.C.; Lee, H.S.; Kim, S.J. Effect of Platelet-Rich Plasma on the Degenerative Rotator Cuff Tendinopathy According to the Compositions. J. Orthop. Surg. Res. 2019, 14, 408. [Google Scholar] [CrossRef]
- Annaniemi, J.A.; Pere, J.; Giordano, S. Platelet-rich plasma injections decrease the need for any surgical procedure for chronic epicondylitis versus conservative treatment—A comparative study with long-term follow-up. J. Clin. Med. 2023, 12, 102. [Google Scholar] [CrossRef]
- Zou, G.; Zheng, M.; Chen, W.; He, X.; Cang, D. Autologous platelet-rich plasma therapy for refractory pain after low-grade medial collateral ligament injury. J. Int. Med. Res. 2020, 48, 1–7. [Google Scholar] [CrossRef]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.J.F.C.; Schott, V.; Balduino, A.; Bastos, R.; Oliveira, J.M.; Reis, R.L.; Rodeo, S.; et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1989–1999. [Google Scholar] [CrossRef]
- Chen, C.-F.; Wu, P.-K.; Fu, Y.-S.; Tsai, S.-W.; Wu, C.-M.; Chen, W.-M.; Wu, H.-H.; Lee, C.-H.; Chang, C.-L.; Lin, P.-C.; et al. Safety and tolerability of intra-articular injection of adipose-derived mesenchymal stem cells GXCPC1 in 11 subjects with knee osteoarthritis: A nonrandomized pilot study without a control arm. Cell Transplant. 2024, 33, e103721. [Google Scholar] [CrossRef]
- Orozco Delclós, L.; Soler Rich, R.; Arriaza Loureda, R.; Moreno García, A.; Gómez Barrena, E. Efficacy and safety of autologous or allogeneic mesenchymal stromal cells from adult adipose tissue expanded and combined with tricalcium phosphate biomaterial for the surgical treatment of atrophic nonunion of long bones: A phase II clinical trial. J. Transl. Med. 2024, 22, 493. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, S.; Wu, J.; Guo, B.; Gao, T.; Shah SZ, A.; Huang, B.; Li, Y.; Zhu, B.; Fan, J.; et al. Immunity-and-matrix-regulatory cells enhance cartilage regeneration for meniscus injuries: A phase I dose-escalation trial. Signal Transduct. Target. Ther. 2023, 8, 417. [Google Scholar] [CrossRef]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? A clinical and magnetic resonance imaging study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-I.; Lee, M.C.; Lee, J.H.; Moon, Y.-W.; Lee, W.-S.; Lee, H.-J.; Hwang, S.-C.; In, Y.; Shon, O.-J.; Bae, K.-C.; et al. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Sports Med. 2023, 51, 1–11. [Google Scholar] [CrossRef]
- Liebergall, M.; Schroeder, J.; Mosheiff, R.; Gazit, Z.; Zilberman, Y.; Rasooly, L.; Daskal, A.; Khoury, A.; Weil, Y.; Beyth, S. Stem Cell–Based Therapy for Prevention of Delayed Fracture Union: A Randomized and Prospective Preliminary Study. Mol. Ther. 2013, 21, 1631–1638. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef]
- Park, Y.-B.; Ha, C.-W.; Lee, C.-H.; Yoon, Y.C.; Park, Y.-G. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: Results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Pintore, A.; Notarfrancesco, D.; Zara, A.; Oliviero, A.; Migliorini, F.; Oliva, F.; Maffulli, N. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: A prospective comparative clinical trial. J. Orthop. Surg. Res. 2023, 18, 350. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.S.; Cucchi, D.; Fossati, C.; Boerci, L.; Nocerino, E.; Ambrogi, F.; Menon, A. Arthroscopic Rotator Cuff Repair Augmentation With Autologous Microfragmented Lipoaspirate Tissue Is Safe and Effectively Improves Short-term Clinical and Functional Results: A Prospective Randomized Controlled Trial With 24-Month Follow-up. Am. J. Sports Med. 2022, 50, 1344–1357. [Google Scholar] [CrossRef]
- Toan, D.D.; Binh, N.T.; Dung, T.T.; Thuy, L.Q.; Hoa, N.D.; Long, N.H.; Tung, P.S. The effectiveness of knee osteoarthritis treatment by arthroscopic microfracture technique in combination with autologous bone marrow stem cells transplantation. J. Back. Musculoskelet. Rehabil. 2020, 33, 397–403. [Google Scholar] [CrossRef]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Vigano’, M.; Lanfranchi, L.; Alfieri Montrasio, U.; de Girolamo, L. Intratendinous Adipose-Derived Stromal Vascular Fraction (SVF) Injection Provides a Safe, Efficacious Treatment for Achilles Tendinopathy: Results of a Randomized Controlled Clinical Trial at a 6-Month Follow-Up. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Bao, R. Intra-articular mesenchymal stem cell injection for knee osteoarthritis: Mechanisms and clinical evidence. Int. J. Mol. Sci. 2023, 24, 59. [Google Scholar] [CrossRef]
- Widodo, W.; Hadisoebroto Dilogo, I.; Kamal, A.F.; Antarianto, R.D.; Wuyung, P.E.; Siregar, N.C.; Octaviana, F.; Kekalih, A.; Suroto, H.; Latief, W.; et al. Functional outcome and histologic analysis of late onset total type brachial plexus injury treated with intercostal nerve transfer to median nerve with local umbilical cord-derived mesenchymal stem cells or secretome injection: A double-blinded, randomized control study. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 4073–4082. [Google Scholar] [CrossRef]
- Chaverri, D.; Gallardo-Villares, S.; Pinto, J.A.; Rodríguez, L.; Codinach, M.; García-López, J.; Querol, S.; Coll, R.; Vives, J.; Granell-Escobar, F. Treatment of non-hypertrophic pseudoarthrosis of long bones with a Tissue Engineered Product loaded with autologous bone marrow-derived Mesenchymal Stromal Cells: Results from a phase IIa, prospective, randomized, parallel, pilot clinical trial comparing to iliac crest autograft. Injury 2024, 55, 111596. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: A phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.-M.; Soler-Rich, R.; Vadalà, G.; Ferreira, R.; Duflos, C.; Picot, M.-C.; Herman, F.; Broussous, S.; Sánchez, A.; Noriega, D.; et al. Allogenic bone marrow–derived mesenchymal stromal cell–based therapy for patients with chronic low back pain: A prospective, multicentre, randomised placebo-controlled trial (RESPINE study). Ann. Rheum. Dis. 2024, 83, 1572–1583. [Google Scholar] [CrossRef]
- Toosi, S.; Naderi-Meshkin, H.; Moradi, A.; Daliri, M.; Moghimi, V.; Majd, H.M.; Sahebkar, A.H.; Heirani-Tabasi, A.; Behravan, J. Scaphoid Bone Nonunions: Clinical and Functional Outcomes of Collagen/PGA Scaffolds and Cell-Based Therapy. ACS Biomater. Sci. Eng. 2023, 9, 1928–1939. [Google Scholar] [CrossRef]
- Vieira, M.H.C.; Schweich-Adami, L.C.; Oliveira, R.J.; Antoniolli-Silva, A.C.M.B. Effect of Cell Therapy with Adipose-Derived Stem Cells in the Treatment of Acute Rupture of the Achilles Tendon in Humans. Cell Tissue Bank. 2024, 25, 831–838. [Google Scholar] [CrossRef]
- Czajka, A.; Kania, E.M.; Genovese, L.; Corbo, A.; Merone, G.; Luci, C.; Sibilla, S. Daily Oral Supplementation with Collagen Peptides Combined with Vitamins and Other Bioactive Compounds Improves Skin Elasticity and Has a Beneficial Effect on Joint and General Wellbeing. Nutr. Res. 2018, 57, 97–108. [Google Scholar] [CrossRef]
- Eckstein, F.; Maschek, S.; Wirth, W.; Ladel, C.; Bihlet, A.R.; Knight, C.; Somberg, K.; Zhao, L. Unbiased Analysis of Knee Cartilage Thickness Change Over Three Years After Sprifermin vs. Placebo Treatment—A Post-Hoc Analysis from the Phase 2B Forward Study. Osteoarthr. Cartil. Open 2024, 6, 100513. [Google Scholar] [CrossRef] [PubMed]
- Hagino, H.; Sugimoto, T.; Tanaka, S.; Sasaki, K.; Sone, T.; Nakamura, T.; Soen, S.; Mori, S. A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: Primary results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos. Int. 2021, 32, 2301–2311. [Google Scholar] [CrossRef]
- Kuwaba, K.; Kusubata, M.; Taga, Y.; Igarashi, H.; Nakazato, K.; Mizuno, K. Dietary collagen peptides alleviate exercise-induced muscle soreness in healthy middle-aged males: A randomized double-blinded crossover clinical trial. J. Int. Soc. Sports Nutr. 2023, 20, 2206392. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, F.; Ramonda, R.; Hoxha, A.; Scanu, A.; Galozzi, P.; Favero, M.; Frallonardo, P.; Punzi, L. Effect of an oral preparation containing hyaluronic acid, chondroitin sulfate, hydrolyzed collagen type II, and hydrolyzed keratin on synovial fluid features and clinical indices in knee osteoarthritis: A pilot study. Reumatismo 2020, 72, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Schunck, M.; Zdzieblik, D.; Oesser, S. Impact of specific bioactive collagen peptides on joint discomforts in the lower extremity during daily activities: A randomized controlled trial. Int. J. Environ. Res. Public. Health 2024, 21, 687. [Google Scholar] [CrossRef] [PubMed]
- Vrouwe, J.P.M.; Meulenberg, J.J.M.; Klarenbeek, N.B.; Navas Canete, A.; Reijnierse, M.; Ruiterkamp, G.; Bevaart, L.; Lamers, R.J.; Kloppenburg, M.; Nelissen, R.G.H.H.; et al. Administration of an adeno-associated viral vector expressing interferon-beta in patients with inflammatory hand arthritis, results of a phase I/II study. Osteoarthr. Cartil. 2022, 30, 52–60. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Brame, J.; Oesser, S.; Gollhofer, A.; König, D. The Influence of Specific Bioactive Collagen Peptides on Knee Joint Discomfort in Young Physically Active Adults: A Randomized Controlled Trial. Nutrients 2017, 13, 523. [Google Scholar] [CrossRef]
- Devasia, S.; Joseph, J.T.; Stephena, P.S.; Koizumi, S.; Clarke, L.; Sriraam, V.T.; Kailas, A.P.; Madhavan, S. Management and Amelioration of Knee Joint Osteoarthritis in Adults Using a Novel High-Functional Bovine Collagen Peptide as a Nutritional Therapy: A Double-Blind, Prospective, Multicentric, Randomized, Active and Placebo Controlled, Five-Arm, Clinical Study to Evaluate the Efficacy, Safety, and Tolerability. Cartilage 2024, 15, 363–374. [Google Scholar] [CrossRef]
- Sadri, B.; Hassanzadeh, M.; Bagherifard, A.; Mohammadi, J.; Alikhani, M.; Moeinabadi-Bidgoli, K.; Madani, H.; Diaz-Solano, D.; Karimi, S.; Mehrazmay, M.; et al. Cartilage Regeneration and Inflammation Modulation in Knee Osteoarthritis Following Injection of Allogeneic Adipose-Derived Mesenchymal Stromal Cells: A Phase II, Triple-Blinded, Placebo Controlled, Randomized Trial. Stem Cell Res. Ther. 2023, 14, 162. [Google Scholar] [CrossRef]
- Farr, J.; Gomoll, A.H.; Yanke, A.B.; Strauss, E.J.; Mowry, K.C.; ASA Study Group. A Randomized Controlled Single-Blind Study Demonstrating Superiority of Amniotic Suspension Allograft Injection Over Hyaluronic Acid and Saline Control for Modification of Knee Osteoarthritis Symptoms. J. Knee Surg. 2019, 32, 1143–1154. [Google Scholar] [CrossRef]
- Kon, E.; Filardo, G.; Brittberg, M.; Busacca, M.; Condello, V.; Engebretsen, L.; Marlovits, S.; Niemeyer, P.; Platzer, P.; Posthumus, M.; et al. A Multilayer Biomaterial for Osteochondral Regeneration Shows Superiority vs Microfractures for the Treatment of Osteochondral Lesions in a Multicentre Randomized Trial at 2 Years. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 2704–2715. [Google Scholar] [CrossRef]
- Gupta, A.; Maffulli, N.; Rodriguez, H.C.; Lee, C.E.; Levy, H.J.; El-Amin, S.F., III. Umbilical cord-derived Wharton’s jelly for treatment of knee osteoarthritis: Study protocol for a non-randomized, open-label, multi-center trial. J. Orthop. Surg. Res. 2021, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Murrell, W.; Schramme, U.; Maffulli, N. Intra-articular gold induced cytokine (GOLDIC®) injection therapy in patients with osteoarthritis of knee joint: A clinical study. Int. Orthop. 2021, 45, 497–507. [Google Scholar] [CrossRef]
- Kruijntjens, D.S.M.G.; Kjaersgaard-Andersen, P.; Revald, P.; Leonhardt, J.S.; Arts, J.J.C.; ten Broeke, R.H.M. 5-year clinical and radiographic follow-up of the uncemented Symax hip stem in an international study. J. Orthop. Surg. Res. 2018, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Waluyo, Y.; Budu; Bukhari, A.; Adnan, E.; Haryadi, R.D.; Idris, I.; Hamid, F.; Usman, A.; Johan, M.P. Changes in levels of cartilage oligomeric proteinase and urinary C-terminal telopeptide of type II collagen in subjects with knee osteoarthritis after dextrose prolotherapy: A randomized controlled trial. J. Rehabil. Med. 2021, 53, jrm00196. [Google Scholar] [CrossRef]
- Mei-Dan, O.; Lippi, G.; Sánchez, M.; Andia, I.; Maffulli, N. Autologous Platelet-Rich Plasma: A Revolution in Soft Tissue Sports Injury Management? Physician Sportsmed. 2010, 38, 127–135. [Google Scholar] [CrossRef]
- Jamal, M.S.; Hurley, E.T.; Asad, H.; Asad, A.; Taneja, T. The Role of Platelet Rich Plasma and Other Orthobiologics in Bone Healing and Fracture Management: A Systematic Review. J. Clin. Orthop. Trauma. 2022, 25, 101759. [Google Scholar] [CrossRef]
- Awad, M.E.; Hussein, K.A.; Helwa, I.; Abdelsamid, M.F.; Aguilar-Perez, A.; Mohsen, I.; Hunter, M.; Hamrick, M.W.; Isales, C.M.; Elsalanty, M.; et al. Meta-Analysis and Evidence Base for the Efficacy of Autologous Bone Marrow Mesenchymal Stem Cells in Knee Cartilage Repair: Methodological Guidelines and Quality Assessment. Stem Cells Int. 2019, 2019, 3826054. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, G.; Han, Y.; Yang, W.; Xu, N.; Luo, M.; Pan, J.; Liu, J.; Zeng, L.-F. Combination of Mesenchymal Stem Cells (MSCs) and Platelet-Rich Plasma (PRP) in the Treatment of Knee Osteoarthritis: A Meta-Analysis of Randomised Controlled Trials. BMJ Open 2022, 12, e061008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and Advances in Clinical Applications of Mesenchymal Stromal Cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Pennone, V.; Rosini, E.; Mascheroni, E.; Gianola, S.; Castellini, G.; Bargeri, S.; Lovati, A.B. Revolutionizing Orthopedic Healthcare: A Systematic Review Unveiling Recombinant Antimicrobial Peptides. Front. Microbiol. 2024, 15, 1370826. [Google Scholar] [CrossRef]
- Pugliese, R.; Gelain, F. Peptidic Biomaterials: From Self-Assembling to Regenerative Medicine. Trends Biotechnol. 2017, 35, 145–158. [Google Scholar] [CrossRef]
- Hao, Z.; Chen, R.; Chai, C.; Wang, Y.; Chen, T.; Li, H.; Hu, Y.; Feng, Q.; Li, J. Antimicrobial Peptides for Bone Tissue Engineering: Diversity, Effects, and Applications. Front. Bioeng. Biotechnol. 2022, 10, 1030162. [Google Scholar] [CrossRef] [PubMed]
- Abu Owida, H. Recent Biomimetic Approaches for Articular Cartilage Tissue Engineering and Their Clinical Applications: Narrative Review of the Literature. Adv. Orthop. 2022, 2022, 8670174. [Google Scholar] [CrossRef]
- Liang, Q.; Ma, Y.; Yao, X.; Wei, W. Advanced 3D-Printing Bioinks for Articular Cartilage Repair. Int. J. Bioprint. 2022, 8, 511. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yang, Z.; Gao, C.; Li, H.; Yuan, Z.; Wang, F.; Sui, X.; Liu, S.; Guo, Q. Advances and Prospects in Biomimetic Multilayered Scaffolds for Articular Cartilage Regeneration. Regen. Biomater. 2020, 7, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Morya, V.K.; Shahid, H.; Lang, J.; Kwak, M.K.; Park, S.-H.; Noh, K.-C. Advancements in Therapeutic Approaches for Degenerative Tendinopathy: Evaluating Efficacy and Challenges. Int. J. Mol. Sci. 2024, 25, 11846. [Google Scholar] [CrossRef]
- Everts, P.A.; Lana, J.F.; Alexander, R.W.; Dallo, I.; Kon, E.; Ambach, M.A.; van Zundert, A.; Podesta, L. Profound properties of protein-rich, platelet-rich plasma matrices as novel, multi-purpose biological platforms in tissue repair, regeneration, and wound healing. Int. J. Mol. Sci. 2024, 25, 7914. [Google Scholar] [CrossRef]
- Hurley, E.T.; Sherman, S.L.; Stokes, D.J.; Rodeo, S.A.; Shapiro, S.A.; Mautner, K.; Buford, D.A.; Dragoo, J.L.; Mandelbaum, B.R.; Zaslav, K.R.; et al. Experts Achieve Consensus on a Majority of Statements Regarding Platelet-Rich Plasma Treatments for Treatment of Musculoskeletal Pathology. Arthrosc. J. Arthrosc. Relat. Surg. 2024, 40, 470–477.e1. [Google Scholar] [CrossRef]
- Pojala, C.V.; Toma, S.; Costache, C.; Peter, T.; Pojala, C.E.; Roman, N.A.; Dima, L. The potential of intra-articular therapies in managing knee osteoarthritis: A systematic review. Clin. Pract. 2024, 14, 1970–1996. [Google Scholar] [CrossRef]
- Jayaram, P.; Mitchell, P.J.T.; Shybut, T.B.; Moseley, B.J.; Lee, B. Leukocyte-rich platelet-rich plasma is predominantly anti-inflammatory compared with leukocyte-poor platelet-rich plasma in patients with mild-moderate knee osteoarthritis: A prospective, descriptive laboratory study. Am. J. Sports Med. 2023, 51, 2133–2140. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, J.; Yin, L.; Li, Y.; Liu, H. Transplantation of three mesenchymal stem cells for knee osteoarthritis, which cell type is more beneficial? A systematic review and network meta-analysis. J. Orthop. Surg. Res. 2024, 19, 366. [Google Scholar] [CrossRef]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal Stem Cell-Derived Exosomes: A New Therapeutic Approach to Osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Scalzone, A.; Sanjurjo-Rodríguez, C.; Berlinguer-Palmini, R.; Dickinson, A.M.; Jones, E.; Wang, X.N.; Crossland, R.E. Functional and Molecular Analysis of Human Osteoarthritic Chondrocytes Treated with Bone Marrow-Derived MSC-EVs. Bioengineering 2024, 11, 388. [Google Scholar] [CrossRef]
- Piuzzi, N.S.; Emara, A.; Chahla, J.; Mandelbaum, B.R. Ethical and Practical Considerations for Integrating Cellular (“Stem Cell”) Therapy into Clinical Practice. Curr. Rev. Musculoskelet. Med. 2020, 13, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Childs, P.G.; Reid, S.; Salmeron-Sanchez, M.; Dalby, M.J. Hurdles to Uptake of Mesenchymal Stem Cells and Their Progenitors in Therapeutic Products. Biochem. J. 2020, 477, 3349–3366. [Google Scholar] [CrossRef] [PubMed]
- Mashweu, A.R.; Azov, V.A. Nanotechnology in Drug Delivery: Anatomy and Molecular Insight into the Self-Assembly of Peptide-Based Hydrogels. Molecules 2024, 29, 5654. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Li, G.; Lin, Q.; Zhang, W.; Liu, H.; Su, J. The role of collagen-derived peptides in cartilage regeneration and osteoarthritis management. Mater. Today Bio 2024, 24, 100948. [Google Scholar] [CrossRef]
- Pol, S.H. Recent Trends and Scope of Nanotechnology in Orthopaedic Surgery: A Narrative Review. J. Clin. Diagn. Res. 2024, 18, RE01–RE05. [Google Scholar] [CrossRef]
- Montesissa, M.; Sassoni, E.; Boi, M.; Borciani, G.; Boanini, E.; Graziani, G. Synthetic or natural (bio-based) hydroxyapatite? A systematic comparison between biomimetic nanostructured coatings produced by ionized jet deposition. Nanomaterials 2024, 14, 1332. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Q.; Tian, Z.; Yao, Q.; Zhang, M. Recent advances in 3D bioprinted cartilage-mimicking constructs for applications in tissue engineering. Mater. Today Bio 2023, 23, 100870. [Google Scholar] [CrossRef]
- Morwood, A.J.; Lundy, F.T.; El-Karim, I.A.; Clarke, S.A. The Role of Extracellular Matrix (ECM) Adhesion Motifs in Functionalised Hydrogels. Molecules 2023, 28, 4616. [Google Scholar] [CrossRef]
- Kleiderman, E.; Boily, A.; Hasilo, C.; Knoppers, B.M. Overcoming Barriers to Facilitate the Regulation of Multi-Centre Regenerative Medicine Clinical Trials. Stem Cell Res. Ther. 2018, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, P.; Xue, X.; Zhang, Z.; Wang, L.; Jiang, Y.; Zhang, C.; Zhou, H.; Lv, S.; Shen, W.; et al. The role of platelet-rich plasma in biomedicine: A comprehensive overview. iScience 2025, 28, 111705. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef]
- Wu, K.C.; Chang, Y.H.; Ding, D.C.; Lin, S.Z. Mesenchymal Stromal Cells for Aging Cartilage Regeneration: A Review. Int. J. Mol. Sci. 2024, 25, 12911. [Google Scholar] [CrossRef]
- Wei, L.; Yan, W.; Shah, W.; Zhang, Z.; Wang, M.; Liu, B.; Xue, Z.; Cao, Y.; Hou, X.; Zhang, K.; et al. Advancements and Challenges in Stem Cell Transplantation for Regenerative Medicine. Heliyon 2024, 10, e35836. [Google Scholar] [CrossRef]
- Azadi, S.; Yazdanpanah, M.A.; Afshari, A.; Alahdad, N.; Chegeni, S.; Angaji, A.; Rezayat, S.M.; Tavakol, S. Bioinspired Synthetic Peptide-Based Biomaterials Regenerate Bone through Biomimicking of Extracellular Matrix. J. Tissue Eng. 2024, 15, 20417314241303818. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic Natural Biomaterials for Tissue Engineering and Regenerative Medicine: New Biosynthesis Methods, Recent Advances, and Emerging Applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Hashemi-Afzal, F.; Fallahi, H.; Bagheri, F.; Collins, M.N.; Baghaban Eslaminejad, M.; Seitz, H. Advancements in Hydrogel Design for Articular Cartilage Regeneration: A Comprehensive Review. Bioact. Mater. 2025, 43, 1–31. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Pazhouhnia, Z.; Rostami, M.; Rajabi Zangi, A.; Maleki, R.; Kolahi Azar, H.; Zalouli, V.; Rajavand, H.; Farzin, A.; et al. Commercialization and Regulation of Regenerative Medicine Products: Promises, Advances and Challenges. Biomed. Pharmacother. 2022, 153, 113431. [Google Scholar] [CrossRef]
- D’souza, R.S.; Her, Y.F.; Hussain, N.; Karri, J.; Schatman, M.; Calodney, A.K.; Lam, C.; Buchheit, T.; Boettcher, B.J.; Chien, G.C.C.; et al. Evidence-Based Clinical Practice Guidelines on Regenerative Medicine Treatment for Chronic Pain: A Consensus Report from a Multispecialty Working Group. J. Pain. Res. 2024, 17, 2951–3001. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Gupton, M.; Schroeder, F. Regenerative Medicine in Orthopedic Surgery: Expanding Our Toolbox. Cureus 2024, 16, e68487. [Google Scholar] [CrossRef] [PubMed]

| Therapy Type | Study | Study Design | Patient Demo. | Sample Size | Intervention Methods | Outcome Results Data | Overall Risk of Bias |
|---|---|---|---|---|---|---|---|
| PRP | [40] | Randomized Controlled Trial (RTC) | Ankle osteoarthritis patients | n = 100 | PRP vs. placebo injections | PRP resulted in significant pain reduction at 6 months (p < 0.05), but no significant difference at 12 months | Serious Risk (Cochrane RoB) |
| PRP | [41] | RCT | Lumbar herniated nucleus pulposus patients | n = 84 | PRP vs. triamcinolone injections | PRP showed superior pain relief and improved function at 6 months compared to triamcinolone (p = 0.03) | Moderate Risk (Cochrane RoB) |
| PRP | [42] | RCT | Chronic Achilles tendinopathy | n = 240 | PRP vs. sham injections | No significant difference between PRP and sham injections in pain or functional outcomes at 6 and 12 months | Moderate Risk (Cochrane RoB) |
| PRP | [15] | Double-Blind RCT | Partial-thickness rotator cuff tears | n = 92 | PRP vs. corticosteroids | PRP showed superior pain relief and function at 3 months (p = 0.02), but no significant difference at 12 months | Low Risk (Cochrane RoB) |
| PRP | [43] | Multicenter RCT | Acute Achilles tendon rupture | n = 230 | PRP vs. placebo injections | PRP did not significantly improve healing rates or functional recovery compared to placebo | Serious Risk (Cochrane RoB) |
| PRP | [44] | Triple-Blinded Prospective Study | Lateral elbow tendinopathy | n = 64 | PRP vs. steroid injections | PRP group showed greater pain reduction (p < 0.05) and functional improvement compared to steroids | Low Risk (Cochrane RoB) |
| PRP | [45] | RCT | Knee osteoarthritis patients, grade 3 | n = 120 | PRP injections (1, 2, or 3) at 2-week intervals vs. baseline | Three-dose PRP group showed significant pain reduction (p < 0.01) and functional improvement at 12 months | Moderate Risk (Cochrane RoB) |
| PRP | [46] | Placebo-Controlled Trial | Acute Achilles tendon rupture | n = 230 | PRP vs. placebo | No significant difference in healing rates or functional outcomes at 12 months | Serious Risk (Cochrane RoB) |
| PRP | [47] | RCT | Partial-thickness rotator cuff tears | n = 76 | PRP vs. PRP + vitamin C | PRP + vitamin C significantly reduced pain (p < 0.001) and improved function compared to PRP alone | Moderate Risk (Cochrane RoB) |
| PRP | [48] | Double-Blind RCT | Rotator cuff tear patients | n = 96 | Leukocyte-poor PRP | PRP improved pain and functional scores at 6 months (p < 0.05) but not at 12 months | Low Risk (Cochrane RoB) |
| PRP | [49] | Double-Blind RCT | Acute hamstring injuries | n = 80 | PRP vs. placebo injections | No significant difference in return-to-play time or reinjury rates | Moderate Risk (Cochrane RoB) |
| PRP | [50] | Double-Blind RCT | Patients with knee osteoarthritis | n = 102 | Three doses of LP-PRP vs. saline | PRP group showed significant pain reduction (p < 0.05) at 6 and 12 months | Low Risk (Cochrane RoB) |
| PRP | [51] | Prospective Randomized Study | Patients with rotator cuff tears | n = 78 | PRP injections to supraspinatus tendon | PRP improved pain and function (p = 0.04) at 6 months | Serious Risk (Cochrane RoB) |
| PRP | [52] | RCT | Patients with early-stage knee osteoarthritis | n = 90 | PRP + Pulsed Electromagnetic Fields (PEMFs) vs. PRP alone | PRP + PEMFs had superior pain relief and improved joint function (p < 0.05) | Moderate Risk (Cochrane RoB) |
| PRP | [53] | Single-Blind RCT | Shoulder impingement patients | n = 108 | PRP vs. corticosteroids | PRP led to improved ROM and pain reduction compared to steroids (p = 0.012 at 12 months) | Moderate Risk (Cochrane RoB) |
| PRP | [54] | RCT | ACL reconstruction patients | n = 88 | Three doses of PRP post-ACLR | PRP significantly reduced postoperative pain and improved graft healing (p < 0.05) | Moderate Risk (Cochrane RoB) |
| PRP | [55] | Double-Blind RCT | Patients with early-stage knee osteoarthritis | n = 110 | Single PRP vs. hyaluronic acid (HA) injection | PRP provided superior pain relief (p < 0.05) and improved joint function compared to HA | Low Risk (Cochrane RoB) |
| PRP | [56] | RCT | Patients with rotator cuff tendinopathy | n = 74 | PRP injection vs. rotator cuff strengthening exercises | PRP improved American Shoulder and Elbow Surgeons (ASES) and Constant scores, correlated with IL-1β and TGF-β levels | Moderate Risk (Cochrane RoB) |
| PRP | [57] | Comparative Study | Patients with chronic epicondylitis | n = 94 | PRP vs. conservative treatment | PRP group had significantly lower symptom scores at 6, 12, and 24 months, but not at 36 months. PRP group had fewer surgical procedures (0% vs. 20%, p = 0.027) | Serious Risk (ROBINS-I) |
| PRP | [58] | Comparative Study | Low-grade MCL injury patients | n = 52 | Autologous PRP injections | PRP resulted in faster return to sport and reduced pain scores compared to conservative management | Serious Risk (ROBINS-I) |
| Stem Cells | [59] | RCT | Patients with knee osteoarthritis | n = 54 | MSCs from bone marrow vs. placebo | Significant improvement in pain and function at 6 months (p < 0.05) | Low Risk (Cochrane RoB) |
| Stem Cells | [60] | Double-Blind RCT | Rotator cuff tear patients | n = 80 | MSCs vs. corticosteroids | MSCs improved pain and function at 12 months (p < 0.001) | Low Risk (Cochrane RoB) |
| Stem Cells | [61] | RCT | Cartilage defects | n = 92 | MSCs vs. PRP | MSCs showed greater cartilage regeneration on MRI | Low Risk (Cochrane RoB) |
| Stem Cells | [62] | Comparative Study | Osteoarthritis patients | n = 60 | MSCs vs. hyaluronic acid (HA) | MSCs superior in reducing inflammatory markers and improving function | Moderate Risk (Cochrane RoB) |
| Stem Cells | [63] | RCT | Rotator cuff tendinopathy | n = 84 | MSCs vs. conservative therapy | MSCs led to improved Constant and UCLA shoulder rating scores | Serious Risk (Cochrane RoB) |
| Stem Cells | [64] | RCT | Achilles tendinopathy | n = 74 | MSCs vs. platelet-rich fibrin | MSCs showed enhanced collagen repair and reduced pain | Low Risk (Cochrane RoB) |
| Stem Cells | [65] | RCT | Osteochondral defects | n = 60 | MSCs vs. microfracture | MSCs demonstrated superior cartilage repair on MRI | Low Risk (Cochrane RoB) |
| Stem Cells | [66] | RCT | Rotator cuff repair patients | n = 90 | MSCs vs. placebo | MSCs improved tendon healing rates (p = 0.02) | Serious Risk (Cochrane RoB) |
| Stem Cells | [67] | RCT | Knee OA patients | n = 88 | MSCs vs. HA injections | MSCs showed better pain relief at 6 and 12 months | Low Risk (Cochrane RoB) |
| Stem Cells | [68] | RCT | Hip OA patients | n = 78 | MSCs vs. PRP | MSCs resulted in Serious Risker functional recovery at 1-year follow-up | Low Risk (Cochrane RoB) |
| Stem Cells | [69] | RCT | Rotator cuff tear | n = 72 | MSCs vs. placebo | MSCs demonstrated improved short-term clinical outcomes but did not significantly impact tendon integrity or retear rates on MRI at 18 months. | Low Risk (Cochrane RoB) |
| Stem Cells | [70] | RCT | Cartilage defect patients | n = 60 | MSCs vs. microfracture | MSCs led to significantly better cartilage repair | Low Risk (Cochrane RoB) |
| Stem Cells | [71] | RCT | Achilles tendinopathy patients | n = 82 | MSCs vs. PRP | MSCs had greater pain relief and tendon healing rates | Low Risk (Cochrane RoB) |
| Stem Cells | [72] | RCT | Knee OA patients | n = 78 | MSCs vs. placebo | MSCs showed superior pain relief and function improvement | Low Risk (Cochrane RoB) |
| Stem Cells | [73] | Double-Blind RCT | Brachial plexus injury patients | n = 88 | Umbilical cord mesenchymal stem cells (UC-MSC) vs. secretome injection | UC-MSC led to improved SF-36 and DASH scores, but no significant histologic changes | Low Risk (Cochrane RoB) |
| Stem Cells | [74] | Prospective Cohort | Hip osteoarthritis patients | n = 72 | Intra-articular injection of MSCs | Improved Harris Hip Score and reduced pain over 1 year | Serious Risk (ROBINS-I) |
| Stem Cells | [75] | Prospective Study | Patients with knee osteoarthritis | n = 48 | Intra-articular injection of MSCs | Pain reduction and cartilage thickness improvement | Serious Risk (ROBINS-I) |
| Stem Cells | [76] | Cohort Study | Shoulder arthritis patients | n = 82 | MSCs + HA vs. HA alone | MSCs group had significant function and pain relief improvements | Serious Risk (ROBINS-I) |
| Stem Cells | [77] | Comparative Study | Patients with ACL injuries | n = 64 | MSCs vs. conservative rehab | MSCs group had improved function and lower re-tear rate | Moderate Risk (ROBINS-I) |
| Stem Cells | [78] | Prospective Cohort | Osteoarthritis patients | n = 74 | MSCs vs. HA | MSCs led to significant improvement in KOOS scores | Serious Risk (ROBINS-I) |
| Peptides | [79] | RCT | Patients with knee osteoarthritis | n = 88 | Collagen peptide supplementation vs. placebo | Significant reduction in knee pain and improved joint function (p < 0.05) | Low Risk (Cochrane RoB) |
| Peptides | [80] | Double-Blind RCT | Patients with rotator cuff tendinopathy | n = 80 | Peptide injection vs. corticosteroids | Peptide therapy resulted in superior pain relief and functional improvement at 6 months (p < 0.001) | Low Risk (Cochrane RoB) |
| Peptides | [81] | Comparative Study | Athletes with joint overuse injuries | n = 65 | Peptide-based supplementation vs. standard rehabilitation | Peptide group showed faster recovery time and enhanced tissue repair (p = 0.02) | Moderate Risk (Cochrane RoB) |
| Peptides | [82] | RCT | Patients with degenerative knee osteoarthritis | n = 74 | Peptide injections vs. placebo | Statistically significant improvement in joint mobility and reduced inflammatory markers (p < 0.05) | Low Risk (Cochrane RoB) |
| Peptides | [83] | Double-Blind RCT | Patients with Achilles tendinopathy | n = 82 | Peptide-based injections vs. PRP | Peptides performed comparably to PRP in pain reduction but showed greater improvement in collagen synthesis markers | Low Risk (Cochrane RoB) |
| Peptides | [84] | RCT | Patients undergoing ACL reconstruction | n = 90 | Perioperative peptide therapy vs. standard care | Faster post-operative recovery with improved tissue healing (p < 0.01) | Low Risk (Cochrane RoB) |
| Peptides | [85] | Phase I/II Study | Patients with inflammatory hand arthritis | n = 57 | Adeno-associated viral vector expressing interferon-beta | Local administration showed potential benefits but raised safety concerns due to prolonged adverse events | Serious Risk (Cochrane RoB) |
| Peptides | [86] | RCT | Physically active adults | n = 74 | Bioactive collagen peptides vs. placebo | Significant reduction in activity-related knee pain (p = 0.024) | Low Risk (Cochrane RoB) |
| Peptides | [87] | Prospective Cohort Study | Patients with chronic tendinopathy | n = 72 | Peptide-based therapy combined with physiotherapy | Improvement in pain scores and tendon elasticity (p < 0.01) | Moderate Risk (ROBINS-I) |
| Peptides | [88] | Prospective Study | Elderly patients with osteoarthritis | n = 66 | Peptide supplementation for 12 weeks | Pain reduction and improved joint stiffness, but no significant change in MRI findings | Serious Risk (ROBINS-I) |
| Biomimetic | [89] | RCT | Knee osteoarthritis patients | n = 100 | Biomimetic implant vs. microfracture | Biomimetic implant led to superior pain relief and functional improvement (p < 0.05) | Low Risk (Cochrane RoB) |
| Biomimetic | [90] | RCT | Patients with cartilage defects | n = 80 | Autologous protein solution (APS) vs. HA injection | Improved ACL healing rates and functional stability (p < 0.05) | Low Risk (Cochrane RoB) |
| Biomimetic | [91] | RCT | Patients with ACL injuries | n = 80 | Collagen-based biomaterial augmentation | Significant improvement in Knee Injury and Osteoarthritis Outcome Score (KOOS) scores for scaffold group at 12 months (p = 0.02) | Low Risk (Cochrane RoB) |
| Biomimetic | [34] | RCT | Patients with knee osteochondral lesions | n = 90 | Collagen-hydroxyapatite scaffold vs. microfracture | Biomimetic scaffold led to improved knee stability and reduced OA progression | Low Risk (Cochrane RoB) |
| Biomimetic | [92] | Prospective Comparative Study | Patients with meniscal injuries | n = 90 | Biomimetic scaffold vs. meniscectomy | APS group had significantly improved WOMAC scores at 6 and 12 months (p = 0.01) | Serious Risk (ROBINS-I) |
| Biomimetic | [93] | Prospective Case Series | Cartilage repair patients | n = 50 | Biomimetic hydrogel scaffold vs. standard care | Enhanced chondrogenesis and tissue integration (p < 0.05) | Serious Risk (ROBINS-I) |
| Biomimetic | [94] | Prospective Observational Study | Rotator cuff tear patients | n = 60 | Collagen-based scaffold augmentation | Increased tendon healing rates compared to standard repair (p < 0.05) | Serious Risk (ROBINS-I) |
| Therapy Type | Number of Studies | Mean Sample Size | Pain Reduction Percent (Mean ± SD, 95% CI) | Functional Improvement | Cochrane RoB Summary | ROBINS-I Summary | Statistical Comparisons (p-Values) | Meta-Regression (β, p-Value) |
|---|---|---|---|---|---|---|---|---|
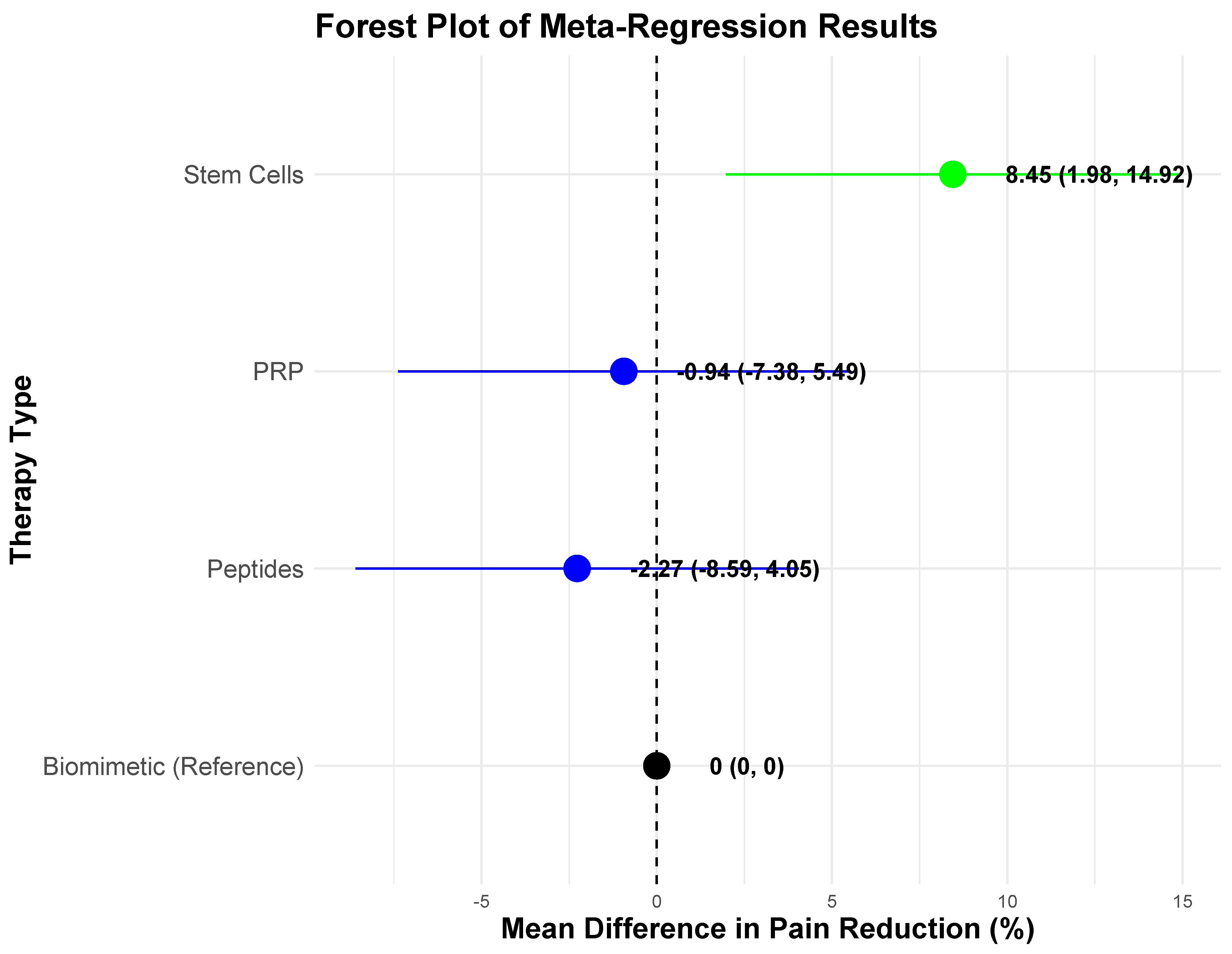
| PRP | 20 | 2022 (101) | 41.0 ± 6.36% (36.5–45.5) | Observed in most studies but inconsistent in long-term follow-ups. | Low Risk: 5 studies (25%) (Cochrane RoB); Moderate Risk: 9 studies (45%) (Cochrane RoB); Serious Risk: 4 studies (20%) (Cochrane RoB) | Serious Risk: 2 studies (10%) (ROBINS-I) | PRP vs. Biomimetic: p > 0.05 PRP vs. Peptides: p > 0.05 PRP vs. Stem Cells: p > 0.05 | β = −0.94, p > 0.05 |
| Stem Cells | 20 | 1579 (78) | 45.0 ± 3.85% (43.3–46.8) | Observed in most studies, superior in cartilage regeneration and tendon healing. | Low Risk: 12 studies (60%) (Cochrane RoB); Moderate Risk: 1 study (5%) (Cochrane RoB); Serious Risk: 2 studies (10%) (Cochrane RoB) | Serious Risk: 4 studies (20%) (ROBINS-I); Moderate Risk: 1 study (5%) (ROBINS-I) | PRP vs. Stem Cells: p > 0.05 Stem Cells vs. Peptides: p < 0.001 *** Stem Cells vs. Biomimetic: p < 0.001 *** | β = 8.45, p < 0.05 * |
| Peptides | 10 | 738 (73) | 35.5 ± 3.03% (33.3–37.7) | Improvement seen in tendon healing and pain management but limited long-term evidence. | Low Risk: 6 studies (60%) (Cochrane RoB); Moderate Risk: 1 study (10%) (Cochrane RoB); Serious Risk: 1 study (10%) (Cochrane RoB) | Serious Risk: 1 study (10%) (ROBINS-I); Moderate Risk: 1 study (10%) (ROBINS-I) | PRP vs. Peptides: p > 0.05 Stem Cells vs. Peptides: p < 0.001 *** Peptides vs. Biomimetic: p > 0.05 | β = −2.27, p > 0.05 |
| Biomimetic | 7 | 530 (75) | 37.4 ± 2.82% (34.8–40.0) | Notable in ACL healing, meniscus repair, and cartilage regeneration, but mixed long-term durability. | Low Risk: 4 studies (57%) (Cochrane RoB) | Serious Risk: 3 studies (43%) (ROBINS-I) | PRP vs. Biomimetic: p > 0.05 Peptides vs. Biomimetic: p > 0.05 Stem Cells vs. Biomimetic: p < 0.001 *** | Reference (β = 0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulian, A.J.; Goldstein, B.; Saad, M.A. Advancements in Regenerative Therapies for Orthopedics: A Comprehensive Review of Platelet-Rich Plasma, Mesenchymal Stem Cells, Peptide Therapies, and Biomimetic Applications. J. Clin. Med. 2025, 14, 2061. https://doi.org/10.3390/jcm14062061
Goulian AJ, Goldstein B, Saad MA. Advancements in Regenerative Therapies for Orthopedics: A Comprehensive Review of Platelet-Rich Plasma, Mesenchymal Stem Cells, Peptide Therapies, and Biomimetic Applications. Journal of Clinical Medicine. 2025; 14(6):2061. https://doi.org/10.3390/jcm14062061
Chicago/Turabian StyleGoulian, Andrew J., Brielle Goldstein, and Maarouf A. Saad. 2025. "Advancements in Regenerative Therapies for Orthopedics: A Comprehensive Review of Platelet-Rich Plasma, Mesenchymal Stem Cells, Peptide Therapies, and Biomimetic Applications" Journal of Clinical Medicine 14, no. 6: 2061. https://doi.org/10.3390/jcm14062061
APA StyleGoulian, A. J., Goldstein, B., & Saad, M. A. (2025). Advancements in Regenerative Therapies for Orthopedics: A Comprehensive Review of Platelet-Rich Plasma, Mesenchymal Stem Cells, Peptide Therapies, and Biomimetic Applications. Journal of Clinical Medicine, 14(6), 2061. https://doi.org/10.3390/jcm14062061






