Anterior Cruciate Ligament Return to Play: “A Framework for Decision Making”
1. Introduction
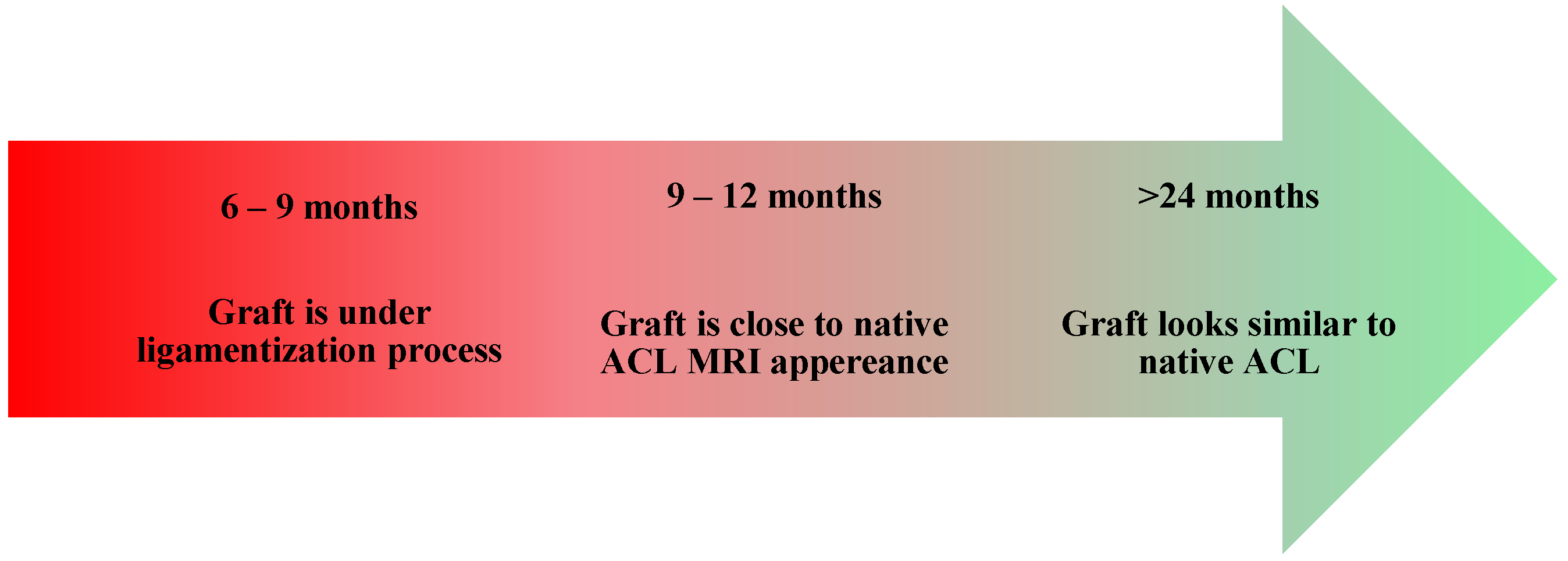
- Healing and Necrosis Phase—The host initiates an inflammatory response, leading to graft necrosis, particularly in the central region.
- Remodeling Phase—The graft loses some of its mechanical properties, including the stiffness and collagen crimp pattern, while increasing its type III collagen content and undergoing cellular repopulation. At the same time, the host initiates an angiogenesis process.
- Maturation Phase—The graft progressively acquires ligamentous properties, and the bone tunnel closes.
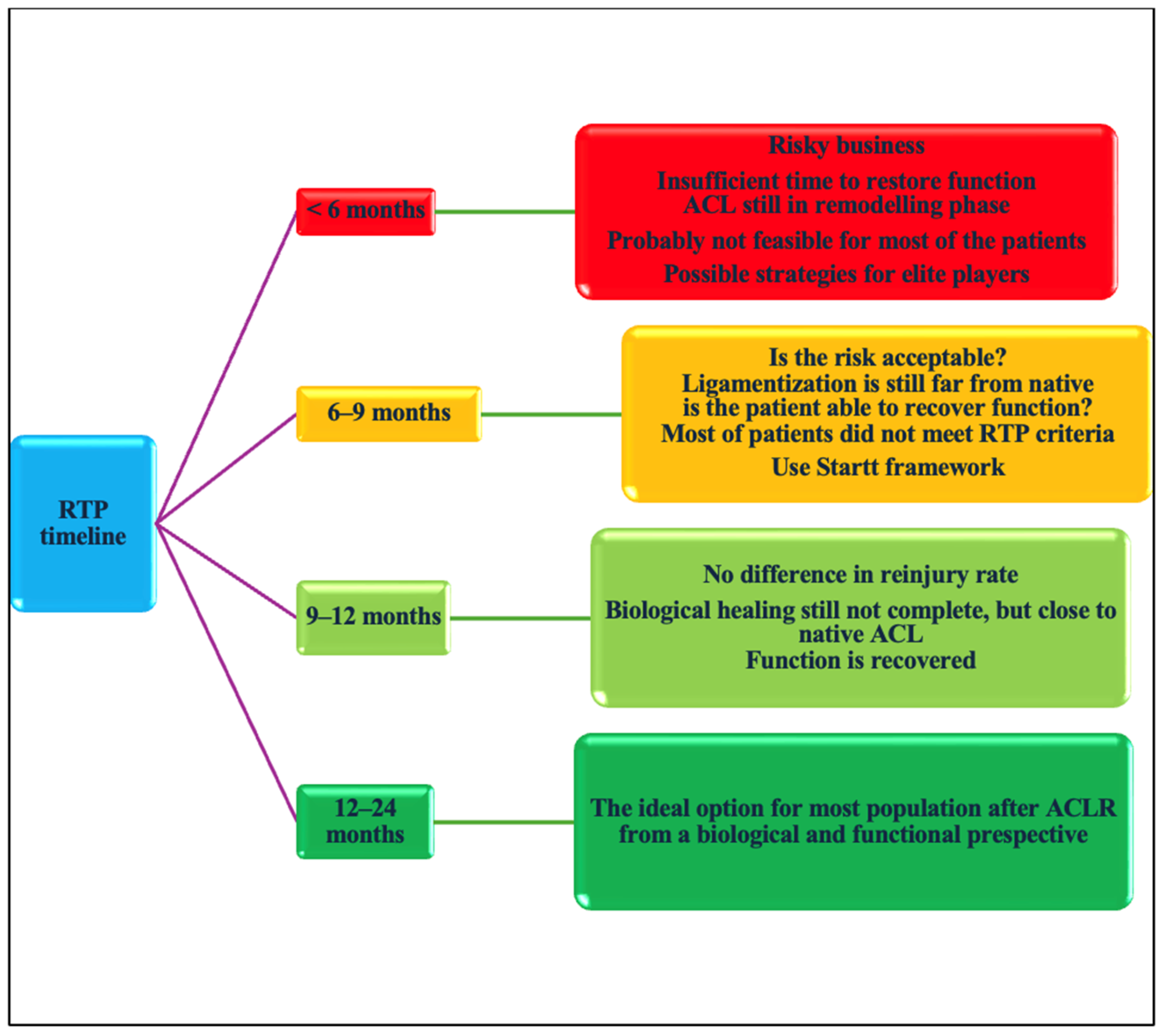
- RTP <6 months;
- RTP 6–9 months;
- 9–12 months;
- >12 months.
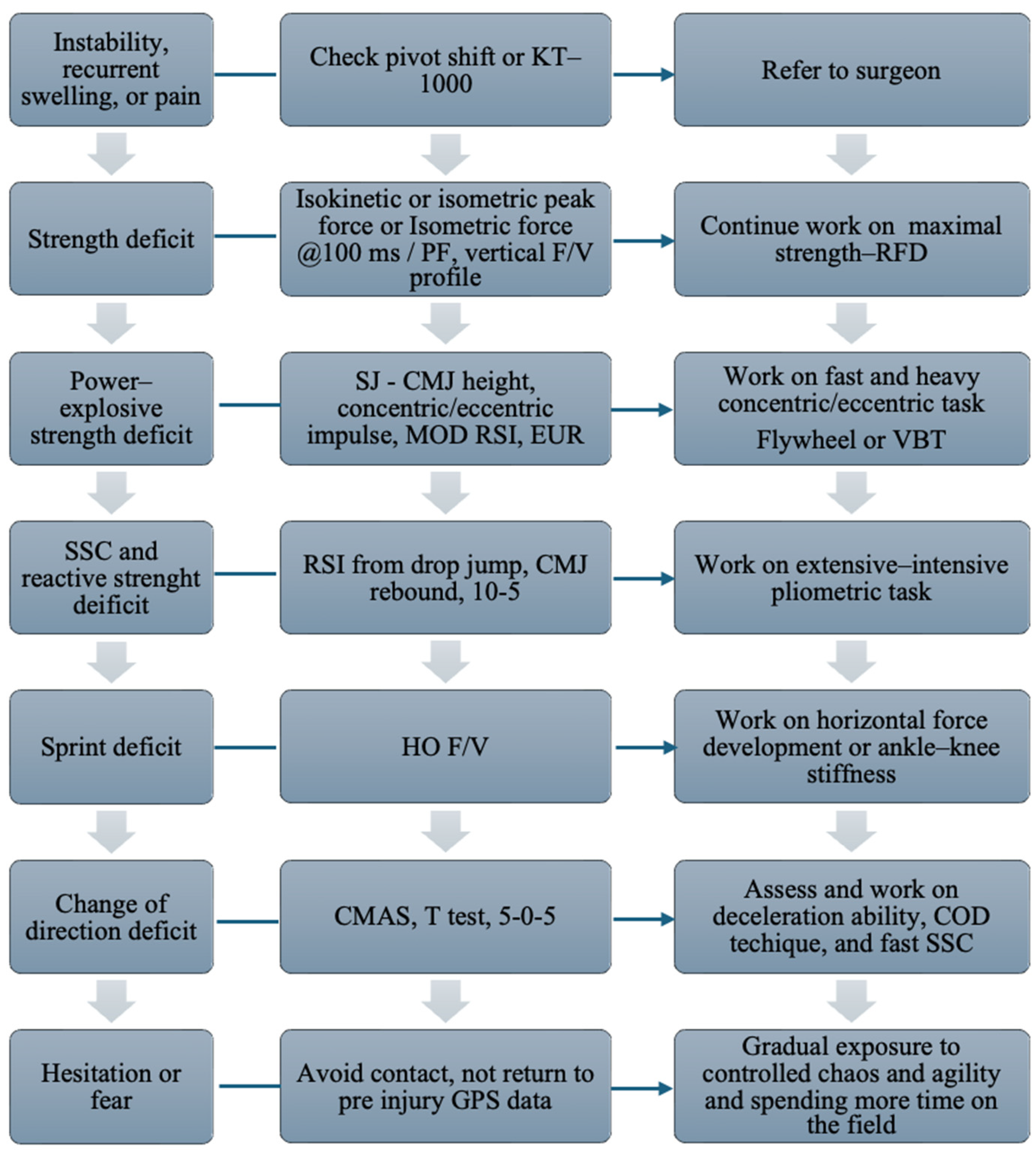
- Tissue Health, which evaluates the injured tissue’s ability to tolerate stress;
- Tissue Stress, which assesses the mechanical load that is imposed by sports activity and the effectiveness of protective measures;
- Risk Tolerance Modifiers, which consider external factors such as the competitive level, financial incentives, and psychological well-being.
2. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACL | anterior cruciate ligament |
| ACL-RSI | Anterior Cruciate Ligament—Return to Sport after Injury Scale |
| ACLR | anterior cruciate ligament reconstruction |
| CMAS | change of direction assessment score |
| CMJ | countermovement jump |
| COD | change of direction |
| DJ | drop jump |
| EPIC | estimated preinjury capacity |
| EUR | eccentric utilization ratio |
| F/V | force velocity profile |
| HO F/v | horizontal force velocity profile |
| IKDC | International Knee Documentation Committee Subjective Knee Form, isometric force @100 ms/PF, vertical ratio between force at 100 ms and peak force |
| KOOS | Knee Injury and Osteoarthritis Outcome score |
| KPI | key performance indicator |
| LSI | limb symmetry index |
| MOD RSI | reactive strength index modified |
| MRI | magnetic resonance imaging |
| PF | peak force |
| PROMS | patient-reported outcomes |
| RFD | rate of force development |
| RSI | reactive strength index |
| RTP | return to play |
| SJ | squat jump |
| SSC | stretch-shortening cycle |
| SNQ | signal–noise quotient |
| T test | agility T test |
| V F/v | vertical force velocity profile |
| VBT | velocity-based training |
| vGRF | vertical ground reaction force |
References
- Niederer, D.; Engeroff, T.; Wilke, J.; Vogt, L.; Banzer, W. Return to play, performance, and career duration after anterior cruciate ligament rupture: A case–control study in the five biggest football nations in Europe. Scand. J. Med. Sci. Sports. 2018, 28, 2226–2233. [Google Scholar] [PubMed]
- Manojlovic, M.; Ninkovic, S.; Matic, R.; Versic, S.; Modric, T.; Sekulic, D.; Drid, P. Return to Play and Performance After Anterior Cruciate Ligament Reconstruction in Soccer Players: A Systematic Review of Recent Evidence. Sports Med. 2024, 54, 2097–2108. [Google Scholar] [PubMed]
- Farinelli, L.; Abermann, E.; Meena, A.; Ueblacker, P.; Hahne, J.; Fink, C. Return to Play and Pattern of Injury After ACL Rupture in a Consecutive Series of Elite UEFA Soccer Players. Orthop. J. Sports Med. 2023, 11, 23259671231153629. [Google Scholar] [PubMed]
- van Melick, N.; Cingel, R.E.H.v.; Brooijmans, F.; Neeter, C.; van Tienen, T.; Hullegie, W.; der Sanden, M.W.G.N.-V. Evidence-based clinical practice update: Practice guidelines for anterior cruciate ligament rehabilitation based on a systematic review and multidisciplinary consensus. Br. J. Sports Med. 2016, 50, 1506–1515. [Google Scholar]
- Longstaffe, R.; Leiter, J.; Gurney-Dunlop, T.; McCormack, R.; MacDonald, P. Return to Play and Career Length After Anterior Cruciate Ligament Reconstruction Among Canadian Professional Football Players. Am. J. Sports Med. 2020, 48, 1682–1688. [Google Scholar]
- Lai, C.C.; Ardern, C.L.; Feller, J.A.; Webster, K.E. Eighty-three per cent of elite athletes return to pre-injury sport after anterior cruciate ligament reconstruction: A systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br. J. Sports Med. 2018, 52, 128–138. [Google Scholar]
- Ardern, C.L.; Glasgow, P.; Schneiders, A.; Witvrouw, E.; Clarsen, B.; Cools, A.; Gojanovic, B.; Griffin, S.; Khan, K.M.; Moksnes, H.; et al. 2016 Consensus statement on return to sport from the First World Congress in Sports Physical Therapy, Bern. Br. J. Sports Med. 2016, 50, 853–864. [Google Scholar]
- Borque, K.A.; Laughlin, M.S.; Pinheiro, V.H.; Ngo, D.; Kent, M.; Balendra, G.; Jones, M.; Williams, A. The Effect of Primary ACL Reconstruction on Career Longevity in English Premier League and Championship Soccer Players Compared with Uninjured Controls: A Matched Cohort Analysis. Am. J. Sports Med. 2024, 52, 1183–1188. [Google Scholar] [CrossRef]
- Pinheiro, V.H.; Borque, K.A.; Laughlin, M.S.; Jones, M.; Balendra, G.; Kent, M.R.; Ajgaonkar, R.; Williams, A. Determinants of Performance in Professional Soccer Players at 2 and 5 Years After ACL Reconstruction. Am. J. Sports Med. 2023, 51, 3649–3657. [Google Scholar]
- Leathers, M.P.; Merz, A.; Wong, J.; Scott, T.; Wang, J.C.; Hame, S.L. Trends and demographics in anterior cruciate ligament reconstruction in the United States. J. Knee Surg. 2015, 28, 390–394. [Google Scholar]
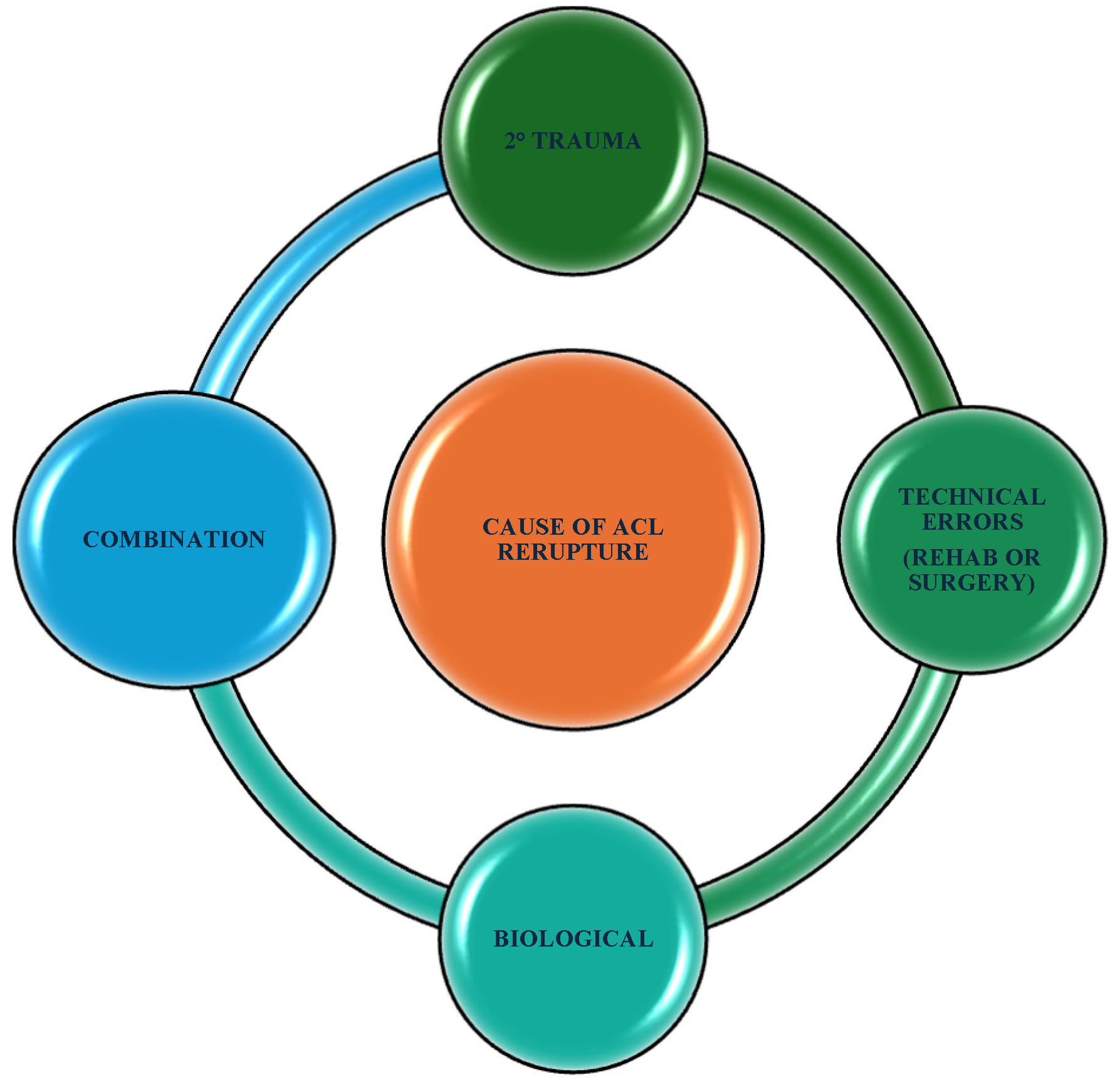
- Grassi, A.; Macchiarola, L.; Lucidi, G.A.; Stefanelli, F.; Neri, M.; Silvestri, A.; Della Villa, F.; Zaffagnini, S. More Than a 2-Fold Risk of Contralateral Anterior Cruciate Ligament Injuries Compared with Ipsilateral Graft Failure 10 Years After Primary Reconstruction. Am. J. Sports Med. 2020, 48, 310–317. [Google Scholar] [PubMed]
- Cronström, A.; Tengman, E.; Häger, C.K. Return to Sports: A Risky Business? A Systematic Review with Meta-Analysis of Risk Factors for Graft Rupture Following ACL Reconstruction. Sports Med. 2023, 53, 91–110. [Google Scholar] [PubMed]
- Della Villa, F.; Hägglund, M.; Della Villa, S.; Ekstrand, J.; Waldén, M. High rate of second ACL injury following ACL reconstruction in male professional footballers: An updated longitudinal analysis from 118 players in the UEFA Elite Club Injury Study. Br. J. Sports Med. 2021, 55, 1350–1357. [Google Scholar] [PubMed]
- McAleese, T.; Welch, N.; King, E.; Roshan, D.; Keane, N.; Moran, K.A.; Jackson, M.; Withers, D.; Moran, R.; Devitt, B.M. Primary Anterior Cruciate Ligament Reconstruction in Level 1 Athletes: Factors Associated with Return to Play, Re-injury, and Knee Function at 5 Years of Follow-up. Am. J. Sports Med. 2025, 53, 777–790. [Google Scholar]
- Paterno, M.V.; Huang, B.; Thomas, S.; Hewett, T.E.; Schmitt, L.C. Clinical factors that predict a second ACL injury after ACL reconstruction and return to sport: Preliminary development of a clinical decision algorithm. Orthop. J. Sports Med. 2017, 5, 2325967117745279. [Google Scholar]
- Barber-Westin, S.; Noyes, F.R. One in 5 athletes sustain re-injury upon return to high-risk sports after ACL reconstruction: A systematic review in 1239 athletes younger than 20 years. Sports Health 2020, 12, 587–597. [Google Scholar]
- Bloch, H.; Reinsberger, C.; Klein, C.; Luig, P.; Krutsch, W. High revision arthroscopy rate after ACL reconstruction in men’s professional team sports. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 142–151. [Google Scholar]
- Samitier, G.; Marcano, A.I.; Alentorn-Geli, E.; Cugat, R.; Farmer, K.W.; Moser, M.W. Failure of Anterior Cruciate Ligament Reconstruction. Arch. Bone Jt. Surg. 2015, 3, 220–240. [Google Scholar]
- Pfeifer, C.E.; Beattie, P.F.; Sacko, R.S.; Hand, A. Risk factors associated with non-contact anterior cruciate ligament injury: A systematic review. Int. J. Sports Phys. Ther. 2018, 13, 575–587. [Google Scholar]
- Li, X.; Yan, L.; Li, D.; Fan, Z.; Liu, H.; Wang, G.; Jiu, J.; Yang, Z.; Li, J.J.; Wang, B. Failure modes after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Int. Orthop. SICOT 2023, 47, 719–734. [Google Scholar]
- Hong, I.S.; Pierpoint, L.A.; Hellwinkel, J.E.; Berk, A.N.; Salandra, J.M.; Meade, J.D.; Piasecki, D.P.; Fleischli, J.E.; Ahmad, C.S.; Trofa, D.P.; et al. Clinical outcomes after ACL reconstruction in soccer (football, futbol) players: A systematic review and meta-analysis. Sports Health 2023, 15, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Szymski, D.; Achenbach, L.; Weber, J.; Huber, L.; Memmel, C.; Kerschbaum, M.; Alt, V.; Krutsch, W. Reduced performance after return to competition in ACL injuries: An analysis on return to competition in the “ACL registry in German Football”. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nwachukwu, B.U.; Anthony, S.G.; Lin, K.M.; Wang, T.; Altchek, D.W.; Allen, A.A. Return to play and performance after anterior cruciate ligament reconstruction in the National Basketball Association: Surgeon case series and literature review. Phys. Sportsmed. 2017, 45, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.; Barton, D.; Khwaja, A.; Latt, L.D. Return to play rates in NFL wide receivers and running backs after ACL reconstruction: An updated analysis. Orthop. J. Sports Med. 2021, 9, 2325967120974743. [Google Scholar] [CrossRef]
- Welling, W.; Benjaminse, A.; Lemmink, K.; Gokeler, A. Passing return to sports tests after ACL reconstruction is associated with greater likelihood for return to sport but fail to identify second injury risk. Knee 2020, 27, 949–957. [Google Scholar] [CrossRef]
- Della Villa, F.; Boldrini, L.; Ricci, M.; Danelon, F.; Snyder-Mackler, L.; Zaffagnini, S. Compliance in post-operative rehabilitation is a key factor for return to sport after revision anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2023. [Google Scholar] [CrossRef]
- van Haren, I.E.; van Cingel, R.E.; Verbeek, A.L.; van Melick, N.; Stubbe, J.H.; Bloo, H.; Groenewoud, J.H.M.; van der Wees, P.J.; Staal, J.B. Predicting readiness for return to sport and performance after anterior cruciate ligament reconstruction rehabilitation. Ann. Phys. Rehabil. Med. 2023, 66, 101689. [Google Scholar] [CrossRef]
- Gill, V.S.; Tummala, S.V.; Sullivan, G.; Han, W.; Haglin, J.M.; Marks, L.; Tokish, J.M. Functional Return-to-Sport Testing Demonstrates Inconsistency in Predicting Short-Term Outcomes Following Anterior Cruciate Ligament Reconstruction: A Systematic Review. Arthroscopy 2024, 40, 2135–2151.e2. [Google Scholar] [CrossRef]
- Webster, K.E.; Feller, J.A. Who passes return-to-sport tests, and which tests are most strongly associated with return to play after anterior cruciate ligament reconstruction? Orthop. J. Sports Med. 2020, 8, 2325967120969425. [Google Scholar] [CrossRef]
- Toole, A.R.; Ithurburn, M.P.; Rauh, M.J.; Hewett, T.E.; Paterno, M.V.; Schmitt, L.C. Young athletes cleared for sports participation after anterior cruciate ligament reconstruction: How many actually meet recommended return-to-sport criterion cutoffs? J. Orthop. Sports Phys. Ther. 2017, 47, 825–833. [Google Scholar] [CrossRef]
- Welling, W.; Benjaminse, A.; Seil, R.; Lemmink, K.; Zaffagnini, S.; Gokeler, A. Low rates of patients meeting return to sport criteria 9 months after anterior cruciate ligament reconstruction: A prospective longitudinal study. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Unverzagt, C.; Andreyo, E.; Tompkins, J. ACL return to sport testing: It’s time to step up our game. Int. J. Sports Phys. Ther. 2021, 16, 1169–1177. [Google Scholar] [PubMed]
- Wood, A.; Hargreaves, M.; Manfredi, J.N.; Harrell, M.; Marks Benson, E.; Rahaman, C.; Momaya, A.M. Anterior Cruciate Ligament Reconstruction Return to Sport Testing Passing Rates for Healthy People: A Systematic Review. Am. J. Sports Med. 2025, 03635465241313194. [Google Scholar] [CrossRef]
- Burgi, C.R.; Peters, S.; Ardern, C.L.; Magill, J.R.; Gomez, C.D.; Sylvain, J.; Reiman, M.P. Which criteria are used to clear patients to return to sport after primary ACL reconstruction? A scoping review. Br. J. Sports Med. 2019, 53, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.T.; Myer, G.D.; Read, P.J. Is It Time We Better Understood the Tests We are Using for Return to Sport Decision Making Following ACL Reconstruction? A Critical Review of the Hop Tests. Sports Med. 2020, 50, 485–495. [Google Scholar]
- Marumo, K.; Saito, M.; Yamagishi, T.; Fujii, K. The “ligamentization” process in human anterior cruciate ligament reconstruction with autogenous patellar and hamstring tendons: A biochemical study. Am. J. Sports Med. 2005, 33, 1166–1173. [Google Scholar]
- Claes, S.; Verdonk, P.; Forsyth, R.; Bellemans, J. The “ligamentization” process in anterior cruciate ligament reconstruction: What happens to the human graft? A systematic review of the literature. Am. J. Sports Med. 2011, 39, 2476–2483. [Google Scholar]
- Yao, S.; Fu BS, C.; Yung PS, H. Graft healing after anterior cruciate ligament reconstruction (ACLR). Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2021, 25, 8–15. [Google Scholar] [CrossRef]
- Zdanowicz, U.; Ciszkowska-Łysoń, B.; Paśnik, M.; Drwięga, M.; Ratajczak, K.; Fulawka, K.; Lee, Y.C.; Śmigielski, R. Evaluation of ACL Graft Remodeling and Prediction of Graft Insufficiency in Sequenced MRI—Two-Year Follow-Up. Appl. Sci. 2021, 11, 5278. [Google Scholar] [CrossRef]
- DeFroda, S.F.; ODonnell, R.M.; Fadale, P.D.; Owens, B.D.; Fleming, B.C. The role of magnetic resonance imaging in evaluating postoperative ACL reconstruction healing and graft mechanical properties: A new criterion for return to play? Physician Sportsmed. 2021, 49, 123–129. [Google Scholar]
- Malahias, M.-A.; Capece, F.M.; Ballarati, C.; Viganò, M.; Marano, M.; Hofbauer, M.; Togninalli, D.; de Girolamo, L.; Denti, M. Sufficient MRI graft structural integrity at 9 months after anterior cruciate ligament reconstruction with hamstring tendon autograft. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 1893–1900. [Google Scholar] [PubMed]
- Li, H.; Chen, J.; Li, H.; Wu, Z.; Chen, S. MRI-based ACL graft maturity does not predict clinical and functional outcomes during the first year after ACL reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3171–3178. [Google Scholar]
- Zhou, T.; Xu, Y.; Zhang, A.; Zhang, X.; Deng, K.; Wu, H.; Xu, W. Association of Graft Maturity on MRI With Return to Sports at 9 Months After Primary Single-Bundle ACL Reconstruction with Autologous Hamstring Graft. Orthop. J. Sports Med. 2024, 12, 23259671241248202. [Google Scholar] [PubMed]
- Ma, Y.; Murawski, C.D.; Rahnemai-Azar, A.A.; Maldjian, C.; Lynch, A.D.; Fu, F.H. Graft maturity of the reconstructed anterior cruciate ligament 6 months postoperatively: A magnetic resonance imaging evaluation of quadriceps tendon with bone block and hamstring tendon autografts. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 661–668. [Google Scholar] [PubMed]
- Biercevicz, A.M.; Akelman, M.R.; Fadale, P.D.; Hulstyn, M.J.; Shalvoy, R.M.; Badger, G.J.; Tung, G.A.; Oksendahl, H.L.; Fleming, B.C. MRI volume and signal intensity of ACL graft predict clinical, functional, and patient-oriented outcome measures after ACL reconstruction. Am. J. Sports Med. 2015, 43, 693–699. [Google Scholar]
- Hofbauer, M.; Soldati, F.; Szomolanyi, P.; Trattnig, S.; Bartolucci, F.; Fu, F.; Denti, M. Hamstring tendon autografts do not show complete graft maturity 6 months postoperatively after anterior cruciate ligament reconstruction. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 130–136. [Google Scholar]
- Li, H.; Chen, S.; Tao, H.; Li, H.; Chen, S. Correlation analysis of potential factors influencing graft maturity after anterior cruciate ligament reconstruction. Orthop. J. Sports Med. 2014, 2, 2325967114553552. [Google Scholar]
- Van Dyck, P.; Clockaerts, S.; Wouters, K. MRI signal intensity changes in ACL grafts: Clinical significance and implications for return to sport. Eur. Radiol. 2021, 31, 4102–4113. [Google Scholar]
- Biercevicz, A.M.; Akelman, M.R.; Fadale, P.D. The role of MRI in evaluating ACL graft healing and its correlation with knee stability. Orthop. J. Sports Med. 2021, 9, 23259671211035684. [Google Scholar]
- Nagell, C.V.; Hewett, T.E. Should Return to Sport be Delayed Until 2 Years After Anterior Cruciate Ligament Reconstruction? Biological and Functional Considerations. Sports Med. 2017, 47, 221–232. [Google Scholar] [CrossRef]
- Mayer, M.A.; Deliso, M.; Hong, I.S.; Saltzman, B.M.; Longobardi, R.S.; DeLuca, P.F.; Rizio, L. Rehabilitation and Return to Play Protocols After Anterior Cruciate Ligament Reconstruction in Soccer Players: A Systematic Review. Am. J. Sports Med. 2025, 53, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gill, V.S.; Tummala, S.V.; Han, W.; Boddu, S.P.; Verhey, J.T.; Marks, L.; Chhabra, A. Athletes Continue to Show Functional Performance Deficits at Return to Sport After Anterior Cruciate Ligament Reconstruction: A Systematic Review. Arthroscopy 2024, 40, 2309–2321.e2. [Google Scholar] [CrossRef] [PubMed]
- Kotsifaki, A.; Korakakis, V.; Whiteley, R.; Van Rossom, S.; Jonkers, I. Measuring only hop distance during single leg hop testing is insufficient to detect deficits in knee function after ACL reconstruction: A systematic review and meta-analysis. Br. J. Sports Med. 2020, 54, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Kotsifaki, A.; Whiteley, R.; Van Rossom, S.; Korakakis, V.; Bahr, R.; Sideris, V.; Jonkers, I. Single leg hop for distance symmetry masks lower limb biomechanics: Time to discuss hop distance as decision criterion for return to sport after ACL reconstruction? Br. J. Sports Med. 2022, 56, 249–256. [Google Scholar] [CrossRef]
- Peebles, A.T.; Renner, K.E.; Miller, T.K.; Moskal, J.T.; Queen, R.M. Associations between Distance and Loading Symmetry during Return to Sport Hop Testing. Med. Sci. Sports Exerc. 2019, 51, 624–629. [Google Scholar] [CrossRef]
- Ithurburn, M.P.; Longfellow, M.A.; Thomas, S.; Paterno, M.V.; Schmitt, L.C. Knee function, strength, and resumption of pre-injury sports participation in young athletes following anterior cruciate ligament reconstruction. J. Orthop. Sports Phys. Ther. 2019, 49, 145–153. [Google Scholar] [CrossRef]
- King, E.; Richter, C.; Franklyn-Miller, A.; Wadey, R.; Moran, R.; Strike, S. Back to normal symmetry? Biomechanical variables remain more asymmetrical than normal during jump and change-of-direction testing 9 months after anterior cruciate ligament reconstruction. Am. J. Sports Med. 2019, 47, 1175–1185. [Google Scholar] [CrossRef]
- Kowalczyk, K.M.; Shumski, E.J.; Lisee, C.; Lynall, R.C. Female collegiate soccer players post anterior cruciate ligament reconstruction utilize aberrant movement strategies to achieve similar performance to uninjured players. Clin. Biomech. 2025, 122, 106424. [Google Scholar] [CrossRef]
- Fältström, A.; Kvist, J.; Hägglund, M. Are We Jumping to the Wrong Conclusions? Longer Jumps and More Hops in Female Football Players Who Went on to Sustain a Primary or Secondary ACL Injury Compared to Those Who Did Not. Sports Med. Open. 2023, 9, 105. [Google Scholar] [CrossRef]
- King, E.; Richter, C.; Daniels, K.; Miller, A.F.; Myer, G.; Strike, S. “Can Biomechanical Testing After ACLR Identify Athletes at Risk for Subsequent ACL Injury to the Contralateral Uninjured Limb?” and “Biomechanical but Not Strength or Performance Measures Differentiate Male Athletes Who Experience ACL Re-injury on Return to Level 1 Sports”: Response. Am. J. Sports Med. 2021, 49, NP36–NP37. [Google Scholar]
- Wellsandt, E.; Failla, M.J.; Snyder-Mackler, L. Limb Symmetry Indexes Can Overestimate Knee Function After Anterior Cruciate Ligament Injury. J. Orthop. Sports Phys. Ther. 2017, 47, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.J.; Bishop, C. Testing limb symmetry and asymmetry after anterior cruciate ligament injury: 4 considerations to increase its utility. Strength. Cond. J. 2024, 46, 406–414. [Google Scholar] [CrossRef]
- Van Melick, N.; Pronk, Y.; Nijhuis-van der Sanden, M.; Rutten, S.; van Tienen, T.; Hoogeboom, T. Meeting movement quantity or quality return to sport criteria is associated with reduced second ACL injury rate. J. Orthop. Res. 2022, 40, 117–128. [Google Scholar] [PubMed]
- Van Melick, N.; van der Weegen, W.; van der Horst, N.; Bogie, R. Double-Leg and Single-Leg Jump Test Reference Values for Athletes with and Without Anterior Cruciate Ligament Reconstruction Who Play Popular Pivoting Sports, Including Soccer and Basketball: A Scoping Review. J. Orthop. Sports Phys. Ther. 2024, 54, 377–390. [Google Scholar]
- Jordan, M.T.; Turner, J.A.; Cameron, K.L.; Padua, D.A. Strength of the Uninvolved Limb Following Return to Activity After ACL Injury: Implications for Symmetry as a Marker of Sufficient Strength. Int. J. Sports Phys. Ther. 2024, 19, 657–669. [Google Scholar]
- Lambricht, N.; Englebert, A.; Pitance, L.; Fisette, P.; Detrembleur, C. Quantifying performance and joint kinematics in functional tasks crucial for anterior cruciate ligament rehabilitation using smartphone video and pose detection. Knee 2025, 52, 171–178. [Google Scholar] [CrossRef]
- Lima, Y.L.; Collings, T.; Hall, M.; Bourne, M.N.; Diamond, L.E. Validity and reliability of trunk and lower-limb kinematics during squatting, hopping, jumping and side-stepping using OpenCap markerless motion capture application. J. Sports Sci. 2024, 42, 1847–1858. [Google Scholar] [CrossRef]
- Aleksic, J.; Kanevsky, D.; Mesaroš, D.; Knezevic, O.M.; Cabarkapa, D.; Bozovic, B.; Mirkov, D.M. Validation of Automated Countermovement Vertical Jump Analysis: Markerless Pose Estimation vs. 3D Marker-Based Motion Capture System. Sensors 2024, 24, 6624. [Google Scholar] [CrossRef]
- Simonsson, R.; Sundberg, A.; Piussi, R.; Högberg, J.; Senorski, C.; Thomeé, R.; Samuelsson, K.; Della Villa, F.; Senorski, E.H. Questioning the rules of engagement: A critical analysis of the use of limb symmetry index for safe return to sport after anterior cruciate ligament reconstruction. Br. J. Sports Med. 2024, 59, 376–384. [Google Scholar] [CrossRef]
- Kotsifaki, R.; King, E.; Bahr, R.; Whiteley, R. Is 9 months the sweet spot for male athletes to return to sport after anterior cruciate ligament reconstruction? Br. J. Sports 2025. [Google Scholar] [CrossRef]
- Kotsifaki, R.; Korakakis, V.; King, E.; Barbosa, O.; Maree, D.; Pantouveris, M.; Bjerregaard, A.; Luomajoki, J.; Wilhelmsen, J.; Whiteley, R. Aspetar clinical practice guideline on rehabilitation after anterior cruciate ligament reconstruction. Br. J. Sports Med. 2023, 57, 500–514. [Google Scholar] [PubMed]
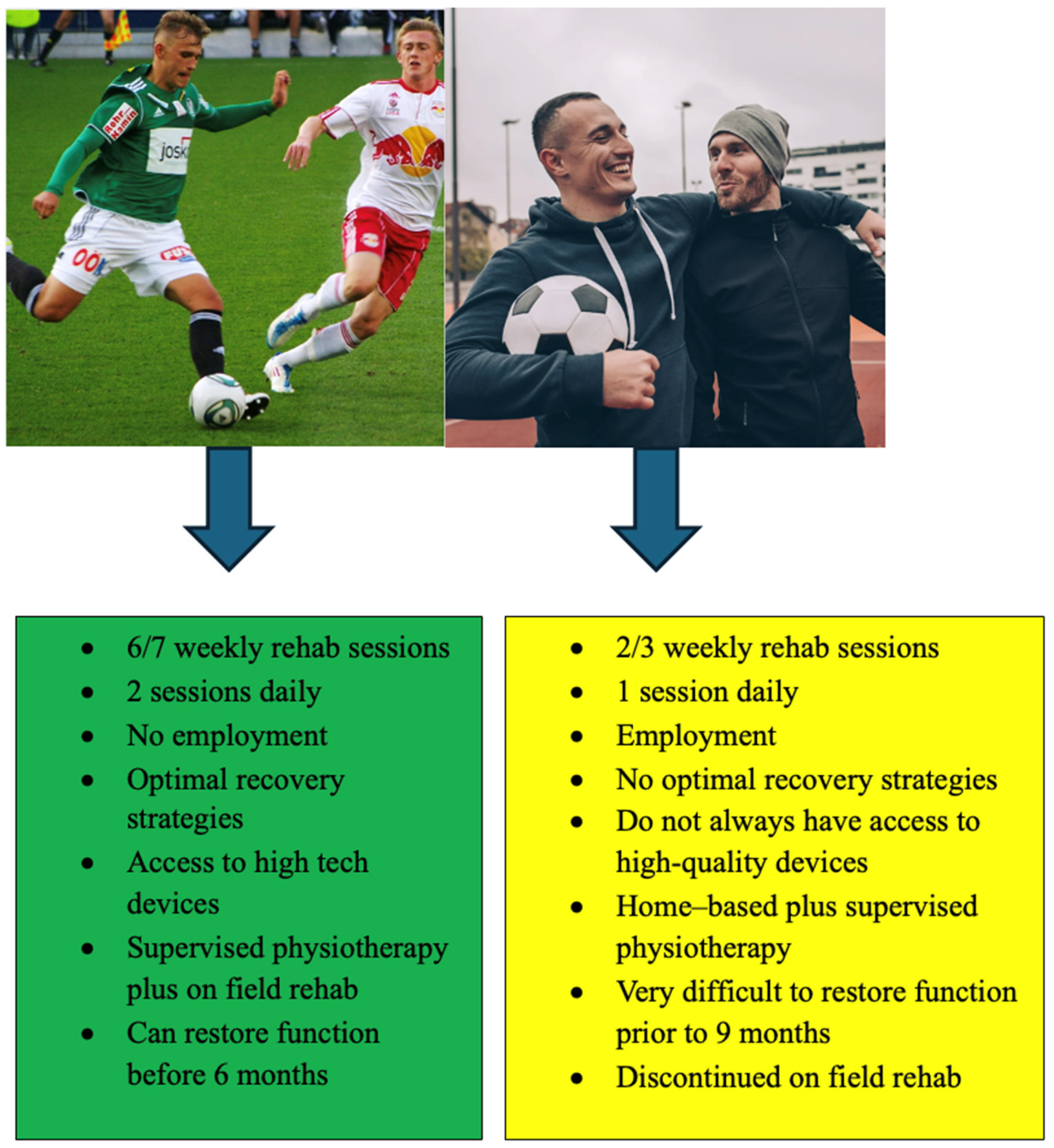
- Buckthorpe, M.; Della Villa, F.; Della Villa, S.; Roi, G.S. On-field rehabilitation part 1: 4 pillars of high-quality on-field rehabilitation are restoring movement quality, physical conditioning, restoring sport-specific skills, and progressively developing chronic training load. J. Orthop. Sports Phys. Ther. 2019, 49, 565–569. [Google Scholar] [PubMed]
- Florelli, F.; Le Coroller, N.; Gaspar, M.; Memain, G.; Kakavas, G.; Miraglia, N.; Marine, P.; Maille, P.; Hewett, T.E.; Rambaud, A.J. Ecological and Specific Evidence-Based Safe Return To Play After Anterior Cruciate Ligament Reconstruction In Soccer Players: A New International Paradigm. Int. J. Sports Phys. Ther. 2023, 18, 526–540. [Google Scholar]
- Disegni, E.; Memain, G.; Bouvet, J.; Gaspar, M.; Maille, R.; Tamalet, B.; Orhant, E.; Maille, P.; Bohu, Y.; Hardy, A. The “11 to Perf Score”, a Test for Professional Players Returning to Soccer After Anterior Cruciate Ligament Reconstruction. J. Clin. Med. 2024, 14, 11. [Google Scholar] [CrossRef]
- Bodkin, S.G.; Hertel, J.; Diduch, D.R.; Saliba, S.A.; Novicoff, W.M.; Brockmeier, S.F.; Miller, M.D.; Gwathmey, F.W.; Werner, B.C.; Hart, J.M.; et al. Predicting anterior cruciate ligament re-injury from return-to-activity assessments at 6 months postsurgery: A prospective cohort study. J. Athl. Train. 2022, 57, 325–333. [Google Scholar]
- Meredith, S.J.; Rauer, T.; Chmielewski, T.L.; Fink, C.; Diermeier, T.; Rothrauff, B.B.; Svantesson, E.; Hamrin Senorski, E.; Hewett, T.E.; Sherman, S.L.; et al. Return to sport after anterior cruciate ligament injury: Panther Symposium ACL Injury Return to Sport Consensus Group. Orthop. J. Sports Med. 2020, 8, 2325967120930829. [Google Scholar]
- Beischer, S.; Gustavsson, L.; Senorski, E.H.; Karlsson, J.; Thomeé, C.; Samuelsson, K.; Thomeé, R. Young athletes who return to sport before 9 months after anterior cruciate ligament reconstruction have a rate of new injury 7 times that of those who delay return. J. Orthop. Sports Phys. Ther. 2020, 50, 83–90. [Google Scholar]
- Grindem, H.; Snyder-Mackler, L.; Moksnes, H.; Engebretsen, L.; Risberg, M.A. Simple decision rules can reduce re-injury risk by 84% after ACL reconstruction: The Delaware-Oslo ACL cohort study. Br. J. Sports Med. 2016, 50, 804–808. [Google Scholar]
- Bodkin, S.G. Time to Reflect on return to spo Timing Following ACL Reconstruction. Sports Med. 2024, 54, 1749–1754. [Google Scholar]
- Webster, K.E.; Feller, J.A.; Klemm, H.J. Second ACL injury rates in younger athletes who were advised to delay return to sport until 12 months after ACL reconstruction. Orthop. J. Sports Med. 2021, 9, 2325967120985636. [Google Scholar]
- Picinini, F.; Della Villa, F.; Tallent, J.; Patterson, S.D.; Galassi, L.; Parigino, M.; La Rosa, G.; Nanni, G.; Olmo, J.; Stride, M.; et al. High Return to Competition Rate After On-Field Rehabilitation in Competitive Male Soccer Players After ACL Reconstruction: GPS Tracking in 100 Consecutive Cases. Orthop. J. Sports Med. 2025, 13, 23259671251320093. [Google Scholar] [PubMed]
- Shrier, I. Strategic Assessment of Risk and Risk Tolerance (StARRT) framework for return-to-play decision-making. Br. J. Sports Med. 2015, 49, 1311–1315. [Google Scholar] [PubMed]
- Högberg, J.; Fridh, E.; Piussi, R.; Simonsson, R.; Cristiani, R.; Samuelsson, K.; Senorski, E.H. Delayed anterior cruciate ligament reconstruction is associated with lower odds of returning to pre-injury physical activity level at 12 months follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2025, in press. [Google Scholar] [CrossRef]
- McGrath, T.M.; Waddington, G.; Scarvell, J.M.; Ball, N.; Creer, R.; Woods, K.; Smith, D.; Adams, R. An Ecological Study of Anterior Cruciate Ligament Reconstruction, Part 1: Clinical Tests Do Not Correlate With Return-to-Sport Outcomes. Orthop. J. Sports Med. 2016, 4, 2325967116672208. [Google Scholar] [CrossRef]
- Taberner, M.; Allen, T.; Cohen, D.D. Progressing rehabilitation after injury: Consider the ‘control-chaos continuum’. Br. J. Sports Med. 2019, 53, 1132–1136. [Google Scholar]
- Taberner, M.; Allen, T.; O’keefe, J.; Chaput, M.; Grooms, D.; Cohen, D.D. Evolving the Control-Chaos Continuum: Part 2–Shifting ‘Attention’ to Progress On-Pitch Rehabilitation. J. Orthop. Sports Phys. Ther. 2025, 55, 162–172. [Google Scholar]






| KPI | Value |
|---|---|
| Knee laxity–swelling–pain | Absent |
| PROMs (IKDC, KOOS, ACL-RSI) | Normalized |
| Isokinetic strength | >90% flexion and extension 60–180°/s LSI |
| Single-leg CMJ impulse and height | >90% LSI |
| Single-leg DJ RSI and height | >90% LSI |
| Hop distance (battery) | >90% LSI |
| Side hop repetition | >90 LSI |
| Jump–hop biomechanics | Symmetry value for moments, angles, and work in vertical and horizontal jumps, especially in sagittal and frontal planes at hip, knee, and ankle |
| Double-leg CMJ and DJ | Symmetry in vGRF |
| Running–sprinting profile (1080 sprint or Myjump ®) and biomechanics | Same vGRF; same knee, hip and ankle angle; no trunk side flexion or lack of pelvis frontal plane control Preinjury horizontal F/V profile |
| Running sprinting curriculum | Preinjury velocity in linear, curvilinear, and multidirectional movements |
| Repeated sprint ability test index | <3% |
| Agility test (T-test, Illinois) | Normative value |
| Adherence to a structured rehabilitation program | More than 50 sessions, more than 30 sessions on field, more than 3 sessions per week |
| 45′ Game simulation—preinjury training load | GPS data |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricupito, R.; Grassi, A.; Mourad, F.; Di Filippo, L.; Gobbo, M.; Maselli, F. Anterior Cruciate Ligament Return to Play: “A Framework for Decision Making”. J. Clin. Med. 2025, 14, 2146. https://doi.org/10.3390/jcm14072146
Ricupito R, Grassi A, Mourad F, Di Filippo L, Gobbo M, Maselli F. Anterior Cruciate Ligament Return to Play: “A Framework for Decision Making”. Journal of Clinical Medicine. 2025; 14(7):2146. https://doi.org/10.3390/jcm14072146
Chicago/Turabian StyleRicupito, Roberto, Alberto Grassi, Firas Mourad, Luigi Di Filippo, Massimiliano Gobbo, and Filippo Maselli. 2025. "Anterior Cruciate Ligament Return to Play: “A Framework for Decision Making”" Journal of Clinical Medicine 14, no. 7: 2146. https://doi.org/10.3390/jcm14072146
APA StyleRicupito, R., Grassi, A., Mourad, F., Di Filippo, L., Gobbo, M., & Maselli, F. (2025). Anterior Cruciate Ligament Return to Play: “A Framework for Decision Making”. Journal of Clinical Medicine, 14(7), 2146. https://doi.org/10.3390/jcm14072146









