Clinical Significance of Extracellular Volume of Myocardium (ECV) Assessed by Computed Tomography: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
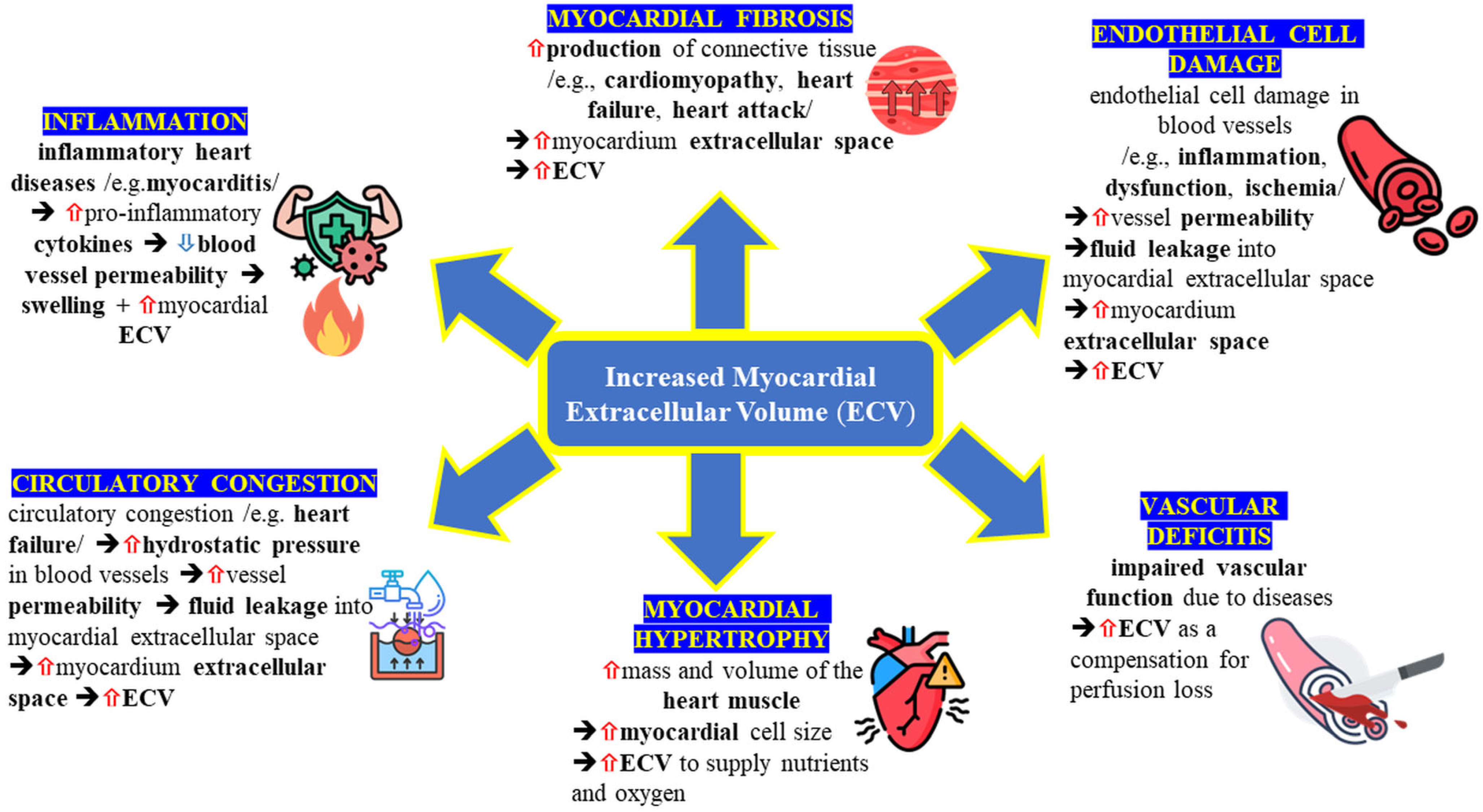
1.1. Myocardial ECV: Morphology and Pathology
1.2. Assessing ECV
2. Materials and Methods
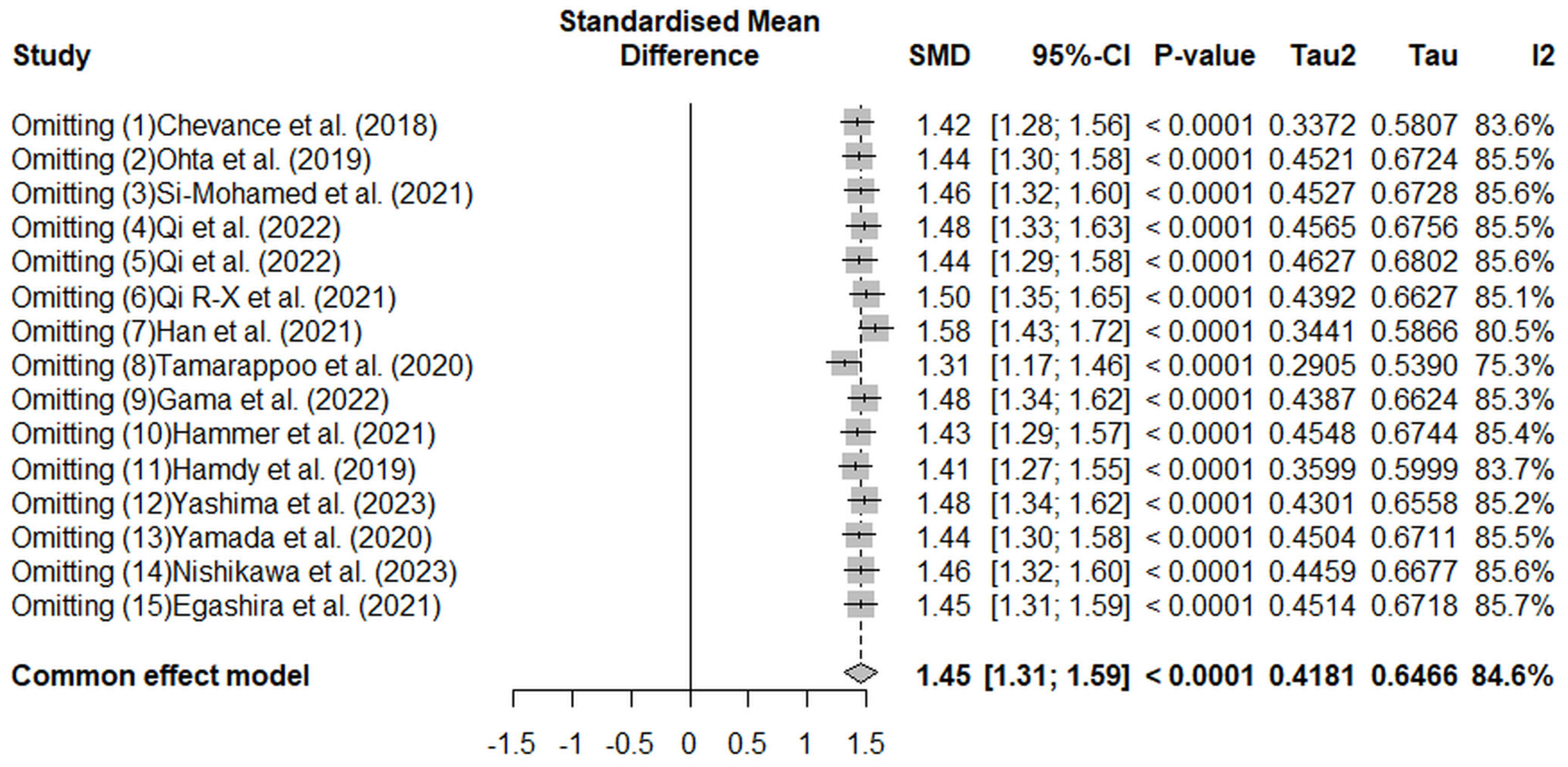
3. Results
| Authors (Year) | Selection | Comparability | Outcome | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Frustaci A. et al. (2007) | * | * | * | * | ** | * | * | * |
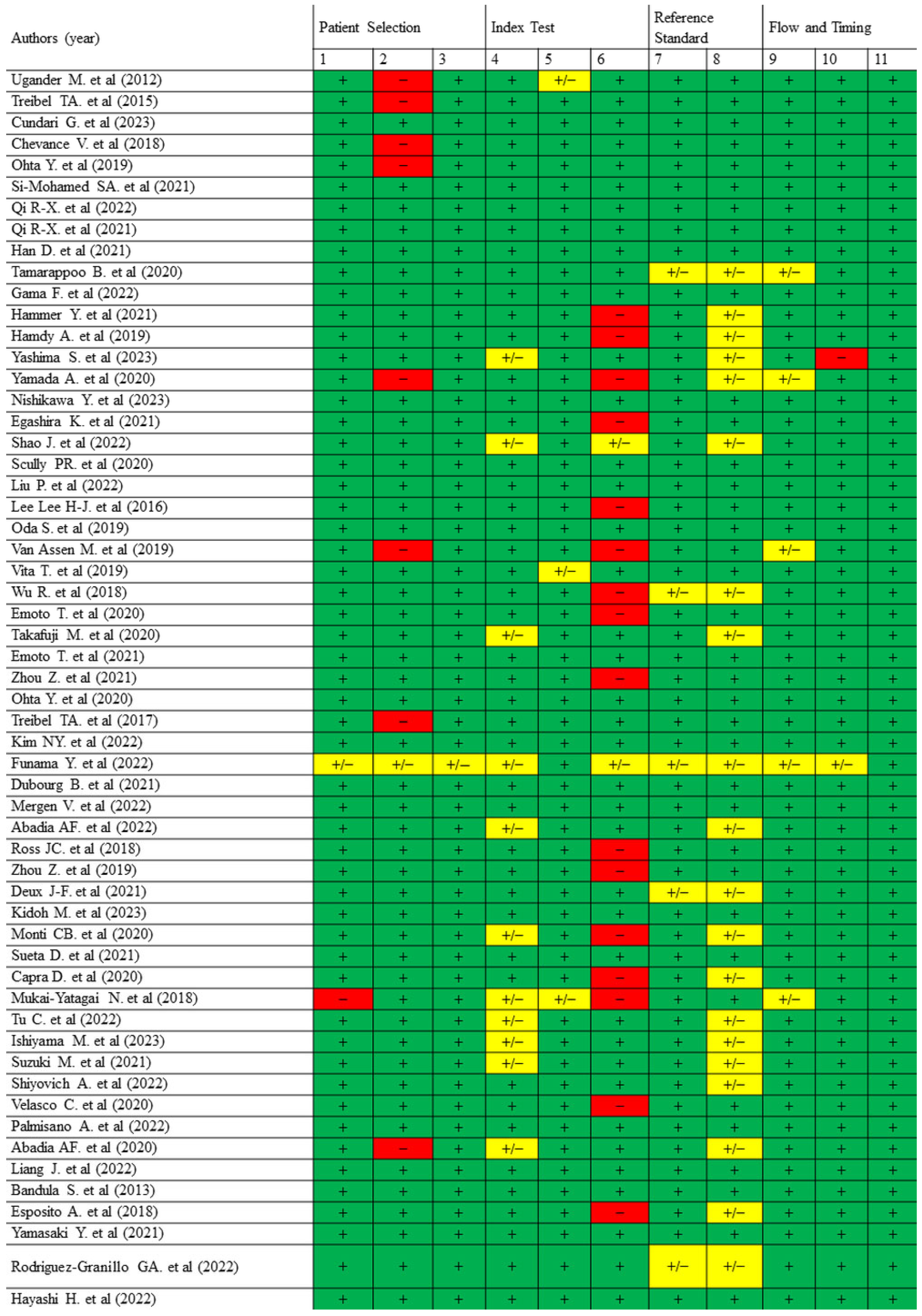
| Authors (Year) | Confounding | Selection Bias | Classification Bias | Deviation from Intervention | Missing Data | Outcome Measurement Bias | Reporting Bias | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Egashira et al. (2022) | M | M | L | L | L | L | L | M |
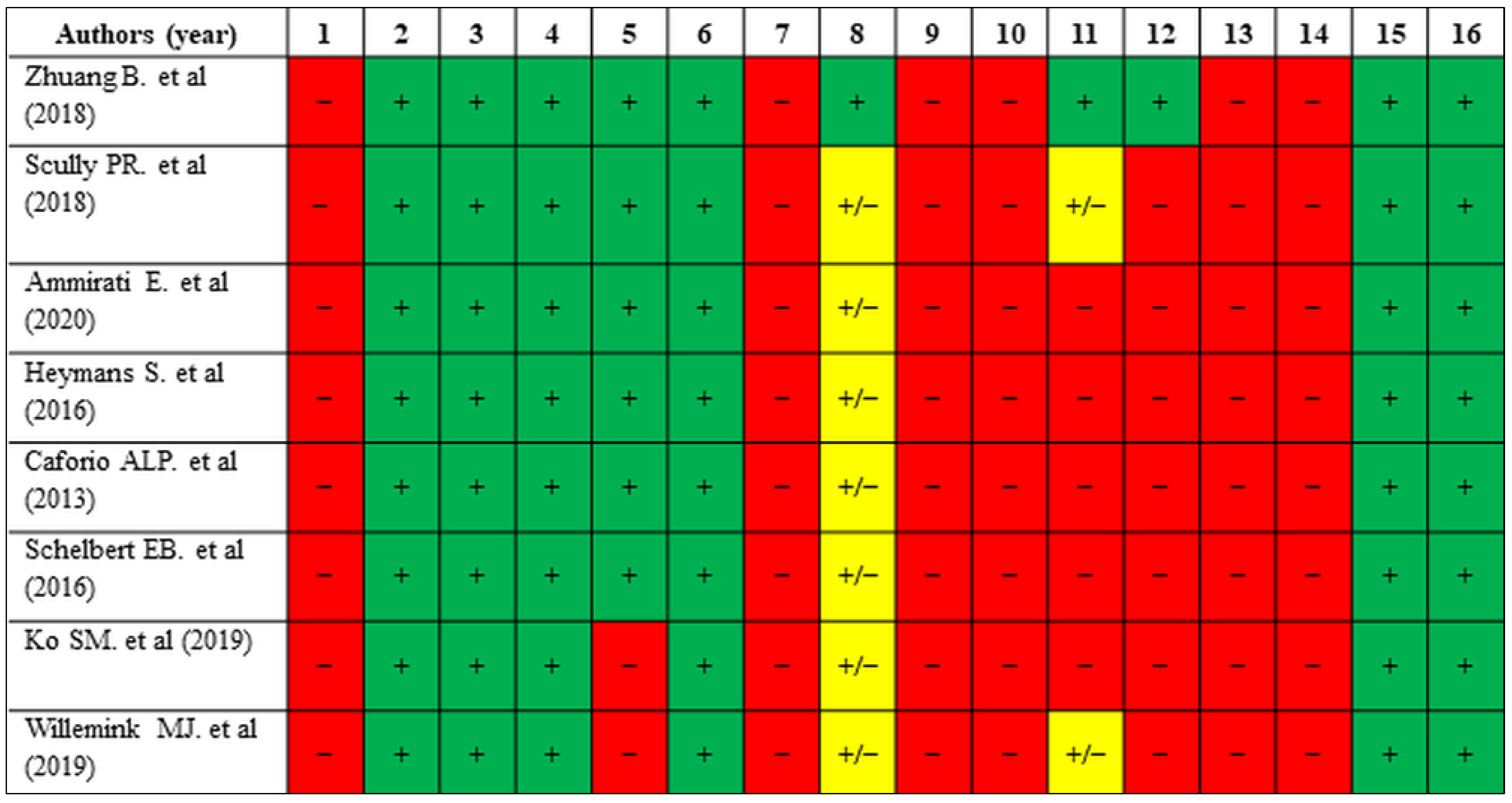
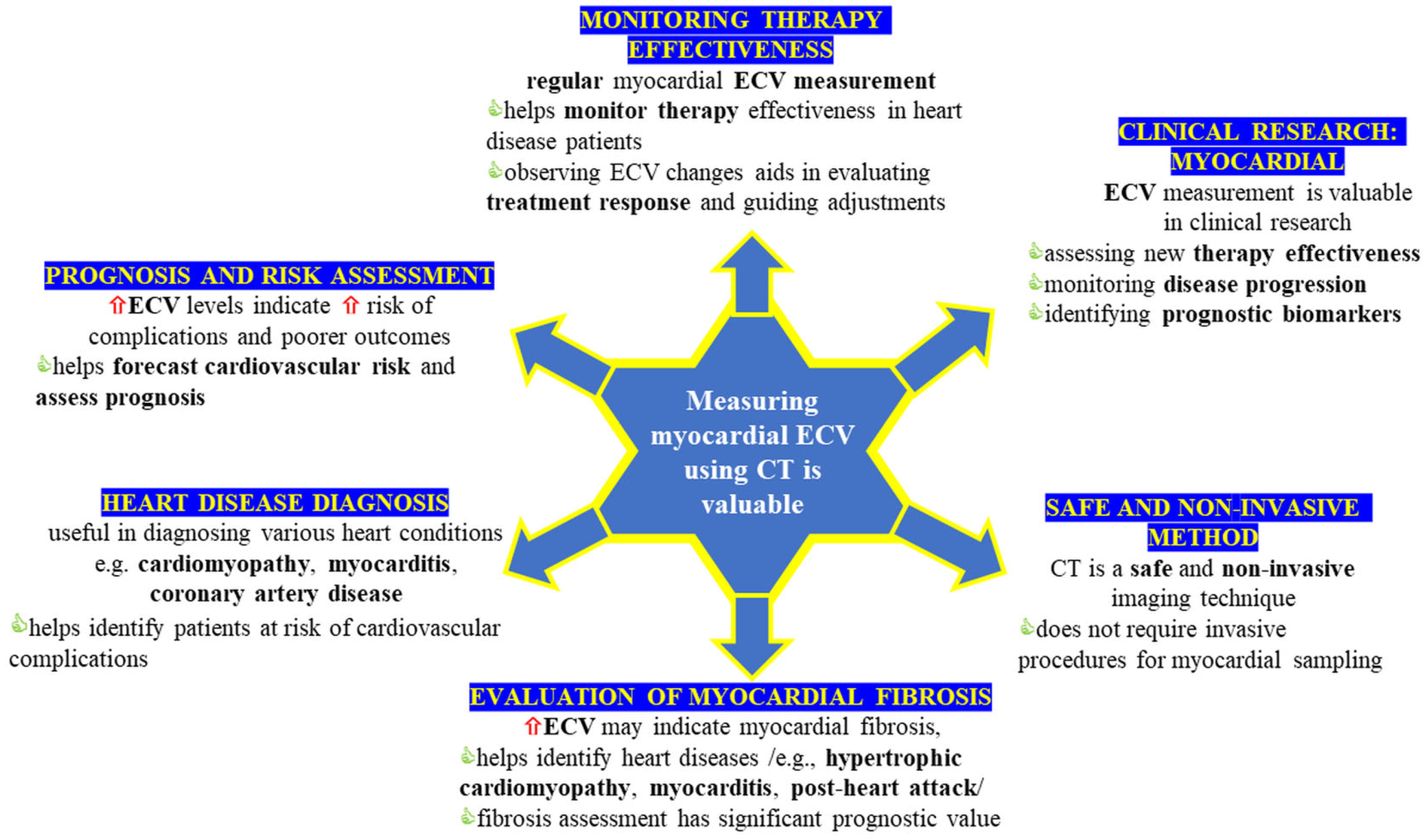
4. Discussion
4.1. Methods of Obtaining ECV from CT
4.2. Amyloidosis
4.3. Oncology
4.4. Transcatheter Aortic Valve Implantation and Aortic Stenosis
4.5. ECV in Myocarditis, Cardiomyopathy, and Other Cardiac Conditions
4.6. Other Studies
4.7. Different Ranges of Radiation Dose
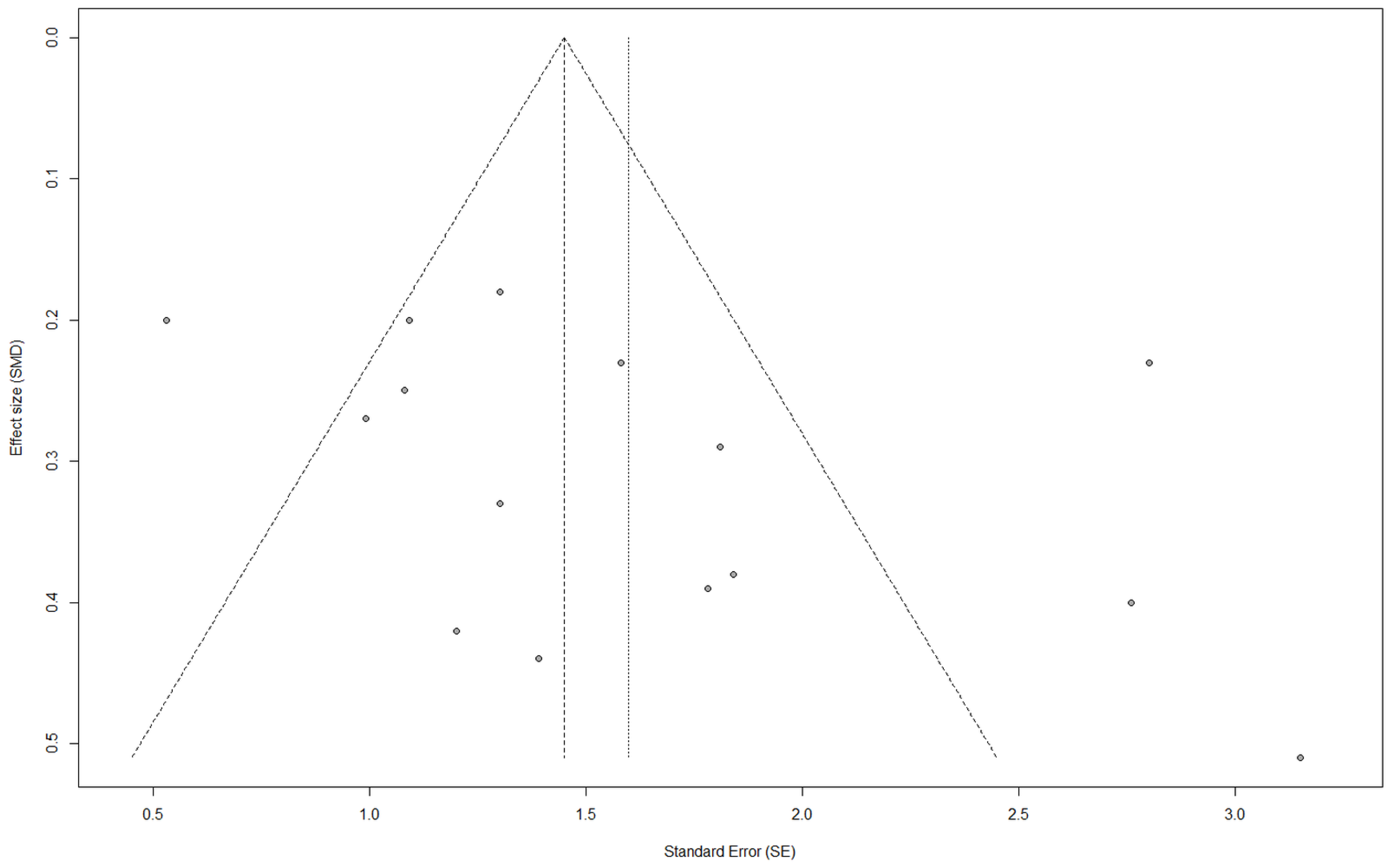
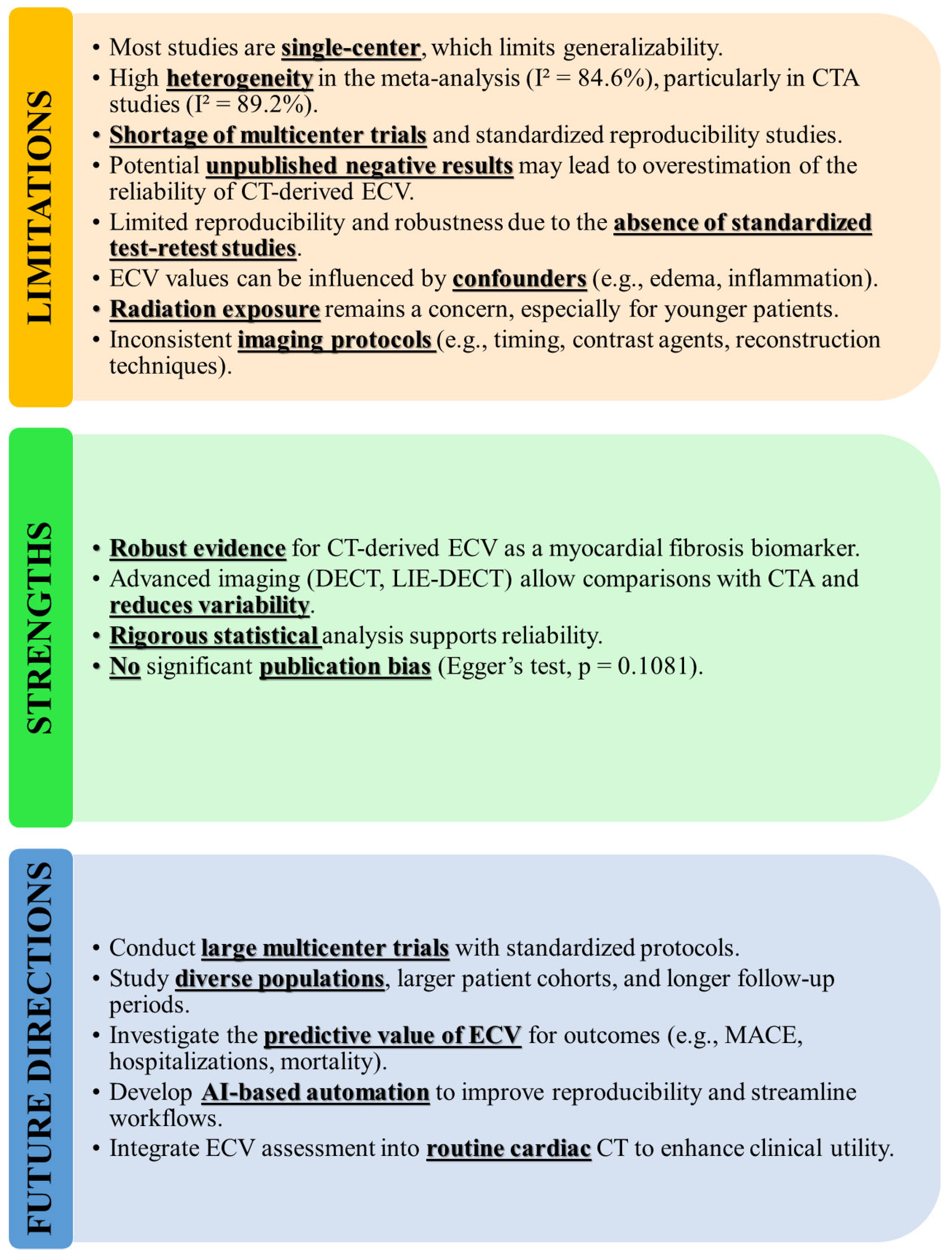
5. Limitations, Strengths, and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| AHA | American Heart Association |
| AL | Amyloidosis |
| AS | Aortic Stenosis |
| ATTR | Amyloid Transthyretin |
| AUC | Area Under The Curve |
| BNP | B-Type Natriuretic Peptide |
| CA | Cardiac Amyloidosis |
| CCT | Cardiac Computed Tomography |
| CCTA | Coronary Computed Tomography Angiography |
| CI | Confidence Interval |
| CTEPH | Chronic Thromboembolic Pulmonary Hypertension |
| CTRCD | Cancer Therapy-Related Cardiac Dysfunction |
| DCM | Dilated Cardiomyopathy |
| DE | Dual Energy |
| DECT | Dual-Energy CT |
| DESCT | Delayed-Enhancement Spectral Computed Tomography |
| DLCT | Dual-Layer Computed Tomography |
| DLP | Dose Length Product |
| ECV | Extracellular Volume |
| ECViodine | Extracellular Volume Calculated Using Iodine Contrast Agent |
| ECVsub | Extracellular Volume Of A Specific Myocardial Subregion |
| FFR | Fractional Flow Reserve |
| GLS | Global Longitudinal Strain |
| HFpEF | Heart Failure With Preserved Ejection Fraction |
| HR | Hazard Ratio |
| HU | Hounsfield Units |
| ICC | Intraclass Correlation Coefficient |
| id-CT | Iodine Density-Derived CT |
| IVST | Interventricular Septal Thickness |
| LAV | Left Atrial Volume |
| LAVI | Left Atrial Volume Index |
| LE | Late Enhancement |
| LE-CT | Late Enhancement CT |
| LGE | Late Gadolinium Enhancement |
| LIE-DECT | Late Iodine Enhancement Dual-Energy Computed Tomography |
| LV | Left Ventricle |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVEDVI | Left Ventricular End-Diastolic Volume Index |
| LVEF | Left Ventricular Ejection Fraction |
| LVESV | Left Ventricular End-Systolic Volume |
| LVESVI | Left Ventricular End-Systolic Volume Index |
| LVID | Left Ventricular Internal Diameter |
| LVM | Left Ventricular Mass |
| LVMM | Left Ventricular Myocardial Mass |
| LVMMI | Left Ventricular Myocardial Mass Index |
| MACE | Major Adverse Cardiovascular Events |
| MIC | Myocardial Iodine Concentration |
| MIF | Myocardial Interstitial Fibrosis |
| mPAP | Mean Pulmonary Artery Pressure |
| NACH | Non-Amyloid Hypertrophic Heart |
| NOS | Newcastle–Ottawa Scale |
| NYHA | New York Heart Association |
| PCD-CT | Photon-Counting Detector Computed Tomography |
| PCI | Percutaneous Coronary Intervention |
| PH | Pulmonary Hypertension |
| PWT | Posterior Wall Thickness |
| ROC | Receiver Operating Characteristic |
| RVIP | Right Ventricular Insertion Point |
| SAVR | Surgical Aortic Valve Replacement |
| SDCT | Spectral Dual Detector CT |
| sd-CT | Subtraction-Derived CT |
| SE | Standard Error |
| SECT | Single-Energy CT |
| SMD | Standard Mean Deviation |
| SSDE-CT | Single Source Dual-Energy CT |
| TAVI | Transcatheter Aortic Valve Implantation |
| TAVR | Transcatheter Aortic Valve Replacement |
| TNF | Tumor Necrosis Factors |
| TRO | Triple-Rule-Out |
| VUE | Virtual Unenhanced Attenuation |
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Sirajuddin, A.; Wang, S.; Arai, A.; Zhao, S.; Lu, M. Prognostic Value of T1 Mapping and Extracellular Volume Fraction in Cardiovascular Disease: A Systematic Review and Meta-Analysis. Heart Fail. Rev. 2018, 23, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Bastarrika, G.; Moon, J.C.; Treibel, T.A. Myocardial Extracellular Volume Quantification by Cardiovascular Magnetic Resonance and Computed Tomography. Curr. Cardiol. Rep. 2018, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Schelbert, E.B.; Messroghli, D.R. State of the Art: Clinical Applications of Cardiac T1 Mapping. Radiology 2016, 278, 658–676. [Google Scholar] [CrossRef]
- Ugander, M.; Oki, A.J.; Hsu, L.-Y.; Kellman, P.; Greiser, A.; Aletras, A.H.; Sibley, C.T.; Chen, M.Y.; Bandettini, W.P.; Arai, A.E. Extracellular Volume Imaging by Magnetic Resonance Imaging Provides Insights into Overt and Sub-Clinical Myocardial Pathology. Eur. Heart J. 2012, 33, 1268–1278. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020, 13, e007405. [Google Scholar] [CrossRef]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Frustaci, A.; Verardo, R.; Caldarulo, M.; Acconcia, M.C.; Russo, M.A.; Chimenti, C. Myocarditis in Hypertrophic Cardiomyopathy Patients Presenting Acute Clinical Deterioration. Eur. Heart J. 2007, 28, 733–740. [Google Scholar] [CrossRef]
- Treibel, T.A.; Bandula, S.; Fontana, M.; White, S.K.; Gilbertson, J.A.; Herrey, A.S.; Gillmore, J.D.; Punwani, S.; Hawkins, P.N.; Taylor, S.A.; et al. Extracellular Volume Quantification by Dynamic Equilibrium Cardiac Computed Tomography in Cardiac Amyloidosis. J. Cardiovasc. Comput. Tomogr. 2015, 9, 585–592. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Cundari, G.; Galea, N.; Mergen, V.; Alkadhi, H.; Eberhard, M. Myocardial Extracellular Volume Quantification with Computed Tomography—Current Status and Future Outlook. Insights Imaging 2023, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Xu, B.; Liu, J.; Wang, G.; An, J.; Zhang, X.; Wang, R.; Dong, W.; Guan, Z. Diagnostic Value of 11C-PIB PET/MR in Cardiac Amyloidosis. Front. Cardiovasc. Med. 2022, 9, 830572. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef]
- Stang, A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane. 2024. Available online: https://training.cochrane.org/handbook/ (accessed on 1 March 2025).
- Chevance, V.; Damy, T.; Tacher, V.; Legou, F.; Ridouani, F.; Luciani, A.; Kobeiter, H.; Rahmouni, A.; Deux, J.-F. Myocardial Iodine Concentration Measurement Using Dual-Energy Computed Tomography for the Diagnosis of Cardiac Amyloidosis: A Pilot Study. Eur. Radiol. 2018, 28, 816–823. [Google Scholar] [CrossRef]
- Ohta, Y.; Kitao, S.; Yunaga, H.; Watanabe, T.; Mukai—Yatagai, N.; Kishimoto, J.; Yamamoto, K.; Ogawa, T. Quantitative Evaluation of Non-Ischemic Dilated Cardiomyopathy by Late Iodine Enhancement Using Rapid kV Switching Dual-Energy Computed Tomography: A Feasibility Study. J. Cardiovasc. Comput. Tomogr. 2019, 13, 148–156. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Restier, L.M.; Branchu, A.; Boccalini, S.; Congi, A.; Ziegler, A.; Tomasevic, D.; Bochaton, T.; Boussel, L.; Douek, P.C. Diagnostic Performance of Extracellular Volume Quantified by Dual-Layer Dual-Energy CT for Detection of Acute Myocarditis. J. Clin. Med. 2021, 10, 3286. [Google Scholar] [CrossRef]
- Qi, R.-X.; Jiang, J.-S.; Shao, J.; Zhang, Q.; Zheng, K.-L.; Xiao, J.; Huang, S.; Gong, S.-C. Measurement of Myocardial Extracellular Volume Fraction in Patients with Heart Failure with Preserved Ejection Fraction Using Dual-Energy Computed Tomography. Eur. Radiol. 2022, 32, 4253–4263. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.-X.; Shao, J.; Jiang, J.-S.; Ruan, X.-W.; Huang, S.; Zhang, Q.; Hu, C.-H. Myocardial Extracellular Volume Fraction Quantitation Using Cardiac Dual-Energy CT with Late Iodine Enhancement in Patients with Heart Failure without Coronary Artery Disease: A Single-Center Prospective Study. Eur. J. Radiol. 2021, 140, 109743. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Tamarappoo, B.; Klein, E.; Tyler, J.; Chakravarty, T.; Otaki, Y.; Miller, R.; Eisenberg, E.; Park, R.; Singh, S.; et al. Computed Tomography Angiography-Derived Extracellular Volume Fraction Predicts Early Recovery of Left Ventricular Systolic Function after Transcatheter Aortic Valve Replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 179–185. [Google Scholar] [CrossRef]
- Tamarappoo, B.; Han, D.; Tyler, J.; Chakravarty, T.; Otaki, Y.; Miller, R.; Eisenberg, E.; Singh, S.; Shiota, T.; Siegel, R.; et al. Prognostic Value of Computed Tomography–Derived Extracellular Volume in TAVR Patients with Low-Flow Low-Gradient Aortic Stenosis. JACC Cardiovasc. Imaging 2020, 13, 2591–2601. [Google Scholar] [CrossRef]
- Gama, F.; Rosmini, S.; Bandula, S.; Patel, K.P.; Massa, P.; Tobon-Gomez, C.; Ecke, K.; Stroud, T.; Condron, M.; Thornton, G.D.; et al. Extracellular Volume Fraction by Computed Tomography Predicts Long-Term Prognosis Among Patients with Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 2082–2094. [Google Scholar] [CrossRef]
- Hammer, Y.; Talmor-Barkan, Y.; Abelow, A.; Orvin, K.; Aviv, Y.; Bar, N.; Levi, A.; Landes, U.; Shafir, G.; Barsheshet, A.; et al. Myocardial Extracellular Volume Quantification by Computed Tomography Predicts Outcomes in Patients with Severe Aortic Stenosis. PLoS ONE 2021, 16, e0248306. [Google Scholar] [CrossRef]
- Hamdy, A.; Kitagawa, K.; Goto, Y.; Yamada, A.; Nakamura, S.; Takafuji, M.; Nagasawa, N.; Sakuma, H. Comparison of the Different Imaging Time Points in Delayed Phase Cardiac CT for Myocardial Scar Assessment and Extracellular Volume Fraction Estimation in Patients with Old Myocardial Infarction. Int. J. Cardiovasc. Imaging 2019, 35, 917–926. [Google Scholar] [CrossRef]
- Yashima, S.; Takaoka, H.; Iwahana, T.; Nishikawa, Y.; Ota, J.; Aoki, S.; Kinoshita, M.; Takahashi, M.; Sasaki, H.; Suzuki-Eguchi, N.; et al. Evaluation of Extracellular Volume by Computed Tomography Is Useful for Prediction of Prognosis in Dilated Cardiomyopathy. Heart Vessels 2023, 38, 185–194. [Google Scholar] [CrossRef]
- Yamada, A.; Kitagawa, K.; Nakamura, S.; Takafuji, M.; Goto, Y.; Okamoto, R.; Dohi, K.; Sakuma, H. Quantification of Extracellular Volume Fraction by Cardiac Computed Tomography for Noninvasive Assessment of Myocardial Fibrosis in Hemodialysis Patients. Sci. Rep. 2020, 10, 15367. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Takaoka, H.; Kanaeda, T.; Takahira, H.; Suzuki, S.; Aoki, S.; Goto, H.; Suzuki, K.; Yashima, S.; Takahashi, M.; et al. A New Composite Indicator Consisting of Left Ventricular Extracellular Volume, N-Terminal Fragment of B-Type Natriuretic Peptide, and Left Ventricular End-Diastolic Volume Is Useful for Predicting Reverse Remodeling after Catheter Ablation for Atrial Fibrillation. Heart Vessels 2023, 38, 721–730. [Google Scholar] [CrossRef]
- Egashira, K.; Sueta, D.; Tomiguchi, M.; Kidoh, M.; Oda, S.; Usuku, H.; Hidaka, K.; Goto-Yamaguchi, L.; Sueta, A.; Komorita, T.; et al. Cardiac Computed Tomography-Derived Extracellular Volume Fraction in Late Anthracycline-Induced Cardiotoxicity. IJC Heart Vasc. 2021, 34, 100797. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Jiang, J.-S.; Wang, X.-Y.; Wu, S.-M.; Xiao, J.; Zheng, K.-L.; Qi, R.-X. Measurement of Myocardial Extracellular Volume Using Cardiac Dual-Energy Computed Tomography in Patients with Ischaemic Cardiomyopathy: A Comparison of Different Methods. Int. J. Cardiovasc. Imaging 2022, 38, 1591–1600. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Patel, K.P.; Saberwal, B.; Klotz, E.; Augusto, J.B.; Thornton, G.D.; Hughes, R.K.; Manisty, C.; Lloyd, G.; Newton, J.D.; et al. Identifying Cardiac Amyloid in Aortic Stenosis. JACC Cardiovasc. Imaging 2020, 13, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lin, L.; Xu, C.; Han, Y.; Lin, X.; Hou, Y.; Lu, X.; Vembar, M.; Jin, Z.; Wang, Y. Quantitative Analysis of Late Iodine Enhancement Using Dual-Layer Spectral Detector Computed Tomography: Comparison with Magnetic Resonance Imaging. Quant. Imaging Med. Surg. 2022, 12, 310–320. [Google Scholar] [CrossRef]
- Lee, H.-J.; Im, D.J.; Youn, J.-C.; Chang, S.; Suh, Y.J.; Hong, Y.J.; Kim, Y.J.; Hur, J.; Choi, B.W. Myocardial Extracellular Volume Fraction with Dual-Energy Equilibrium Contrast-Enhanced Cardiac CT in Nonischemic Cardiomyopathy: A Prospective Comparison with Cardiac MR Imaging. Radiology 2016, 280, 49–57. [Google Scholar] [CrossRef]
- Oda, S.; Emoto, T.; Nakaura, T.; Kidoh, M.; Utsunomiya, D.; Funama, Y.; Nagayama, Y.; Takashio, S.; Ueda, M.; Yamashita, T.; et al. Myocardial Late Iodine Enhancement and Extracellular Volume Quantification with Dual-Layer Spectral Detector Dual-Energy Cardiac CT. Radiol. Cardiothorac. Imaging 2019, 1, e180003. [Google Scholar] [CrossRef]
- Van Assen, M.; De Cecco, C.N.; Sahbaee, P.; Eid, M.H.; Griffith, L.P.; Bauer, M.J.; Savage, R.H.; Varga-Szemes, A.; Oudkerk, M.; Vliegenthart, R.; et al. Feasibility of Extracellular Volume Quantification Using Dual-Energy CT. J. Cardiovasc. Comput. Tomogr. 2019, 13, 81–84. [Google Scholar] [CrossRef]
- Vita, T.; Gräni, C.; Abbasi, S.A.; Neilan, T.G.; Rowin, E.; Kaneko, K.; Coelho-Filho, O.; Watanabe, E.; Mongeon, F.-P.; Farhad, H.; et al. Comparing CMR Mapping Methods and Myocardial Patterns Toward Heart Failure Outcomes in Nonischemic Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2019, 12, 1659–1669. [Google Scholar] [CrossRef]
- Wu, R.; Hori, M.; Onishi, H.; Nakamoto, A.; Fukui, H.; Ota, T.; Nishida, T.; Enchi, Y.; Satoh, K.; Tomiyama, N. Effects of Reconstruction Technique on the Quality of Abdominal CT Angiography: A Comparison between Forward Projected Model-Based Iterative Reconstruction Solution (FIRST) and Conventional Reconstruction Methods. Eur. J. Radiol. 2018, 106, 100–105. [Google Scholar] [CrossRef]
- Emoto, T.; Kidoh, M.; Oda, S.; Nakaura, T.; Nagayama, Y.; Sasao, A.; Funama, Y.; Araki, S.; Takashio, S.; Sakamoto, K.; et al. Myocardial Extracellular Volume Quantification in Cardiac CT: Comparison of the Effects of Two Different Iterative Reconstruction Algorithms with MRI as a Reference Standard. Eur. Radiol. 2020, 30, 691–701. [Google Scholar] [CrossRef]
- Takafuji, M.; Kitagawa, K.; Nakamura, S.; Hamdy, A.; Goto, Y.; Ishida, M.; Sakuma, H. Feasibility of Extracellular Volume Fraction Calculation Using Myocardial CT Delayed Enhancement with Low Contrast Media Administration. J. Cardiovasc. Comput. Tomogr. 2020, 14, 524–528. [Google Scholar] [CrossRef]
- Emoto, T.; Oda, S.; Kidoh, M.; Nakaura, T.; Nagayama, Y.; Sakabe, D.; Kakei, K.; Goto, M.; Funama, Y.; Hatemura, M.; et al. Myocardial Extracellular Volume Quantification Using Cardiac Computed Tomography: A Comparison of the Dual-Energy Iodine Method and the Standard Subtraction Method. Acad. Radiol. 2021, 28, e119–e126. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, Y.; Wang, H.; Wang, W.; Zhang, H.; Wang, S.; Sun, Z.; Xu, L. Myocardial Extracellular Volume Fraction Analysis in Doxorubicin-Induced Beagle Models: Comparison of Dual-Energy CT with Equilibrium Contrast-Enhanced Single-Energy CT. Cardiovasc. Diagn. Ther. 2021, 11, 102–110. [Google Scholar] [CrossRef]
- Ohta, Y.; Kishimoto, J.; Kitao, S.; Yunaga, H.; Mukai-Yatagai, N.; Fujii, S.; Yamamoto, K.; Fukuda, T.; Ogawa, T. Investigation of Myocardial Extracellular Volume Fraction in Heart Failure Patients Using Iodine Map with Rapid-kV Switching Dual-Energy CT: Segmental Comparison with MRI T1 Mapping. J. Cardiovasc. Comput. Tomogr. 2020, 14, 349–355. [Google Scholar] [CrossRef]
- Treibel, T.A.; Fontana, M.; Steeden, J.A.; Nasis, A.; Yeung, J.; White, S.K.; Sivarajan, S.; Punwani, S.; Pugliese, F.; Taylor, S.A.; et al. Automatic Quantification of the Myocardial Extracellular Volume by Cardiac Computed Tomography: Synthetic ECV by CCT. J. Cardiovasc. Comput. Tomogr. 2017, 11, 221–226. [Google Scholar] [CrossRef]
- Kim, N.Y.; Im, D.J.; Youn, J.-C.; Hong, Y.J.; Choi, B.W.; Kang, S.-M.; Lee, H.-J. Synthetic Extracellular Volume Fraction Derived Using Virtual Unenhanced Attenuation of Blood on Contrast-Enhanced Cardiac Dual-Energy CT in Nonischemic Cardiomyopathy. Am. J. Roentgenol. 2022, 218, 454–461. [Google Scholar] [CrossRef]
- Funama, Y.; Oda, S.; Kidoh, M.; Sakabe, D.; Nakaura, T. Effect of Image Quality on Myocardial Extracellular Volume Quantification Using Cardiac Computed Tomography: A Phantom Study. Acta Radiol. 2022, 63, 159–165. [Google Scholar] [CrossRef]
- Dubourg, B.; Dacher, J.-N.; Durand, E.; Caudron, J.; Bauer, F.; Bubenheim, M.; Eltchaninoff, H.; Serfaty, J.-M. Single-Source Dual Energy CT to Assess Myocardial Extracellular Volume Fraction in Aortic Stenosis before Transcatheter Aortic Valve Implantation (TAVI). Diagn. Interv. Imaging 2021, 102, 561–570. [Google Scholar] [CrossRef]
- Mergen, V.; Sartoretti, T.; Klotz, E.; Schmidt, B.; Jungblut, L.; Higashigaito, K.; Manka, R.; Euler, A.; Kasel, M.; Eberhard, M.; et al. Extracellular Volume Quantification With Cardiac Late Enhancement Scanning Using Dual-Source Photon-Counting Detector CT. Invest. Radiol. 2022, 57, 406–411. [Google Scholar] [CrossRef]
- Abadia, A.F.; Aquino, G.J.; Schoepf, U.J.; Wels, M.; Schmidt, B.; Sahbaee, P.; Dargis, D.M.; Burt, J.R.; Varga-Szemes, A.; Emrich, T. Automated Dual-Energy Computed Tomography-Based Extracellular Volume Estimation for Myocardial Characterization in Patients With Ischemic and Nonischemic Cardiomyopathy. J. Thorac. Imaging 2022, 37, 307–314. [Google Scholar] [CrossRef]
- Ross, J.C.; Hutt, D.F.; Burniston, M.; Page, J.; Steeden, J.A.; Gillmore, J.D.; Wechalekar, A.D.; Hawkins, P.N.; Fontana, M. Quantitation of 99m Tc-DPD Uptake in Patients with Transthyretin-Related Cardiac Amyloidosis. Amyloid 2018, 25, 203–210. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, L.; Wang, R.; Varga-Szemes, A.; Durden, J.A.; Joseph Schoepf, U.; Sun, Z.; Fan, Z. Quantification of Doxorubicin-Induced Interstitial Myocardial Fibrosis in a Beagle Model Using Equilibrium Contrast-Enhanced Computed Tomography: A Comparative Study with Cardiac Magnetic Resonance T1-Mapping. Int. J. Cardiol. 2019, 281, 150–155. [Google Scholar] [CrossRef]
- Deux, J.-F.; Nouri, R.; Tacher, V.; Zaroui, A.; Derbel, H.; Sifaoui, I.; Chevance, V.; Ridouani, F.; Galat, A.; Kharoubi, M.; et al. Diagnostic Value of Extracellular Volume Quantification and Myocardial Perfusion Analysis at CT in Cardiac Amyloidosis. Radiology 2021, 300, 326–335. [Google Scholar] [CrossRef]
- Kidoh, M.; Oda, S.; Takashio, S.; Hirakawa, K.; Kawano, Y.; Shiraishi, S.; Hayashi, H.; Nakaura, T.; Nagayama, Y.; Funama, Y.; et al. CT Extracellular Volume Fraction versus Myocardium-to-Lumen Signal Ratio for Cardiac Amyloidosis. Radiology 2023, 306, e220542. [Google Scholar] [CrossRef]
- Monti, C.B.; Zanardo, M.; Bosetti, T.; Alì, M.; De Benedictis, E.; Luporini, A.; Secchi, F.; Sardanelli, F. Assessment of Myocardial Extracellular Volume on Body Computed Tomography in Breast Cancer Patients Treated with Anthracyclines. Quant. Imaging Med. Surg. 2020, 10, 934–944. [Google Scholar] [CrossRef]
- Sueta, D.; Kidoh, M.; Oda, S.; Egashira, K.; Yamamoto, E.; Kaikita, K.; Matsushita, K.; Yamamoto, Y.; Hirai, T.; Tsujita, K. Usefulness of Cardiac Computed Tomography in the Diagnosis of Anti-Cancer Therapy-Related Cardiac Dysfunction—Consistency with Magnetic Resonance Imaging—. Circ. J. 2021, 85, 393–396. [Google Scholar] [CrossRef]
- Capra, D.; Monti, C.B.; Luporini, A.G.; Lombardi, F.; Gumina, C.; Sironi, A.; Asti, E.L.G.; Bonavina, L.; Secchi, F.; Sardanelli, F. Computed Tomography-Derived Myocardial Extracellular Volume: An Early Biomarker of Cardiotoxicity in Esophageal Cancer Patients Undergoing Radiation Therapy. Insights Imaging 2020, 11, 120. [Google Scholar] [CrossRef]
- Mukai-Yatagai, N.; Haruki, N.; Kinugasa, Y.; Ohta, Y.; Ishibashi-Ueda, H.; Akasaka, T.; Kato, M.; Ogawa, T.; Yamamoto, K. Assessment of Myocardial Fibrosis Using T1-Mapping and Extracellular Volume Measurement on Cardiac Magnetic Resonance Imaging for the Diagnosis of Radiation-Induced Cardiomyopathy. J. Cardiol. Cases 2018, 18, 132–135. [Google Scholar] [CrossRef]
- Tu, C.; Shen, H.; Liu, R.; Wang, X.; Li, X.; Yuan, X.; Chen, Q.; Wang, Y.; Ran, Z.; Lan, X.; et al. Myocardial Extracellular Volume Derived from Contrast-Enhanced Chest Computed Tomography for Longitudinal Evaluation of Cardiotoxicity in Patients with Breast Cancer Treated with Anthracyclines. Insights Imaging 2022, 13, 85. [Google Scholar] [CrossRef]
- Ishiyama, M.; Kurita, T.; Takafuji, M.; Sato, K.; Sugiura, E.; Nakamori, S.; Fujimoto, N.; Kitagawa, K.; Sakuma, H.; Dohi, K. The Cardiac Computed Tomography-Derived Extracellular Volume Fraction Predicts Patient Outcomes and Left Ventricular Mass Reductions after Transcatheter Aortic Valve Implantation for Aortic Stenosis. J. Cardiol. 2023, 81, 476–484. [Google Scholar] [CrossRef]
- Suzuki, M.; Toba, T.; Izawa, Y.; Fujita, H.; Miwa, K.; Takahashi, Y.; Toh, H.; Kawamori, H.; Otake, H.; Tanaka, H.; et al. Prognostic Impact of Myocardial Extracellular Volume Fraction Assessment Using Dual-Energy Computed Tomography in Patients Treated With Aortic Valve Replacement for Severe Aortic Stenosis. J. Am. Heart Assoc. 2021, 10, e020655. [Google Scholar] [CrossRef] [PubMed]
- Shiyovich, A.; Plakht, Y.; Hammer, Y.; Aviv, Y.; Wiessman, M.; Shafir, G.; Assa, H.V.; Kornowski, R.; Hamdan, A. Relation of Serum Albumin Levels to Myocardial Extracellular Volume in Patients With Severe Aortic Stenosis. Am. J. Cardiol. 2022, 163, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Velasco, C.; Mota-Cobián, A.; Mota, R.A.; Pellico, J.; Herranz, F.; Galán-Arriola, C.; Ibáñez, B.; Ruiz-Cabello, J.; Mateo, J.; España, S. Quantitative Assessment of Myocardial Blood Flow and Extracellular Volume Fraction Using 68Ga-DOTA-PET: A Feasibility and Validation Study in Large Animals. J. Nucl. Cardiol. 2020, 27, 1249–1260. [Google Scholar] [CrossRef]
- Palmisano, A.; Vignale, D.; Tadic, M.; Moroni, F.; De Stefano, D.; Gatti, M.; Boccia, E.; Faletti, R.; Oppizzi, M.; Peretto, G.; et al. Myocardial Late Contrast Enhancement CT in Troponin-Positive Acute Chest Pain Syndrome. Radiology 2022, 302, 545–553. [Google Scholar] [CrossRef]
- Abadia, A.F.; Van Assen, M.; Martin, S.S.; Vingiani, V.; Griffith, L.P.; Giovagnoli, D.A.; Bauer, M.J.; Schoepf, U.J. Myocardial Extracellular Volume Fraction to Differentiate Healthy from Cardiomyopathic Myocardium Using Dual-Source Dual-Energy CT. J. Cardiovasc. Comput. Tomogr. 2020, 14, 162–167. [Google Scholar] [CrossRef]
- Liang, J.; Li, H.; Xie, J.; Yu, H.; Chen, W.; Yin, K.; Chen, X.; Sheng, Z.; Zhang, X.; Mu, D. Iodine-Based Extracellular Volume for Evaluating Myocardial Status in Patients Undergoing Percutaneous Coronary Intervention for Acute Myocardial Infarction by Using Dual-Layer Spectral Detector Computed Tomography: A Comparison Study with Magnetic Resonance. Quant. Imaging Med. Surg. 2022, 12, 4502–4511. [Google Scholar] [CrossRef]
- Bandula, S.; White, S.K.; Flett, A.S.; Lawrence, D.; Pugliese, F.; Ashworth, M.T.; Punwani, S.; Taylor, S.A.; Moon, J.C. Measurement of Myocardial Extracellular Volume Fraction by Using Equilibrium Contrast-Enhanced CT: Validation against Histologic Findings. Radiology 2013, 269, 396–403. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Antunes, S.; Colantoni, C.; Rancoita, P.M.V.; Vignale, D.; Baratto, F.; Della Bella, P.; Del Maschio, A.; De Cobelli, F. Assessment of Remote Myocardium Heterogeneity in Patients with Ventricular Tachycardia Using Texture Analysis of Late Iodine Enhancement (LIE) Cardiac Computed Tomography (cCT) Images. Mol. Imaging Biol. 2018, 20, 816–825. [Google Scholar] [CrossRef]
- Yamasaki, Y.; Abe, K.; Kamitani, T.; Sagiyama, K.; Hida, T.; Hosokawa, K.; Matsuura, Y.; Hioki, K.; Nagao, M.; Yabuuchi, H.; et al. Right Ventricular Extracellular Volume with Dual-Layer Spectral Detector CT: Value in Chronic Thromboembolic Pulmonary Hypertension. Radiology 2021, 298, 589–596. [Google Scholar] [CrossRef]
- Rodriguez-Granillo, G.A.; Cirio, J.J.; Ciardi, C.; Caballero, M.L.; Fontana, L.A.; Buezas, M.D.; Diluca, P.; Lylyk, P. Hyperacute Incidental Late Myocardial Enhancement in Ischemic Stroke Using Chest Spectral CT: Relationship with Etiology. Rev. Cardiovasc. Med. 2022, 23, 093. [Google Scholar] [CrossRef]
- Hayashi, H.; Oda, S.; Emoto, T.; Kidoh, M.; Nagayama, Y.; Nakaura, T.; Sakabe, D.; Tokuyasu, S.; Hirakawa, K.; Takashio, S.; et al. Myocardial Extracellular Volume Quantification by Cardiac CT in Pulmonary Hypertension: Comparison with Cardiac MRI. Eur. J. Radiol. 2022, 153, 110386. [Google Scholar] [CrossRef] [PubMed]
- Egashira, K.; Sueta, D.; Kidoh, M.; Tomiguchi, M.; Oda, S.; Usuku, H.; Hidaka, K.; Goto-Yamaguchi, L.; Sueta, A.; Komorita, T.; et al. Cardiac Computed Tomography-derived Myocardial Tissue Characterization after Anthracycline Treatment. ESC Heart Fail. 2022, 9, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Hwang, S.H.; Lee, H.-J. Role of Cardiac Computed Tomography in the Diagnosis of Left Ventricular Myocardial Diseases. J. Cardiovasc. Imaging 2019, 27, 73. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Noël, P.B. The Evolution of Image Reconstruction for CT—From Filtered Back Projection to Artificial Intelligence. Eur. Radiol. 2019, 29, 2185–2195. [Google Scholar] [CrossRef]
- Nishihara, T.; Oda, S.; Sueta, D.; Izumiya, Y.; Kaikita, K.; Tsujita, K.; Utsunomiya, D.; Nakaura, T.; Yamashita, Y. Clinical Usefulness of Dual-Energy Cardiac Computed Tomography in Acute Coronary Syndrome Using a Dual-Layer Spectral Detector Scanner. Circ. Cardiovasc. Imaging 2018, 11, e007277. [Google Scholar] [CrossRef]
- Esposito, A.; Palmisano, A.; Barbera, M.; Vignale, D.; Benedetti, G.; Spoladore, R.; Ancona, M.B.; Giannini, F.; Oppizzi, M.; Del Maschio, A.; et al. Cardiac Computed Tomography in Troponin-Positive Chest Pain. JACC Cardiovasc. Imaging 2019, 12, 745–748. [Google Scholar] [CrossRef]
- Bergamaschi, L.; Pavon, A.G.; Angeli, F.; Tuttolomondo, D.; Belmonte, M.; Armillotta, M.; Sansonetti, A.; Foà, A.; Paolisso, P.; Baggiano, A.; et al. The Role of Non-Invasive Multimodality Imaging in Chronic Coronary Syndrome: Anatomical and Functional Pathways. Diagnostics 2023, 13, 2083. [Google Scholar] [CrossRef]


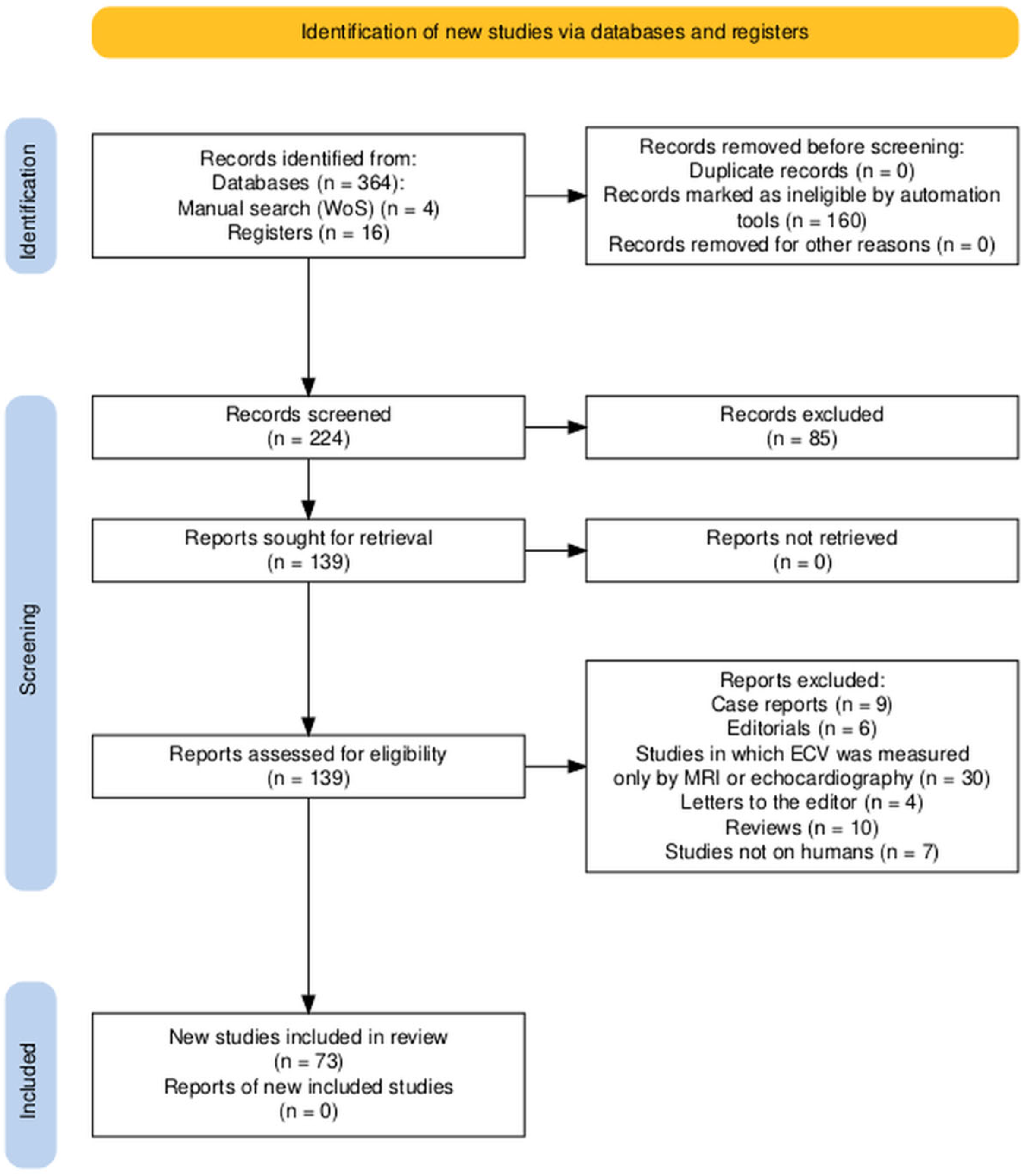


| NCT Number (Acronym) | Title | Condition | Estimated Enrollment | Status | Location | Study Type |
|---|---|---|---|---|---|---|
| NCT06308094 | 320-detector Computed Tomography to Assess Myocardial Extracellular Volume Fraction in Patients With Atrial Fibrillation Before AF Ablation | Atrial Fibrillation | 100 | Not yet recruiting | Not provided | Interventional |
| NCT05717998 | Imaging and Blood-Based Biomarkers for the Evaluation of Early Signs of Myocardial Injury After Thoracic Radiation Therapy | ECV calculated from MRI | ||||
| NCT04880317 | Validation of Quantitative Myocardial Tissue Characterization Through Non-gated CT | Cardiac Disease; COVID-19; Cardiotoxicity; Extracellular Space Alteration | 188 | Recruiting | Italy | Interventional |
| NCT05877768 (EPIPHANY) | Evaluation of PCD-CT Based Image Parameters in the Assessment and Quantification of Coronary Artery Disease | Coronary Artery Disease | 3000 | Not yet recruiting | Germany | Observational |
| NCT06029400 (CTMyoC) | CT-based Myocardial Characterization (CTMyoC) | Different Cardiomyopathy and Major Cardiovascular Adverse Events | 2200 | Recruiting | Italy | Observational |
| NCT03704701 (TICKER) | The Interrogation of the Cardiomyopathy of Chronic Kidney Disease With advancEd caRdiac Imaging (TICKER) | Chronic Kidney Diseases; Cardiomyopathies; Cardiovascular Diseases; Cardiac Disease | 28 | Terminated | United Kingdom | Observational |
| NCT05326126 | Microvascular Function in Patients Undergoing Transcatheter Aortic Valve Implant (TAVI) for Severe Symptomatic Aortic Stenosis: Association With Myocardial Fibrosis | Severe Symptomatic Aortic Stenosis | 75 | Recruiting | Italy | Interventional |
| NCT04392960 | Novel Imaging Tools in Newly-diagnosed Patients With Cardiac AL Amyloidosis | ECV calculated from MRI | ||||
| NCT03029026 (ATTRact-AS) | The Role of Occult Cardiac Amyloid in the Elderly With Aortic Stenosis | Cardiac Amyloidosis; Aortic Stenosis | 250 | Unknown status | United Kingdom | Observational |
| NCT06020209 (CT-STEMI) | CCT for Comprehensive Risk Stratification Following STEMI | Myocardial Infarction | 200 | Recruiting | Italy | Interventional |
| NCT04625075 (MEMORY-COVID) | Manganese-Enhanced Magnetic Resonance Imaging of MyOcardial injuRY in COVID 19 (COVID-19) (MEMORY-COVID) | ECV calculated from MRI | ||||
| NCT05069168 | Predictors Of Left Ventricular Systolic Function Recovery After Transcatheter Aortic Valve Replacement | Left Ventricular Systolic Function | 30 | Recruiting | Egypt | Observational |
| NCT05758493 | Characterizing Iodine-124 Evuzumitide (AT-01) in Systemic Amyloidosis | ECV calculated mainly from MRI | ||||
| NCT06048458 (PC-TOX) | Cancer Treatment Related Cardiovascular Toxicity: Comprehensive Myocardial and Vascular Phenotyping | ECV calculated from MRI | ||||
| NCT02316587 (AMFAST) | Assessment of Myocardial Fibrosis in Aortic STenosis (AMFAST) | Endomyocardial Fibrosis; Aortic Valve Stenosis | 112 | Completed | Denmark | Observational |
| NCT05479669 (VIP-HF2) | Value of Intense Phenotyping in Heart Failure With Preserved Ejection Fraction | ECV calculated from MRI | ||||
| Authors (Year) [Ref.] | ECV Measurement Method | n1 | n2 | %ECV1 [Mean (SD)] | %ECV2 [Mean (SD)] | Group 1 (Higher ECV or Pathological Condition) | Group 2 (Lower ECV or Control) | Effect Size (SMD) | 95% CI (Lower, Upper) | SE | 1/SE (Precision) | ICC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Chevance et al. (2018) [19] | DECT | 22 | 13 | 56.0 (7.0) | 36.0 (5.0) | CA | CH | 3.15 | (2.14, 4.16) | 0.51 | 1.95 | NA |
| (2) Ohta et al. (2019) [20] | DECT | 11 | 35 | 31.35 (2.53) | 26.63 (2.69) | Non-ischemic DCM | Healthy control | 1.78 | (1.01, 2.55) | 0.39 | 2.55 | NA |
| (3) Si-Mohamed et al. (2021) [21] | DECT | 60 | 12 | 34.18 (3.33) | 30.04 (2.25) | Myocarditis | Healthy control | 1.30 | (0.64, 1.96) | 0.33 | 2.99 | NA |
| (4) Qi et al. (2022) [22] | LIE-DECT | 77 | 80 | 31.5 (4.3) | 26.8 (2.8) | Non-ischemic HFpEF | Healthy control | 1.30 | (0.96, 1.64) | 0.18 | 5.69 | NA |
| (5) Qi et al. (2022) [22] | LIE-DECT | 35 | 31.7 (3.7) | Ischemic HFpEF | 1.58 | (1.13, 2.03) | 0.23 | 4.39 | ||||
| (6) Qi R-X et al. (2021) [23] | LIE-DECT | 60 | 60 | 31.3 (4.0) | 27.1 (3.7) | HF without CAD | Healthy control | 1.09 | (0.71, 1.47) | 0.20 | 5.11 | NA |
| (7) Han et al. (2021) [24] | CTA | 70 | 39 | 33.2 (7.7) | 29.4 (6.1) | AS with no LVEF improvement post-TAVR | AS with LVEF improvement post-TAVR | 0.53 | (0.13, 0.93) | 0.20 | 4.93 | 0.91 |
| (8) Tamarappoo et al. (2020) [25] | CTA | 57 | 93 | 40.1 (6.0) | 25.6 (4.6) | Patients with ECV > 33%, higher risk of death and HF hospitalization | Patients with ECV ≤ 33%, lower risk of death and HF hospitalization | 2.80 | (2.34, 3.26) | 0.23 | 4.28 | 0.94 |
| (9) Gama et al. (2022) [26] | CTA | 37 | 35 | 56.0 (11.0) | 43.0 (13.0) | ATTR | AL | 1.08 | (0.59, 1.58) | 0.25 | 3.96 | NA |
| (10) Hammer et al. (2021) [27] | CTA | 75 | 19 | 40.0 (11.0) | 21.6 (5.6) | AS | Healthy control | 1.81 | (1.24, 2.37) | 0.29 | 3.46 | 0.93 |
| (11) Hamdy et al. (2019) [28] | CTA | 35 | 17 | 39.6 (5.3) | 27.1 (2.1) | MI | Healthy control | 2.76 | (1.97, 3.55) | 0.40 | 2.50 | NA |
| (12) Yashima et al. (2023) [29] | CTA | 21 | 49 | 37.16 (5.91) | 32.59 (3.95) | DCM with MACE | DCM without MACE | 0.99 | (0.45, 1.53) | 0.27 | 3.65 | NA |
| (13) Yamada et al. (2020) [30] | CTA | 20 | 20 | 33.8 (4.7) | 26.6 (2.9) | HD | Healthy control | 1.84 | (1.10, 2.58) | 0.38 | 2.65 | 0.96 |
| (14) Nishikawa et al. (2023) [31] | CTA | 9 | 24 | 39 (5) | 33 (5) | After CatAbl due to AF without RR | After CatAbl due to AF with RR | 1.20 | (0.38, 2.02) | 0.42 | 2.39 | NA |
| (15) Egashira et al. (2021) [32] | CTA | 7 | 37 | 30.3 (4.8) | 26.2 (2.5) | CTRCD (anthracycline) | After anthracycline therapy without CTRCD | 1.39 | (0.54, 2.25) | 0.44 | 2.28 | NA |
| Disease | Clinical Role of CT-Derived ECV | Supporting Evidence |
|---|---|---|
| Myocarditis | Diagnostic (inflammation, fibrosis detection); prognostic (risk stratification) | Elevated ECV significantly distinguishes myocarditis patients from controls (e.g., 34.18% vs. 30.04%), aiding diagnosis and prognosis [21] |
| Cardiac Amyloidosis | Diagnostic (amyloid burden quantification); prognostic (severity, mortality) | Consistently elevated ECV (>40–50%) strongly correlates with myocardial amyloid deposition, disease severity, and increased mortality risk [19,26] |
| Dilated Cardiomyopathy (DCM) | Prognostic (predicting adverse cardiac events) | Higher ECV (>32%) associated with increased risk of major adverse cardiac events and worse clinical outcomes [29] |
| Heart Failure with Preserved EF (HFpEF) | Prognostic (cardiac remodeling, risk stratification) | Increased ECV (>31%) associated with higher NT-proBNP, worse NYHA class, and adverse outcomes, predicting HF progression [22] |
| Aortic Stenosis (AS) | Prognostic (post-TAVI outcomes, myocardial fibrosis severity) | High pre-TAVI ECV (>33%) strongly predicts poor clinical outcomes, reduced LV functional recovery, and increased HF hospitalizations [24,27] |
| Anthracycline-induced Cardiotoxicity | Diagnostic and monitoring (early detection of myocardial injury) | Elevated ECV post-anthracycline (e.g., >30%) identifies early myocardial injury, preceding LVEF reduction, enabling early intervention [32] |
| Chronic Kidney Disease (CKD) | Monitoring (cardiovascular remodeling, fibrosis) | ECV elevation correlates with LV remodeling, higher cardiovascular risk, and fibrosis extent in CKD patients undergoing dialysis [30] |
| Pulmonary Hypertension (PH) | Monitoring and prognostic (severity, response to therapy) | ECV correlates positively with pulmonary artery pressure and right ventricular remodeling, effectively monitoring treatment response and disease severity [70,72] |
| Atrial Fibrillation (AF) | Prognostic (reverse remodeling prediction post-ablation) | Patients with lower baseline ECV have significantly better reverse remodeling outcomes post-ablation [31] |
| Authors [Ref.] | Scanner Model | Vendor | Protocol | Effective Dose (mSv) | Notes |
|---|---|---|---|---|---|
| Treibel et al. [9] | Somatom Sensation 64 | Siemens Healthineers | DynEQ-CT | 1.56 ± 0.58 | Prospective gating; chest-specific conversion factor. |
| Cundari et al. [11] | Various | Multiple | Various | 1.2–25 | Photon-counting detectors significantly lower doses. |
| Chevance et al. [19] | DECT (model not specified) | Not specified | DECT | 7.9 ± 3.1 to 8.4 ± 2.4 | Prospective ECG triggering; ASIR technology. |
| Liu et al. [35] | iQon Spectral CT | Philips Healthcare | DECT | 2.6 ± 0.9 | Standardized dual-layer spectral detector CT protocol. |
| Monti et al. [56] | Somatom Definition/Emotion 16 | Siemens Healthineers | Body CT | DLP 2093–5680 mGy·cm | Significant variability due to scanner capabilities and settings. |
| Abadia et al. [51] | Somatom Definition Flash/Force | Siemens Healthineers | DECT | Not reported | Dose reductions with tin filtration and advanced dual-energy features. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martuszewski, A.; Paluszkiewicz, P.; Poręba, R.; Gać, P. Clinical Significance of Extracellular Volume of Myocardium (ECV) Assessed by Computed Tomography: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2066. https://doi.org/10.3390/jcm14062066
Martuszewski A, Paluszkiewicz P, Poręba R, Gać P. Clinical Significance of Extracellular Volume of Myocardium (ECV) Assessed by Computed Tomography: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(6):2066. https://doi.org/10.3390/jcm14062066
Chicago/Turabian StyleMartuszewski, Adrian, Patrycja Paluszkiewicz, Rafał Poręba, and Paweł Gać. 2025. "Clinical Significance of Extracellular Volume of Myocardium (ECV) Assessed by Computed Tomography: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 6: 2066. https://doi.org/10.3390/jcm14062066
APA StyleMartuszewski, A., Paluszkiewicz, P., Poręba, R., & Gać, P. (2025). Clinical Significance of Extracellular Volume of Myocardium (ECV) Assessed by Computed Tomography: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(6), 2066. https://doi.org/10.3390/jcm14062066







