Predicting the Hamstring Graft Size for ACL Reconstruction Using a 3D Tendon Model in Preoperative MRI
Abstract
1. Introduction
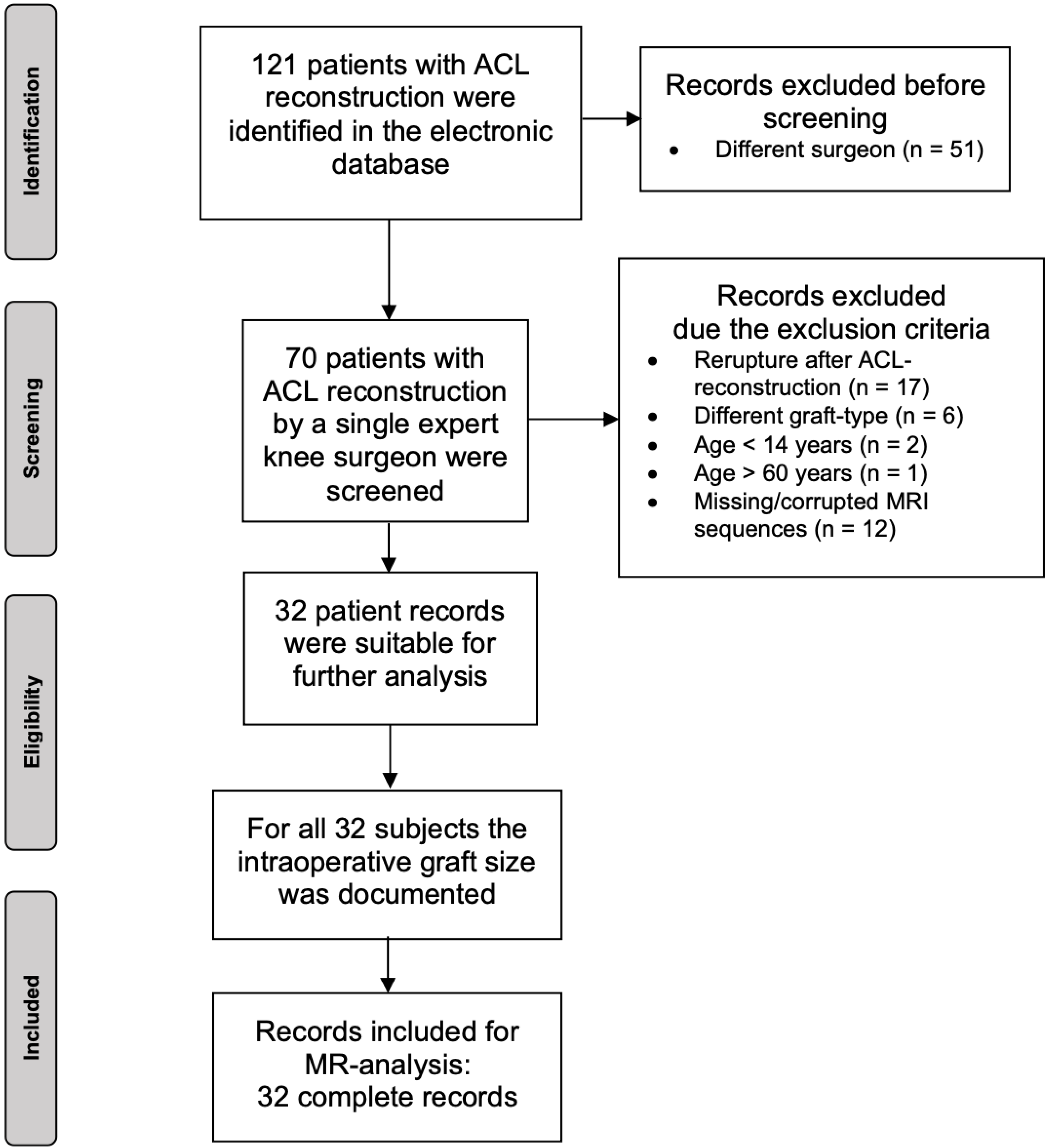
2. Materials and Methods
2.1. Patients
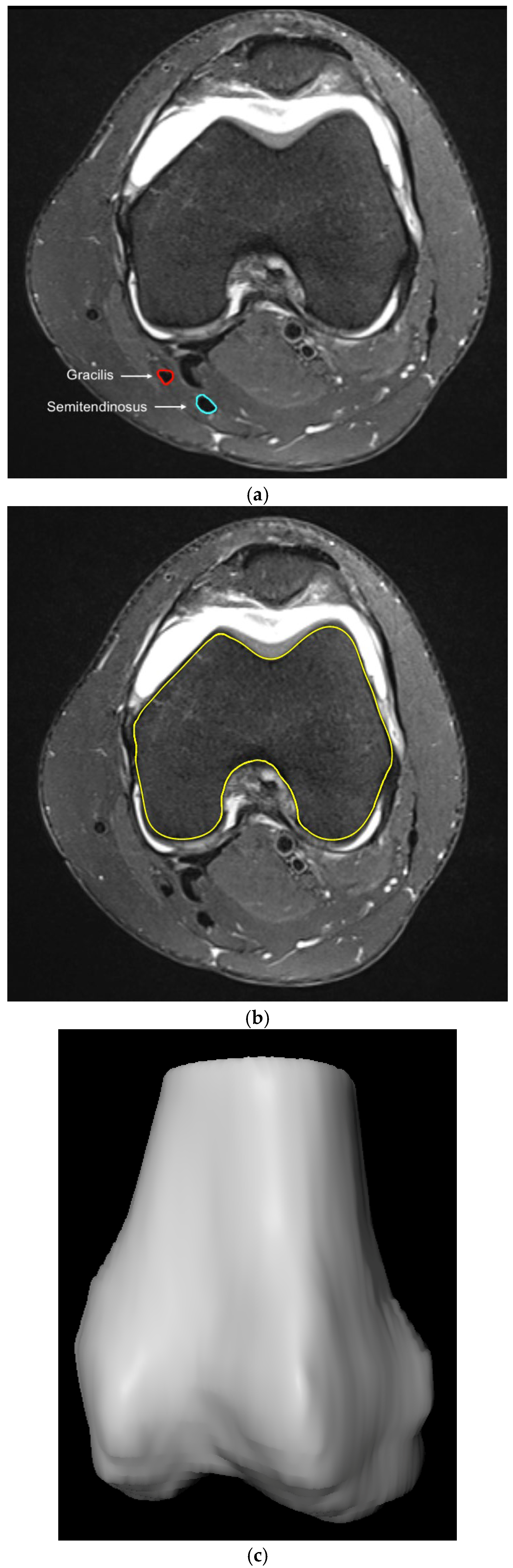
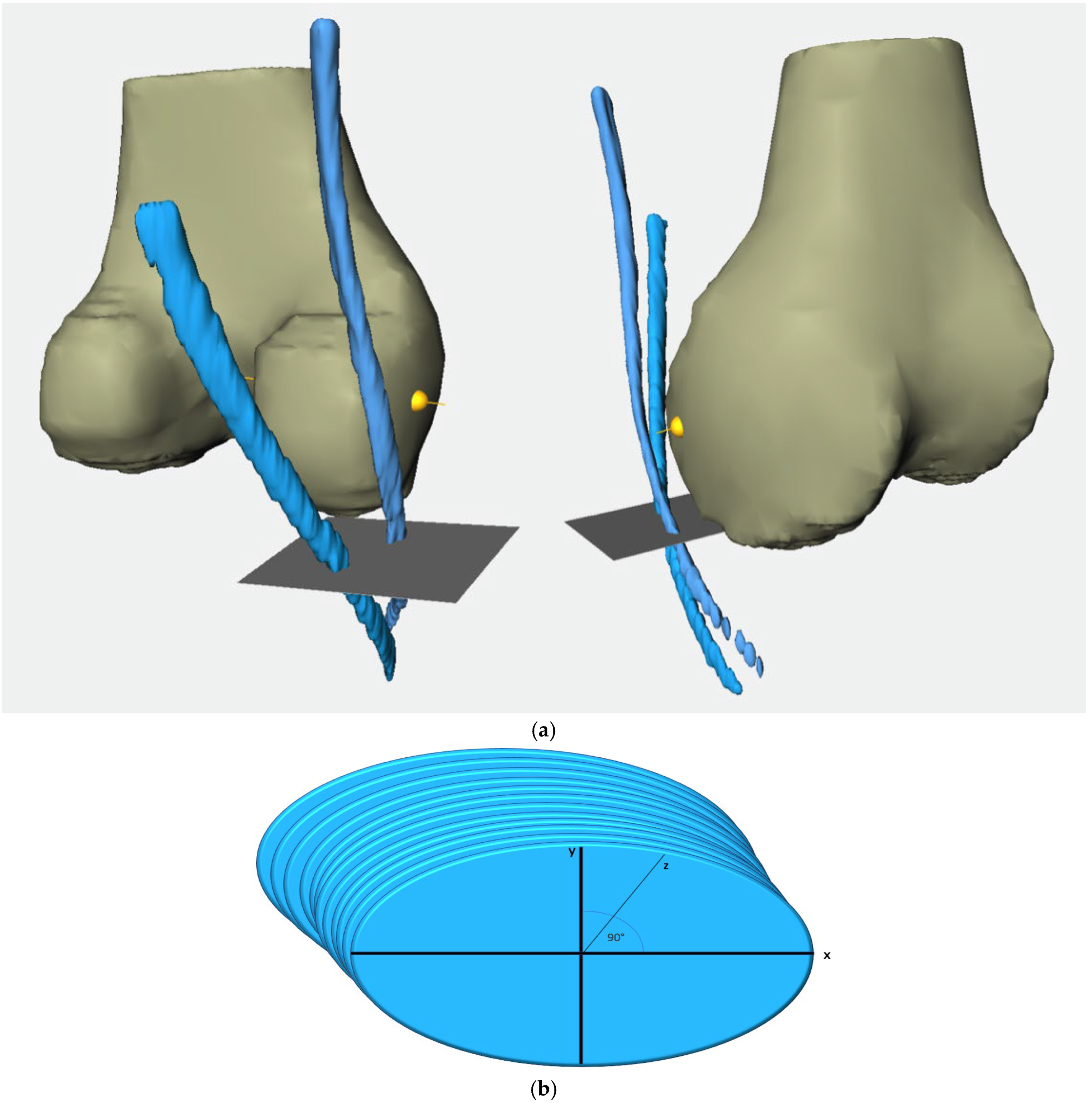
2.2. Methodological Approach
- From 20 mm above the joint line to the joint line (20 mm–0).
- From 10 mm above the joint line to the joint line (10 mm–0).
- From 10 mm below the joint line to 10 mm above the joint line (−10 mm–10 mm).
- From 10 mm below the joint line to the joint line (−10 mm–0).
- From 20 mm below the joint line to the joint line (−20 mm–0).
- From the widest intercondylar diameter to the joint line (0–WID).
2.3. Statistical Analysis
3. Results
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sutton, K.M.; Bullock, J.M. Anterior Cruciate Ligament Rupture: Differences between Males and Females. J. Am. Acad. Orthop. Surg. 2013, 21, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-D.; Gao, S.-J.; Zhang, Y.-Z. Hamstring Autograft Versus Hybrid Graft for Anterior Cruciate Ligament Reconstruction: A Systematic Review. Am. J. Sports Med. 2020, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.M.; Boyle, C.; Marom, N.; Marx, R.G. Graft Selection in Anterior Cruciate Ligament Reconstruction. Sports Med. Arthrosc. Rev. 2020, 28, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.J.; Choi, Y.S.; Shin, J.H.; Yoon, T.H.; Kim, T.W.; Chang, C.B.; Kang, S.-B. Comparison of Failure Rates and Functional Outcomes between Hamstring Autografts and Hybrid Grafts in Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Orthop. Traumatol. Surg. Res. OTSR 2023, 109, 103499. [Google Scholar] [CrossRef]
- Mouarbes, D.; Menetrey, J.; Marot, V.; Courtot, L.; Berard, E.; Cavaignac, E. Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis of Outcomes for Quadriceps Tendon Autograft Versus Bone-Patellar Tendon-Bone and Hamstring-Tendon Autografts. Am. J. Sports Med. 2019, 47, 3531–3540. [Google Scholar] [CrossRef]
- Zhao, D.; Pan, J.-K.; Lin, F.-Z.; Luo, M.-H.; Liang, G.-H.; Zeng, L.-F.; Huang, H.-T.; Han, Y.-H.; Xu, N.-J.; Yang, W.-Y.; et al. Risk Factors for Revision or Rerupture After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Am. J. Sports Med. 2023, 51, 3053–3075. [Google Scholar] [CrossRef]
- Vivekanantha, P.; Grzela, P.; Wong, S.; Mansour, F.; Khalik, H.A.; Johnson, J.; Hantouly, A.; de Sa, D. Tendon Cross-Sectional Area on Magnetic Resonance Imaging and Anthropometric Characteristics Can Be Used to Predict Insufficient Four-Strand Hamstring Autograft Diameter in Anterior Cruciate Ligament Reconstruction: A Systematic Review. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2024, 32, 1470–1491. [Google Scholar] [CrossRef]
- Thomas, S.; Bhattacharya, R.; Saltikov, J.B.; Kramer, D.J. Influence of Anthropometric Features on Graft Diameter in ACL Reconstruction. Arch. Orthop. Trauma Surg. 2013, 133, 215–218. [Google Scholar] [CrossRef]
- Goyal, S.; Matias, N.; Pandey, V.; Acharya, K. Are Pre-Operative Anthropometric Parameters Helpful in Predicting Length and Thickness of Quadrupled Hamstring Graft for ACL Reconstruction in Adults? A Prospective Study and Literature Review. Int. Orthop. 2016, 40, 173–181. [Google Scholar] [CrossRef]
- Pontoh, L.A.; Rahyussalim, A.J.; Widodo, W.; Fiolin, J.; Rhatomy, S. Anthropometric Study as a Predictor of Anterior Cruciate Ligament Sizes in Asian Indonesian Population. J. Orthop. Surg. Hong Kong 2021, 29, 23094990211000462. [Google Scholar] [CrossRef]
- Domnick, C.; Herbort, M.; Raschke, M.J.; Schliemann, B.; Siebold, R.; Śmigielski, R.; Fink, C. Converting Round Tendons to Flat Tendon Constructs: Does the Preparation Process Have an Influence on the Structural Properties? Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2017, 25, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Stirling, C.E.; Neeteson, N.J.; Walker, R.E.A.; Boyd, S.K. Deep Learning-Based Automated Detection and Segmentation of Bone and Traumatic Bone Marrow Lesions from MRI Following an Acute ACL Tear. Comput. Biol. Med. 2024, 178, 108791. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zheng, H.; Liang, P.; Zhang, L.; Zaman, F.; Wu, X.; Sonka, M.; Chen, D.Z. KCB-Net: A 3D Knee Cartilage and Bone Segmentation Network via Sparse Annotation. Med. Image Anal. 2022, 82, 102574. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Z.; Wang, Y.; Fang, K.-B.; Zheng, H.-Z.; Lai, Q.-Q.; Xia, Y.-F.; Chen, J.-Y.; Dai, Z.-S. Automated Meniscus Segmentation and Tear Detection of Knee MRI with a 3D Mask-RCNN. Eur. J. Med. Res. 2022, 27, 247. [Google Scholar] [CrossRef]
- Hoshino, Y.; Wang, J.H.; Lorenz, S.; Fu, F.H.; Tashman, S. Gender Difference of the Femoral Kinematics Axis Location and Its Relation to Anterior Cruciate Ligament Injury: A 3D-CT Study. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 1282–1288. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Conte, E.J.; Hyatt, A.E.; Gatt, C.J.; Dhawan, A. Hamstring Autograft Size Can Be Predicted and Is a Potential Risk Factor for Anterior Cruciate Ligament Reconstruction Failure. Arthrosc. J. Arthrosc. Relat. Surg. Off. Publ. Arthrosc. Assoc. N. Am. Int. Arthrosc. Assoc. 2014, 30, 882–890. [Google Scholar] [CrossRef]
- Kuruvilla, R.S.; Gunasekaran, C.; Jepegnanam, T.S.; Kandagaddala, M.; Panwar, J. Estimation of Diameter of Quadrupled Hamstring Graft for ACL Reconstruction Using Pre-Operative MRI Measurement as a Predictive Tool. Malays. Orthop. J. 2024, 18, 91–98. [Google Scholar] [CrossRef]
- Galanis, N.; Savvidis, M.; Tsifountoudis, I.; Gkouvas, G.; Alafropatis, I.; Kirkos, J.; Kellis, E. Correlation between Semitendinosus and Gracilis Tendon Cross-Sectional Area Determined Using Ultrasound, Magnetic Resonance Imaging and Intraoperative Tendon Measurements. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2016, 26, 44–51. [Google Scholar] [CrossRef]
- Leiter, J.; Elkurbo, M.; McRae, S.; Chiu, J.; Froese, W.; MacDonald, P. Using Pre-Operative MRI to Predict Intraoperative Hamstring Graft Size for Anterior Cruciate Ligament Reconstruction. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2017, 25, 229–235. [Google Scholar] [CrossRef]
- Hollnagel, K.; Johnson, B.M.; Whitmer, K.K.; Hanna, A.; Miller, T.K. Prediction of Autograft Hamstring Size for Anterior Cruciate Ligament Reconstruction Using MRI. Clin. Orthop. 2019, 477, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Singh Thapa, S.; Mahara, D.P.; Paudel, S.; Lamichhane, A.; Saud, T. Prediction of Hamstring Autograft Sizes for Anterior Cruciate Ligament Reconstruction Using Preoperative Magnetic Resonance Imaging. J. Nepal Health Res. Counc. 2023, 21, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mayr, M.F.; Siegel, M.; Taghizadeh, E.; Obid, P.; Schmal, H.; Izadpanah, K. Prediction of the Hamstring Graft Size for ACL Reconstruction Using Different Axial Layers in Preoperative MRI. J. Pers. Med. 2024, 14, 582. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Hollnagel, K.; Whitmer, K.; John, C.; Johnson, B.; Godin, J.; Miller, T. Reliability of Magnetic Resonance Imaging Prediction of Anterior Cruciate Ligament Autograft Size and Comparison of Radiologist and Orthopaedic Surgeon Predictions. Orthop. J. Sports Med. 2019, 7, 2325967119889593. [Google Scholar] [CrossRef]
- Bhamare, D.S.; Sirasala, S.; Jivrajani, P.; Nair, A.; Taori, S. Preoperative MRI Assessment of Hamstring Tendons to Predict the Quadruple Hamstring Graft Diameter in Anterior Cruciate Ligament Reconstruction. Cureus 2022, 14, e21753. [Google Scholar] [CrossRef]
- Hodges, C.T.; Shelton, T.J.; Bateni, C.P.; Henrichon, S.S.; Skaggs, A.W.; Boutin, R.D.; Lee, C.A.; Haus, B.M.; Marder, R.A. The Medial Epicondyle of the Distal Femur Is the Optimal Location for MRI Measurement of Semitendinosus and Gracilis Tendon Cross-Sectional Area. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2019, 27, 3498–3504. [Google Scholar] [CrossRef]
- Erquicia, J.I.; Gelber, P.E.; Doreste, J.L.; Pelfort, X.; Abat, F.; Monllau, J.C. How to Improve the Prediction of Quadrupled Semitendinosus and Gracilis Autograft Sizes with Magnetic Resonance Imaging and Ultrasonography. Am. J. Sports Med. 2013, 41, 1857–1863. [Google Scholar] [CrossRef]
- Kremen, T.J.; Arnold, M.T.; Trivellas, M.; Shi, B.Y.; Jones, K.J.; Garcia-Mansilla, I. Combined Assessments of Patellar Tendon and Hamstring Tendon Parameters on Preoperative Magnetic Resonance Imaging Can Improve Predictability of Hamstring Tendon Autograft Diameter in the Setting of Anterior Cruciate Ligament Reconstruction. Arthrosc. Sports Med. Rehabil. 2022, 4, e1913–e1921. [Google Scholar] [CrossRef]
- Serino, J.; Murray, R.; Argintar, E.H. Use of Magnetic Resonance Imaging to Predict Quadrupled Semitendinosus Graft Diameter in All-Inside Anterior Cruciate Ligament Reconstruction. Orthopedics 2017, 40, e617–e622. [Google Scholar] [CrossRef]
- An, V.V.; Scholes, C.; Mhaskar, V.A.; Parker, D.A.; Fritsch, B.A. Regression Modelling Combining MRI Measurements and Patient Anthropometry for Patient Screening and Prediction of Graft Diameter in Hamstring Autograft Arthroscopic ACL Reconstruction. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017, 8, 24. [Google Scholar] [CrossRef]
|
Female Mean ± SD |
Male Mean ± SD | p-Value |
Total Mean ± SD | |
|---|---|---|---|---|
| Number of patients n | 12 | 20 | 32 | |
| Age (years) | 28.5 ± 13.7 | 27.4 ± 10.0 | 0.82 | 27.8 ± 11.4 |
| Height (cm) | 163.1 ± 7.4 | 180.6 ± 6.2 | 0.001 | 174.1 ± 10.9 |
| Body weight (kg) | 66.3 ± 8.5 | 79.0 ± 8.1 | 0.001 | 74.8 ± 10.5 |
| BMI (kg/cm2) | 25.1 ± 4.0 | 24.5 ± 2.6 | 0.96 | 24.7 ± 3.1 |
| Graft diameter * (mm) | 7.9 ± 0.5 | 8.2 ± 0.6 | 0.29 | 8.1 ± 0.5 |
| Level | CSA ST | CSA GT | CSA STGT |
|---|---|---|---|
| 20 mm | 0.95 | 0.94 | 0.95 |
| 10 mm | 0.96 | 0.93 | 0.96 |
| Joint Line | 0.84 | 0.95 | 0.92 |
| −10 mm | 0.95 | 0.91 | 0.95 |
| −20 mm | 0.89 | 0.60 | 0.85 |
| WID | 0.92 | 0.87 | 0.94 |
| Segment | n |
Female Mean ± SD | n |
Male Mean ± SD | p-Value | n |
Total Mean ± SD |
|---|---|---|---|---|---|---|---|
| 20 mm–0 | 12 | 14.9 ± 5.7 | 20 | 17.9 ± 6.3 | 0.12 | 32 | 16.8 ± 6.1 |
| 10 mm–0 | 12 | 15.3 ± 6.2 | 20 | 17.7 ± 6.1 | 0.15 | 32 | 16.8 ± 6.1 |
| −10 mm–10 mm | 12 | 15.1 ± 6.3 | 20 | 17.3 ± 5.9 | 0.22 | 32 | 16.5 ± 6.1 |
| −10 mm–0 | 12 | 15.2 ± 6.3 | 20 | 16.7 ± 6.0 | 0.31 | 32 | 16.2 ± 6.1 |
| −20 mm–0 | 12 | 14.2 ± 6.2 | 20 | 16.2 ± 6.1 | 0.21 | 32 | 15.4 ± 6.1 |
| 0–WID | 12 | 14.7 ± 5.2 | 20 | 18.1 ± 6.2 | 0.08 | 32 | 16.8 ± 6.0 |
| Segment | n | Correlation r | p-Value |
|---|---|---|---|
| 20 mm–0 | 20 | 0.478 | 0.033 |
| 10 mm–0 | 20 | 0.537 | 0.015 |
| −10 mm–10 mm | 20 | 0.511 | 0.021 |
| −10 mm–0 | 20 | 0.489 | 0.029 |
| −20 mm–0 | 20 | 0.468 | 0.038 |
| 0–WID | 20 | 0.487 | 0.029 |
| Segment | n | Correlation r | p-Value |
|---|---|---|---|
| 20 mm–0 | 12 | 0.593 | 0.042 |
| 10 mm–0 | 12 | 0.603 | 0.038 |
| −10 mm–10 mm | 12 | 0.597 | 0.040 |
| −10 mm–0 | 12 | 0.576 | 0.050 |
| −20 mm–0 | 12 | 0.493 | 0.104 |
| 0–WID | 12 | 0.629 | 0.028 |
| Correlation Coefficient B | Standard Error | p-Value | |
|---|---|---|---|
| BMI | 0.404 | 0.247 | 0.113 |
| Height | 0.129 | 0.073 | 0.097 |
| Body Weight | −0.135 | 0.085 | 0.122 |
| Segment | n | Correlation r | p-Value |
|---|---|---|---|
| 20 mm–0 | 32 | 0.511 | 0.001 |
| 10 mm–0 | 32 | 0.552 | 0.001 |
| −10 mm–10 mm | 32 | 0.537 | 0.001 |
| −10 mm–0 | 32 | 0.514 | 0.001 |
| −20 mm–0 | 32 | 0.479 | 0.003 |
| 0–WID | 32 | 0.528 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frodl, A.; Mayr, M.; Siegel, M.; Meine, H.; Taghizadeh, E.; Bendak, S.; Schmal, H.; Izadpanah, K. Predicting the Hamstring Graft Size for ACL Reconstruction Using a 3D Tendon Model in Preoperative MRI. J. Clin. Med. 2025, 14, 2128. https://doi.org/10.3390/jcm14062128
Frodl A, Mayr M, Siegel M, Meine H, Taghizadeh E, Bendak S, Schmal H, Izadpanah K. Predicting the Hamstring Graft Size for ACL Reconstruction Using a 3D Tendon Model in Preoperative MRI. Journal of Clinical Medicine. 2025; 14(6):2128. https://doi.org/10.3390/jcm14062128
Chicago/Turabian StyleFrodl, Andreas, Moritz Mayr, Markus Siegel, Hans Meine, Elham Taghizadeh, Sebastian Bendak, Hagen Schmal, and Kaywan Izadpanah. 2025. "Predicting the Hamstring Graft Size for ACL Reconstruction Using a 3D Tendon Model in Preoperative MRI" Journal of Clinical Medicine 14, no. 6: 2128. https://doi.org/10.3390/jcm14062128
APA StyleFrodl, A., Mayr, M., Siegel, M., Meine, H., Taghizadeh, E., Bendak, S., Schmal, H., & Izadpanah, K. (2025). Predicting the Hamstring Graft Size for ACL Reconstruction Using a 3D Tendon Model in Preoperative MRI. Journal of Clinical Medicine, 14(6), 2128. https://doi.org/10.3390/jcm14062128








