Age-Related Variations and Seasonal Influences: A Network Analysis of Comorbidities in Asthma Hospitalizations (2013–2023)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
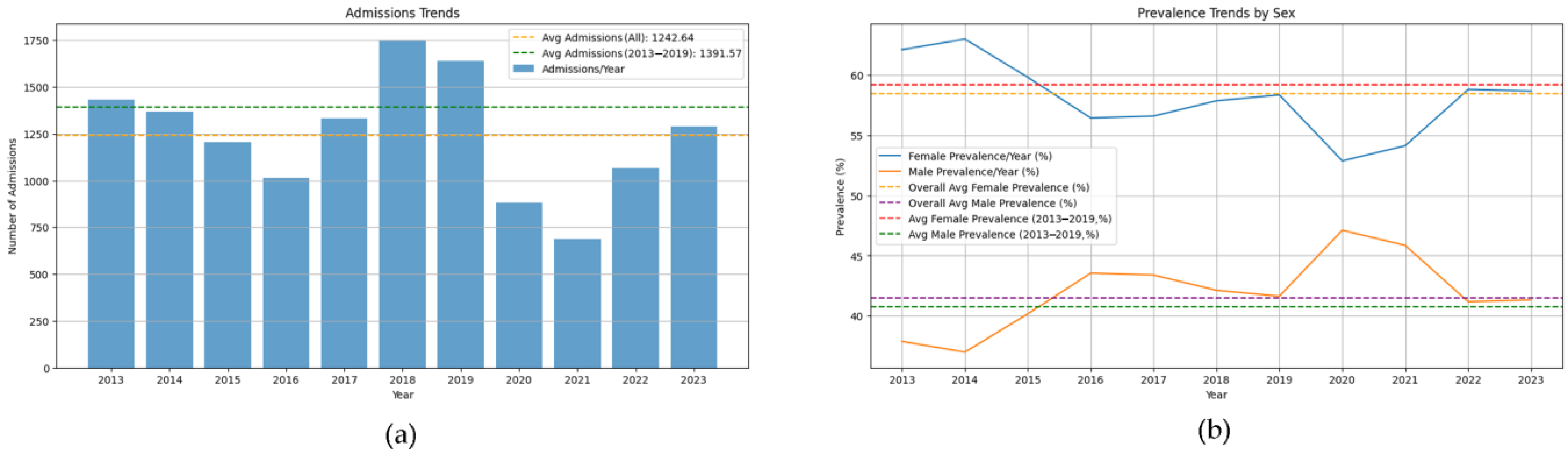
4.1. Seasonal and Demographic Trends
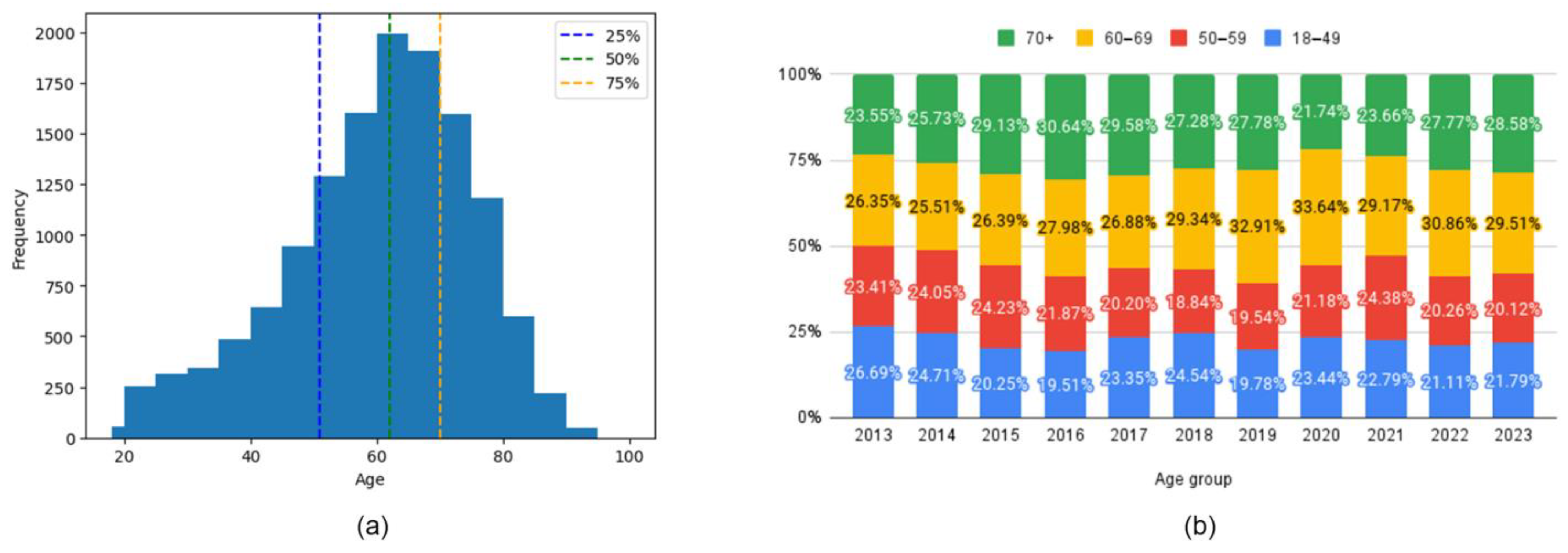
4.2. Age Groups and Comorbidities
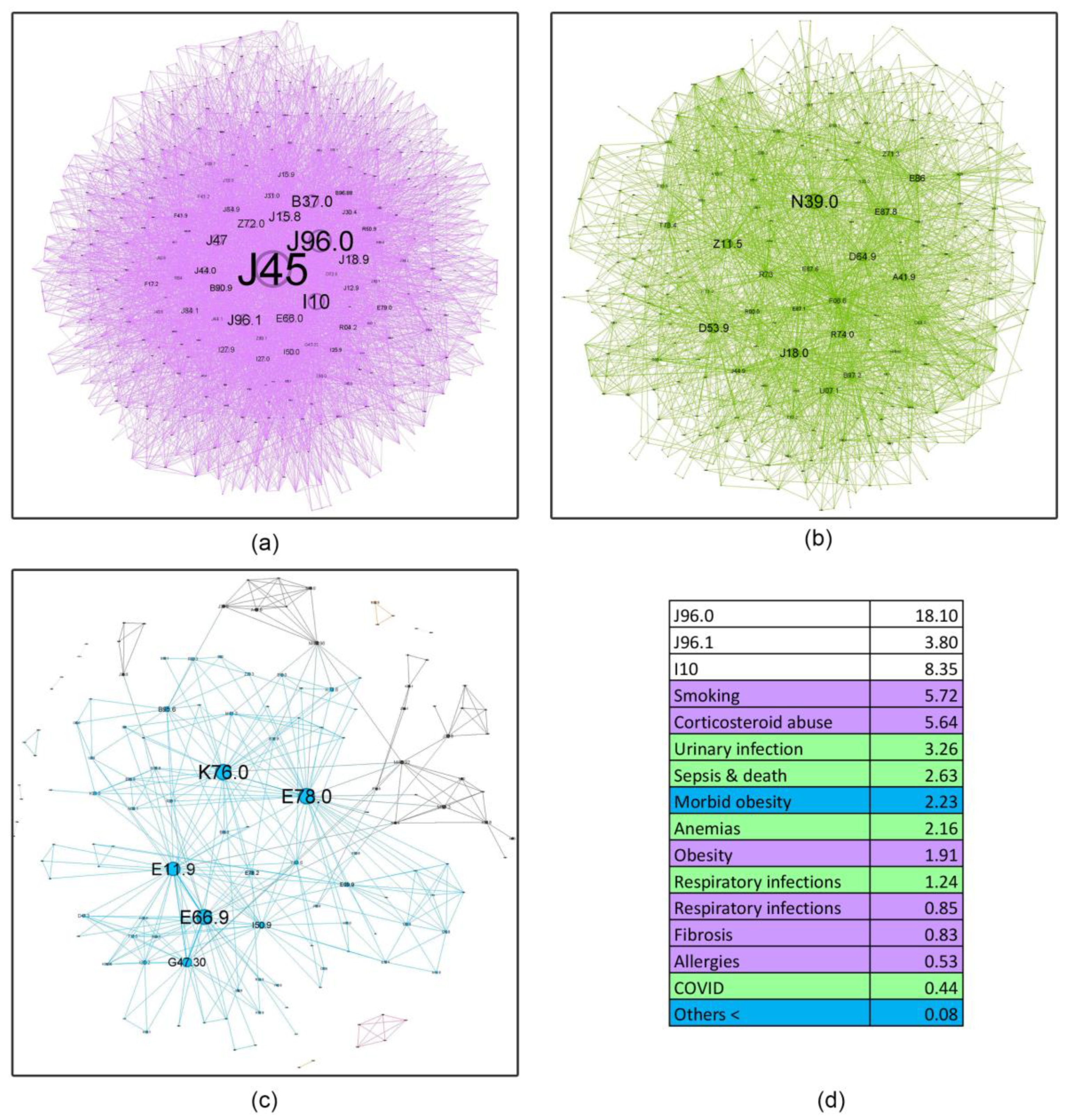
4.3. The Impact of Comorbidities
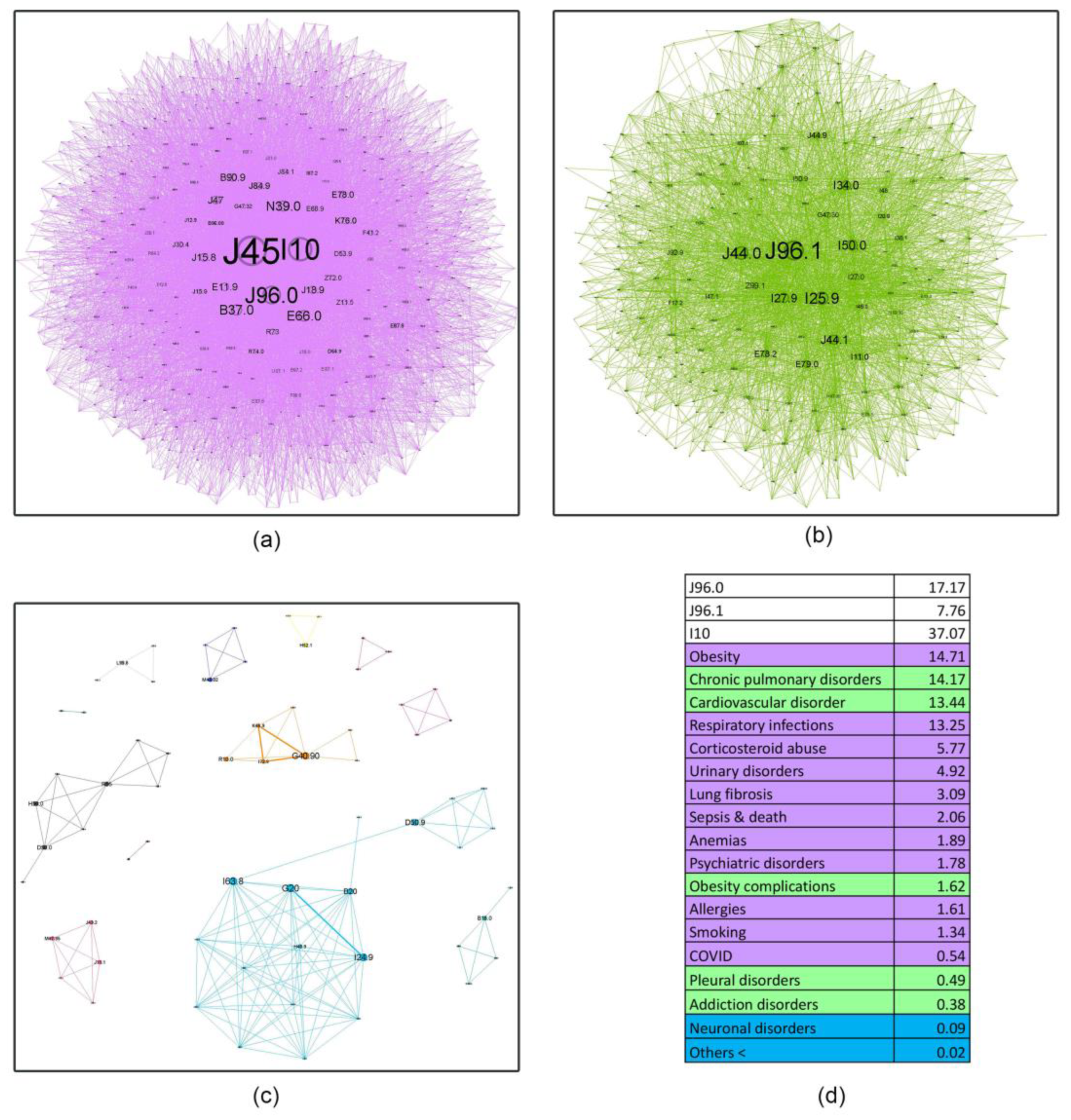
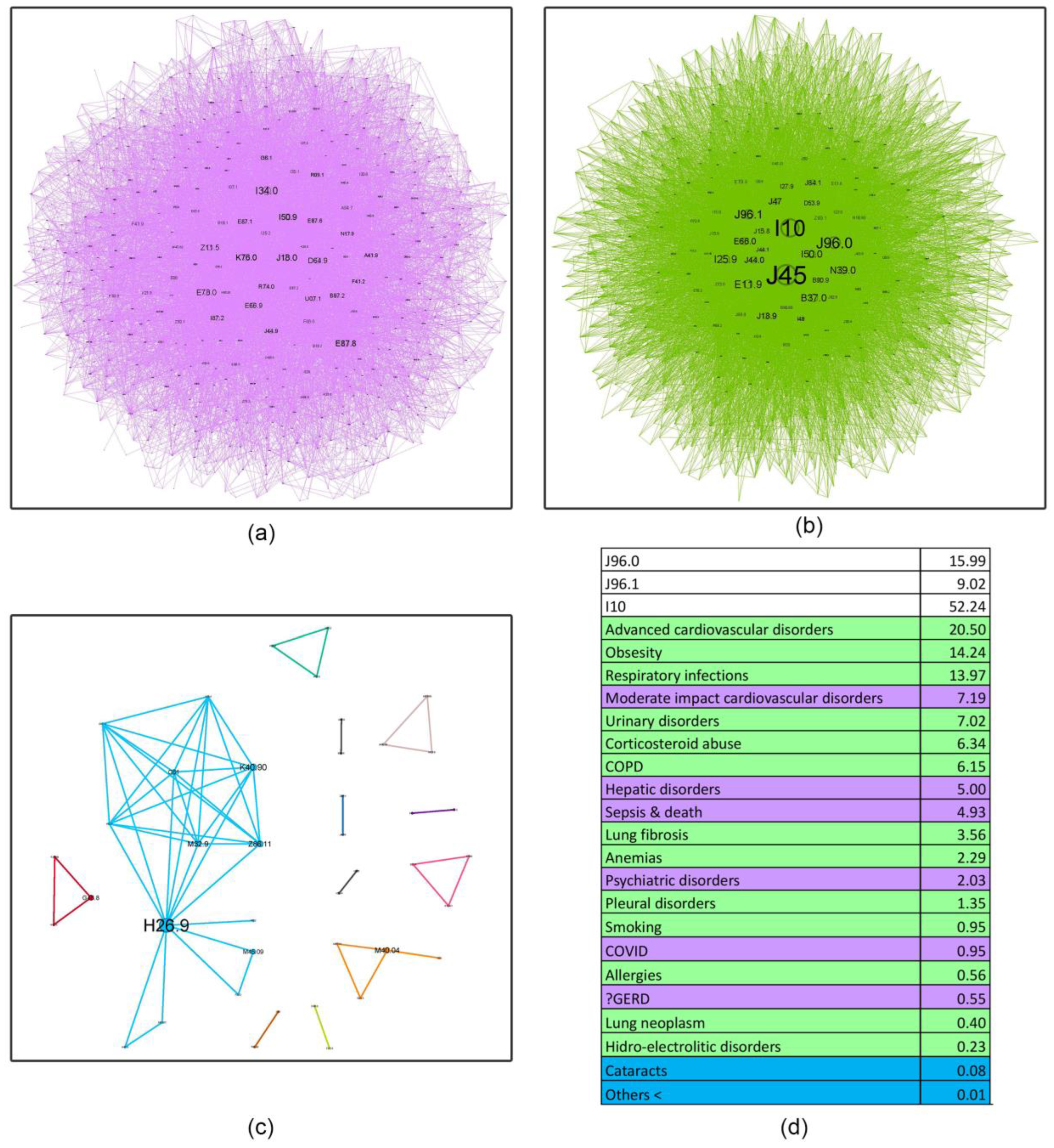
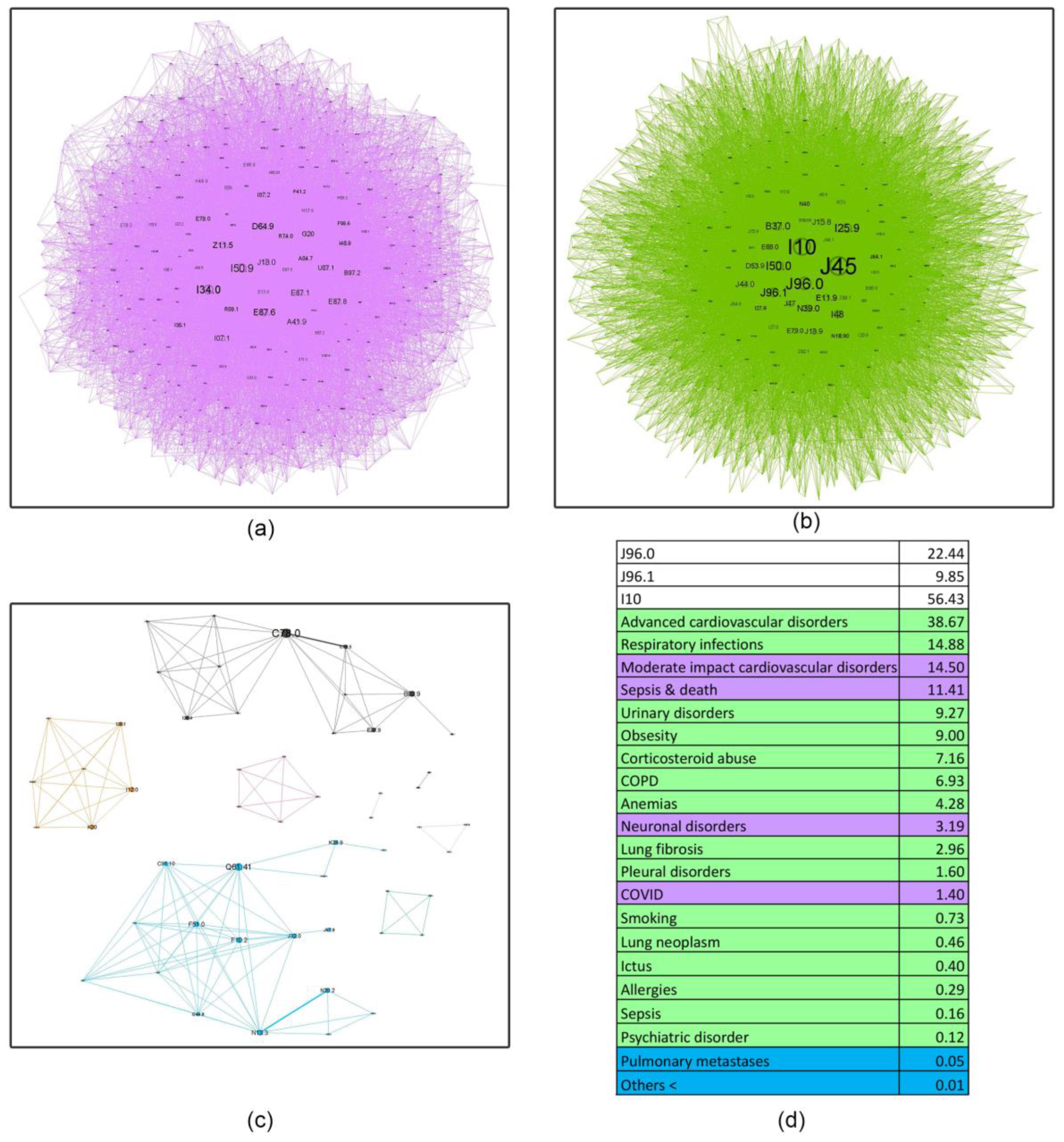
- Asthma influence in the network as a whole, decreases with age, almost halving in the last age bracket compared to the first. This finding shows the importance of managing comorbidities in older patients and how complex geriatric pathology is, possibly explaining the challenges encountered in treating this age group. Recent literature supports this affirmation, confirming that a comprehensive, multidisciplinary approach is needed to better understand and treat the elderly [46].
- Respiratory failure (J96 code), a rational asthma comorbidity, presents an upward relative influence trend with age (21.89%, 24.93%, 25.01%, 32.29%), but the acute (J96.0) versus the chronic component (J96.1) have different patterns. Young patients are approximately 4.77 times more likely to experience acute respiratory failure (ARF) compared to chronic respiratory failure (CRF). Several studies show a higher prevalence of ARF in younger patients compared to older ones, with small changes in the diameter of the airways increasing the resistance and reducing airflow significantly, making smaller children more susceptible to this acute condition [47]. In contrast, older patients exhibit a smaller differential, with a roughly twofold increase in acute respiratory failure relative to chronic respiratory failure (2.21, 1.77, and 2.28, respectively). It is a known fact that as we age, our organ systems begin to decline in function slowly. These changes make older patients more susceptible to organ failure; therefore, CRF is found predominantly in this age group. Also, a longer amount of environmental exposure, such as household air pollution and occupational hazards, may influence this mechanism [48].
- Hypertension (I10) is always close to the J45 node, with a pervasive relative influence (8.35%, 37.07%, 52.24%, 56.43%), and a clear influence increase from the first group to the last. Corresponding to the recent literature, hypertension is a common comorbidity in asthma and other chronic conditions, and it shows a continuous rise from age 35 to 79 [49]. It is also associated with increased asthma severity, emphasizing the importance of managing hypertension as it may be a crucial factor in optimizing asthma care [50].
- Inadequate inhaler techniques or abuse of corticosteroid devices, leading to oral and, in the later stages, pulmonary candidiasis (B37.0, B37.1), have a slow upward trend (5.64%, 5.77%, 6.34%, 7.16%), making the elderly more susceptible to not using the medication correctly. However, this rather low prevalence may be biased due to physicians’ underreporting of this diagnosis in discharge summaries.
- Respiratory infections, as expected, play a triggering role in asthma pathology; however, their influence is minimal in the young age group (0.85%) and slightly higher in subsequent age cohorts (13.25%, 13.97%, 14.88%). This information is discordant with the literature on respiratory infections, particularly viral etiology, which play an important role in asthma exacerbation in younger patients [51]. A possible explanation might be that young adults in the first age group may experience milder infectious exacerbations, which do not require hospitalization compared to older patients.
- Surprisingly, allergies do not seem to have an important influence (0.53%, 1.61%, 0.56%, 0.29%). A potential reason for these findings is the lack of very young patients in our healthcare facility (children and adolescents), knowing that allergic asthma is more prevalent in this age group [52].
- Obesity shows a reverse U-shape trend, with the lowest percentage found in the younger group (1.91%), an upward trend for the 50–59 and 60–69 age groups (14.71%, 14.24%), and an abrupt decline in the elderly group 70+ (9%). Patients from these middle groups may have a higher percentage of obesity due to longer exposure to traditional cooking practices in our region (oil-fried meat, sauces, and processed food). A possible explanation for the decrease in the 70+ group may be the onset of frailty syndrome, a condition observed usually in the elderly, characterized by weight loss, sarcopenia, osteoporosis, and an altered state of nutrition, which can increase the risk of hospitalization and mortality [53].
- A downward trend is seen in smoking behavior (5.72%, 1.34%, 0.95%, 0.73%), with younger patients showing a tendency to exhibit a higher percentage of unhealthy habits like this. International statistics demonstrate similar results, stating that UK citizens over 65 have the lowest proportion of current smokers [54].
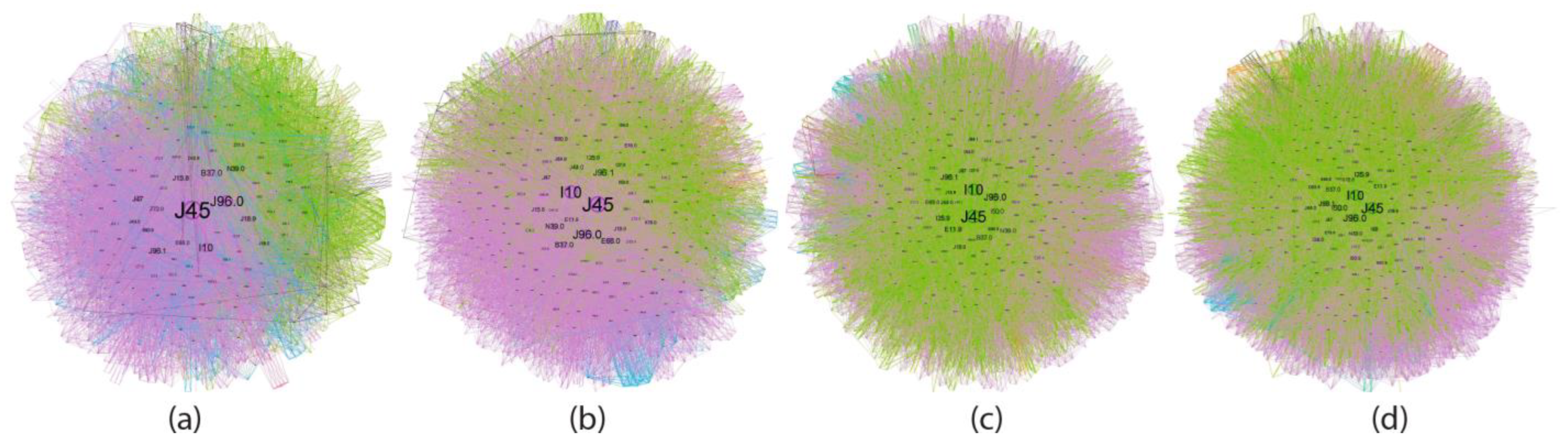
4.4. Asthma ICD-10 Complex Networks (Node Characteristic Tables in Age Groups of Asthma Patients)
4.5. Asthma Treatment and Management Implications
- Prioritizing multidisciplinary care: Effective management of asthma in older adults often requires a coordinated approach involving pulmonologists, cardiologists, geriatricians, and other specialists.
- Minimizing the risk of drug interactions: Polypharmacy is common in older adults with multiple comorbidities, increasing the potential for adverse drug reactions.
- Improving long-term prognosis: By personalizing treatment and consistent prospective screening, for patients with co-existing conditions.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GINA-2024-Strategy-Report-24_05_22_WMS.pdf [Internet]. Available online: https://ginasthma.org/wp-content/uploads/2024/05/GINA-2024-Strategy-Report-24_05_22_WMS.pdf (accessed on 8 December 2024).
- WHO Asthma [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 8 December 2024).
- Finland: EU Country with Highest Share of Asthmatics [Internet]. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20210924-1 (accessed on 12 January 2025).
- Pate, C.A.; Zahran, H.S.; Qin, X.; Johnson, C.; Hummelman, E.; Malilay, J. Asthma Surveillance—United States, 2006–2018. MMWR Surveill. Summ. 2021, 70, 1–32. [Google Scholar] [PubMed]
- Mortimer, K.; Lesosky, M.; García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; El Sony, A.; Ellwood, P.; Marks, G.B.; et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur. Respir. J. 2022, 60, 2102865. [Google Scholar]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Lond Engl. 2020, 396, 1204–1222. [Google Scholar]
- Kankaanranta, H.; Kauppi, P.; Tuomisto, L.E.; Ilmarinen, P. Emerging Comorbidities in Adult Asthma: Risks, Clinical Associations, and Mechanisms. Mediat. Inflamm. 2016, 2016, 3690628. [Google Scholar]
- Ilmarinen, P.; Tuomisto, L.E.; Kankaanranta, H. Phenotypes, Risk Factors, and Mechanisms of Adult-Onset Asthma. Mediat. Inflamm. 2015, 2015, 514868. [Google Scholar]
- Trivedi, M.; Denton, E. Asthma in Children and Adults—What Are the Differences and What Can They Tell us About Asthma? Front. Pediatr. 2019, 7, 256. Available online: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2019.00256/full (accessed on 13 January 2025).
- Kong, D.L.; Qin, Z.; Shen, H.; Jin, H.Y.; Wang, W.; Wang, Z.F. Association of Obstructive Sleep Apnea with Asthma: A Meta-Analysis. Sci. Rep. 2017, 7, 4088. [Google Scholar]
- Ledford, D.K.; Lockey, R.F. Asthma and comorbidities. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 78. [Google Scholar]
- Akmatov, M.K.; Ermakova, T.; Holstiege, J.; Steffen, A.; von Stillfried, D.; Bätzing, J. Comorbidity profile of patients with concurrent diagnoses of asthma and COPD in Germany. Sci. Rep. 2020, 10, 17945. [Google Scholar]
- Zhang, S.Q.; Xiong, X.F.; Wu, Z.H.; Huang, T.T.; Cheng, D.Y. Clinical features of asthma with comorbid bronchiectasis. Medicine 2021, 100, e23858. [Google Scholar]
- Cazzola, M.; Rogliani, P.; Ora, J.; Calzetta, L.; Matera, M.G. Asthma and comorbidities: Recent advances. Pol. Arch. Intern. Med. 2022, 132, 16250. Available online: https://www.mp.pl/paim/issue/article/16250 (accessed on 11 January 2025). [CrossRef] [PubMed]
- Pollevick, M.E.; Xu, K.Y.; Mhango, G.; Federmann, E.G.; Vedanthan, R.; Busse, P.; Holguin, F.; Federman, A.D.; Wisnivesky, J.P. The Relationship Between Asthma and Cardiovascular Disease. Chest 2021, 159, 1338–1345. [Google Scholar]
- Forte, G.C.; Grutcki, D.M.; Menegotto, S.M.; Pereira, R.P.; de Tarso Roth Dalcin, P. Prevalence of obesity in asthma and its relations with asthma severity and control. Rev. Assoc. Médica. Bras. 2013, 59, 594–599. [Google Scholar]
- Kauppi, P.; Linna, M.; Jantunen, J.; Martikainen, J.E.; Haahtela, T.; Pelkonen, A.; Mäkelä, M. Chronic Comorbidities Contribute to the Burden and Costs of Persistent Asthma. Mediat. Inflamm. 2015, 2015, 819194. [Google Scholar]
- Cunha, M.S.; Amaral, R.; Pereira, A.M.; Almeida, R.; Alves-Correia, M.; Loureiro, C.C.; Lopes, C.; Carvalho, J.; Ribeiro, C.; Vidal, C.; et al. Symptoms of anxiety and depression in patients with persistent asthma: A cross-sectional analysis of the INSPIRERS studies. BMJ Open 2023, 13, e068725. [Google Scholar]
- Baan, E.J.; De Roos, E.W.; Engelkes, M.; De Ridder, M.; Pedersen, L.; Berencsi, K.; Prieto-Alhambra, D.; Lapi, F.; Van Dyke, M.K.; Rijnbeek, P.; et al. Characterization of Asthma by Age of Onset: A Multi-Database Cohort Study. J. Allergy Clin. Immunol. Pract. 2022, 10, 1825–1834.e8. [Google Scholar]
- Mendy, A.; Mersha, T.B. Comorbidities in childhood-onset and adult-onset asthma. Ann. Allergy Asthma. Immunol. 2022, 129, 327–334. [Google Scholar]
- Perry, T.T.; Grant, T.L.; Dantzer, J.A.; Udemgba, C.; Jefferson, A.A. Impact of socioeconomic factors on allergic diseases. J. Allergy Clin. Immunol. 2024, 153, 368–377. [Google Scholar]
- Sinharoy, A.; Mitra, S.; Mondal, P. Socioeconomic and Environmental Predictors of Asthma-Related Mortality. J. Environ. Public. Health. 2018, 2018, 9389570. [Google Scholar]
- Grant, T.; Croce, E.; Matsui, E.C. Asthma and the social determinants of health. Ann. Allergy Asthma. Immunol. 2022, 128, 5–11. [Google Scholar]
- Szefler, S.J.; Raphiou, I.; Zeiger, R.S.; Stempel, D.; Kral, K.; Pascoe, S. Seasonal variation in asthma exacerbations in the AUSTRI and VESTRI studies. ERJ. Open Res. 2019, 5, 00153–2018. Available online: https://publications.ersnet.org/content/erjor/5/2/00153-2018 (accessed on 10 January 2025).
- Dounce-Cuevas, C.A.; Flores-Flores, A.; Bazán, M.S.; Portales-Rivera, V.; Morelos-Ulíbarri, A.A.; Bazán-Perkins, B. Asthma and COVID-19: A controversial relationship. Virol. J. 2023, 20, 207. [Google Scholar] [PubMed]
- Chen, Y.L.; Lin, Y.Y.; Chin, P.W.; Chen, C.C.; Cheng, C.G.; Cheng, C.A. Analyzing COVID-19 and Air Pollution Effects on Pediatric Asthma Emergency Room Visits in Taiwan. Toxics 2024, 12, 79. [Google Scholar] [CrossRef]
- Karcher, H.; Schoenberger, M.; Rayban, T.; Kelly, C.; Heaney, A.; Mackay, A. Impact of COVID-19 measures on exacerbation rates and healthcare visits in US asthma patients. Allergy Asthma. Proc. 2023, 44, 422–428. [Google Scholar] [CrossRef]
- Sykes, D.L.; Faruqi, S.; Holdsworth, L.; Crooks, M.G. Impact of COVID-19 on COPD and asthma admissions, and the pandemic from a patient’s perspective. ERJ. Open Res. 2021, 7, 00822–2020. Available online: https://publications.ersnet.org/content/erjor/7/1/00822-2020 (accessed on 9 January 2025). [PubMed]
- Imlach, F.; McKinlay, E.; Kennedy, J.; Pledger, M.; Middleton, L.; Cumming, J.; McBride-Henry, K. Seeking Healthcare During Lockdown: Challenges, Opportunities and Lessons for the Future. Int. J. Health Policy Manag. 2021, 11, 1316–1324. [Google Scholar] [PubMed]
- Lee, S.C.; Son, K.J.; Han, C.H.; Jung, J.Y.; Park, S.C. Impact of comorbid asthma on severity of coronavirus disease (COVID-19). Sci. Rep. 2020, 10, 21805. [Google Scholar]
- Caminati, M.; Vultaggio, A.; Matucci, A.; Senna, G.; Almerigogna, F.; Bagnasco, D.; Chieco-Bianchi, F.; Cosini, F.; Girelli, D.; Guarnieri, G.; et al. Asthma in a large COVID-19 cohort: Prevalence, features, and determinants of COVID-19 disease severity. Respir. Med. 2021, 176, 106261. Available online: https://www.resmedjournal.com/article/S0954-6111(20)30401-7/fulltext (accessed on 9 January 2025).
- Khalid, M.; Almasri, T.; Goble, S.; Johnson, D.; Gilbertson, D.; Linzer, M.; Strykowski, R. Seasonal variations and social disparities in asthma hospitalizations and outcomes. J. Asthma. Off J. Assoc. Care Asthma. 2024, 61, 1781–1789. [Google Scholar]
- utilizarea_timpului_r13.pdf [Internet]. Available online: https://insse.ro/cms/files/statistici/comunicate/com_anuale/util_timpul/utilizarea_timpului_r13.pdf (accessed on 10 January 2025).
- Sood, A.; Assad, N.A.; Barnes, P.J.; Churg, A.; Gordon, S.B.; Harrod, K.S.; Irshad, H.; Kurmi, O.P.; Martin, W.J., 2nd; Meek, P.; et al. ERS/ATS workshop report on respiratory health effects of household air pollution. Eur. Respir. J. 2018, 51, 1700698. Available online: https://publications.ersnet.org/content/erj/51/1/1700698 (accessed on 10 January 2025).
- Dai, X.; Bui, D.S.; Perret, J.L.; Lowe, A.J.; Frith, P.A.; Bowatte, G.; Thomas, P.S.; Giles, G.G.; Hamilton, G.S.; Tsimiklis, H.; et al. Exposure to household air pollution over 10 years is related to asthma and lung function decline. Eur. Respir. J. 2021, 57, 2000602. Available online: https://publications.ersnet.org/content/erj/57/1/2000602 (accessed on 10 January 2025). [CrossRef] [PubMed]
- He, L.; Evans, S.; Norris, C.; Barkjohn, K.; Cui, X.; Li, Z.; Zhou, X.; Li, F.; Zhang, Y.; Black, M.; et al. Associations between personal apparent temperature exposures and asthma symptoms in children with asthma. PLoS ONE 2023, 18, e0293603. [Google Scholar]
- Fuseini, H.; Newcomb, D.C. Mechanisms driving gender differences in asthma. Curr. Allergy Asthma. Rep. 2017, 17, 19. [Google Scholar]
- MacIntyre, C.R.; Ridda, I.; Seale, H.; Gao, Z.; Ratnamohan, V.M.; Donovan, L.; Zeng, F.; Dwyer, D.E. Respiratory viruses transmission from children to adults within a household. Vaccine 2012, 30, 3009–3014. [Google Scholar] [PubMed]
- Yang, F.; Zhou, J.; Xiao, H.; Wu, X.; Cui, Y.; Huang, H.; Zheng, S.; Li, H. Caregiver burden among parents of school-age children with asthma: A cross-sectional study. Front. Public. Health 2024, 12, 1368519. Available online: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2024.1368519/full (accessed on 10 January 2025). [CrossRef]
- Briciu, V.; Topan, A.; Calin, M.; Dobrota, R.; Leucuta, D.C.; Lupse, M. Comparison of COVID-19 Severity in Vaccinated and Unvaccinated Patients during the Delta and Omicron Wave of the Pandemic in a Romanian Tertiary Infectious Diseases Hospital. Healthcare 2023, 11, 373. [Google Scholar] [CrossRef]
- Trusculescu, A.A.; Ancusa, V.M.; Pescaru, C.C.; Wellmann, N.; Fira-Mladinescu, C.; Oancea, C.I.; Fira-Mladinescu, O. A Multifaceted Exploration of Status Asthmaticus: A Retrospective Analysis in a Romanian Hospital. J. Clin. Med. 2024, 13, 6615. [Google Scholar] [CrossRef]
- Hong, S.N.; Lai, F.T.T.; Wang, B.; Choi, E.P.H.; Wong, I.C.K.; Lam, C.L.K.; Wan, E.Y.F. Age-specific Multimorbidity Patterns and Burden on All-Cause Mortality and Public Direct Medical Expenditure: A Retrospective Cohort Study. J. Epidemiol. Glob. Health 2024, 14, 1077–1088. [Google Scholar] [CrossRef]
- State of Health in the EU, Romania.pdf [Internet]. Available online: https://health.ec.europa.eu/system/files/2023-12/2023_chp_ro_english.pdf (accessed on 10 January 2025).
- Khosa, J.K.; Louie, S.; Moreno, P.L.; Abramov, D.; Rogstad, D.K.; Alismail, A.; Matus, M.J.; Tan, L.D. Asthma Care in the Elderly: Practical Guidance and Challenges for Clinical Management—A Framework of 5 “Ps”. J. Asthma. Allergy. 2023, 16, 33–43. [Google Scholar]
- Battaglia, S.; Benfante, A.; Spatafora, M.; Scichilone, N. Asthma in the elderly: A different disease? Breathe 2016, 12, 18–28. [Google Scholar]
- Warm, K.; Hedman, L.; Stridsman, C.; Lindberg, A.; Rönmark, E.; Backman, H. Age-related differences in associations between uncontrolled asthma, comorbidities and biomarkers in adult-onset asthma. J Asthma. 2023, 60, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Jiao, A.; Yin, J.; Zou, H.; Liu, Y.; Li, Z.; Wang, Q.; Wu, J.; Shen, K. Analysis of risk factors for acute attacks complicated by respiratory failure in children with asthma. Front. Pediatr. 2024, 11, 1335540. Available online: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2023.1335540/full (accessed on 11 January 2025). [CrossRef]
- Joshi, P.R. Pulmonary Diseases in Older Patients: Understanding and Addressing the Challenges. Geriatrics 2024, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Du, Y.; Zhang, Q.; Wang, X.; He, C.; He, J.; Jing, F.; Ren, H.; Guo, M.; Tian, J.; et al. Age-related changes in the risk of high blood pressure. Front. Cardiovasc. Med. 2022, 9, 939103. [Google Scholar]
- Zolotareva, O.; Saik, O.V.; Königs, C.; Bragina, E.Y.; Goncharova, I.A.; Freidin, M.B.; Dosenko, V.E.; Ivanisenko, V.A.; Hofestädt, R. Comorbidity of asthma and hypertension may be mediated by shared genetic dysregulation and drug side effects. Sci. Rep. 2019, 9, 16302. [Google Scholar]
- Mthembu, N.; Ikwegbue, P.; Brombacher, F.; Hadebe, S. Respiratory Viral and Bacterial Factors That Influence Early Childhood Asthma. Front. Allergy 2021, 2, 692841. Available online: https://www.frontiersin.org/journals/allergy/articles/10.3389/falgy.2021.692841/full (accessed on 20 January 2025).
- Schiffers, C.; Wouters, E.F.; Breyer-Kohansal, R.; Buhl, R.; Pohl, W.; Irvin, C.G.; Breyer, M.-K.; Hartl, S. Asthma Prevalence and Phenotyping in the General Population: The LEAD (Lung, hEart, sociAl, boDy) Study. J. Asthma. Allergy. 2023, 16, 367. [Google Scholar]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2019.00255/full (accessed on 28 January 2025).
- Adult Smoking Habits in the UK—Office for National Statistics [Internet]. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2022 (accessed on 21 January 2025).
- Gaffin, J.M.; Castro, M.; Bacharier, L.B.; Fuhlbrigge, A.L. The Role of Comorbidities in Difficult-to-Control Asthma in Adults and Children. J. Allergy Clin. Immunol. Pract. 2022, 10, 397–408. [Google Scholar] [CrossRef]
- STEPAND; DOPD; MOROŞANUA; VINTILESCUB; NICULESCUC. Implications of the Iron Deficiency in Lower Tract Respiratory Acute Infections in Toddlers. Curr. Health Sci. J. 2018, 44, 362–367. [Google Scholar]
- Jayamanna, U.; Jayaweera, J.A.A.S. Childhood Anemia and Risk for Acute Respiratory Infection, Gastroenteritis, and Urinary Tract Infection: A Systematic Review. J. Pediatr. Infect. Dis. 2023, 18, 061–070. [Google Scholar]
- Matsusaka, K.; Kawakami, G.; Kamekawa, H.; Momma, H.; Nagatomi, R.; Itoh, J.; Yamaya, M. Pneumonia risks in bedridden patients receiving oral care and their screening tool: Malnutrition and urinary tract infection-induced inflammation. Geriatr. Gerontol. Int. 2018, 18, 714–722. [Google Scholar] [PubMed]
- de Frel, D.L.; Atsma, D.E.; Pijl, H.; Seidell, J.C.; Leenen, P.J.M.; Dik, W.A.; van Rossum, E.F.C. The Impact of Obesity and Lifestyle on the Immune System and Susceptibility to Infections Such as COVID-19. Front. Nutr. 2020, 7, 597600. Available online: https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2020.597600/full (accessed on 24 January 2025).
- Frasca, D.; Blomberg, B.B.; Paganelli, R. Aging, Obesity, and Inflammatory Age-Related Diseases. Front. Immunol. 2017, 8, 1745. [Google Scholar]
- Yan, X.; Xu, L.; Shi, B.; Wang, H.; Xu, X.; Xu, G. Epidemiology and risk factors of chronic obstructive pulmonary disease in Suzhou: A population-based cross-sectional study. J. Thorac. Dis. 2020, 12, 5347–5356. [Google Scholar] [PubMed]
- Mihaltan, F.D.; Furtunescu, F.; Nemes, R.M.; Farcasanu, D.; Daramus, I.M. COPD prevalence in Romania and possible influence of social and household characteristics. Eur. Respir. J. 2014, 40 (Suppl. 56), 4740. [Google Scholar]
- Feigin, V.L.; Vos, T.; Nichols, E.; O Owolabi, M.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar]
- Mankowski, R.T.; Yende, S.; Angus, D.C. Long-Term Impact of Sepsis on Cardiovascular Health. Intensive Care Med. 2019, 45, 78–81. [Google Scholar]
- Shen, Q.; Mikkelsen, D.H.; Luitva, L.B.; Song, H.; Kasela, S.; Aspelund, T.; Bergstedt, J.; Lu, Y.; Sullivan, P.F.; Ye, W.; et al. Psychiatric disorders and subsequent risk of cardiovascular disease: A longitudinal matched cohort study across three countries. eClinicalMedicine 2023, 61, 102063. [Google Scholar] [CrossRef]
- Melani, A.S. Management of asthma in the elderly patient. Clin. Interv. Aging. 2013, 8, 913–922. [Google Scholar]
- Gadó, K.; Szabo, A.; Markovics, D.; Virág, A. Most common cardiovascular diseases of the elderly—A review article. Dev. Health Sci. 2022, 4, 27–32. [Google Scholar] [CrossRef]
- Stancu, P.; Hentsch, L.; Seeck, M.; Zekry, D.; Graf, C.; Fleury, V.; Assal, F. Neurology of Aging: Adapting Neurology Provision for an Aging Population. Neurodegener. Dis. 2024, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.C.; Lin, C.L.; Shen, T.C.; Tu, C.Y.; Hsia, T.C.; Hsu, W.H. Risk of Pleural Empyema in Adult Patients With Asthma: A Nationwide Retrospective Cohort Study. Front. Med. 2022, 9, 851573. Available online: https://www.frontiersin.org/journals/medicine/articles/10.3389/fmed.2022.851573/full (accessed on 28 January 2025). [CrossRef] [PubMed]

| Criteria | Asthma Cohort Variation |
|---|---|
| Age (years, mean ± SD) | 59.6 ± 14.8 |
| Female (%) | 57.2 |
| Length of Stay (Days, mean ± SD) | 4.5 ± 5.9 |
| Age Category 18–49 (%) | 22.7 |
| Age Category 50–59 (%) | 21.4 |
| Age Category 60–69 (%) | 28.8 |
| Age Category 70+ (%) | 27.1 |
| Year 2013 (%) | 10.5 |
| Year 2014 (%) | 10 |
| Year 2015 (%) | 8.8 |
| Year 2016 (%) | 7.4 |
| Year 2017 (%) | 9.7 |
| Year 2018 (%) | 12.8 |
| Year 2019 (%) | 12 |
| Year 2020 (%) | 6.5 |
| Year 2021 (%) | 5 |
| Year 2022 (%) | 7.8 |
| Year 2023 (%) | 9.4 |
| 18–49 y | 50–59 y | 60–69 y | 70 y + | |
|---|---|---|---|---|
| PURPLE (main community) | Smoking Cs abuse (Candidosis) Obesity Resp. Inf Lung Fibrosis Allergies | Obesity Resp. Infections Cs Abuse (Candidosis) Urinary D. Lung Fibrosis Sepsis and Death Anemia Psychiatric D. Allergies Smoking COVID-19 | Moderate-impact CVD Hepatic D. Sepsis and Death Psychiatric D. COVID-19 GERD | Moderate-impact CVD Sepsis and death Neuronal D. COVID-19 |
| GREEN (secondary community) | Urinary Infection Sepsis and Death Anemia Resp Inf COVID-19 | Chronic pulm.D. CVD. Obesity Complications Pleural D. Addiction D. | Adv CVD Obesity Resp Inf Urinary D. Cs Abuse (Candidosis) COPD Lung Fibrosis Anemia Pleural. D. Smoking Allergies Lung Neo Hydro/electro D. | Adv CVD Resp Inf Urinary D. Obesity Cs Abuse (Candidosis) COPD Anemia Lung Fibrosis Pleural D. Smoking Lung Neo Ictus Allergies Sepsis Psych D. |
| BLUE (tertiary community) | Morbid Obesity Others | Neuronal D. Others | Cataracts Others | Pulmonary metastases Others |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trusculescu, A.A.; Ancusa, V.M.; Burducescu, A.; Pescaru, C.C.; Trăilă, D.; Wellmann, N.; Fira-Mladinescu, O.; Oancea, C.I. Age-Related Variations and Seasonal Influences: A Network Analysis of Comorbidities in Asthma Hospitalizations (2013–2023). J. Clin. Med. 2025, 14, 2350. https://doi.org/10.3390/jcm14072350
Trusculescu AA, Ancusa VM, Burducescu A, Pescaru CC, Trăilă D, Wellmann N, Fira-Mladinescu O, Oancea CI. Age-Related Variations and Seasonal Influences: A Network Analysis of Comorbidities in Asthma Hospitalizations (2013–2023). Journal of Clinical Medicine. 2025; 14(7):2350. https://doi.org/10.3390/jcm14072350
Chicago/Turabian StyleTrusculescu, Ana Adriana, Versavia Maria Ancusa, Alexandra Burducescu, Camelia Corina Pescaru, Daniel Trăilă, Norbert Wellmann, Ovidiu Fira-Mladinescu, and Cristian Iulian Oancea. 2025. "Age-Related Variations and Seasonal Influences: A Network Analysis of Comorbidities in Asthma Hospitalizations (2013–2023)" Journal of Clinical Medicine 14, no. 7: 2350. https://doi.org/10.3390/jcm14072350
APA StyleTrusculescu, A. A., Ancusa, V. M., Burducescu, A., Pescaru, C. C., Trăilă, D., Wellmann, N., Fira-Mladinescu, O., & Oancea, C. I. (2025). Age-Related Variations and Seasonal Influences: A Network Analysis of Comorbidities in Asthma Hospitalizations (2013–2023). Journal of Clinical Medicine, 14(7), 2350. https://doi.org/10.3390/jcm14072350











