Beyond Cholesterol: Emerging Risk Factors in Atherosclerosis
Abstract
1. Introduction
2. Inflammatory Markers and Atherosclerosis
| Biomarker | Mechanism and Role | Clinical Relevance | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| C-reactive protein (CRP) | Produced by the liver in response to IL-6; marker of systemic inflammation; associated with increased cardiovascular risk. | Used in risk stratification; high-sensitivity CRP (hs-CRP) is a strong predictor of cardiovascular events. | Widely available; non-invasive test; cost-effective. | Non-specific; elevated in various inflammatory and infectious conditions. | [23] |
| Interleukin-6 (IL-6) | Pro-inflammatory cytokine that amplifies vascular inflammation, promotes CRP production, and facilitates immune cell recruitment. | Correlates with plaque instability and adverse cardiovascular outcomes; potential therapeutic target. | Directly involved in inflammatory cascade; potential target for anti-inflammatory therapies. | Short half-life; highly variable levels; affected by multiple inflammatory conditions. | [22] |
| Tumor necrosis factor-alpha (TNF-α) | Enhances endothelial activation, increases vascular permeability, promotes leukocyte adhesion, and contributes to plaque formation and rupture. | Elevated TNF-α levels are associated with increased atherosclerosis severity and cardiovascular mortality. | Well-established role in inflammation; TNF-α inhibitors exist and are widely used in autoimmune diseases. | Systemic effects can lead to immunosuppression; TNF-α inhibitors can have severe side effects such as infections and malignancies. | [27] |
| Monocyte chemoattractant protein-1 (MCP-1) | Key player in monocyte recruitment to atherosclerotic plaques, promoting foam cell formation and chronic inflammation. | Elevated MCP-1 levels correlate with increased plaque burden and cardiovascular events. | Plays a crucial role in early-stage atherogenesis; potential biomarker for identifying high-risk individuals. | Not widely used in routine clinical practice; limited availability of standardized assays. | [29,30] |
| Interleukin-1 beta (IL-1β) | Central mediator of inflammation; activates endothelial cells and smooth muscle proliferation, promoting plaque growth and instability. | Targeted in the CANTOS, where IL-1β inhibition (canakinumab) significantly reduced cardiovascular events. | Strong potential for therapeutic targeting; IL-1β inhibitors have shown clinical benefits beyond lipid lowering. | Expensive treatment; inhibition can weaken immune defenses, increasing infection risk. | [31] |
| Intercellular adhesion molecule-1 (ICAM-1) | Facilitates leukocyte adhesion to the endothelium, aiding immune cell infiltration into plaques. | High ICAM-1 levels are linked to increased endothelial dysfunction and cardiovascular risk. | Useful in studying vascular inflammation; potential marker for endothelial activation. | Lacks specificity for atherosclerosis; limited role in routine clinical settings. | [42] |
| Vascular cell adhesion molecule-1 (VCAM-1) | Promotes monocyte and T-cell adhesion to endothelial cells, accelerating plaque development. | Higher VCAM-1 levels correlate with early atherosclerosis and plaque progression. | Strongly associated with vascular inflammation; may help identify subclinical disease. | Not routinely measured in clinical practice; affected by various inflammatory conditions. | [43] |
| Myeloperoxidase (MPO) | Enzyme released by neutrophils that promotes oxidative stress, endothelial dysfunction, and LDL oxidation. | Elevated MPO levels predict future cardiovascular events and plaque vulnerability. | Provides insights into oxidative stress-driven inflammation; could serve as a marker for plaque instability. | Less commonly used; testing is not standardized in routine cardiovascular screening. | [44] |
| Lipopolysaccharide-binding protein (LBP) | Indicator of bacterial endotoxin activity; linked to gut microbiota dysbiosis and systemic inflammation. | Higher LBP levels are associated with metabolic syndrome, obesity, and increased atherosclerotic risk. | Highlights gut-immune interactions in atherosclerosis; potential target for novel therapies. | Research is still emerging; clinical applications are limited. | [45] |
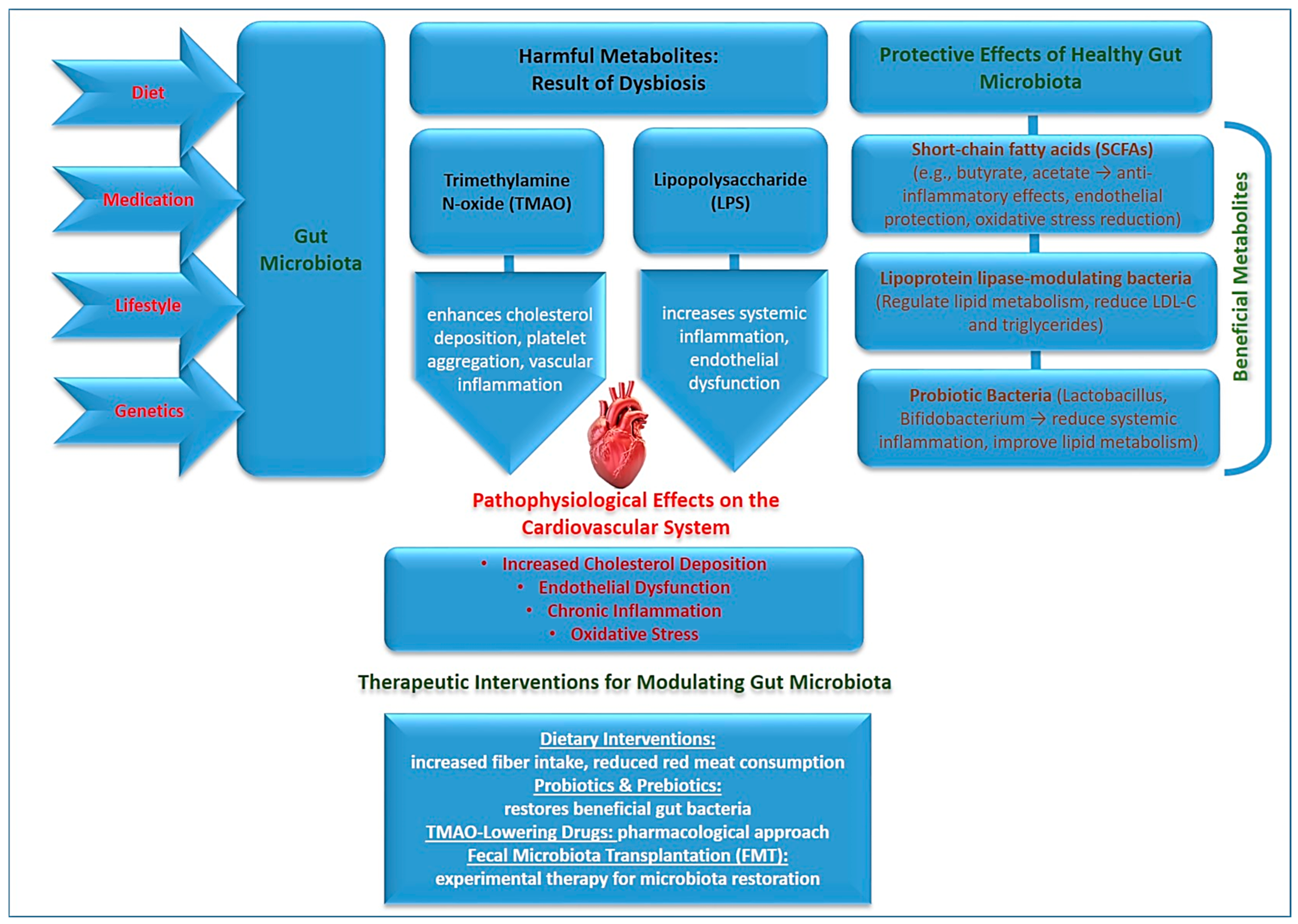
3. The Role of Gut Microbiota in Atherosclerosis
4. Environmental Exposures and Cardiovascular Risk
| Environmental Factor | Mechanism of Action | Cardiovascular Impact | Sources/ Exposure | Mitigation Strategies | Ref. |
|---|---|---|---|---|---|
| Air Pollution (PM2.5, NO2, O3, CO, SO2) | Fine particulate matter (PM2.5) and gaseous pollutants induce oxidative stress, systemic inflammation, and endothelial dysfunction. | Increased risk of hypertension, myocardial infarction, stroke, atherosclerosis progression, and arterial stiffness. | Vehicle emissions, industrial pollution, biomass combustion, household cooking fuels. | Air quality regulations, urban green spaces, air purifiers, minimizing outdoor activities in high-pollution areas. | [80] |
| Heavy Metals (Arsenic, Lead, Cadmium, Mercury) | Promote oxidative stress, disrupt mitochondrial function, impair vascular elasticity, and interfere with lipid metabolism. | Hypertension, endothelial dysfunction, increased atherosclerosis risk, neurotoxicity. | Contaminated water, industrial emissions, tobacco smoke, lead-based paint, seafood (mercury exposure). | Water filtration systems, stringent environmental policies, safer industrial waste disposal, smoking cessation. | [85] |
| Endocrine-Disrupting Chemicals (BPA, Phthalates, Dioxins, PCBs) | Interfere with hormonal regulation, disrupt lipid metabolism, increase systemic inflammation, and impair insulin signaling. | Increased risk of obesity, insulin resistance, dyslipidaemia, endothelial dysfunction, and cardiovascular disease. | Plastics, food packaging, industrial solvents, personal care products, pesticides. | Using BPA-free products, reducing plastic use, stricter chemical regulations, promoting eco-friendly materials. | [87,88] |
| Chronic Psychological Stress (Work, Financial, Social, PTSD) | Activates the hypothalamic–pituitary–adrenal (HPA) axis, increasing cortisol levels, enhancing sympathetic nervous system activity, and promoting inflammation. | Hypertension, increased heart rate variability, metabolic syndrome, dyslipidaemia, gut microbiota disruption. | Workplace stress, financial instability, social isolation, trauma, sleep disorders. | Stress management (meditation, yoga, therapy), social support programs, workplace mental health initiatives. | [89] |
| Climate Change (Extreme Temperatures, Wildfires, Natural Disasters) | Heat stress, dehydration, and air pollution increase systemic inflammation and cardiovascular strain. | Higher incidence of heatstroke, dehydration-related arrhythmias, stroke, and cardiovascular events. | Global warming, increased frequency of extreme weather events, habitat destruction. | Climate adaptation policies, improved disaster preparedness, hydration strategies, cooling centers in urban areas. | [90,91] |
5. The Role of Imaging in Atherosclerosis: Coronary and Vascular Calcifications
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2025, 151, e41–e660. [Google Scholar] [PubMed]
- Buja, M.; Mcdonald, M.M.; Zhao, B.H.; Narula, N.; Narula, J.; Barth, R.F. Insights from autopsy-initiated pathological studies of the pathogenesis and clinical manifestations of atherosclerosis and ischemic heart disease: Part I. Atherosclerosis. Cardiovasc. Pathol. 2025, 76, 107727. [Google Scholar]
- Ji, L.X.; Ravi, S.; Wright, L.; Nguyen, V.; Wiley, J.; Vukelic, M.; Kim, S. Psoriasis treatments in the stabilization of atherosclerosis: A systematic review. Arch. Dermatol. Res. 2024, 317, 159. [Google Scholar] [CrossRef]
- Song, J.S.; Cao, C.; Wang, Z.Y.; Li, H.R.; Yang, L.L.; Kang, J.; Meng, H.X.; Li, L.; Liu, J.X. Mechanistic insights into the regression of atherosclerotic plaques. Front. Physiol. 2024, 15, 1473709. [Google Scholar]
- Khosravi, M.; Sheikhnia, F.; Pashaei, M.R.; Karimi-Dehkordi, M.; Alizadeh-Fanalou, S. Association between small dense low-density lipoprotein and carotid intima-media thickness. J. Cardiovasc. Thorac. 2024, 16, 202–210. [Google Scholar]
- Sciahbasi, A.; Russo, P.; Zuccanti, M.; Chiorazzo, L.; Castelli, F.M.; Granatelli, A. Management of Hypercholesterolemia in Patients with Coronary Artery Disease: A Glimpse into the Future. J. Clin. Med. 2024, 13, 7420. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M.; Di Giovanni, G.; Nicholls, S.J. New Approaches to Lipoproteins for the Prevention of Cardiovascular Events. J. Atheroscler. Thromb. 2025, 32, 265–280. [Google Scholar]
- Björnson, E.; Adiels, M.; Borén, J.; Packard, C.J. Lipoprotein(a) is a highly atherogenic lipoprotein: Pathophysiological basis and clinical implications. Curr. Opin. Cardiol. 2024, 39, 503–510. [Google Scholar]
- Garagoli, F.; Masson, W.; Barbagelata, L. Association between elevated lipoprotein(a) levels and vulnerability of carotid atherosclerotic plaque: A systematic review. J. Stroke Cerebrovasc. Dis. 2024, 33, 108020. [Google Scholar]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar]
- Annink, M.E.; Kraaijenhof, J.M.; Beverloo, C.Y.Y.; Oostveen, R.F.; Verberne, H.J.; Stroes, E.S.G.; Nurmohamed, N.S. Estimating inflammatory risk in atherosclerotic cardiovascular disease: Plaque over plasma? Eur. Heart J. Cardiovasc. Imaging 2024, 26, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Klosowicz, M.; Leksa, D.; Bartusik-Aebisher, D.; Mysliwiec, A.; Dynarowicz, K.; Aebisher, D. Biomarkers That Seem to Have the Greatest Impact on Promoting the Formation of Atherosclerotic Plaque in Current Scientific Research. Curr. Issues Mol. Biol. 2024, 46, 9503–9522. [Google Scholar] [CrossRef] [PubMed]
- Netala, V.R.; Teertam, S.K.; Li, H.Z.; Zhang, Z.J. A Comprehensive Review of Cardiovascular Disease Management: Cardiac Biomarkers, Imaging Modalities, Pharmacotherapy, Surgical Interventions, and Herbal Remedies. Cells 2024, 13, 1471. [Google Scholar] [CrossRef]
- Latif, F.; Mubbashir, A.; Khan, M.S.; Shaikh, Z.; Memon, A.; Alvares, J.; Azhar, A.; Jain, H.; Ahmed, R.; Kanagala, S.G. Trimethylamine N-oxide in cardiovascular disease: Pathophysiology and the potential role of statins. Life Sci. 2025, 361, 123304. [Google Scholar] [CrossRef]
- Li, Z.; He, X.Y.; Fang, Q.; Yin, X.L. Gut Microbe-Generated Metabolite Trimethylamine-N-Oxide and Ischemic Stroke. Biomolecules 2024, 14, 1463. [Google Scholar] [CrossRef]
- Zhu, W.Q.; Al-Kindi, S.G.; Rajagopalan, S.; Rao, X.Q. Air Pollution in Cardio-Oncology and Unraveling the Environmental Nexus JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2024, 6, 347–362. [Google Scholar] [CrossRef]
- Pan, Z.W.; Gong, T.Y.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Dong, Q.L.; Meng, X.; Gong, J.C.; Zhu, T. A review of advances in black carbon exposure assessment and health effects. Chin. Sci. Bull. 2024, 69, 703–716. [Google Scholar] [CrossRef]
- Melo, M.G.; von Eckardstein, A.; Robert, J. Modeling human atherosclerotic lesions in the test tube: Are we there yet? Atherosclerosis 2024, 398, 118560. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.T.; Chen, Y.J.; Hou, L.J.; Yu, Y.L.; Ma, D.; Jiang, T.; Zhao, G.J. Immune cell-mediated features of atherosclerosis. Front. Cardiovasc. Med. 2024, 11, 1450737. [Google Scholar] [CrossRef]
- Mackay, C.D.A.; Meechem, M.B.; Patel, V.B. Macrophages in vascular disease: Roles of mitochondria and metabolic mechanisms. Vasc. Pharmacol. 2024, 156, 107419. [Google Scholar]
- Mccabe, J.J.; Walsh, C.; Gorey, S.; Arnold, M.; Demarchis, G.M.; Harris, K.; Hervella, P.; Iglesias-Rey, R.; Jern, C.; Katan, M.; et al. Interleukin-6, C-Reactive Protein, and Recurrence After Stroke: A Time-Course Analysis of Individual-Participant Data. Stroke 2024, 55, 2825–2834. [Google Scholar] [CrossRef]
- Wang, Y.F.; Wang, L.; Zhao, Z.Q.; Yin, S.; Tang, X.J.; Zhang, K.R. The predictive role of the hs-CRP/HDL-C ratio for long-term mortality in the general population: Evidence from a cohort study. BMC Cardiovasc. Disord. 2024, 24, 758. [Google Scholar] [CrossRef]
- Omran, F.; Kyrou, I.; Osman, F.; Lim, V.G.; Randeva, H.S.; Chatha, K. Cardiovascular Biomarkers: Lessons of the Past and Prospects for the Future. Int. J. Mol. Sci. 2022, 23, 5680. [Google Scholar] [CrossRef]
- Arroyo-Espliguero, R.; Viana-Llamas, M.C.; Silva-Obregón, A.; Avanzas, P. The Role of C-reactive Protein in Patient Risk Stratification and Treatment. Eur. Cardiol. Rev. 2021, 16, e28. [Google Scholar]
- Mehta, N.N.; Degoma, E.; Shapiro, M.D. IL-6 and Cardiovascular Risk: A Narrative Review. Curr. Atheroscler. Rep. 2024, 27, 12. [Google Scholar] [PubMed]
- Desouky, D.A.; Nosair, N.A.; Salama, M.K.; El-Magd, M.A.; Desouky, M.A.; Sherif, D.E. PCSK9 and its relationship with HMGB1, TLR4, and TNFα in non-statin and statin-treated coronary artery disease patients. Mol. Cell Biochem. 2024. [Google Scholar] [CrossRef]
- Kosyakovsky, L.B.; de Boer, R.A.; Ho, J.E. Screening for Heart Failure: Biomarkers to Detect Heightened Risk in the General Population. Curr. Heart Fail. Rep. 2024, 21, 591–603. [Google Scholar]
- Mao, W.R.; Zhang, X.Y.; Yin, Y.Y.; Tang, X.H.; Jiang, Q.Q.; Chen, X.; Chen, X.L. Electrochemiluminescence ratio sensor for detecting MCP-1 based on s-PdNS. Sens. Bio-Sens. Res. 2025, 47, 100723. [Google Scholar]
- Nyárády, B.B.; Dósa, E.; Kohidai, L.; Pállinger, E.; Gubán, R.; Szonyi, A.; Kiss, L.Z.; Bagyura, Z. Associations between Various Inflammatory Markers and Carotid Findings in a Voluntary Asymptomatic Population Sample. Int. J. Mol. Sci. 2024, 25, 9656. [Google Scholar] [CrossRef]
- Mai, W.Q.; Liao, Y.H. Targeting IL-1β in the Treatment of Atherosclerosis. Front. Immunol. 2020, 11, 589654. [Google Scholar]
- Ortega-Paz, L.; Capodanno, D.; Angiolillo, D.J. Canakinumab for secondary prevention of coronary artery disease. Future Cardiol. 2021, 17, 427–442. [Google Scholar] [PubMed]
- Wachsmann-Maga, A.; Kaszuba, M.; Maga, M.; Włodarczyk, A.; Krężel, J.; Kaczmarczyk, P.; Bogucka, K.; Maga, P. Leukotrienes in the atherosclerotic cardiovascular diseases—A systematic review. Acta Angiol. 2022, 28, 147–153. [Google Scholar]
- Zhang, Y.W.; Qu, J.; Wang, Z.; Liu, W.F.; Xu, W.H. Leukotriene B4 (LTB4) Aggravates Myocardial Ischemia-Reperfusion Injury through BLT2/JAK1/STAT1 Pathway. J. Biol. Regul. Homeost. Agents 2024, 38, 3419–3433. [Google Scholar]
- Corbin, A.; Aromolaran, K.A.; Aromolaran, A.S. Leukotriene B4 is elevated in diabetes and promotes ventricular arrhythmogenesis in guinea pig. J. Cell. Physiol. 2025, 240, e31467. [Google Scholar] [CrossRef]
- Nunes, V.S.; Rogério, A.P.; Abrahao, O.; Serhan, C.N. Leukotriene B4 receptor 1 (BLT1) activation by leukotriene B4 (LTB4) and E resolvins (RvE1 and RvE2). Comput. Biol. Chem. 2024, 113, 108236. [Google Scholar]
- Vén, K.; Besztercei, B.; Janovicz, A.; Karsai, N.; Chun, J.; Tigyi, G.; Benyó, Z.; Ruisanchez, E. LPA-Induced Thromboxane A2-Mediated Vasoconstriction Is Limited to Poly-Unsaturated Molecular Species in Mouse Aortas. Int. J. Mol. Sci. 2024, 25, 6872. [Google Scholar] [CrossRef]
- Fejes, R.; Draxler, A.; Bragagna, L.; Woodman, R.J.; Croft, K.D.; Bondonno, C.P.; Hodgson, J.; Wolzt, M.; Wagner, K.H.; Neubauer, O. Dietary Nitrate from Beetroot Juice Reduces Oxidized Ldl, Ldl/Nox Ratio and Ldl Concentrations in Adults with Grade 1 Hypertension. Free Radic. Biol. Med. 2024, 218, 33–34. [Google Scholar]
- Douglas, J.; Gonski, S.; Bogart, A.M.; Bastarache, J.A.; Sinha, P.; Ware, L.B.; Meegan, J.E. Higher Levels of Soluble LOX-1 and Oxidized LDL Are Associated With the Hypoinflammatory LCA Phenotype in Sepsis. Am. J. Resp. Crit. Care 2024, 209, A5493. [Google Scholar]
- Wachsmann-Maga, A.; Maga, M.; Polczyk, R.; Włodarczyk, A.; Pasieka, P.; Terlecki, K.; Maga, P. Vascular Inflammatory Markers as Predictors of Peripheral Arterial Disease Patients’ Quality-of-Life Changes after Endovascular Treatment. J. Clin. Med. 2023, 12, 3412. [Google Scholar] [CrossRef]
- Barros, W.R.G.; Gonsalves, J.J.r. Deficiency of interleukin-1 receptor antagonist: A systematic review. Arch. Rheumatol. 2024, 39, 566–578. [Google Scholar]
- Thangasparan, S.; Kamisah, Y.; Ugusman, A.; Anuar, N.N.M.; Ibrahim, N.I. Unravelling the Mechanisms of Oxidised Low-Density Lipoprotein in Cardiovascular Health: Current Evidence from In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 13292. [Google Scholar] [CrossRef]
- Castro, R.; Adair, J.H.; Mastro, A.M.; Neuberger, T.; Matters, G.L. VCAM-1-targeted nanoparticles to diagnose, monitor and treat atherosclerosis. Nanomedicine 2024, 19, 723–735. [Google Scholar] [CrossRef]
- Quinn, M.; Zhang, R.Y.K.; Bello, I.; Rye, K.A.; Thomas, S.R. Myeloperoxidase as a Promising Therapeutic Target after Myocardial Infarction. Antioxidants 2024, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Narum, M.; Seljeflot, I.; Bratseth, V.; Berg, T.J.; Sveen, K.A. Intestinal fatty acid binding protein is associated with coronary artery disease in long-term type 1 diabetes-the Dialong study. Cardiovasc. Diabetol. 2024, 23, 419. [Google Scholar] [CrossRef]
- Yoshida, K.; Glynn, R.J.; Choi, H.K.; Everett, B.M.; Li, Y.; MacFadyen, J.G.; Ridker, P.M.; Solomon, D.H. Canakinumab’s Effect Against Subsequent Gout Flares and High-Sensitivity C-Reactive Protein Levels: A Causal Mediation Analysis. Arthritis Care Res. 2023, 75, 817–824. [Google Scholar] [CrossRef]
- Mohammadnia, N.; Opstal, T.S.J.; El Messaoudi, S.; Bax, W.A.; Cornel, J.H. An Update on Inflammation in Atherosclerosis: How to Effectively Treat Residual Risk. Clin. Ther. 2023, 45, 1055–1059. [Google Scholar]
- Hsia, J.; MacFadyen, J.G.; Monyak, J.; Ridker, P.M. Cardiovascular Event Reduction and Adverse Events Among Subjects Attaining Low-Density Lipoprotein Cholesterol <50 mg/dl With Rosuvastatin. J. Am. Coll. Cardiol. 2011, 57, 1666–1675. [Google Scholar] [PubMed]
- Younas, A.; Awan, Z.; Khan, T.; Mehta, S.; Munir, A.; Raja, H.A.A.; Jain, H.; Raza, A.; Sehar, A.; Ahmed, R.; et al. The effect of colchicine on myocardial infarction: An updated systematic review and meta-analysis of randomized controlled trials. Curr. Probl. Cardiol. 2025, 50, 102878. [Google Scholar] [PubMed]
- Tucker, B.; Goonetilleke, N.; Patel, S.; Keech, A. Colchicine in atherosclerotic cardiovascular disease. Heart 2024, 110, 618–625. [Google Scholar] [CrossRef]
- Giordano, S.; Camera, M.; Brambilla, M.; Sarto, G.; Spadafora, L.; Bernardi, M.; Iaconelli, A.; D’Amario, D.; Biondi-Zoccai, G.; Celia, A.I.; et al. Combining Colchicine and Antiplatelet Therapy to Tackle Atherothrombosis: A Paradigm in Transition? Int. J. Mol. Sci. 2025, 26, 1136. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnia, N.; van Broekhoven, A.; Bax, W.A.; Eikelboom, J.W.; Mosterd, A.; Fiolet, A.T.L.; Tijssen, J.G.P.; Thompson, P.L.; de Kleijn, D.P.; Tsimikas, S.; et al. The effects of colchicine on lipoprotein(a)- and oxidized phospholipid-associated cardiovascular disease risk. Eur. J. Prev. Cardiol. 2024, zwae355. [Google Scholar] [CrossRef]
- Ezeamuzie, C.I.; Rao, M.S.; El-Hashim, A.Z.; Philip, E.; Phillips, O.A. Anti-allergic, anti-asthmatic and anti-inflammatory effects of an oxazolidinone hydroxamic acid derivative (PH-251)-A novel dual inhibitor of 5-lipoxygenase and mast cell degranulation. Int. Immunopharmacol. 2022, 105, 108558. [Google Scholar] [PubMed]
- Muthukrishnan, P.T.; Nouraie, M.; Parikh, A.; Holguin, F. Zileuton use and phenotypic features in asthma. Pulm. Pharmacol. Ther. 2020, 60, 101872. [Google Scholar]
- Szczuko, M.; Kozioł, I.; Kotlęga, D.; Brodowski, J.; Drozd, A. The Role of Thromboxane in the Course and Treatment of Ischemic Stroke: Review. Int. J. Mol. Sci. 2021, 22, 11644. [Google Scholar] [CrossRef]
- Safdar, M.; Ullah, M.; Hamayun, S.; Wahab, A.; Khan, S.U.; Abdikakhorovich, S.A.; Ul Haq, Z.; Mehreen, A.; Naeem, M.; Mustopa, A.Z.; et al. Microbiome miracles and their pioneering advances and future frontiers in cardiovascular disease. Curr. Probl. Cardiol. 2024, 49, 102686. [Google Scholar]
- Zhang, H.H.; Xie, Y.D.; Cao, F.; Song, X.Y. Gut microbiota-derived fatty acid and sterol metabolites: Biotransformation and immunomodulatory functions. Gut Microbes 2024, 16, 2382336. [Google Scholar]
- Zhang, Y.S.; Wang, H.; Sang, Y.W.; Liu, M.; Wang, Q.; Yang, H.J.; Li, X.Y. Gut microbiota in health and disease: Advances and future prospects. Medcomm 2024, 5, e70012. [Google Scholar]
- Lu, L.J.; Jing, W.W.; Qian, W.M.; Fan, L.; Cheng, J.F. Association between dietary patterns and cardiovascular diseases: A review. Curr. Probl. Cardiol. 2024, 49, 102412. [Google Scholar] [CrossRef]
- Mederle, A.L.; Dima, M.; Stoicescu, E.R.; Capastraru, B.F.; Levai, C.M.; Hategan, O.A.; Maghiari, A.L. Impact of Gut Microbiome Interventions on Glucose and Lipid Metabolism in Metabolic Diseases: A Systematic Review and Meta-Analysis. Life 2024, 14, 1485. [Google Scholar] [CrossRef]
- Bratseth, V.; Nendl, A.; Raju, S.C.; Holm, K.; Broch, K.; Hov, J.R.; Seljeflot, I.; Troseid, M.; Awoyemi, A. Gut dysbiosis and neutrophil extracellular traps in chronic heart failure. Int. J. Cardiol. 2025, 419, 132689. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.M.; Wu, Y.; Peng, M.J.; Xiao, N.Q.; Lei, Z.J.; Tan, Z.J. Decreasing of Trimethylamine N-Oxide by Cecal Microbiota and Choline-Trimethylamine Lyase are Associated with Sishen Pill on Diarrhea with Kidney-Yang Deficiency Syndrome. J. Inflamm. Res. 2024, 17, 7275–7294. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Suceveanu, A.P.; Stanigut, A.M.; Tofolean, D.E.; Axelerad, A.D.; Iordache, I.E.; Herlo, A.; Twakor, A.N.; Nicoara, A.D.; Tocia, C.; et al. Intestinal Insights: The Gut Microbiome’s Role in Atherosclerotic Disease: A Narrative Review. Microorganisms 2024, 12, 2341. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.H.; Li, H.T.; Li, J.P. Circulating trimethylamine N-oxide is correlated with high coronary artery atherosclerotic burden in individuals with newly diagnosed coronary heart disease. BMC Cardiovasc. Disord. 2024, 24, 265. [Google Scholar] [CrossRef]
- Ghahfarrokhi, S.S.M.; Mohamadzadeh, M.; Samadi, N.; Fazeli, M.R.; Khaki, S.; Khameneh, B.; Bagheri, R.K. Management of Cardiovascular Diseases by Short-Chain Fatty Acid Postbiotics. Curr. Nutr. Rep. 2024, 13, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Devi, K.; Coutinho, H.D.M.; Mishra, A.P. Exploration of gut microbiome and inflammation: A review on key signalling pathways. Cell. Signal. 2024, 118, 111140. [Google Scholar] [CrossRef]
- Yang, T.Y.; Wu, C.P.; Li, Y.Q.; Wang, C.J.; Mao, Z.X.; Huo, W.Q.; Li, J.; Li, Y.; Xing, W.G.; Li, L.L. Association of short-chain fatty acids and the gut microbiome with type 2 diabetes: Evidence from the Henan Rural Cohort. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1619–1630. [Google Scholar] [CrossRef]
- Trehan, S.; Singh, G.; Bector, G.; Jain, P.; Mehta, T.; Goswami, K.; Chawla, A.; Jain, A.; Puri, P.; Garg, N. Gut Dysbiosis and Cardiovascular Health: A Comprehensive Review of Mechanisms and Therapeutic Potential. Cureus J. Med. Sci. 2024, 16, e67010. [Google Scholar] [CrossRef]
- Theofilis, P.; Vlachakis, P.K.; Oikonomou, E.; Tsioufis, K.; Tousoulis, D. Targeting the Gut Microbiome to Treat Cardiometabolic Disease. Curr. Atheroscler. Rep. 2024, 26, 25–34. [Google Scholar] [CrossRef]
- Datta, S.; Pasham, S.; Inavolu, S.; Boini, K.M.; Koka, S. Role of Gut Microbial Metabolites in Cardiovascular Diseases-Current Insights and the Road Ahead. Int. J. Mol. Sci. 2024, 25, 10208. [Google Scholar] [CrossRef]
- Abeltino, A.; Hatem, D.; Serantoni, C.; Riente, A.; De Giulio, M.M.; De Spirito, M.; De Maio, F.; Maulucci, G. Unraveling the Gut Microbiota: Implications for Precision Nutrition and Personalized Medicine. Nutrients 2024, 16, 3806. [Google Scholar] [CrossRef] [PubMed]
- Cienkowski, K.; Cienkowska, A.; Kupczynska, K.; Bielecka-Dabrowa, A. The Role of Gut Microbiota and Its Metabolites in Patients with Heart Failure. Biomedicines 2024, 12, 894. [Google Scholar] [CrossRef]
- Hameed, S.; Karim, N.; Wasay, M.; Venketasubramanian, N. Emerging Stroke Risk Factors: A Focus on Infectious and Environmental Determinants. J. Cardiovasc. Dev. Dis. 2024, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kim, H.I.; Park, J.; Guo, J.L.; Huang, W. The role of immune cells in different stages of atherosclerosis. Int. J. Med. Sci. 2024, 21, 1129–1143. [Google Scholar] [CrossRef]
- Neto, J.P.R.C.; Freire, M.O.D.; Lemos, D.E.D.; Alves, R.M.F.R.; Cardoso, E.F.D.; Balarini, C.D.; Duman, H.; Karav, S.; de Souza, E.L.; Alves, J.L.D. Targeting Gut Microbiota with Probiotics and Phenolic Compounds in the Treatment of Atherosclerosis: A Comprehensive Review. Foods 2024, 13, 2886. [Google Scholar] [CrossRef]
- Higashiyama, A.; Kohsaka, S.; Fujiyoshi, A. Primary Prevention of Coronary and Other Cardiovascular Diseases: A Focused Review. J. Atheroscler. Thromb. 2024, 31, 1113–1128. [Google Scholar] [PubMed]
- Mitsis, A.; Khattab, E.; Christodoulou, E.; Myrianthopoulos, K.; Myrianthefs, M.; Tzikas, S.; Ziakas, A.; Fragakis, N.; Kassimis, G. From Cells to Plaques: The Molecular Pathways of Coronary Artery Calcification and Disease. J. Clin. Med. 2024, 13, 6352. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Valladares, J.R.; Martinez-Jimenez, Y.I.; Morillon-Torres, V.; Rivera-Maya, O.B.; Gómez, R.; Calderon-Aranda, E.S. Bisphenol A and Its Emergent Substitutes: State of the Art of the Impact of These Plasticizers on Oxidative Stress and Its Role in Vascular Dysfunction. Antioxidants 2024, 13, 1468. [Google Scholar] [CrossRef]
- Kim, H.J.; Oh, Y.H.; Park, S.J.; Song, J.; Kim, K.; Choi, D.; Jeong, S.; Park, S.M. Combined Effects of Air Pollution and Changes in Physical Activity With Cardiovascular Disease in Patients With Dyslipidemia. J. Am. Heart Assoc. 2024, 13, e035933. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Shin, T.H.; Park, C.B.; Lee, W.-S.; Kim, J.; Lee, G. The Impact of Fine Particulate Matter 2.5 on the Cardiovascular System: A Review of the Invisible Killer. Nanomaterials 2022, 12, 2656. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, G.D. Particulate Matter-Induced Emerging Health Effects Associated with Oxidative Stress and Inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chan, K.H.; Kwok, T.; Wu, S.W.; Man, C.L.; Ho, K.F. Effects of long-term indoor air purification intervention on cardiovascular health in elderly: A parallel, double-blinded randomized controlled trial in Hong Kong. Environ. Res. 2024, 247, 118284. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Hu, S.Y.; Kishida, M. Exposure to nitrate and nitrite disrupts cardiovascular development through estrogen receptor in zebrafish embryos and larvae. Fish Physiol. Biochem. 2024, 50, 2165–2178. [Google Scholar] [CrossRef]
- Donzelli, G.; Sera, F.; Morales, M.A.; Vozzi, F.; Roos, T.; Schaffert, A.; Paparella, M.; Murugadoss, S.; Mertens, B.; Gehring, R.; et al. A systematic review and meta-analysis of human population studies on the association between exposure to toxic environmental chemicals and left ventricular dysfunction (LVD). Environ. Res. 2024, 249, 118429. [Google Scholar]
- Jomova, K.; Alomar, S.Y.; Nepovimova, E.; Kuca, K.; Valko, M. Heavy metals: Toxicity and human health effects. Arch. Toxicol. 2025, 99, 153–209. [Google Scholar]
- Mahadik, S.R.; Reddy, A.R.T.; Choudhary, K.; Nama, L.; Jamdade, M.S.; Singh, S.; Murti, K.; Kumar, N. Arsenic induced cardiotoxicity: An approach for molecular markers, epigenetic predictors and targets. Environ. Toxicol. Pharmacol. 2024, 111, 104558. [Google Scholar]
- Ahmad, I.; Kaur, M.; Tyagi, D.; Singh, T.B.; Kaur, G.; Afzal, S.M.; Jauhar, M. Exploring novel insights into the molecular mechanisms underlying Bisphenol A-induced toxicity: A persistent threat to human health. Environ. Toxicol. Pharmacol. 2024, 108, 104467. [Google Scholar]
- Gupta, A.; Singh, A.; Mishra, V.K. Impact of Bisphenol-A in the environment and its removal through biological agents: A review. Environ. Qual. Manag. 2024, 34, e22246. [Google Scholar] [CrossRef]
- Cairns, M.; Odendaal, C.; O’Brien, C.; Marais, E.; Oestlund, I.; Storbeck, K.H.; Sishi, B.; Joseph, D.; Smith, C.; Essop, M.F. Effects of chronic stress on rat heart function following regional ischemia: A sex-dependent investigation. Am. J. Physiol.-Heart Circ. Physiol. 2024, 327, H880–H895. [Google Scholar] [CrossRef]
- Sliwa, K.; Viljoen, C.A.; Stewart, S.; Miller, M.R.; Prabhakaran, D.; Kumar, R.K.; Thienemann, F.; Piniero, D.; Prabhakaran, P.; Narula, J.; et al. Cardiovascular disease in low- and middle-income countries associated with environmental factors. Eur. J. Prev. Cardiol. 2024, 31, 688–697. [Google Scholar]
- Alharbi, H.A.; Rushdi, A.I.; Bazeyad, A.; Al-Mutlaq, K.F. Temporal Variations, Air Quality, Heavy Metal Concentrations, and Environmental and Health Impacts of Atmospheric PM and PM in Riyadh City, Saudi Arabia. Atmosphere 2024, 15, 1448. [Google Scholar] [CrossRef]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef]
- Charles, D.A.; Prince, S.E. Deciphering the molecular mechanism of NLRP3 in BPA-mediated toxicity: Implications for targeted therapies. Heliyon 2024, 10, e28917. [Google Scholar]
- Cheng, F.; Wang, J.L. Biological strategies for Bisphenol A degradation: Mechanisms and pathways. Rev. Environ. Sci. Bio-Technol. 2024, 23, 601–632. [Google Scholar]
- Alghoraibi, A.A.; Alnumair, M.A.M.; Faqeeh, H.S.A.; Al-Araibi, F.S.S.; Al Hawsawi, J.H.Y.; Alrafie, A.A.; Bukhary, A.M.; Almutairi, M.M.H.; Al Harbi, S.T.A.; Alotibi, M.M.F.; et al. The Impact of Psychological and Social Stress on General and Public Health. Egypt. J. Chem. 2024, 67, 717–728. [Google Scholar]
- Munir, L.Z.; du Toit, E.F. Impact of Chronic Psychological Stress on Cardiovascular Disease Risk: A Narrative Review. Heart Mind 2024, 8, 268–278. [Google Scholar]
- Martins-Silva, T.; Martins, R.C.; Murray, J.; Carvalho, A.M.; Rickes, L.N.; Corrêa, B.D.; Fraga, B.B.; Brum, C.B.; Freitas, D.F.; Carpena, M.X.; et al. Hair cortisol measurement: A systematic review of current practices and a proposed checklist for reporting standards. Psychoneuroendocrinology 2025, 171, 107185. [Google Scholar]
- Künzel, R.G.; Elgazzar, M.; Bain, P.A.; Kirschbaum, C.; Papatheodorou, S.; Gelaye, B. The association between maternal prenatal hair cortisol concentration and preterm birth: A systematic review and meta-analysis. Psychoneuroendocrinology 2024, 165, 107041. [Google Scholar] [CrossRef]
- Cortiana, V.; Vaghela, H.; Bakhle, R.; Santhosh, T.; Kaiwan, O.; Tausif, A.; Goel, A.; Suhail, M.K.; Patel, N.; Akram, O.; et al. Beyond the Heart: The Predictive Role of Coronary Artery Calcium Scoring in Non-Cardiovascular Disease Risk Stratification. Diagnostics 2024, 14, 2349. [Google Scholar] [CrossRef]
- Onnis, C.; Virmani, R.; Kawai, K.; Nardi, V.; Lerman, A.; Cademartiri, F.; Scicolone, R.; Boi, A.; Congiu, T.; Faa, G.; et al. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation 2024, 149, 251–266. [Google Scholar]
- Ji, B.; Liu, X.B. Coronary artery calcification: Concepts and clinical applications. Ann. Med. Surg. 2024, 86, 2848–2855. [Google Scholar] [CrossRef]
- Streiber, A.M.; van den Beukel, T.C.; vom Hofe, I.; Neitzel, J.; Vernooij, M.W.; Bos, D.; Vinke, E.J. Arterial calcification in the heart-brain axis and cognitive performance over time. Alzheimers Dement. 2025, 21, e14374. [Google Scholar] [CrossRef] [PubMed]
- Elvas, L.B.; Gomes, S.; Ferreira, J.C.; Rosário, L.B.; Brandao, T. Deep learning for automatic calcium detection in echocardiography. Biodata Min. 2024, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Feldle, P.; Scheuber, M.; Grunz, J.P.; Heidenreich, J.F.; Pannenbecker, P.; Nora, C.; Huflage, H.; Bley, T.A.; Petritsch, B. Virtual non-iodine photon-counting CT-angiography for aortic valve calcification scoring. Sci. Rep. 2024, 14, 4724. [Google Scholar] [CrossRef] [PubMed]
- Bujny, M.; Jesionek, K.; Nalepa, J.; Bartczak, T.; Miszalski-Jamka, K.; Kostur, M. Seeing the Invisible: On Aortic Valve Reconstruction in Non-contrast CT. Med. Image Comput. Comput. Assist. Interv.-Miccai 2024, 15009, 572–581. [Google Scholar]
- Chang, Y.X.; Li, Y.X.; Duan, X.F.; Lv, N.; Meng, Y.F.; Zhou, F.D.; Chen, L.Z.; Zhang, H.; Zhang, Y.; Li, J.P. Assessment of renal artery stenosis using renal fractional flow reserve and correlation with angiography and color Doppler ultrasonography: Data from FAIR-pilot trial. Hypertens. Res. 2025, 48, 702–709. [Google Scholar] [CrossRef]
- Sanchez, S.; Mossa-Basha, M.; Anagnostakou, V.; Liebeskind, D.S.; Samaniego, E.A. Comprehensive imaging analysis of intracranial atherosclerosis. J. Neurointerv. Surg. 2024, 17, 311–320. [Google Scholar] [CrossRef]
- Yang, J.; Lee, K.B.; Kim, H.; Kim, S.W.; Kim, Y.H.; Sung, S.A.; Kim, J.; Oh, K.H.; Jung, J.Y.; Hyun, Y.Y. Statin Use and the Progression of Coronary Artery Calcification in CKD: Findings From the KNOW-CKD Study. Kidney Int. Rep. 2024, 9, 3027–3034. [Google Scholar] [CrossRef]
- Bosco, G.; Mszar, R.; Piro, S.; Sabouret, P.; Gallo, A. Cardiovascular Risk Estimation and Stratification Among Individuals with Hypercholesterolemia. Curr. Atheroscler. Rep. 2024, 26, 537–548. [Google Scholar] [CrossRef]
- Aromiwura, A.A.; Kalra, D.K. Artificial Intelligence in Coronary Artery Calcium Scoring. J. Clin. Med. 2024, 13, 3453. [Google Scholar] [CrossRef]
- Baskaran, L.; Leng, S.; Dutta, U.; Teo, L.; Yew, M.S.; Sia, C.H.; Chew, N.W.; Huang, W.M.; Lee, H.K.; Vaughan, R.; et al. Cohort profile: AI-driven national Platform for CCTA for clinicaL and industriaL applicatiOns (APOLLO). BMJ Open 2024, 14, e089047. [Google Scholar] [CrossRef] [PubMed]
- Seavasine, V.C.; Stoliar, G.A.; Teixeira, B.C.D.; Zétola, V.D.F.; Lange, M.C. Automated evaluation of collateral circulation for outcome prediction in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2024, 33, 107584. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekbossynova, M.; Saliev, T.; Ivanova-Razumova, T.; Andossova, S.; Kali, A.; Myrzakhmetova, G. Beyond Cholesterol: Emerging Risk Factors in Atherosclerosis. J. Clin. Med. 2025, 14, 2352. https://doi.org/10.3390/jcm14072352
Bekbossynova M, Saliev T, Ivanova-Razumova T, Andossova S, Kali A, Myrzakhmetova G. Beyond Cholesterol: Emerging Risk Factors in Atherosclerosis. Journal of Clinical Medicine. 2025; 14(7):2352. https://doi.org/10.3390/jcm14072352
Chicago/Turabian StyleBekbossynova, Makhabbat, Timur Saliev, Tatyana Ivanova-Razumova, Saltanat Andossova, Aknur Kali, and Gulzhan Myrzakhmetova. 2025. "Beyond Cholesterol: Emerging Risk Factors in Atherosclerosis" Journal of Clinical Medicine 14, no. 7: 2352. https://doi.org/10.3390/jcm14072352
APA StyleBekbossynova, M., Saliev, T., Ivanova-Razumova, T., Andossova, S., Kali, A., & Myrzakhmetova, G. (2025). Beyond Cholesterol: Emerging Risk Factors in Atherosclerosis. Journal of Clinical Medicine, 14(7), 2352. https://doi.org/10.3390/jcm14072352





