Identification of Perioperative Risk Factors for Early Sacral Nerve Stimulator Explantation: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Cohort Demographics
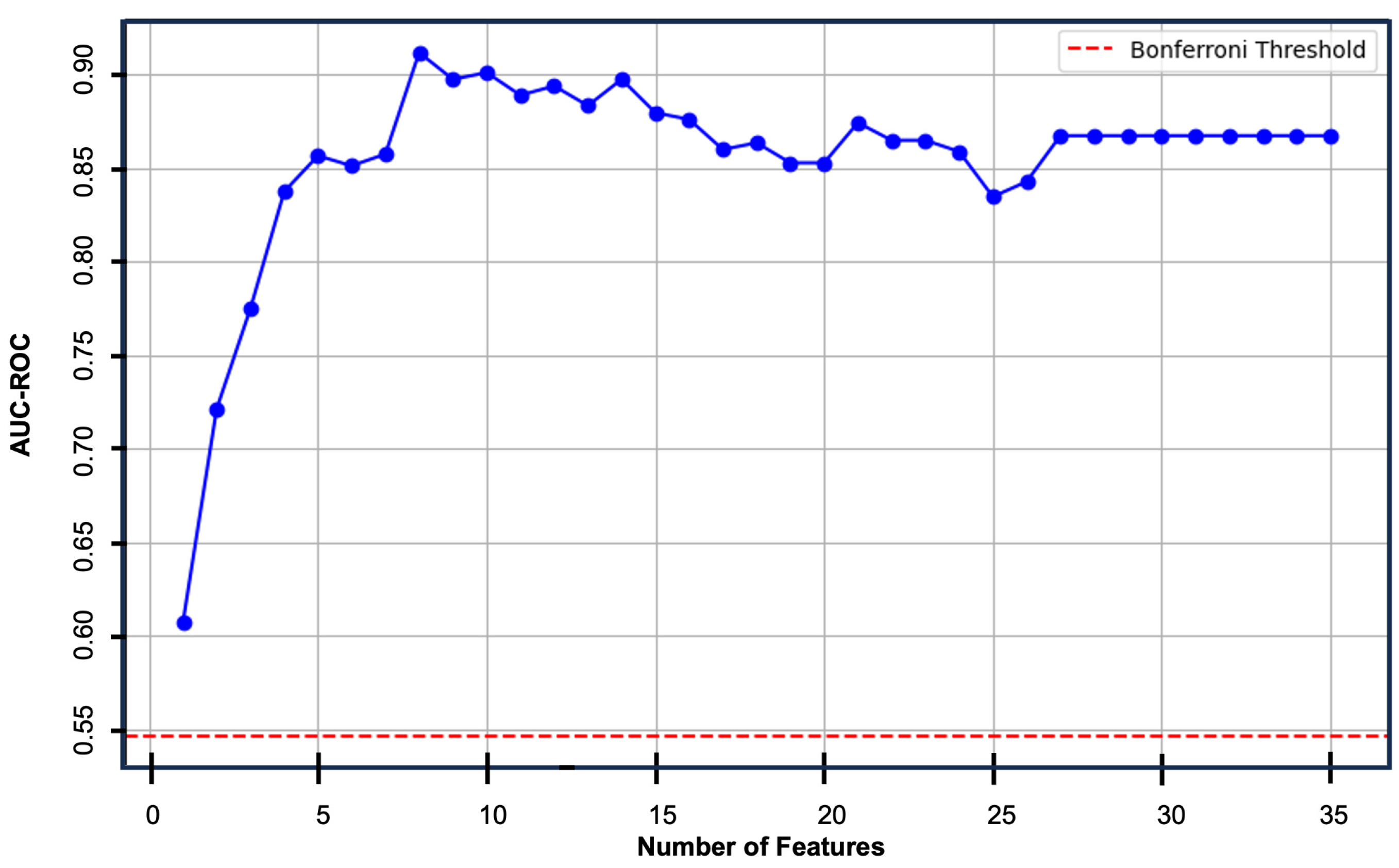
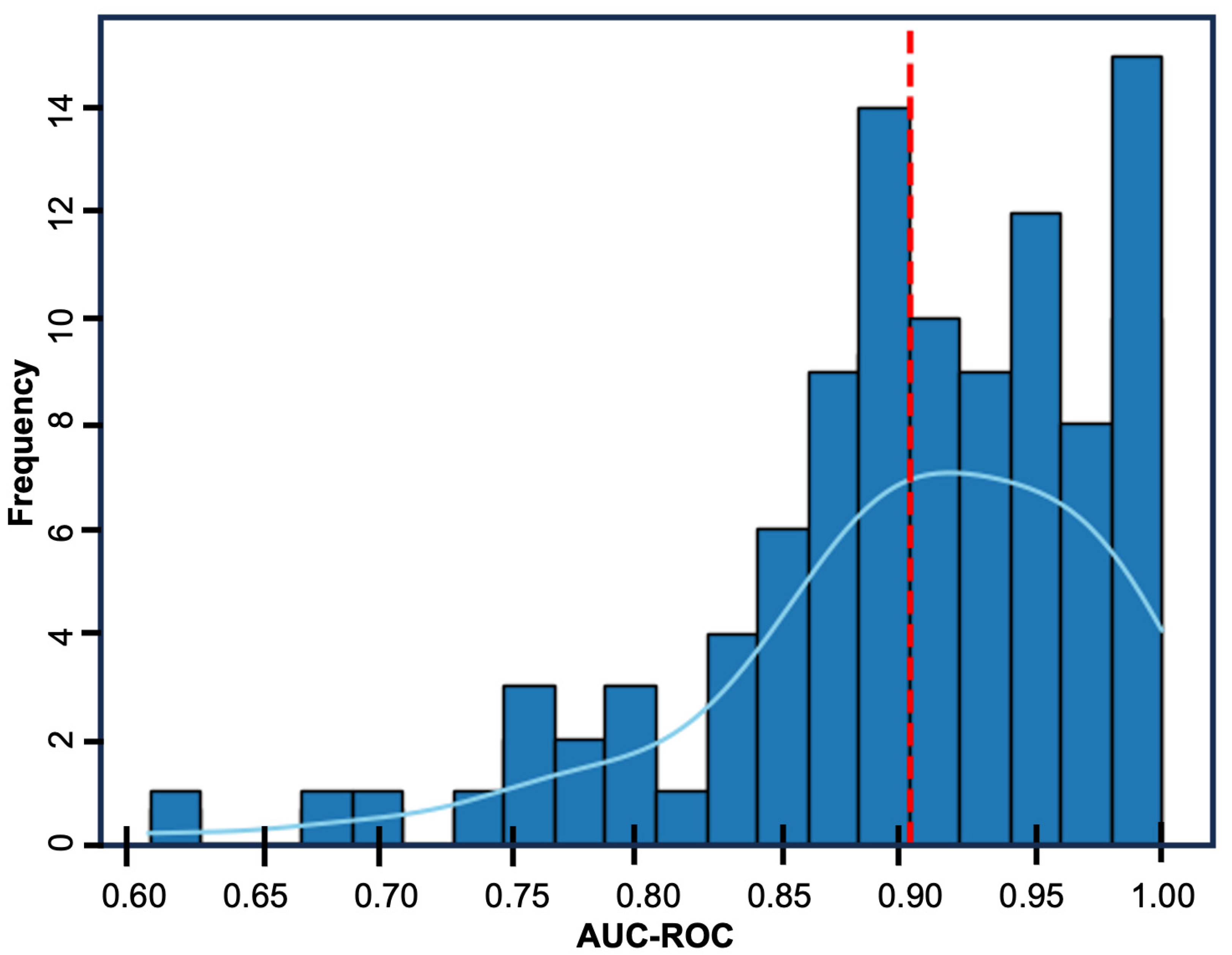
3.2. Multivariate Logistic Regression Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| AUC | area under the curve |
| CI | confidence interval |
| CKD | chronic kidney disease |
| EHR | electronic health records |
| ICD | International Classification of Diseases |
| ICU | intensive care unit |
| IQR | interquartile range |
| MOVER | Medical Informatics Operating room Vitals and Events Repository |
| OR | odds ratio |
| ROC | receiver operating characteristic |
| SCS | spinal cord stimulator |
| SD | standard deviation |
| SMOTE | Synthetic Minority Oversampling Technique |
| SNS | sacral nerve stimulator |
References
- Lukacz, E.S.; Santiago-Lastra, Y.; Albo, M.E.; Brubaker, L. Urinary Incontinence in Women: A Review. JAMA 2017, 318, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, J.B.; Schurch, B.; Hamid, R.; Averbeck, M.; Sakakibara, R.; Agro, E.F.; Dickinson, T.; Payne, C.K.; Drake, M.J.; Haylen, B.T. An International Continence Society (ICS) report on the terminology for adult neurogenic lower urinary tract dysfunction (ANLUTD). Neurourol. Urodyn. 2018, 37, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Griffiths, D.; de Groat, W.C. The neural control of micturition. Nat. Rev. Neurosci. 2008, 9, 453–466. [Google Scholar] [CrossRef]
- Das, A.K.; White, M.D.; Longhurst, P.A. Sacral nerve stimulation for the management of voiding dysfunction. Rev. Urol. 2000, 2, 43–60. [Google Scholar]
- Brazzelli, M.; Murray, A.; Fraser, C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: A systematic review. J. Urol. 2006, 175, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Brusciano, L.; Brillantino, A.; Pellino, G.; Marinello, F.; Baeten, C.I.; Digesu, A.; Naldini, G.; Gambardella, C.; Lucido, F.S.; Sturiale, A.; et al. Sacral nerve modulation for patients with fecal incontinence: Long-term outcome and effects on sexual function. Updates Surg. 2023, 75, 1187–1195. [Google Scholar] [CrossRef]
- Liechti, M.D.; van der Lely, S.; Knupfer, S.C.; Abt, D.; Kiss, B.; Leitner, L.; Mordasini, L.; Tornic, J.; Wollner, J.; Mehnert, U.; et al. Sacral Neuromodulation for Neurogenic Lower Urinary Tract Dysfunction. NEJM Evid. 2022, 1, EVIDoa2200071. [Google Scholar] [CrossRef]
- Assmann, R.; Douven, P.; Kleijnen, J.; van Koeveringe, G.A.; Joosten, E.A.; Melenhorst, J.; Breukink, S.O. Stimulation Parameters for Sacral Neuromodulation on Lower Urinary Tract and Bowel Dysfunction-Related Clinical Outcome: A Systematic Review. Neuromodulation 2020, 23, 1082–1093. [Google Scholar] [CrossRef]
- Zeiton, M.; Faily, S.; Nicholson, J.; Telford, K.; Sharma, A. Sacral nerve stimulation--hidden costs (uncovered). Int. J. Colorectal Dis. 2016, 31, 1005–1010. [Google Scholar] [CrossRef]
- Hounsome, N.; Roukas, C. Cost-effectiveness of sacral nerve stimulation and percutaneous tibial nerve stimulation for faecal incontinence. Ther. Adv. Gastroenterol. 2018, 11, 1–22. [Google Scholar] [CrossRef]
- Jairam, R.; Drossaerts, J.; Marcelissen, T.; van Koeveringe, G.; Vrijens, D.; van Kerrebroeck, P. Predictive Factors in Sacral Neuromodulation: A Systematic Review. Urol. Int. 2022, 106, 323–343. [Google Scholar] [CrossRef]
- Gevelinger, M.M.; Sanderson, D.J.; Jaworski, E.; Doyle, P.J. Evaluation of Sacral Nerve Stimulation Device Revision and Explantation in a Single Center, Multidisciplinary Study. Neuromodulation 2020, 23, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Malde, S.; Marcelissen, T.; Vrijens, D.; Apostilidis, A.; Rahnama, I.S.; Cardozo, L.; Lovick, T. Sacral nerve stimulation for refractory OAB and idiopathic urinary retention: Can phenotyping improve the outcome for patients: ICI-RS 2019? Neurourol. Urodyn. 2020, 39 (Suppl. S3), S96–S103. [Google Scholar] [CrossRef]
- Samad, M.; Angel, M.; Rinehart, J.; Kanomata, Y.; Baldi, P.; Cannesson, M. Medical Informatics Operating Room Vitals and Events Repository (MOVER): A public-access operating room database. JAMIA Open 2023, 6, ooad084. [Google Scholar] [CrossRef]
- Akpala, A.; Lezama, T.; Jinadu, K.; Belal, M.; King, T. A Five-Year Retrospective Study on the Clinical Outcomes of Sacral Nerve Stimulation for Neuromodulation of the Lower Urinary Tract in a Tertiary Hospital. Cureus 2024, 16, e73626. [Google Scholar] [CrossRef] [PubMed]
- Noblett, K.; Siegel, S.; Mangel, J.; Griebling, T.L.; Sutherland, S.E.; Bird, E.T.; Comiter, C.; Culkin, D.; Bennett, J.; Zylstra, S.; et al. Results of a prospective, multicenter study evaluating quality of life, safety, and efficacy of sacral neuromodulation at twelve months in subjects with symptoms of overactive bladder. Neurourol. Urodyn. 2016, 35, 246–251. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 51–56. [Google Scholar]
- Waskom, M.L. seaborn: Statistical data visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Prasada, S.; Desai, M.Y.; Saad, M.; Smilowitz, N.R.; Faulx, M.; Menon, V.; Moudgil, R.; Chaudhury, P.; Hussein, A.A.; Taigen, T.; et al. Preoperative Atrial Fibrillation and Cardiovascular Outcomes After Noncardiac Surgery. J. Am. Coll. Cardiol. 2022, 79, 2471–2485. [Google Scholar] [CrossRef]
- Tateosian, V.S.; Richman, D.C. Preoperative Cardiac Evaluation for Noncardiac Surgery. Anesthesiol. Clin. 2018, 36, 509–521. [Google Scholar] [CrossRef]
- Cameron, A.P.; Anger, J.T.; Madison, R.; Saigal, C.S.; Clemens, J.Q. The Urologic Diseases in America Project. Battery explantation after sacral neuromodulation in the Medicare population. Neurourol. Urodyn. 2013, 32, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Myer, E.N.B.; Petrikovets, A.; Slocum, P.D.; Lee, T.G.; Carter-Brooks, C.M.; Noor, N.; Carlos, D.M.; Wu, E.; Van Eck, K.; Fashokun, T.B.; et al. Risk factors for explantation due to infection after sacral neuromodulation: A multicenter retrospective case-control study. Am. J. Obstet. Gynecol. 2018, 219, 78.e1–78.e9. [Google Scholar] [CrossRef] [PubMed]



| Variable | Total | Explant | No Explant | p Value |
|---|---|---|---|---|
| Number of patients | 54 | 38 | 16 | |
| Sex | ||||
| Male | 7 (13.0%) | 2 (5.3%) | 4 (25.0%) | 0.0563 |
| Female | 47 (87.0%) | 36 (94.7%) | 12 (75.0%) | 0.0563 |
| Age (years ± SD) 1 | 63.7 ± 15.9 | 63.8 ± 16.1 | 63.3 ± 15.8 | 0.8548 |
| ASA score (IQR) 1 | 3 (2–3) | 3 (2–3) | 2 (2–3) | 0.1608 |
| Anesthesia type | ||||
| Monitored anesthesia care | 39 (72.2%) | 27 (71.1%) | 12 (75.0%) | >0.9999 |
| General anesthesia | 15 (27.8%) | 11 (28.9%) | 4 (25.0%) | >0.9999 |
| Length of stay (days ± SD) 1 | 0.1 ± 0.5 | 0.2 ± 0.5 | 0.0 ± 0.0 | 0.5465 |
| Indications for SNS 2 | ||||
| Peripheral neuropathy | 6 (11.1%) | 5 (13.2%) | 1 (6.3%) | 0.6570 |
| Low back pain | 6 (11.1%) | 4 (10.5%) | 2 (12.5%) | >0.9999 |
| Cervical pain | 3 (5.6%) | 2 (5.3%) | 1 (6.3%) | >0.9999 |
| Urinary dysfunction | 53 (98.1%) | 37 (97.4%) | 16 (100.0%) | >0.9999 |
| Past Medical History | ||||
| Cerebrovascular disease | 1 (2.0%) | 0 (0.0%) | 1 (6.3%) | 0.2963 |
| Obstructive sleep apnea | 9 (16.7%) | 4 (10.5%) | 5 (31.3%) | 0.1056 |
| Sleep disorder | 13 (24.1%) | 8 (21.1%) | 5 (31.3%) | 0.4934 |
| Hypertension | 9 (16.7%) | 6 (15.8%) | 3 (18.8%) | >0.9999 |
| Hyperlipidemia | 8 (14.8%) | 3 (7.9%) | 5 (31.4%) | 0.0413 * |
| Atrial fibrillation | 3 (5.6%) | 1 (2.6%) | 2 (12.5%) | 0.2064 |
| Diabetes mellitus | 5 (9.3%) | 3 (7.9%) | 2 (12.5%) | 0.6265 |
| Chronic kidney disease | 1 (1.9%) | 0 (0.0%) | 1 (6.3%) | 0.2963 |
| Anxiety | 9 (16.7%) | 7 (18.4%) | 2 (12.5%) | 0.7093 |
| Depression | 5 (9.3%) | 5 (13.2%) | 0 (0.0%) | 0.3064 |
| Obesity | 3 (5.6%) | 2 (5.3%) | 1 (6.3%) | >0.9999 |
| Migraine | 4 (7.4%) | 4 (10.5%) | 0 (0.0%) | 0.3064 |
| Musculoskeletal pain | 15 (27.8%) | 9 (23.7%) | 6 (37.5%) | 0.3332 |
| Arthritis | 11 (20.4%) | 8 (21.1%) | 3 (18.8%) | >0.9999 |
| Malignancy | 8 (14.8%) | 8 (21.1%) | 0 (0.0%) | 0.0883 |
| Social History | ||||
| Opioid use disorder | 1 (1.9%) | 1 (2.6%) | 0 (0.0%) | >0.9999 |
| Illicit substance use | 1 (1.9%) | 1 (2.6%) | 0 (0.0%) | >0.9999 |
| Alcohol use | 1 (1.9%) | 1 (2.6%) | 0 (0.0%) | >0.9999 |
| Tobacco products | 2 (3.7%) | 0 (0.0%) | 2 (12.5%) | 0.0839 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murin, P.J.; Murin, P.J.; Lima de Mendonça, Y.; Martins, Y.C. Identification of Perioperative Risk Factors for Early Sacral Nerve Stimulator Explantation: A Single-Center Retrospective Cohort Study. J. Clin. Med. 2025, 14, 2363. https://doi.org/10.3390/jcm14072363
Murin PJ, Murin PJ, Lima de Mendonça Y, Martins YC. Identification of Perioperative Risk Factors for Early Sacral Nerve Stimulator Explantation: A Single-Center Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(7):2363. https://doi.org/10.3390/jcm14072363
Chicago/Turabian StyleMurin, Peyton J., Patrick J. Murin, Yara Lima de Mendonça, and Yuri Chaves Martins. 2025. "Identification of Perioperative Risk Factors for Early Sacral Nerve Stimulator Explantation: A Single-Center Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 7: 2363. https://doi.org/10.3390/jcm14072363
APA StyleMurin, P. J., Murin, P. J., Lima de Mendonça, Y., & Martins, Y. C. (2025). Identification of Perioperative Risk Factors for Early Sacral Nerve Stimulator Explantation: A Single-Center Retrospective Cohort Study. Journal of Clinical Medicine, 14(7), 2363. https://doi.org/10.3390/jcm14072363







