Thoracic Ultrasound for Pre-Procedural Dynamic Assessment of Non-Expandable Lung: A Non-Invasive, Real-Time and Multifaceted Diagnostic Tool
Abstract
:1. Introduction
2. Methods
3. Non-Expandable Lung: Clinical Significance of an Understudied Topic
4. Pre-, Peri-, and Post-Procedural Non-Expandable Lung Assessment
4.1. Clinical Symptoms
4.2. Pleural Manometry
4.3. Radiologic Findings
4.4. Multimodal Diagnostic Approach
5. Thoracic Ultrasound Pre-Procedural Non-Expandable Lung Assessment
5.1. M-Mode Sinusoid Sign Assessment
5.2. Additional Emerging Preliminary Experimental Methods
5.2.1. 2D Shear Wave Elastography (SWE)
5.2.2. Speckle Tracking Imaging (STI) Strain Analysis
5.2.3. Lung/Liver Echogenicity (LLE) Ratio
5.2.4. The Dynamic Air Bronchogram
5.2.5. Pleural Thickening
6. Management of Non-Expandable Lung in Malignant Pleural Effusion: Treatment Strategies and Patient-Centered Care
6.1. Treatment Strategies for Non-Expandable Lung
6.2. Patient Education, Shared Decision-Making, and Coordinated Care
7. Medical Consequences, Complications, and Healthcare Costs of Inadequate NEL Management
7.1. Impact on Quality of Life and Clinical Outcomes
7.2. Procedural Complications and Adverse Events
7.3. Cost-Effectiveness Considerations
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NEL | Non-expandable lung |
| PE | Pleural effusion |
| CXR | Chest X-ray |
| CT | Computed tomography |
| TUS | Thoracic ultrasound |
| M-mode | Motion mode |
| STI | Speckle tracking imaging |
| SWE | Shear wave elastography |
| MPE | Malignant pleural effusion |
| IPC | Indwelling pleural catheter |
| PEL | Pleural elastance |
| LLE | Lung/liver echogenicity ratio |
| MT | Medical thoracoscopy |
| AUC | Area under the curve |
| ROI | Region of interest |
| QoL | Quality of life |
| AI | Artificial intelligence |
| ATS | American Thoracic Society |
| ERS | European Respiratory Society |
| EACTS | European Association for Cardio-Thoracic Surgery |
| SECT | Spanish Society of Pulmonology and Thoracic Surgery |
| BTS | British Thoracic Society |
References
- Bibby, A.C.; Halford, P.; de Fonseka, D.; Morley, A.J.; Smith, S.; Maskell, N.A. The Prevalence and Clinical Relevance of Nonexpandable Lung in Malignant Pleural Mesothelioma. A Prospective, Single-Center Cohort Study of 229 Patients. Ann. Am. Thorac. Soc. 2019, 16, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Upadrista, P.K.; Sabbula, B.R.; Akella, J. Trapped Lung. Semin. Respir. Crit. Care Med. 2023, 22, 631–635. [Google Scholar] [CrossRef]
- Huggins, J.T.; Doelken, P.; Sahn, S.A. The Unexpandable Lung. F1000 Med. Rep. 2010, 2, 77. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Rahman, N.M.; Maskell, N.A.; Bibby, A.C.; Blyth, K.G.; Corcoran, J.P.; Edey, A.; Evison, M.; De Fonseka, D.; Hallifax, R.; et al. British Thoracic Society Guideline for Pleural Disease. Thorax 2023, 78, s1–s42. [Google Scholar] [CrossRef]
- Roberts, M.E.; Neville, E.; Berrisford, R.G.; Antunes, G.; Ali, N.J. Management of a Malignant Pleural Effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65, ii32–ii40. [Google Scholar] [CrossRef]
- Hassan, M.; Touman, A.A.; Grabczak, E.M.; Faber, K.; Blyth, K.G.; Pochepnia, S.; Skaarup, S.H. Imaging of Pleural Disease. Breathe 2024, 20, 230172. [Google Scholar] [CrossRef]
- Doelken, P.; Huggins, J.T.; Pastis, N.J.; Sahn, S.A. Pleural Manometry: Technique and Clinical Implications. Chest 2004, 126, 1764–1769. [Google Scholar] [CrossRef]
- Pannu, J.; De Pew, Z.S.; Mullon, J.J.; Daniels, C.E.; Hagen, C.E.; Maldonado, F. Impact of Pleural Manometry on the Development of Chest Discomfort during Thoracentesis: A Symptom-Based Study. J. Bronchol. Interv. Pulmonol. 2014, 21, 306–313. [Google Scholar] [CrossRef]
- Grabczak, E.M.; Krenke, R.; Zielinska-Krawczyk, M.; Light, R.W. Pleural Manometry in Patients with Pleural Diseases—The Usefulness in Clinical Practice. Respir. Med. 2018, 145, 230–236. [Google Scholar] [CrossRef]
- Feller-Kopman, D. Therapeutic Thoracentesis: The Role of Ultrasound and Pleural Manometry. Curr. Opin. Pulm. Med. 2007, 13, 312–318. [Google Scholar] [CrossRef]
- Hassan, M.; Mercer, R.M.; Rahman, N.M. Thoracic Ultrasound in the Modern Management of Pleural Disease. Eur. Respir. Rev. 2020, 29, 190136. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Mathis, G.; Cui, X.W.; Ignee, A.; Hocke, M.; Hirche, T.O. Ultrasound of the Pleurae and Lungs. Ultrasound Med. Biol. 2015, 41, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure: The BLUE Protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D. Lung Ultrasound in the Critically Ill. Curr. Opin. Crit. Care 2014, 20, 315–322. [Google Scholar] [CrossRef]
- Salamonsen, M.R.; Lo, A.K.C.; Ng, A.C.T.; Bashirzadeh, F.; Wang, W.Y.S.; Fielding, D.I.K. Novel Use of Pleural Ultrasound Can Identify Malignant Entrapped Lung Prior to Effusion Drainage. Chest 2014, 146, 1286–1293. [Google Scholar] [CrossRef]
- Ultrasound as a Noninvasive Tool to Diagnose Trapped Lung|C80-C. Imaging Methodology and Application to Lung Disease. Available online: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A6518?download=true (accessed on 20 February 2025).
- Leemans, J.; Dooms, C.; Ninane, V.; Yserbyt, J. Success Rate of Medical Thoracoscopy and Talc Pleurodesis in Malignant Pleurisy: A Single-Centre Experience. Respirology 2018, 23, 613–617. [Google Scholar] [CrossRef]
- Wong, A.; Patail, H.; Ahmad, S. The Absent Sinusoid Sign. Ann. Am. Thorac. Soc. 2019, 16, 506–508. [Google Scholar] [CrossRef]
- Herman, D.D.; Cooper, A.Z.; Esguerra, V. Is the Finding of an Absent “Sinusoid Sign” on Lung Ultrasound Meaningful? Ann. Am. Thorac. Soc. 2019, 16, 1075. [Google Scholar] [CrossRef]
- Patail, H.; Wong, A.; Ahmad, S. Reply: Is the Finding of an Absent “Sinusoid Sign” on Lung Ultrasound Meaningful? Ann. Am. Thorac. Soc. 2019, 16, 1076. [Google Scholar] [CrossRef]
- Hassan, M.; El-Shaarawy, B.; Al-Qaradawi, M.Y.; Gadallah, M.; Reda, M. Ultrasound Predictors of Lung Re-Expansion Following Pleural Effusion Drainage. Eur. Respir. J. 2021, 58, OA4342. [Google Scholar] [CrossRef]
- Khatim, I.; Albaba, I.; Hu, K.; Huggins, T.; Chopra, A. Diagnosis of Non-Expandable Lung Using Thoracic Ultrasound. Respir. Med. Case Rep. 2022, 40, 101749. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.K.; Fjaellegaard, K.; Rasmussen, D.B.; Alstrup, G.; Høegholm, A.; Sidhu, J.S.; Sivapalan, P.; Gerke, O.; Bhatnagar, R.; Clementsen, P.F.; et al. Ultrasound in the Diagnosis of Non-Expandable Lung: A Prospective Observational Study of M-Mode, B-Mode, and 2D-Shear Wave Elastography. Diagnostics 2024, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, H.; Ak, G.; Yılmaz, S.; Alatas, F.; Metintas, M. The Dynamic Air Bronchogram; a New Parameter of Thoracic Ultrasound in Predicting Non-Expandable Lung. Eur. Respir. J. 2024, 64, PA3454. [Google Scholar] [CrossRef]
- Huggins, J.T.; Sahn, S.A.; Heidecker, J.; Ravenel, J.G.; Doelken, P. Characteristics of Trapped Lung: Pleural Fluid Analysis, Manometry, and Air-Contrast Chest CT. Chest 2007, 131, 206–213. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 26, 5. [Google Scholar] [CrossRef]
- Matthews, C.; Freeman, C.; Sharples, L.D.; Fox-Rushby, J.; Tod, A.; Maskell, N.A.; Edwards, J.G.; Coonar, A.S.; Sivasothy, P.; Hughes, V.; et al. MesoTRAP: A Feasibility Study That Includes a Pilot Clinical Trial Comparing Video-Assisted Thoracoscopic Partial Pleurectomy Decortication with Indwelling Pleural Catheter in Patients with Trapped Lung Due to Malignant Pleural Mesothelioma Designed to Address Recruitment and Randomisation Uncertainties and Sample Size Requirements for a Phase III Trial. BMJ Open Respir. Res. 2019, 6, e000368. [Google Scholar] [CrossRef]
- Gillett, D.; Mitchell, M.A.; Dhaliwal, I. Avoid the Trap: Nonexpanding Lung. Chest 2021, 160, 1131–1136. [Google Scholar] [CrossRef]
- Woolhouse, I.; Bishop, L.; Darlison, L.; De Fonseka, D.; Edey, A.; Edwards, J.; Faivre-Finn, C.; Fennell, D.A.; Holmes, S.; Kerr, K.M.; et al. British Thoracic Society Guideline for the Investigation and Management of Malignant Pleural Mesothelioma. Thorax 2018, 73, i1–i30. [Google Scholar] [CrossRef]
- Desai, N.R.; Lee, H.J. Diagnosis and Management of Malignant Pleural Effusions: State of the Art in 2017. J. Thorac. Dis. 2017, 9, S1111–S1122. [Google Scholar] [CrossRef]
- Tanrikulu, A.C.; Abakay, A.; Kaplan, M.A.; Küçüköner, M.; Palanci, Y.; Evliyaoglu, O.; Sezgi, C.; Sen, H.; Carkanat, A.I.; Kirbas, G. A Clinical, Radiographic and Laboratory Evaluation of Prognostic Factors in 363 Patients with Malignant Pleural Mesothelioma. Respiration 2010, 80, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Hollen, P.J.; Gralla, R.J.; Liepa, A.M.; Symanowski, J.T.; Rusthoven, J.J. Adapting the Lung Cancer Symptom Scale (LCSS) to Mesothelioma: Using the LCSS-Meso Conceptual Model for Validation. Cancer 2004, 101, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Feller-Kopman, D.; Walkey, A.; Berkowitz, D.; Ernst, A. The Relationship of Pleural Pressure to Symptom Development during Therapeutic Thoracentesis. Chest 2006, 129, 1556–1560. [Google Scholar] [CrossRef] [PubMed]
- Faber, K.; Krenke, R. Pleural Manometry—Basics for Clinical Practice. Curr. Pulmonol. Rep. 2021, 10, 111–120. [Google Scholar] [CrossRef]
- Heidecker, J.; Huggins, J.T.; Sahn, S.A.; Doelken, P. Pathophysiology of Pneumothorax Following Ultrasound-Guided Thoracentesis. Chest 2006, 130, 1173–1184. [Google Scholar] [CrossRef]
- Martin, G.A.; Tsim, S.; Kidd, A.C.; Foster, J.E.; McLoone, P.; Chalmers, A.; Blyth, K.G. Pre-EDIT: A Randomized Feasibility Trial of Elastance-Directed Intrapleural Catheter or Talc Pleurodesis in Malignant Pleural Effusion. Chest 2019, 156, 1204–1213. [Google Scholar] [CrossRef]
- Masoud, H.H.; El-Zorkany, M.M.; Ahmed, A.A.; Assal, H.H. Pleural Space Elastance and Its Relation to Success Rates of Pleurodesis in Malignant Pleural Effusion. Tuberc. Respir. Dis. 2021, 84, 67–73. [Google Scholar] [CrossRef]
- Lentz, R.J.; Lerner, A.D.; Pannu, J.K.; Merrick, C.M.; Roller, L.; Walston, C.; Valenti, S.; Goddard, T.; Chen, H.; Huggins, J.T.; et al. Routine Monitoring with Pleural Manometry during Therapeutic Large-Volume Thoracentesis to Prevent Pleural-Pressure-Related Complications: A Multicentre, Single-Blind Randomised Controlled Trial. Lancet Respir. Med. 2019, 7, 447–455. [Google Scholar] [CrossRef]
- Feller-Kopman, D.; Berkowitz, D.; Boiselle, P.; Ernst, A. Large-Volume Thoracentesis and the Risk of Reexpansion Pulmonary Edema. Ann. Thorac. Surg. 2007, 84, 1656–1661. [Google Scholar] [CrossRef]
- Feller-Kopman, D. Nonexpandable Lung: More Than Just a Call from Radiology. Ann. Am. Thorac. Soc. 2019, 16, 1240–1242. [Google Scholar] [CrossRef]
- Saha, B.K.; Hu, K.; Shkolnik, B. Non-Expandable Lung: An Underappreciated Cause of Post-Thoracentesis Basilar Pneumothorax. BMJ Case Rep. 2020, 13, e238292. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.G. Expanding Knowledge on Non-Expandable Lungs. Respirology 2020, 25, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Judson, M.A.; Doelken, P.; Maldonado, F.; Rahman, N.M.; Huggins, J.T. The Relationship of Pleural Manometry With Postthoracentesis Chest Radiographic Findings in Malignant Pleural Effusion. Chest 2020, 157, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Light, R.W.; Jenkinson, S.G.; Minh, V.D.; George, R.B. Observations on Pleural Fluid Pressures as Fluid Is Withdrawn during Thoracentesis. Am. Rev. Respir. Dis. 1980, 121, 799–804. [Google Scholar] [CrossRef]
- Boshuizen, R.C.; Sinaasappel, M.; Vincent, A.D.; Goldfinger, V.; Farag, S.; Van Den Heuvel, M.M. Pleural Pressure Swing and Lung Expansion after Malignant Pleural Effusion Drainage: The Benefits of High-Temporal Resolution Pleural Manometry. J. Bronchology Interv. Pulmonol. 2013, 20, 200–205. [Google Scholar] [CrossRef]
- Skaarup, S.H.; Løkke, A.; Laursen, C.B. The Area Method: A New Method for Ultrasound Assessment of Diaphragmatic Movement. Crit. Ultrasound J. 2018, 10, 15. [Google Scholar] [CrossRef]
- Gennisson, J.L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound Elastography: Principles and Techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Wen, X.; Yu, X.; Tian, Y.; Liu, Z.; Cheng, W.; Li, H.; Kang, J.; Wei, T.; Yuan, S.; Tian, J. Quantitative Shear Wave Elastography in Primary Invasive Breast Cancers, Based on Collagen-S100A4 Pathology, Indicates Axillary Lymph Node Metastasis. Quant. Imaging Med. Surg. 2020, 10, 624–633. [Google Scholar] [CrossRef]
- Ozgokce, M.; Yavuz, A.; Akbudak, I.; Durmaz, F.; Uney, I.; Aydin, Y.; Yildiz, H.; Batur, A.; Arslan, H.; Dundar, I. Usability of Transthoracic Shear Wave Elastography in Differentiation of Subpleural Solid Masses. Ultrasound Q. 2018, 34, 233–237. [Google Scholar] [CrossRef]
- Wang, B.; Guo, Q.; Wang, J.Y.; Yu, Y.; Yi, A.J.; Cui, X.W.; Dietrich, C.F. Ultrasound Elastography for the Evaluation of Lymph Nodes. Front. Oncol. 2021, 11, 714660. [Google Scholar] [CrossRef]
- Bibby, A.C.; Dorn, P.; Psallidas, I.; Porcel, J.M.; Janssen, J.; Froudarakis, M.; Subotic, D.; Astoul, P.; Licht, P.; Schmid, R.; et al. ERS/EACTS Statement on the Management of Malignant Pleural Effusions. Eur. Respir. J. 2018, 52, 1800349. [Google Scholar] [CrossRef] [PubMed]
- Porcel, J.M. Ultrasound-Based Elastography: “Hard” to Implement in the Pleural Effusion Work-Up? Eur. Respir. J. 2019, 54, 1901587. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, C.; Jenderka, K.V.; Moran, C.M.; Draghi, F.; Jimenez Diaz, J.F.; Sande, R. EFSUMB Clinical Safety Statement for Diagnostic Ultrasound—(2019 Revision). Ultraschall Med. 2020, 41, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Mandoli, G.E.; Pastore, M.C.; Procopio, M.C.; Pica, A.; Vigna, M.; Benfari, G.; Diviggiano, E.E.; Martini, L.; Lunghetti, S.; Focardi, M.; et al. Unveiling the Reliability of Left Atrial Strain Measurement: A Dedicated Speckle Tracking Software Perspective in Controls and Cases. Eur. Heart J. Imaging Methods Pr. 2024, 2, qyae061. [Google Scholar] [CrossRef]
- Cameli, M.; Mandoli, G.E.; Nistor, D.; Lisi, E.; Massoni, A.; Crudele, F.; Stricagnoli, M.; Lunghetti, S.; Mondillo, S. Left Heart Longitudinal Deformation Analysis in Mitral Regurgitation. Int. J. Cardiovasc. Imaging 2018, 34, 1741–1751. [Google Scholar] [CrossRef]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ Ecosystem: Open-Source Software for Image Visualization, Processing, and Analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef]
- Harris-Love, M.O.; Seamon, B.A.; Teixeira, C.; Ismail, C. Ultrasound Estimates of Muscle Quality in Older Adults: Reliability and Comparison of Photoshop and ImageJ for the Grayscale Analysis of Muscle Echogenicity. PeerJ 2016, 4, e1721. [Google Scholar] [CrossRef]
- Spinnato, S.; De Biase, A.; Bilardo, C.M.; Elvan-Taşpınar, A. Fetal Echogenic Bowel: What Is Real Echogenicity? A Quantitative Method Based on Histogram Analysis of the Grayscale. Fetal Diagn. Ther. 2024, 51, 145–153. [Google Scholar] [CrossRef]
- Di Matteo, A.; Moscioni, E.; Lommano, M.G.; Cipolletta, E.; Smerilli, G.; Farah, S.; Airoldi, C.; Aydin, S.Z.; Becciolini, A.; Bonfiglioli, K.; et al. Reliability Assessment of Ultrasound Muscle Echogenicity in Patients with Rheumatic Diseases: Results of a Multicenter International Web-Based Study. Front. Med. 2023, 9, 1090468. [Google Scholar] [CrossRef]
- Lichtenstein, D.; Mezière, G.; Seitz, J. The Dynamic Air Bronchogram. A Lung Ultrasound Sign of Alveolar Consolidation Ruling out Atelectasis. Chest 2009, 135, 1421–1425. [Google Scholar] [CrossRef]
- Gillman, L.M.; Panebianco, N.; Alkadi, A.; Blaivas, M.; Kirkpatrick, A.W. The Dynamic Sonographic Air Bronchogram: A Simple and Immediate Bedside Diagnosis of Alveolar Consolidation in Severe Respiratory Failure. J. Trauma. 2011, 70, 760. [Google Scholar] [CrossRef] [PubMed]
- Davide, N.; Adelaide, M.; Alfredo, C.; Rosa, R. Ultrasound Diagnosis of Pneumonia: A Case Report. J. Clin. Stud. Rev. Rep. 2024, 6, 1. [Google Scholar] [CrossRef]
- Mongodi, S.; De Vita, N.; Salve, G.; Bonaiti, S.; Daverio, F.; Cavagnino, M.; Siano, G.; Amatu, A.; Maggio, G.; Musella, V.; et al. The Role of Lung Ultrasound Monitoring in Early Detection of Ventilator-Associated Pneumonia in COVID-19 Patients: A Retrospective Observational Study. J. Clin. Med. 2022, 11, 3001. [Google Scholar] [CrossRef]
- Magdy, S.; Youssef, A.; Mohamed, A.A.; Eldin, D.; Amin, A.; Amin, N.A. Role of Pleural Manometry and Transthoracic Ultrasonography to Predict Entrapped Lung. Egypt. J. Bronchol. 2024, 18, 75. [Google Scholar] [CrossRef]
- Martin, G.A.; Kidd, A.C.; Tsim, S.; Halford, P.; Bibby, A.; Maskell, N.A.; Blyth, K.G. Inter-Observer Variation in Image Interpretation and the Prognostic Importance of Non-Expansile Lung in Malignant Pleural Effusion. Respirology 2020, 25, 298–304. [Google Scholar] [CrossRef]
- Feller-Kopman, D.J.; Reddy, C.B.; Gould, M.K.; Balekian, A.A.; DeCamp, M.M.; Diekemper, R.L.; Henry, T.; Iyer, N.P.; Lee, Y.C.G.; Lewis, S.Z.; et al. Management of Malignant Pleural Effusions. An Official ATS/STS/STR Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, 839–849. [Google Scholar] [CrossRef]
- Recuero Díaz, J.L.; Figueroa Almánzar, S.; Gálvez Muñoz, C.; Lázaro Sierra, J.; López Porras, M.; Márquez Medina, D.; Nabal Vicuña, M.; Sánchez Moreno, L.; González Cantalejo, M.; Porcel, J.M. Recommendations of the Spanish Society of Thoracic Surgery for the Management of Malignant Pleural Effusion. Cir. Esp. 2022, 100, 673–683. [Google Scholar] [CrossRef]
- Qureshi, R.A.; Collinson, S.L.; Powell, R.J.; Froeschle, P.O.; Berrisford, R.G. Management of Malignant Pleural Effusion Associated with Trapped Lung Syndrome. Asian Cardiovasc. Thorac. Ann. 2008, 16, 120–123. [Google Scholar] [CrossRef]
- Warren, W.H.; Kim, A.W.; Liptay, M.J. Identification of Clinical Factors Predicting Pleurx Catheter Removal in Patients Treated for Malignant Pleural Effusion. Eur. J. Cardiothorac. Surg. 2008, 33, 89–94. [Google Scholar] [CrossRef]
- Cardillo, G.; Facciolo, F.; Carbone, L.; Regal, M.; Corzani, F.; Ricci, A.; Di Martino, M.; Martelli, M. Long-Term Follow-up of Video-Assisted Talc Pleurodesis in Malignant Recurrent Pleural Effusions. Eur. J. Cardiothorac. Surg. 2002, 21, 302–306. [Google Scholar] [CrossRef]
- Clive, A.O.; Kahan, B.C.; Hooper, C.E.; Bhatnagar, R.; Morley, A.J.; Zahan-Evans, N.; Bintcliffe, O.J.; Boshuizen, R.C.; Fysh, E.T.H.; Tobin, C.L.; et al. Predicting Survival in Malignant Pleural Effusion: Development and Validation of the LENT Prognostic Score. Thorax 2014, 69, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Pien, G.W.; Gant, M.J.; Washam, C.L.; Sterman, D.H. Use of an Implantable Pleural Catheter for Trapped Lung Syndrome in Patients with Malignant Pleural Effusion. Chest 2001, 119, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Havelock, T.; Teoh, R.; Laws, D.; Gleeson, F. Pleural Procedures and Thoracic Ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010, 65, i61–i76. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, M.F.; Ferreiro, L.; Valdés, L. Unexpandable Lung. Arch. Bronconeumol. 2013, 49, 63–69. [Google Scholar] [CrossRef]
- Thomas, R.; Fysh, E.T.H.; Smith, N.A.; Lee, P.; Kwan, B.C.H.; Yap, E.; Horwood, F.C.; Piccolo, F.; Lam, D.C.L.; Garske, L.A.; et al. Effect of an Indwelling Pleural Catheter vs Talc Pleurodesis on Hospitalization Days in Patients With Malignant Pleural Effusion: The AMPLE Randomized Clinical Trial. JAMA 2017, 318, 1903–1912. [Google Scholar] [CrossRef]
- Boshuizen, R.C.; vd Noort, V.; Burgers, J.A.; Herder, G.J.M.; Hashemi, S.M.S.; Hiltermann, T.J.N.; Kunst, P.W.; Stigt, J.A.; van den Heuvel, M.M. A Randomized Controlled Trial Comparing Indwelling Pleural Catheters with Talc Pleurodesis (NVALT-14). Lung Cancer 2017, 108, 9–14. [Google Scholar] [CrossRef]
- Wang, L.; Deng, H.; Chen, X.; Li, C.; Yi, F.; Wei, Y.; Zhang, W. Talc Pleurodesis versus Indwelling Pleural Catheter among Patients with Malignant Pleural Effusion: A Meta-Analysis of Randomized Controlled Trials. World J. Surg. Oncol. 2020, 18, 184. [Google Scholar] [CrossRef]
- Porcel, J.M.; Torres, M.; Pardina, M.; Civit, C.; Salud, A.; Bielsa, S. Predictors of Indwelling Pleural Catheter Removal and Infection: A Single-Center Experience With 336 Procedures. J. Bronchology Interv. Pulmonol. 2020, 27, 86–94. [Google Scholar] [CrossRef]
- Sivakumar, P.; Fitzgerald, D.B.; Ip, H.; Rao, D.; West, A.; Noorzad, F.; Wallace, D.; Haris, M.; Prudon, B.; Hettiarachchi, G.; et al. The Impact of Outpatient versus Inpatient Management on Health-Related Quality of Life Outcomes for Patients with Malignant Pleural Effusion: The OPTIMUM Randomised Clinical Trial. Eur. Respir. J. 2024, 63, 2201215. [Google Scholar] [CrossRef]
- Janssen, J.P.; Collier, G.; Astoul, P.; Tassi, G.F.; Noppen, M.; Rodriguez-Panadero, F.; Loddenkemper, R.; Herth, F.J.; Gasparini, S.; Marquette, C.H.; et al. Safety of Pleurodesis with Talc Poudrage in Malignant Pleural Effusion: A Prospective Cohort Study. Lancet 2007, 369, 1535–1539. [Google Scholar] [CrossRef]
- Egharevba, H.O. A Comparison of Healthcare Funding Systems between Low-/Medium-Income and High-Income Countries: Equity, Equality, and Fairness in the Rationing of Healthcare Resources. J. Health Med. Sci. 2024, 7, 23–31. [Google Scholar] [CrossRef]
- Brakema, E.A.; Tabyshova, A.; Van Der Kleij, R.M.J.J.; Sooronbaev, T.; Lionis, C.; Anastasaki, M.; An, P.L.; Nguyen, L.T.; Kirenga, B.; Walusimbi, S.; et al. The Socioeconomic Burden of Chronic Lung Disease in Low-Resource Settings across the Globe—An Observational FRESH AIR Study. Respir. Res. 2019, 20, 291. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Simkovich, S.; Hossen, S.; Feller-Kopman, D.J. Indwelling Pleural Catheter Drainage Strategy for Malignant Effusion: A Cost-Effectiveness Analysis. Ann. Am. Thorac. Soc. 2020, 17, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Vakalopoulou, M.; Paragios, N.; Revel, M.P. Artificial Intelligence Applications for Thoracic Imaging. Eur. J. Radiol. 2020, 123, 108774. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.Y.; Hwang, E.J.; Lee, S.M.; Park, C.M. Applications of Artificial Intelligence in the Thorax: A Narrative Review Focusing on Thoracic Radiology. J. Thorac. Dis. 2021, 13, 6943. [Google Scholar] [CrossRef]
- Viswanathan, V.S.; Toro, P.; Corredor, G.; Mukhopadhyay, S.; Madabhushi, A. The State of the Art for Artificial Intelligence in Lung Digital Pathology. J. Pathol. 2022, 257, 413. [Google Scholar] [CrossRef]
- Sindhu, A.; Jadhav, U.; Ghewade, B.; Bhanushali, J.; Yadav, P. Revolutionizing Pulmonary Diagnostics: A Narrative Review of Artificial Intelligence Applications in Lung Imaging. Cureus 2024, 16, e57657. [Google Scholar] [CrossRef]
- Cucchiara, F.; Petrini, I.; Romei, C.; Crucitta, S.; Lucchesi, M.; Valleggi, S.; Scavone, C.; Capuano, A.; De Liperi, A.; Chella, A.; et al. Combining Liquid Biopsy and Radiomics for Personalized Treatment of Lung Cancer Patients. State of the Art and New Perspectives. Pharmacol. Res. 2021, 169, 105643. [Google Scholar] [CrossRef]
- Natarajan, A.; Su, H.W.; Heneghan, C.; Blunt, L.; O’Connor, C.; Niehaus, L. Measurement of Respiratory Rate Using Wearable Devices and Applications to COVID-19 Detection. npj Digit. Med. 2021, 4, 136. [Google Scholar] [CrossRef]
- Vitazkova, D.; Foltan, E.; Kosnacova, H.; Micjan, M.; Donoval, M.; Kuzma, A.; Kopani, M.; Vavrinsky, E. Advances in Respiratory Monitoring: A Comprehensive Review of Wearable and Remote Technologies. Biosensors 2024, 14, 90. [Google Scholar] [CrossRef]
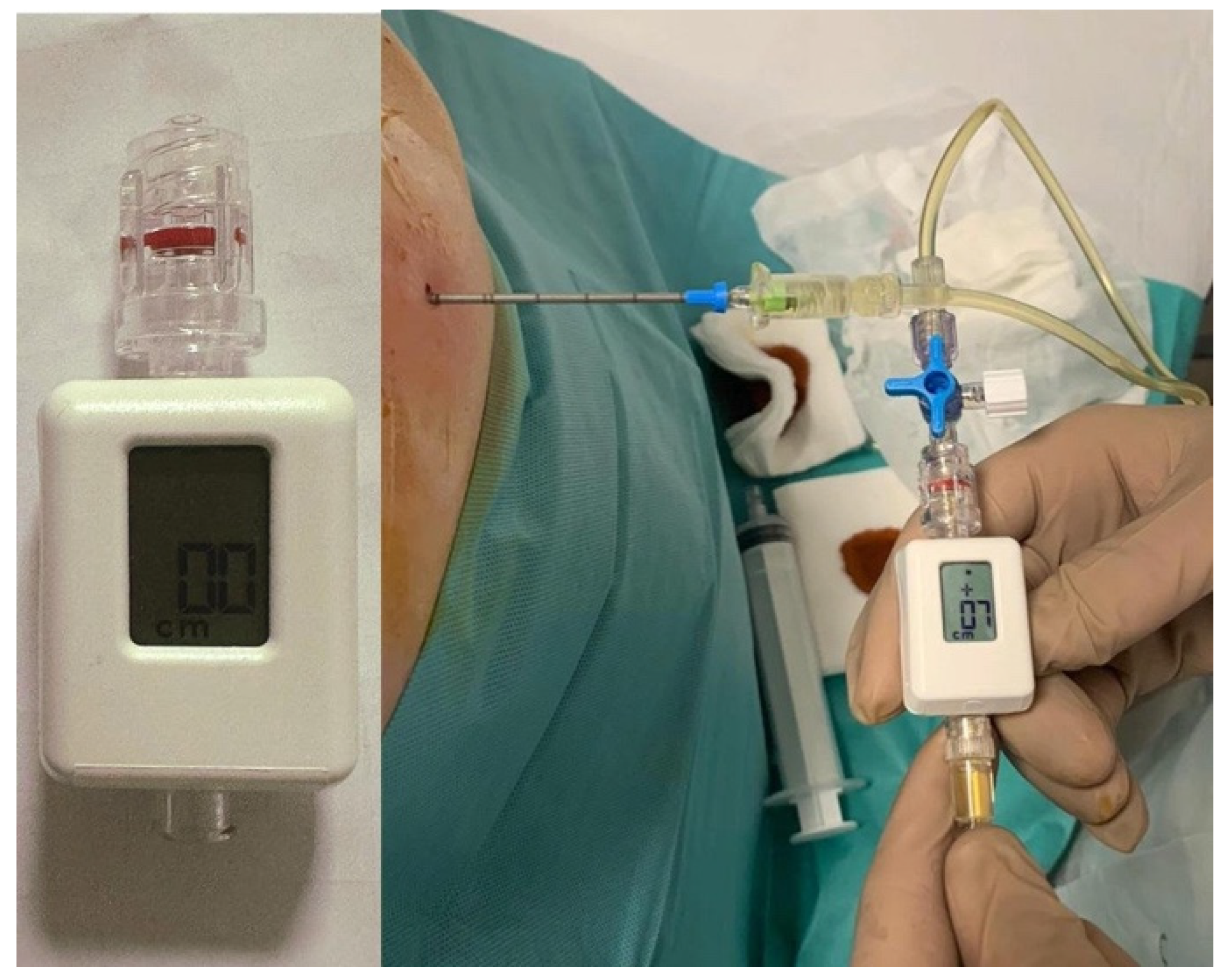
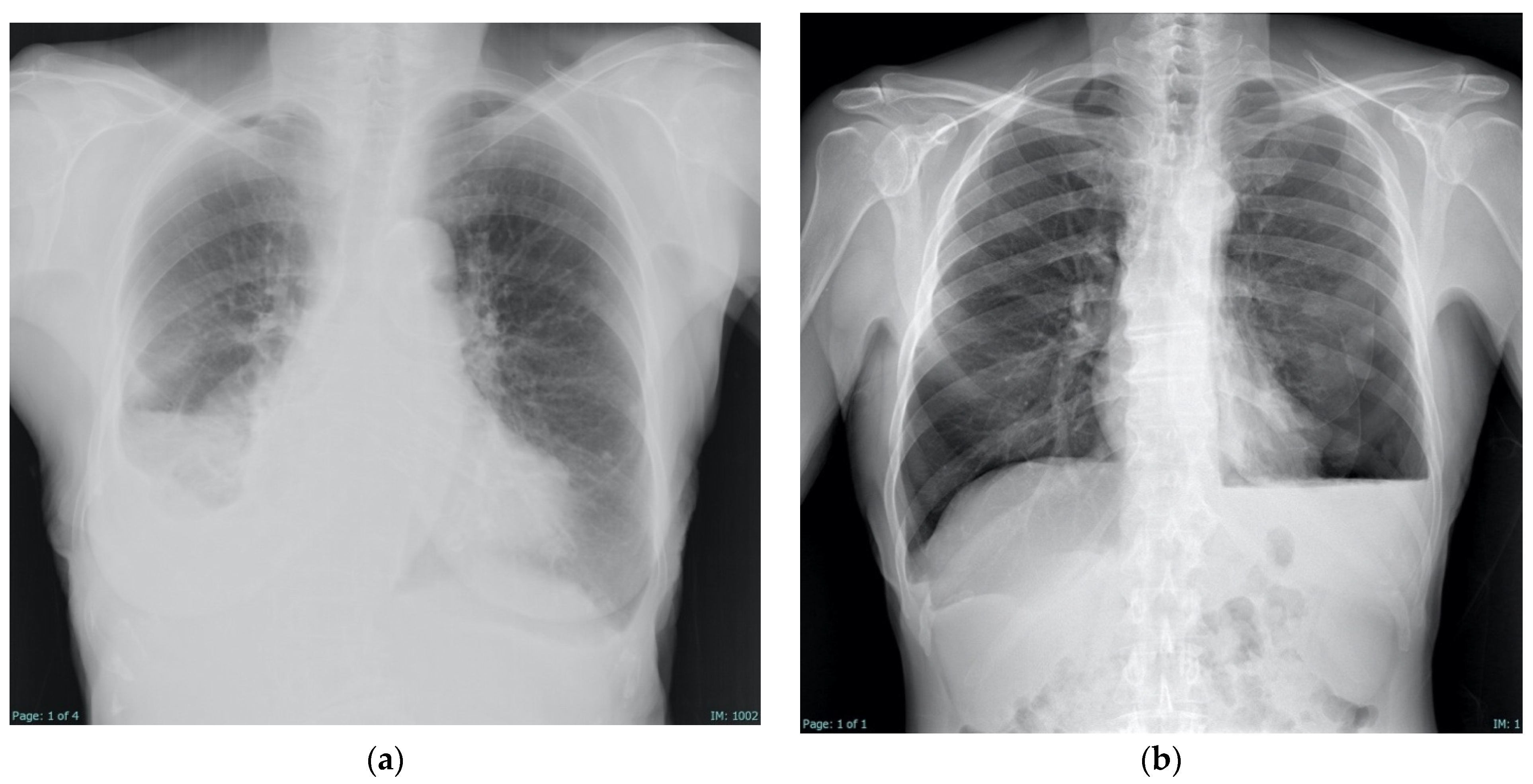


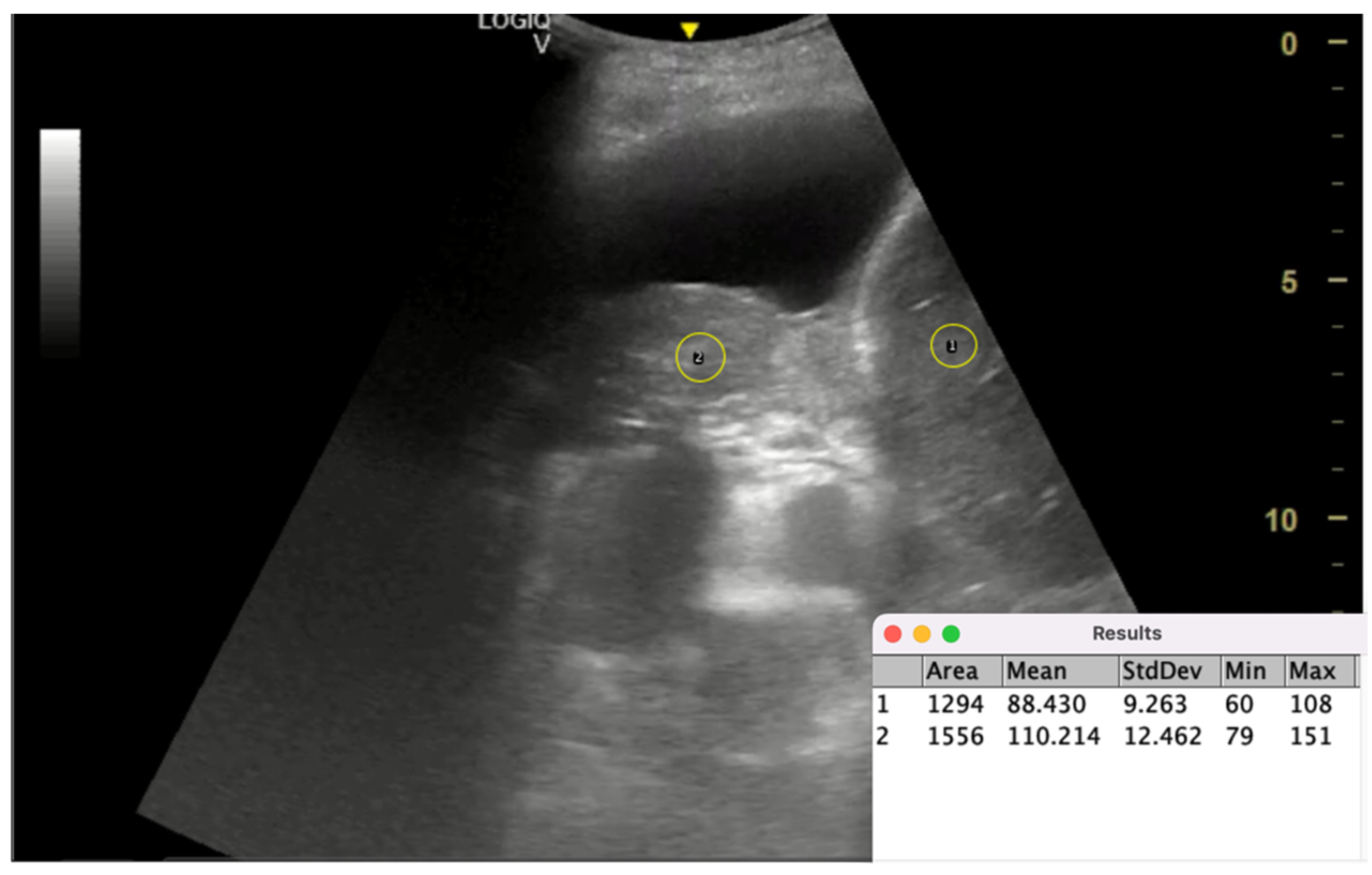
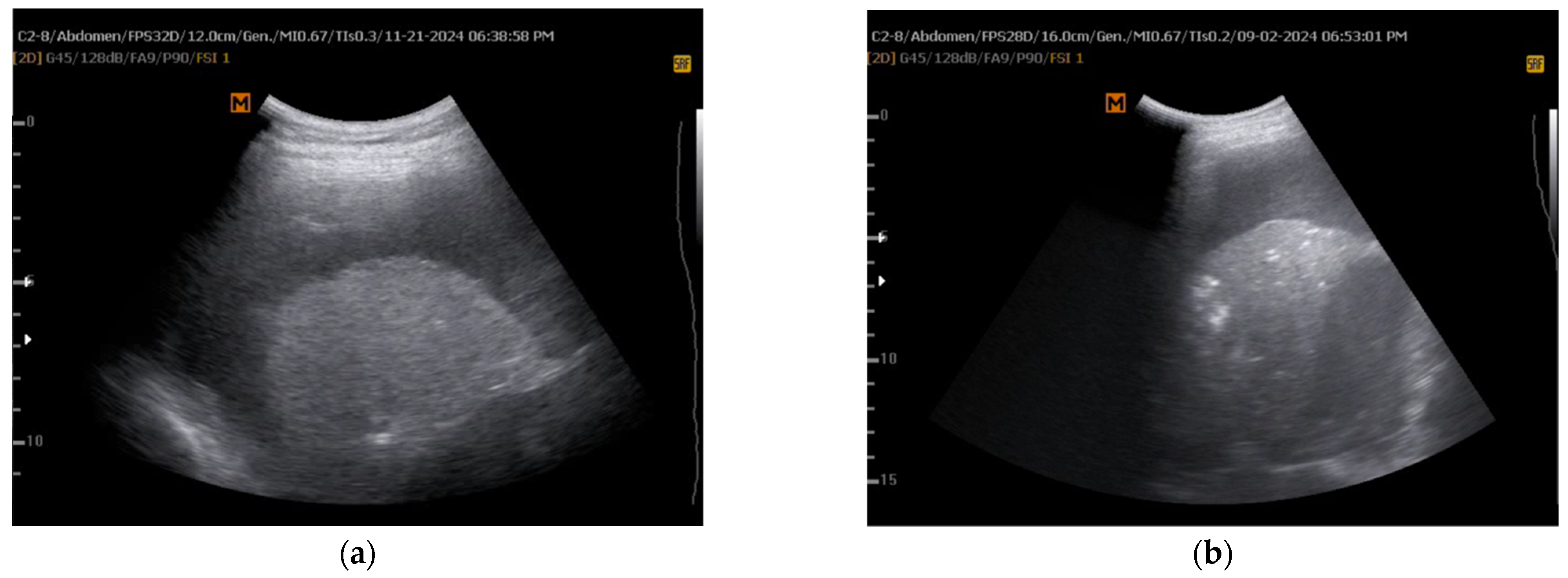
| Aspect | Pre- and Peri-Procedural Evaluation | Post-Procedural Evaluation |
|---|---|---|
| Imaging modalities |
|
|
| Key radiologic findings |
|
|
| Aims |
|
|
| Clinical impact and management |
|
|
| Limitations |
| |
| Solutions |
| |
| Study | Year | Objective | Study Design | Population | Ultrasound Technique | Main Findings | Clinical Implications | Main Limitations |
|---|---|---|---|---|---|---|---|---|
| Lichtenstein et al. [13] | 2008 | Evaluate TUS in acute respiratory failure (BLUE protocol) | Prospective observational study | Critically ill patients with respiratory distress | M-mode, B-mode | Identified sinusoid sign as indicative of lung expansion | Standardized lung ultrasound use in emergency settings | Limited generalizability beyond emergency care |
| Salamonsen et al. [15] | 2014 | Assess M-mode US for predicting NEL | Prospective observational study | 81 patients with MPE | M-mode, speckle tracking imaging (STI) | M-mode displacement <0.8 mm predictive of NEL | Allowed pre-drainage identification of entrapped lung | Some misclassification due to incomplete drainage |
| Flora et al. [16] | 2017 | Correlate sinusoid sign with pleural manometry | Prospective observational study | 10 patients undergoing thoracentesis | M-mode | Presence of sinusoid sign correlated with lung re-expansion | Suggested US as non-invasive alternative to pleural manometry | Small sample size |
| Leemans et al. [17] | 2018 | Investigate predictors of successful talc pleurodesis in malignant pleurisy | Retrospective study | 155 patients undergoing talc pleurodesis | M-mode | M-mode displacement <2 mm correlated with pleurodesis success (91% sensitivity, 88% specificity) | Highlighted role of M-mode in predicting pleurodesis success | Retrospective design, limited validation cohort |
| Wong et al. [18] | 2019 | Case report on absent sinusoid sign and trapped lung | Case report | 1 patient with metastatic breast cancer | M-mode, B-mode | Absent sinusoid sign correlated with high pleural elastance | Suggested pre-thoracentesis US assessment | Single case, needs validation |
| Hassan et al. [21] | 2021 | Validate TUS predictors of NEL | Prospective cohort | 29 patients with pleural effusion | M-mode, lung/liver echogenicity (LLE) ratio | M-mode alone was a poor predictor (AUC = 0.48), LLE ratio was better (AUC = 0.77) | LLE may serve as a better predictor for NEL than M-mode alone | Small sample size, single-center study |
| Khatim et al. [22] | 2022 | Diagnosis of NEL using TUS | Case report | 1 patient with small cell lung cancer and recurrent MPE | M-mode | Blunted cardiophasic variability indicated NEL, confirmed by post-drainage imaging | Highlighted importance of pre-procedural TUS assessment for NEL | Single case, limited generalizability |
| Petersen et al. [23] | 2024 | Compare M-mode, B-mode, and SWE for NEL prediction | Prospective observational | 49 patients with suspected MPE | M-mode, B-mode, 2D shear wave elastography (SWE) | M-mode had highest AUC (0.81) for NEL prediction | Reinforced M-mode as core tool for pleural disease assessment | Small sample, single-center variability |
| Method | Principle | Applications | Key Findings | Strengths | Limitations | Comparison to Gold-Standard Imaging | Standardization and Feasibility | Potential Future Directions |
|---|---|---|---|---|---|---|---|---|
| 2D shear wave elastography (SWE) | Measures tissue stiffness by analyzing shear wave propagation | Assessing hilar lymph nodes, subpleural masses | Less effective than B-mode and M-mode in diagnosing NEL (AUC = 0.57–0.65) | Non-invasive, quantitative, widely used in other fields (e.g., liver, breast imaging) | Poor diagnostic performance for NEL, influenced by measurement variability | Inferior for lung tissue assessment; lacks histopathological confirmation | Requires specialized ultrasound equipment and training, ROI selection critical | Optimization of ROI selection, combination with other ultrasound markers, integration with AI algorithms |
| speckle tracking imaging (STI) strain analysis | Measures myocardial and lung tissue deformation using ultrasound speckle tracking | Assessing entrapped lung in MPE | High diagnostic accuracy (AUC = 0.86), superior to M-mode and pleural elastance | Quantitative, non-invasive, better than traditional ultrasound markers for entrapped lung | Affected by lung location, motion artifacts, out-of-plane movement | Superior to pleural elastance but lacks direct comparison to CT | Requires expertise in software analysis; not widely available outside cardiology settings | Standardization of acquisition protocol, use in combination with manometry and TUS |
| lung/liver echogenicity (LLE) ratio | Compares grayscale pixel density of lung and liver via ImageJ software | Predicting lung re-expansion after pleural drainage | LLE > 1.6 predicts NEL with AUC = 0.77 | Simple, reproducible, quantitative | Limited validation, small study population, dependent on image quality | Lacks direct validation against; potential role as adjunct to manometry | Requires software (ImageJ) and proper grayscale calibration, moderately operator-dependent | Larger, multicenter studies, AI-based echogenicity analysis, validation in different effusion types |
| Dynamic air bronchogram | Visualization of air-filled bronchi moving in consolidated lung tissue | Differentiating pneumonia from atelectasis, predicting NEL | Sensitivity 92.5%, specificity 83.3% for NEL | High specificity, easily performed at bedside | Potential for false positives, limited by poor lung ultrasound window | Comparable to CT but more accessible; lacks histopathological confirmation | Widely feasible with standard ultrasound equipment, easily performed by trained operators | Further validation in different pleural disease contexts, integration with other TUS signs |
| Pleural thickening | Measurement of pleural thickness on TUS | Predicting entrapped lung and complex pleural effusions | Thickness >0.5 cm strongly associated with entrapped lung | Strong association with pathophysiology | Lacks specificity, influenced by chronic inflammation | Inferior to CT in evaluating visceral pleural thickening; lacks correlation with biopsy findings | Easy to perform, requires high-frequency probe, operator-dependent | Integration with pleural manometry, evaluation of histopathological correlation, potential role in guiding biopsy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchi, G.; Cucchiara, F.; Gregori, A.; Biondi, G.; Guglielmi, G.; Serradori, M.; Gherardi, M.; Gabbrielli, L.; Pistelli, F.; Carrozzi, L. Thoracic Ultrasound for Pre-Procedural Dynamic Assessment of Non-Expandable Lung: A Non-Invasive, Real-Time and Multifaceted Diagnostic Tool. J. Clin. Med. 2025, 14, 2062. https://doi.org/10.3390/jcm14062062
Marchi G, Cucchiara F, Gregori A, Biondi G, Guglielmi G, Serradori M, Gherardi M, Gabbrielli L, Pistelli F, Carrozzi L. Thoracic Ultrasound for Pre-Procedural Dynamic Assessment of Non-Expandable Lung: A Non-Invasive, Real-Time and Multifaceted Diagnostic Tool. Journal of Clinical Medicine. 2025; 14(6):2062. https://doi.org/10.3390/jcm14062062
Chicago/Turabian StyleMarchi, Guido, Federico Cucchiara, Alessio Gregori, Giulia Biondi, Giacomo Guglielmi, Massimiliano Serradori, Marco Gherardi, Luciano Gabbrielli, Francesco Pistelli, and Laura Carrozzi. 2025. "Thoracic Ultrasound for Pre-Procedural Dynamic Assessment of Non-Expandable Lung: A Non-Invasive, Real-Time and Multifaceted Diagnostic Tool" Journal of Clinical Medicine 14, no. 6: 2062. https://doi.org/10.3390/jcm14062062
APA StyleMarchi, G., Cucchiara, F., Gregori, A., Biondi, G., Guglielmi, G., Serradori, M., Gherardi, M., Gabbrielli, L., Pistelli, F., & Carrozzi, L. (2025). Thoracic Ultrasound for Pre-Procedural Dynamic Assessment of Non-Expandable Lung: A Non-Invasive, Real-Time and Multifaceted Diagnostic Tool. Journal of Clinical Medicine, 14(6), 2062. https://doi.org/10.3390/jcm14062062









