Temperature Dynamics of Porcine and Human Lungs During Static Ice Storage: Ice Is Not 4 °C †
Abstract
:1. Introduction
2. Methods
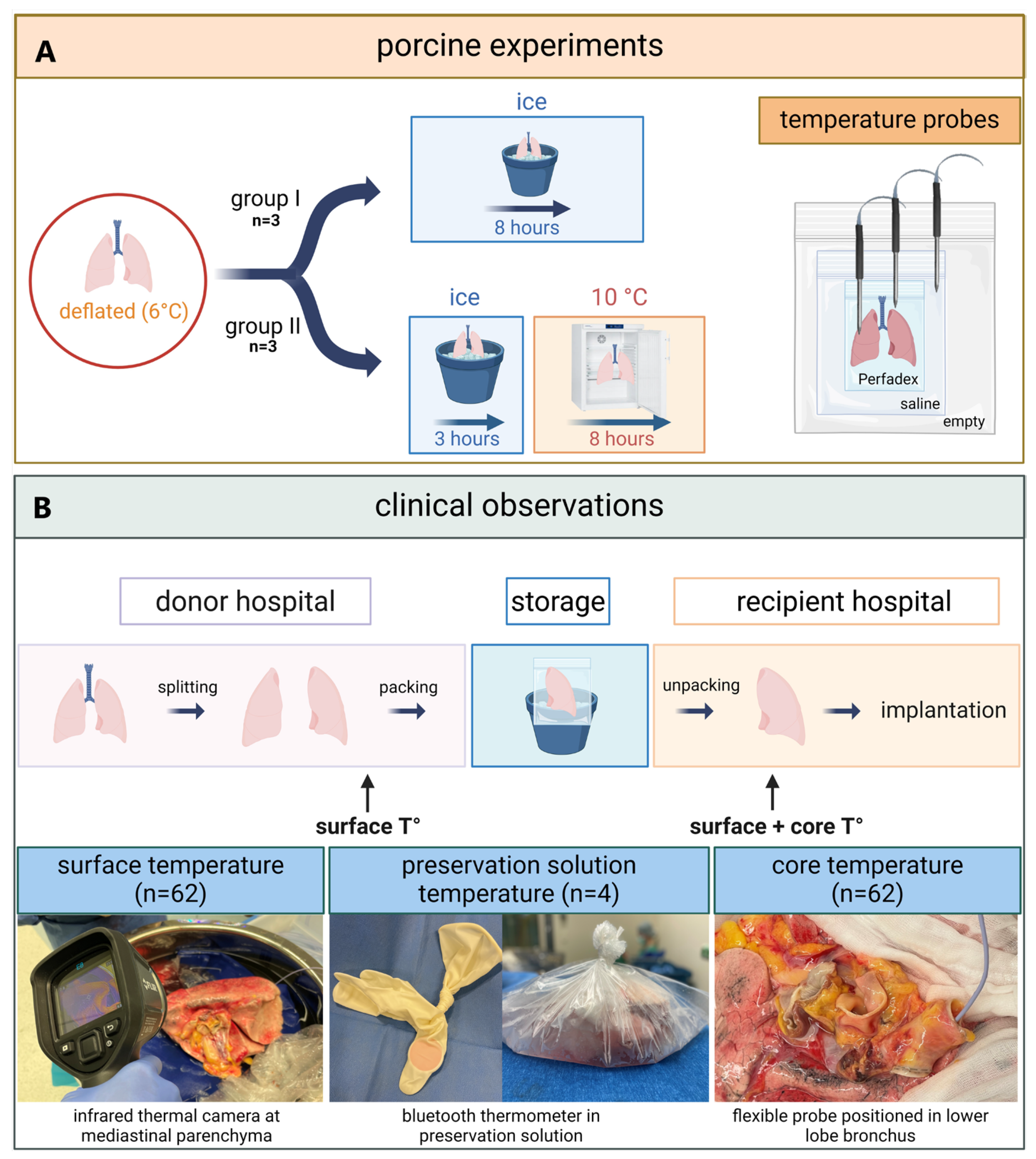
2.1. Porcine
2.2. Clinical
2.2.1. Preservation Solution Temperature During Static Ice Storage in Clinical Lung Transplantation
2.2.2. Surface and Core Temperature of Human Donor Lungs Before and After Static Ice Storage
2.3. Statistics
3. Results
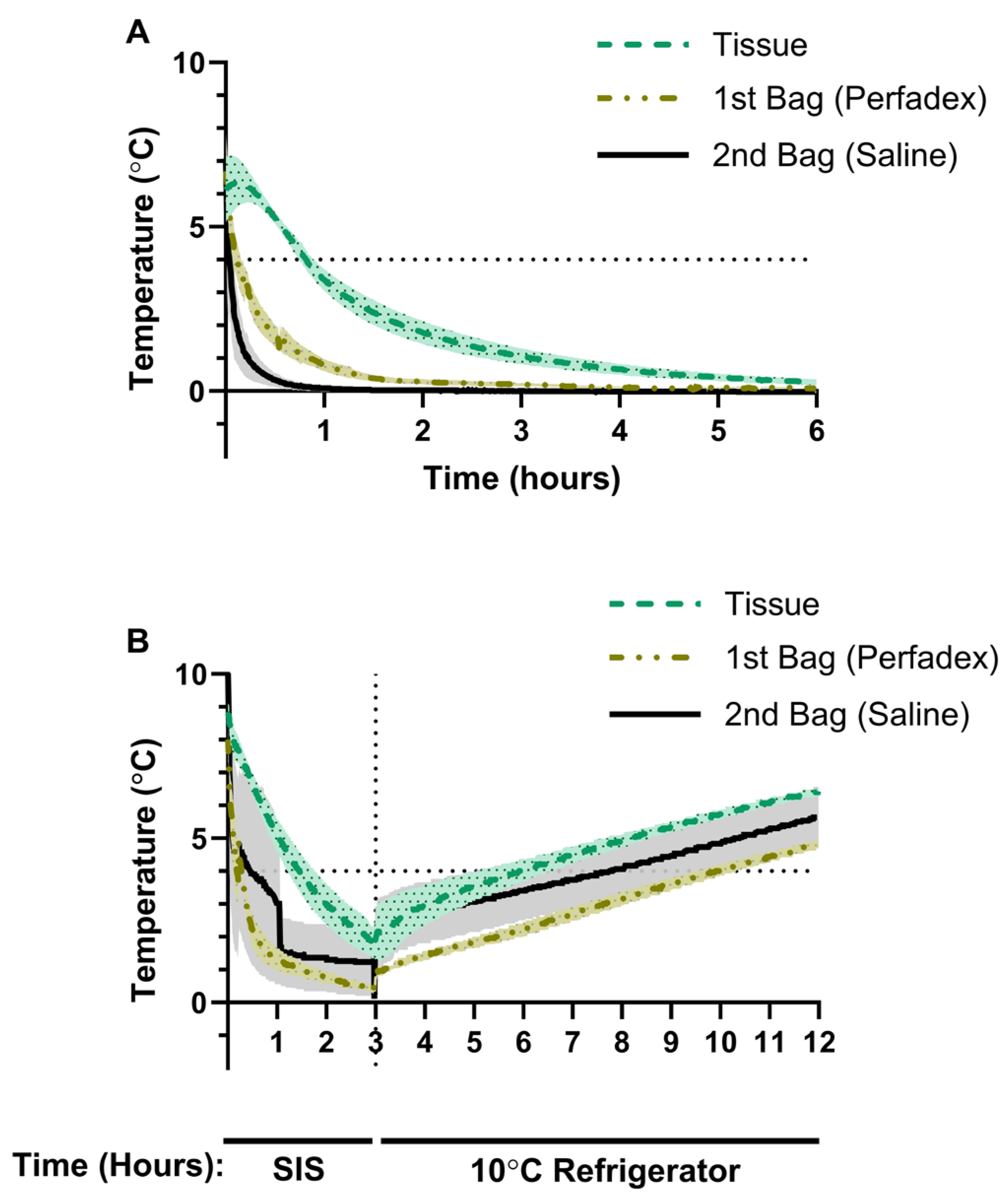
3.1. Porcine Lung Temperature Evolution During Static Ice Storage
3.2. Porcine Lung Temperature During Static Ice Storage Followed by Storage at 10 °C
3.3. Longitudinal Monitoring of Preservation Solution Temperature in Human Donor Lungs
3.4. Human Donor Lung Temperature Before and After Static Ice Storage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHS | Controlled hypothermic storage |
| coreT° | Core temperature |
| FiO2 | Fraction of inspired oxygen |
| ISHLT | International Society for Heart and Lung Transplantation |
| LTx | Lung transplantation |
| OCS | Organ Care System preservation solution |
| psT° | Preservation solution temperature |
| PaO2 | Partial pressure of arterial oxygen |
| PGD3 | Primary graft dysfunction grade 3 |
| ROS | Reactive oxygen species |
| salineT° | Saline temperature |
| SIS | Static ice storage |
| surfaceT° | Surface temperature |
| tissueT° | Tissue temperature |
References
- Venuta, F.; Van Raemdonck, D. History of lung transplantation. J. Thorac. Dis. 2017, 9, 5458–5471. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, D.; Van Slambrouck, J.; Ceulemans, L.J. Donor lung preservation for transplantation—Where do we go from here? J. Thorac. Dis. 2022, 14, 3125–3130. [Google Scholar] [CrossRef] [PubMed]
- Daily, P.O.; Adamson, R.M.; Jones, B.H.; Dembitsky, W.P.; Moreno-Cabral, R.J. Comparisons of methods of myocardial hypothermia for cardiac transplantation. Ann. Thorac. Surg. 1996, 61, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.D.; Pearson, F.G.; Patterson, G.A.; Todd, T.R.; Ginsberg, R.J.; Goldberg, M.; DeMajo, W.A. Technique of successful lung transplantation in humans. J. Thorac. Cardiovasc. Surg. 1987, 93, 173–181. [Google Scholar]
- Jing, L.; Yao, L.; Zhao, M.; Peng, L.P.; Liu, M. Organ preservation: From the past to the future. Acta Pharmacol. Sin. 2018, 39, 845–857. [Google Scholar] [CrossRef]
- Keon, W.J.; Hendry, P.J.; Taichman, G.C.; Mainwood, G.W. Cardiac Transplantation: The Ideal Myocardial Temperature for Graft Transport. Ann. Thorac. Surg. 1988, 46, 337–341. [Google Scholar] [CrossRef]
- Ingemansson, R.; Budrikis, A.; Bolys, R.; Sjöberg, T.; Steen, S. Effect of temperature in long-term preservation of vascular endothelial and smooth muscle function. Ann. Thorac. Surg. 1996, 61, 1413–1417. [Google Scholar] [CrossRef]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ Preservation: Current Concepts and New Strategies for the Next Decade. Transfus. Med. Hemotherapy 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Courtwright, A.; Cantu, E. Evaluation and Management of the Potential Lung Donor. Clin. Chest Med. 2017, 38, 751–759. [Google Scholar] [CrossRef]
- Mulvihill, M.S.; Yerokun, B.A.; Davis, R.P.; Ranney, D.N.; Daneshmand, M.A.; Hartwig, M.G. Extracorporeal membrane oxygenation following lung transplantation: Indications and survival. J. Heart Lung Transplant. 2018, 37, 259–267. [Google Scholar] [CrossRef]
- Thabut, G.; Mal, H.; Cerrina, J.; Dartevelle, P.; Dromer, C.; Velly, J.-F.; Stern, M.; Loirat, P.; Lesèche, G.; Bertocchi, M.; et al. Graft Ischemic Time and Outcome of Lung Transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Pirenne, J. Time to think out of the (ice) box. Curr. Opin. Organ Transplant. 2010, 15, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; O’Brien, M.E.; Yu, J.; Xu, C.; Zhang, Q.; Lu, S.; Liang, L.; An, X.; McDyer, J.F.; Mallampalli, R.K. Prolonged Cold Ischemia Induces Necroptotic Cell Death in Ischemia–Reperfusion Injury and Contributes to Primary Graft Dysfunction After Lung Transplantation. Am. J. Respir. Cell Mol. Biol. 2019, 61, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Yoshikawa, K.; Miyoshi, S.; Nakamoto, K.; Hsieh, C.-M.; Yamazaki, F.; Cardoso, P.F.G.; Schaefers, G.H.-J.; Brito, J.; Keshavjee, S.H.; et al. The effect of ischemic time and temperature on lung preservation in a simple ex vivo rabbit model used for functional assessment. J. Thorac. Cardiovasc. Surg. 1989, 98, 333–342. [Google Scholar] [CrossRef]
- Copeland, H.; Hayanga, J.W.A.; Neyrinck, A.; MacDonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor heart and lung procurement: A consensus statement. J. Heart Lung Transplant. 2020, 39, 501–517. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 85th ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 990–1003. [Google Scholar]
- Robicsek, F.; Duncan, G.D.; Rice, H.E.; Robicsek, S.A. Experiments with a bowl of saline: The hidden risk of hypothermic-osmotic damage during topical cardiac cooling. J. Thorac. Cardiovasc. Surg. 1989, 97, 461–466. [Google Scholar]
- Horch, D.F.; Mehlitz, T.; Laurich, O.; Abel, A.; Reuter, S.; Pratschke, H.; Neuhaus, P.; Wesslau, C. Organ transport temperature box: Multicenter study on transport temperature of organs. Transplant. Proc. 2002, 34, 2320. [Google Scholar] [CrossRef]
- Hendry, P.J.; Walley, V.M.; Koshal, A.; Masters, R.G.; Keon, W.J. Are temperatures attained by donor hearts during transport too cold? J. Thorac. Cardiovasc. Surg. 1989, 98, 517–522. [Google Scholar]
- Ali, A.; Wang, A.; Ribeiro, R.V.P.; Beroncal, E.L.; Baciu, C.; Galasso, M.; Gomes, B.; Mariscal, A.; Hough, O.; Brambate, E.; et al. Static lung storage at 10 °C maintains mitochondrial health and preserves donor organ function. Sci. Transl. Med. 2021, 13, eabf7601. [Google Scholar] [CrossRef]
- Dias, C.L.; Ala-Nissila, T.; Wong-ekkabut, J.; Vattulainen, I.; Grant, M.; Karttunen, M. The hydrophobic effect and its role in cold denaturation. Cryobiology 2010, 60, 91–99. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Hu, Y.; Chen, X.; Tan, S. Cryopreservation of tissues and organs: Present, bottlenecks, and future. Front. Vet. Sci. 2023, 10, 1201794. [Google Scholar] [CrossRef] [PubMed]
- Cenik, I.; Van Slambrouck, J.; Provoost, A.L.; Barbarossa, A.; Vanluyten, C.; Boelhouwer, C.; Vanaudenaerde, B.M.; Vos, R.; Pirenne, J.; Van Raemdonck, D.E.; et al. Controlled Hypothermic Storage for Lung Preservation: Leaving the Ice Age Behind. Transpl. Int. 2024, 37, 12601. [Google Scholar] [CrossRef]
- Novysedlak, R.; Provoost, A.L.; Langer, N.B.; Van Slambrouck, J.; Barbarossa, A.; Cenik, I.; Van Raemdonck, D.; Vos, R.; Vanaudenaerde, B.M.; Rabi, S.A.; et al. Extended Ischemic Time (>15 Hours) Using Controlled Hypothermic Storage in Lung Transplantation: A Multicenter Experience. J. Heart Lung Transplant. 2024, 43, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Provoost, A.-L.; Novysedlak, R.; Van Raemdonck, D.; Van Slambrouck, J.; Prisciandaro, E.; M Vandervelde, C.; Barbarossa, A.; Jin, X.; Denaux, K.; De Leyn, P.; et al. Lung transplantation following controlled hypothermic storage with a portable lung preservation device: First multicenter European experience. Front Cardiovasc Med. 2024, 11, 1370543. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Hoetzenecker, K.; Luis Campo-Cañaveral de la Cruz, J.; Schwarz, S.; Barturen, M.G.; Tomlinson, G.; Yeung, J.; Donahoe, L.; Yasufuku, K.; Pierre, A.; et al. Extension of Cold Static Donor Lung Preservation at 10 °C. NEJM Evid. 2023, 2, EVIDoa2300008. [Google Scholar] [CrossRef]
- Date, H.; Matsumura, A.; Manchester, J.K.; Cooper, J.M.; Lowry, O.H.; Cooper, J.D. Changes in alveolar oxygen and carbon dioxide concentration and oxygen consumption during lung preservation: The maintenance of aerobic metabolism during lung preservation. J. Thorac. Cardiovasc. Surg. 1993, 105, 492–501. [Google Scholar] [CrossRef]
- Date, H.; Lima, O.; Matsumura, A.; Tsuji, H.; d’Avignon, D.A.; Cooper, J.D. In a canine model, lung preservation at 10 °C is superior to that at 4 °C: A comparison of two preservation temperatures on lung function and on adenosine triphosphate level measured by phosphorus 31-nuclear magnetic resonance. J. Thorac. Cardiovasc. Surg. 1992, 103, 773–780. [Google Scholar] [CrossRef]
- Date, H.; Matsumura, A.; Manchester, J.K.; Obo, H.; Lima, O.; Cooper, J.M.; Sundaresan, S.; Lowry, O.H.; Cooper, J.D. Evaluation of lung metabolism during successful twenty-four-hour canine lung preservation. J. Thorac. Cardiovasc. Surg. 1993, 105, 480–491. [Google Scholar] [CrossRef]


| Transplant cases using SIS | 31 |
| Number of lungs stored in ice | 62 |
| Age (years) | 59.0 (48–68) |
| Sex (%male) | 54.8 |
| Donor type (DCD III) | 48.4 |
| Body mass index (kg/m2) | 25.0 (23–29) |
| Ventilator time (hours) | 146.0 (75–246) |
| Donor P/F ratio (PaO2/FiO2 100%) | 431 (386–507) |
| Cold ischemic time (hours) | 4 h 12 (2 h 43–5 h 19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cenik, I.; Van Slambrouck, J.; Barbarossa, A.; Jin, X.; Provoost, A.-L.; Patel, P.; Churchill, L.; Bulka, B.; Haney, J.; Ceulemans, L.J. Temperature Dynamics of Porcine and Human Lungs During Static Ice Storage: Ice Is Not 4 °C. J. Clin. Med. 2025, 14, 2127. https://doi.org/10.3390/jcm14062127
Cenik I, Van Slambrouck J, Barbarossa A, Jin X, Provoost A-L, Patel P, Churchill L, Bulka B, Haney J, Ceulemans LJ. Temperature Dynamics of Porcine and Human Lungs During Static Ice Storage: Ice Is Not 4 °C. Journal of Clinical Medicine. 2025; 14(6):2127. https://doi.org/10.3390/jcm14062127
Chicago/Turabian StyleCenik, Ismail, Jan Van Slambrouck, Annalisa Barbarossa, Xin Jin, An-Lies Provoost, Pratik Patel, Lucas Churchill, Ben Bulka, John Haney, and Laurens J. Ceulemans. 2025. "Temperature Dynamics of Porcine and Human Lungs During Static Ice Storage: Ice Is Not 4 °C" Journal of Clinical Medicine 14, no. 6: 2127. https://doi.org/10.3390/jcm14062127
APA StyleCenik, I., Van Slambrouck, J., Barbarossa, A., Jin, X., Provoost, A.-L., Patel, P., Churchill, L., Bulka, B., Haney, J., & Ceulemans, L. J. (2025). Temperature Dynamics of Porcine and Human Lungs During Static Ice Storage: Ice Is Not 4 °C. Journal of Clinical Medicine, 14(6), 2127. https://doi.org/10.3390/jcm14062127






