Compatibility of Post-Kidney Transplant Immunosuppression Therapy with Lactation
Abstract
1. Introduction
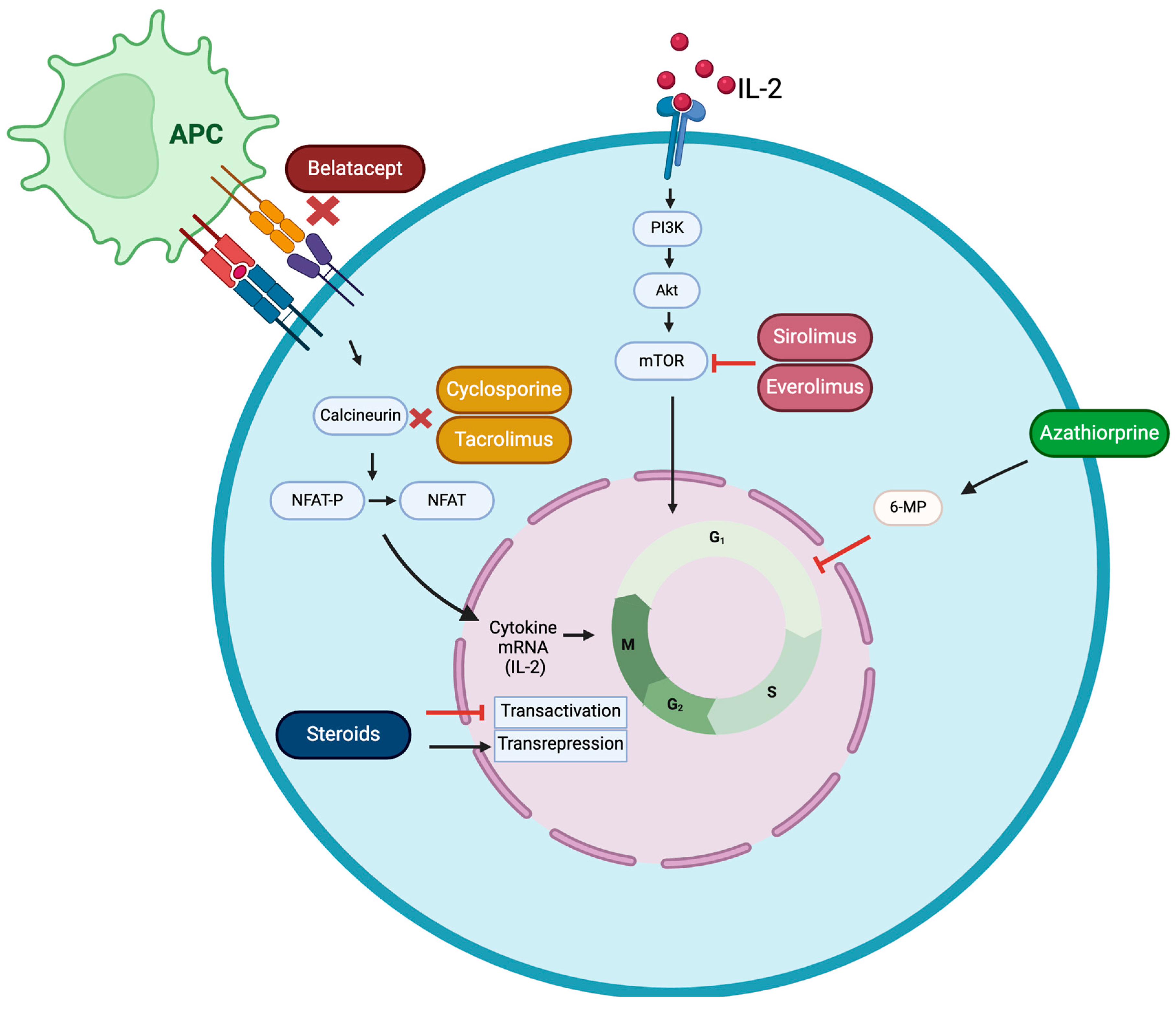
2. Immunosuppression After Kidney Transplant
3. Post-Kidney Transplant Immunosuppressive Treatments
4. Immunosuppressive Drugs and Breastfeeding
4.1. Calcineurin Inhibitors
4.1.1. Cyclosporine
4.1.2. Tacrolimus
4.2. Thiopurine Drugs (Antimetabolites)
Azathioprine
| Immunosuppressive Agent | Dose | Subject Group | Drug Levels in Breast Milk | Effects on the Infant | Year | Reference |
|---|---|---|---|---|---|---|
| Cyclosporine | 450 mg/day during pregnancy | 1 | 101, 109, and 263 μg/L in breastmilk on days 2, 3, and 4 postpartum | Not specified. | 1983 | [53] |
| 325 mg 2 h before the onset of labor | 1 | 16 μg/L | Undetectable (<3 μg//L) cyclosporine blood levels. | 1985 | [54] | |
| 225 mg/day | 1 | Not reported | Estimated intake of 6 μg/kg daily (0.01% maternal weight-adjusted dose); the infant remained healthy and normal. | 1995 | [55] | |
| 3 mg/kg twice daily | 1 | 596 μg/L 5 weeks postpartum; the infant would receive less than 0.1 mg/kg per day or no more than 1.7% of the maternal weight-adjusted dose | At 5 weeks of age, normal renal function and a blood cyclosporin concentration below 3 μg/L; estimated levels taken through milk (150 mL/Kg/day) < 0.1 mg/kg of cyclosporin. | 1997 | [56] | |
| Not reported | 5 | 50 to 227 ng/mL | All infants had levels below the detection limit of 30 ng/mL. Breastfed infants of mothers on cyclosporine received less than 300 μg/day, with absorption amounts being undetectable. No nephrotoxic effects or other side effects were observed. | 1998 | [57] | |
| 1 | 25 to 120 μg/L | |||||
| 1 | 87 to 440 μg/L | |||||
| 300 mg twice daily | 1 | 79 to 286 μg/L on three separate occasions over a 10-week period | The breast milk/maternal blood level ratio was 84%, but the infant had undetectable levels. The infant grew and developed normally. | 2001 | [58] | |
| 5.3 mg/kg per day | 1 | 403 μg/L | The estimated dose the infant would ingest through breast milk was 0.06 mg/kg/day (1.1% of the weight-adjusted maternal dose), 1% of the therapeutic dose on a weight basis. | 2003 | [59] | |
| 225 mg/day | 1 | 465 μg/L in foremilk and 564 μg/L in hindmilk | The estimated dose the infant would ingest through breast milk was 0.08 mg/kg/day (2.1% of the weight-adjusted maternal dose). The cyclosporine concentration in the infant’s blood was below the detection limit of 25 μg/L. | |||
| 250 mg/day | 1 | 97.6 μg/L | The estimated dose the infant would ingest through breast milk was 0.01 mg/kg per day (0.2% of the weight-adjusted maternal dose). Cyclosporine levels in the infant’s blood were below the detection limit. | |||
| 250 mg/day | 1 | 117.7 μg/L ranged from 75 to 150 μg/L | The infant received a dose of 0.4% of the weight-adjusted maternal dose. The infant’s blood concentration was below the detection limit of 25 μg/L. | |||
| 150 mg twice daily | 1 | Two points measurement on day 8 yielded 84 and 144 μg/L | Infant exposure is estimated to be 0.5% of the maternal weight-adjusted dose. Breastfeeding continued without any adverse effects observed. | |||
| 100 mg in the morning and 75 mg in the evening | 1 | 46 μg/L | The estimated dose the infant would ingest through breast milk was 0.007 mg/kg, or 0.33% of the weight-adjusted maternal dose. The infant’s blood levels were undetectable (<10 μg/L), and no apparent clinical adverse effects from cyclosporine were observed. | 2011 | [60] | |
| 200 mg/day | 1 | Not reported | The infant’s serum cyclosporine level was undetectable (with an assay lower limit of 15 mg/L). The mother continued breastfeeding for 5 months, during which her infant remained healthy and had normal renal function. | 2011 | [61] | |
| 120 mg/day | 1 | The infant’s serum cyclosporine level was undetectable, with a lower assay limit of 15 mg/L. | ||||
| 5 mg/kg | 1 | Not reported | The infant’s serum cyclosporine concentrations after the morning feed were consistently undetectable (<30 μg/L). | 2011 | [62] | |
| 200 mg/day | 1 | 128 μg/L, 200 μg/L, and 364 μg/L on days 10, 30, and 50 after morning dose: on day 40, before her morning dose, was 207 μg/L | By 12 months of age, the infant was developing normally and showed no noticeable adverse effects from the drug in the breast milk. | 2014 | [63] | |
| 1.5 mg/kg/day | 1 | 15.5 μg/L in colostrum | In the newborn, cyclosporine disappeared within 2 days. No immediate complications were observed with this pregnancy. | 2016 | [64] | |
| 200 mg/day | 7 | 22.4 μg/L | The mean cyclosporine concentration in the colostrum was 22.40 ± 9.43 μg/L, with an estimated mean daily dose of 1049.22 ± 397.41 ng/kg/24 h. The average daily infant dosage was estimated to be 1.05 μg/kg. | 2020 | [65] | |
| 125 mg in the morning and 100 mg at night, totaling 3 mg/kg/day | 1 | 0.443 μg/L to 5.3 μg/L | At the three-month follow-up, both twin infants were growing and developing normally, with no adverse effects observed. | 2022 | [66] | |
| Tacrolimus | 3 mg/day | 1 | 0.57 μg//L one hour after the dose | The average amount of tacrolimus that neonates would ingest through maternal milk was 151.4 ng/kg/24 h. The peak tacrolimus concentration in colostrum was observed 8 h after an oral dose, reaching 3.219 ng/mL. The low concentrations of tacrolimus in colostrum indicate that the neonates would ingest only trace amounts of the drug. The infant was developing normally, both physically and neurologically. | 2003 | [67] |
| 2 mg twice daily | 1 | Average 1.8 μg/L, with a milk-to-blood ratio of 0.23 | The baby ingested about 0.5% of the maternal weight-adjusted dose. The authors calculated that an exclusively breastfed infant would receive a daily dose of 0.27 μg/kg, which is approximately 0.5% of the maternal weight-adjusted dose and less than 0.2% of the pediatric dose for organ transplant rejection. | 2006 | [68] | |
| Not reported | 6 | 0.3 to 1.9 μg/L, with average 1.7 μg/L | Normal prenatal growth for the gestational age and postnatal growth for the infant’s postpartum age. | 2010 | [69] | |
| 9.6 mg/day (range from 4.5 to 15 mg/day) | 4 | Not reported | Whole-blood drug concentrations between day 15 and day 27 after delivery were undetectable, with a lower limit of detection of <1.9 μg/L. | 2012 | [70] | |
| 6 mg twice daily | 1 | Not reported | Infant’s drug blood level was less than 1 μg/L. | 2012 | [71] | |
| Dose not specified, but assumed to be 6 mg/day | 14 | 0.8 μg//L average (range 0.1 to 1.6 μg//L) | All infants experienced a 15% daily decline in tacrolimus levels. The maximum estimated absorption from breast milk was 0.23% of the maternal dose (weight-adjusted). The highest dosage an exclusively breastfed infant would receive is 0.56 μg/day, which is equivalent to 0.23% of the maternal weight-adjusted dose. | 2013 | [72] | |
| Average 7.5 mg/day | 8 | Average 0.93 ng/mL | Infants were exposed to less than 0.3% of the mother’s weight-adjusted tacrolimus dose through breast milk. With such low levels of neonatal drug exposure, it is considered unlikely to pose any health risk to the breastfeeding infant. | 2013 | [73] | |
| 4 to 14 mg/day | 14 | Average 3.2 μg/L | The average amount of tacrolimus ingested by neonates through maternal milk was 151.4 ng/kg/24 h. The highest concentration was observed 8 h after an oral dose, reaching 3.219 ng/mL. The low concentrations of tacrolimus indicate that neonates are exposed to only trace amounts of the drug. | 2013 | [74] | |
| 3 mg daily | 2 | Not reported | At 1 hour after breastfeeding, the first infant’s blood drug level was 0.2 μg/L at 10 days of age, while the second infant’s level was 0.5 μg/L at 7 days of age. | 2014 | [75] | |
| 3.2 mg/day (range 2 to 5.5 mg) | 13 | The median levels were 3 μg/L at trough, 3.8 μg/L at 2 h, and 3.7 μg/L at 12 h | The relative infant dose in breastfed infants was less than 1%, and the drug levels in the infant’s blood were below detectable limits. | 2018 | [76] | |
| 1.5 mg twice daily | 1 | At 4 days postpartum, the milk level was 1.1 μg/L; at 21 days postpartum, the milk levels were 1.4 μg/L at the time of the morning dose, 1.3 μg/L 4 h after the dose, 1.6 μg/L 8 h after the dose, and 1.4 μg/L 12 h after the dose | The breast milk-to-maternal blood ratio ranged from 0.40 to 0.64. Tacrolimus was undetectable in the neonate three weeks after birth. The authors estimated that a fully breastfed infant would receive 0.4% of the mother’s weight-adjusted dose. | 2021 | [77] | |
| Not reported | 1 | 0.5 μg/L | The infant showed normal weight gain and motor development, with no indications of metabolic disorders or significant infections. The child’s exposure to the drug was extremely low, with the blood concentration approximately 90 times lower than the mother’s. | 2024 | [78] | |
| Everolimus | 2 mg daily during pregnancy | 1 | Undetectable levels (<0.5 μg/L) in colostrum 1 day postpartum | Estimated elimination half-life of everolimus was estimated at 86 h in the newborn. | 2016 | [64] |
| 0.5 mg/day | 1 | Highest level was 66 ng/L | The estimated infant dose of the drug was 4.224 ng/kg/24 h, which accounted for 0.38% of the mother’s dose. | 2017 | [79] | |
| Azathioprine | 75 mg/day | 1 | Peak colostrum levels (2 days postpartum) 2 and 8 h after oral dose, being 3.4 and 4.5 μg/L, respectively | The milk levels in these two mothers were equivalent to 0.05% and 0.6% of the maternal weight-adjusted doses, respectively. Infant serum levels were not assessed. | 1982 | [80] |
| 25 mg oral dose | 1 | Peak 6-MP milk level of 18 μg/L occurred 2 h after oral dose (7 days postpartum) | ||||
| Not reported | 2 | Not reported | The infants exhibited normal blood cell counts, no increase in infections, and an above-average growth rate. | 2008 | [81] | |
| 100 mg/day | 1 | 6-MP was not detected five weeks after birth | With a detection limit of 5 μg/L, the infant would have ingested a maximum of 0.09% of the mother’s weight-adjusted dose. The child remained healthy and breastfed for 12 months. | 1995 | [55] | |
| 1.2 to 2.1 mg/kg/day | 4 | Not reported | At 3 to 3.5 months of age, none of the infants had detectable blood levels of 6-TGNs and 6-MP. | 2006 | [82] | |
| 100 mg/day | 2 | In 5 and 6 milk samples of each subject collected over a 24 h period, 6-MP was undetectable (<5 μg/L) | The absolute relative infant dose would have been under 0.09% of the maternal weight-adjusted dose, and no adverse effects were observed in the infants. | 2006 | [83] | |
| 75 mg/day | 1 | Not reported | At the 1-month follow-up, the growth and development of the breastfeeding infant were reported as normal. | |||
| 50 mg/day | 1 | Not reported | No adverse effects were observed in this child during the 2-month follow-up. | |||
| 75 to 150 mg/day | 10 | Only one woman on 100 mg/day of azathioprine had detectable 6-MP in her milk; on day 28 postpartum, milk concentrations were 1.2 μg/L at 3 h and 7.6 μg/L at 6 h after the dose; no 6-MP was found in any of the other 29 milk samples | 6-MP and 6-TGN were undetectable in the neonatal blood. None of the ten neonates showed clinical or hematological signs of immunosuppression during the first 28 days postpartum. One infant had a slightly low neutrophil count, but the overall white cell count remained normal. | 2007 | [84] | |
| 75–200 mg/day | 8 | Peak 6-MP concentrations in milk were observed within the first 4 h after the dose, ranging from 2 to 50 μg/L | The estimated infant intake was less than 0.008 mg/kg body weight per 24 h, representing less than 1% of the maternal weight-adjusted dose. | 2008 | [85] | |
| 100 mg (1.4 mg/kg) daily | 1 | Not reported | At both 8-days and 3-months postpartum, 6-TGNs were undetectable in the infant’s blood. Over the 6-month follow-up period, the child thrived and experienced no infections. | 2009 | [86] | |
| Median dose 150 mg/day (range 100 to 250 mg/day) | 11 | Not reported | There were no differences in mental or physical development between the two groups of infants, nor was there any variation in the incidence of other infections between the groups. | 2011 | [87] | |
| 1.93 mg/Kg (AZA), 0.94–1.32 mg/Kg (6-MP) | Mothers taking either azathioprine (n = 28) or 6-MP (n = 2) | Not reported | In this cohort, nine infants were breastfed for an average of 7 months (ranging from 3 to 13 months). No statistically significant differences were observed between breastfed and formula-fed infants across any of the 12 survey domains. | 2013 | [88] | |
| Prednisone | Single 10 mg oral dose | 1 | 28.3 μg/L | N.A. | 1975 | [89] |
| Single 20 mg oral dose | 1 | 102 μg/L | N.A. | 1981 | [90] | |
| 10 to 80 mg/day | 6 | Milk concentrations were 5% to 25% of those in serum. | At a daily dose of 80 mg of prednisolone, the infant would consume less than 0.1% of that dose, which is equivalent to less than 10% of the infant’s natural cortisol production. | 1985 | [91] | |
| Single 50 mg (intravenous dose) | 3 | Only 0.025% of the prednisolone dose (ranging from 0.010% to 0.049%) was found in the milk | N.A. | 1993 | [92] | |
| 2 mg every 12 h | 1 | Prednisone levels in milk were undetectable (<4 μg/L) after 12 h, while prednisolone levels were undetectable after 6 h | The weight-adjusted infant dosages were 0.58% and 0.35% of the maternal prednisone dose and 0.18% and 0.09% of the maternal prednisolone dose. | 2019 | [93] | |
| 15 mg every 24 h | 1 | |||||
| Belatacept | 10 mg/kg monthly | 1 | Not reported | Normal growth and cognitive development. | 2020 | [94] |
| Not reported | 5 | Not reported | No reports of problems breastfeeding or issues in the children. | 2023 | [95] |
4.3. mTOR Inhibitors
4.3.1. Sirolimus
4.3.2. Everolimus
4.4. Corticosteroids
4.5. Belatacept
5. Additional Considerations
6. Discussion
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ESRD | End-stage renal disease |
| NTPR | National Transplant Registry |
| AMR | Antibody-mediated rejection |
| TCMR | T-cell mediated rejection |
| M/P | Milk-to-plasma concentration ratio of drugs |
| RID | Relative infant dose |
| CNIs | Calcineurin inhibitors |
| 6-MP | 6-mercaptopurine |
| 6-TG | 6-thioguanine |
| mTOR | mammalian target Of rapamycin |
References
- Armenti, V.T.; Daller, J.A.; Constantinescu, S.; Silva, P.; Radomski, J.S.; Moritz, M.J.; Gaughan, W.J.; McGrory, C.H.; Coscia, L.A. CT06 Chapter 4: Report from the National Transplantation Pregnancy Registry: Outcomes of Pregnancy After Transplantation. Clin. Transpl. 2006, 4, 57–70. [Google Scholar]
- Sgro, M.D.; Barozzino, T.; Mirghani, H.M.; Sermer, M.; Moscato, L.; Akoury, H.; Koren, G.; Chitayat, D.A. Pregnancy outcome post renal transplantation. Teratology 2002, 65, 5–9. [Google Scholar] [CrossRef]
- Colon, M.d.M.; Hibbard, J.U. Obstetric considerations in the management of pregnancy in kidney transplant recipients. Adv. Chronic Kidney Dis. 2007, 14, 168–177. [Google Scholar] [CrossRef]
- Sibanda, N.; Briggs, J.D.; Davison, J.M.; Johnson, R.J.; Rudge, C.J. Pregnancy after organ transplantation: A report from the UK transplant pregnancy registry. Transplantation 2007, 83, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.B.; Josephson, M.A. Pregnancy after Kidney Transplantation. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S2), S117–S125. [Google Scholar] [CrossRef] [PubMed]
- Caretto, A.; Caldara, R.; Castiglioni, M.T.; Scavini, M.; Secchi, A. Pregnancy after pancreas-kidney transplantation. J. Nephrol. 2020, 33, 1009–1018. [Google Scholar] [CrossRef]
- WHO. Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services. 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/259386/9789241550086-eng.pdf (accessed on 15 February 2025).
- Sankar, M.J.; Sinha, B.; Chowdhury, R.; Bhandari, N.; Taneja, S.; Martines, J.; Bahl, R. Optimal breastfeeding practices and infant and child mortality: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 3–13. [Google Scholar] [CrossRef]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef]
- Weng, S.F.; A Redsell, S.; A Swift, J.; Yang, M.; Glazebrook, C.P. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch. Dis. Child. 2012, 97, 1019–1026. [Google Scholar] [CrossRef]
- Ip, S.; Chung, M.; Raman, G.; Trikalinos, T.A.; Lau, J. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed. Med. 2009, 4 (Suppl. S1), S17. [Google Scholar] [CrossRef]
- Hylander, M.A.; Strobino, D.M.; Dhanireddy, R. Human Milk Feedings and Infection Among Very Low Birth Weight Infants. Pediatrics 1998, 102, e38. [Google Scholar] [CrossRef] [PubMed]
- Schanler, R.J. The use of human milk for premature infants. Pediatr. Clin. N. Am. 2001, 48, 207–219. [Google Scholar] [CrossRef]
- Okamoto, T.; Shirai, M.; Kokubo, M.; Takahashi, S.; Kajino, M.; Takase, M.; Sakata, H.; Oki, J. Human milk reduces the risk of retinal detachment in extremely low-birthweight infants. Pediatr. Int. 2007, 49, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Vohr, B.R.; Poindexter, B.B.; Dusick, A.M.; McKinley, L.T.; Wright, L.L.; Langer, J.C.; Poole, W.K.; NICHD Neonatal Research Network. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics 2006, 118, e115–e123. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Jacobs, J.; Hall, R.; Adamkin, D.; Auestad, N.; Castillo, M.; Connor, W.E.; Connor, S.L.; Fitzgerald, K.; Groh-Wargo, S.; et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J. Pediatr. Gastroenterol. Nutr. 2003, 37, 437–446. [Google Scholar] [CrossRef]
- El-Khuffash, A.; Lewandowski, A.J.; Jain, A.; Hamvas, A.; Singh, G.K.; Levy, P.T. Cardiac Performance in the First Year of Age Among Preterm Infants Fed Maternal Breast Milk. JAMA Netw. Open 2021, 4, e2121206. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21, 21–137. [Google Scholar] [CrossRef]
- Halloran, P.F. T Cell-mediated rejection of kidney transplants: A personal viewpoint. Am. J. Transplant. 2010, 10, 1126–1134. [Google Scholar] [CrossRef]
- Loupy, A.; Lefaucheur, C. Antibody-Mediated Rejection of Solid-Organ Allografts. N. Engl. J. Med. 2018, 379, 1150–1160. [Google Scholar] [CrossRef]
- Balani, S.S.; Jensen, C.J.; Kouri, A.M.; Kizilbash, S.J. Induction and maintenance immunosuppression in pediatric kidney transplantation—Advances and controversies. Pediatr. Transplant. 2021, 25, e14077. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Russ, G.R.; Chadban, S.J.; Campbell, S.B.; McDonald, S.P. Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation 2007, 84, 611–618. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.-U.; Schold, J.D.; Kaplan, B. Long-term renal allograft survival: Have we made significant progress or is it time to rethink our analytic and therapeutic strategies? Am. J. Transplant. 2004, 4, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F. Immunosuppressive Drugs for Kidney Transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Szumilas, K.; Wilk, A.; Wiśniewski, P.; Gimpel, A.; Dziedziejko, V.; Kipp, M.; Pawlik, A. Current Status Regarding Immunosuppressive Treatment in Patients after Renal Transplantation. Int. J. Mol. Sci. 2023, 24, 10301. [Google Scholar] [CrossRef]
- Broen, J.C.A.; Van Laar, J.M. Mycophenolate mofetil, azathioprine and tacrolimus: Mechanisms in rheumatology. Nat. Rev. Rheumatol. 2020, 16, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory Action of Glucocorticoids—New Mechanisms for Old Drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef]
- McKay, D.B.; Josephson, M.A. Reproduction and Transplantation: Report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am. J. Transplant. 2005, 5, 1592–1599. [Google Scholar] [CrossRef]
- Klein, C.L.; Josephson, M.A. Post-Transplant Pregnancy and Contraception. Clin. J. Am. Soc. Nephrol. 2022, 17, 114–120. [Google Scholar] [CrossRef]
- Ponticelli, C.; Zaina, B.; Moroni, G. Planned Pregnancy in Kidney Transplantation. A Calculated Risk. J. Pers. Med. 2021, 11, 956. [Google Scholar] [CrossRef]
- Shah, S.; Venkatesan, R.L.; Gupta, A.; Sanghavi, M.K.; Welge, J.; Johansen, R.; Kean, E.B.; Kaur, T.; Gupta, A.; Grant, T.J.; et al. Pregnancy outcomes in women with kidney transplant: Metaanalysis and systematic review. BMC Nephrol. 2019, 20, 24. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Verma, P. Overview of Pregnancy in Renal Transplant Patients. Int. J. Nephrol. 2016, 2016, 4539342. [Google Scholar] [CrossRef]
- Newton, E.R.; Hale, T.W. Drugs in breast milk. Clin. Obstet. Gynecol. 2015, 58, 868–884. [Google Scholar] [CrossRef]
- Drugs and Lactation Database (LactMed). Available online: https://www.ncbi.nlm.nih.gov/books/NBK547442/ (accessed on 3 March 2025).
- Ziegenhagen, D.J.; Crombach, G.; Dieckmann, M.; Zehnter, E.; Wienand, P.; Baldamus, C.A. Pregnancy during cyclosporin medication following a kidney transplant. Dtsch Med. Wochenschr. 1988, 113, 260–263. [Google Scholar] [CrossRef]
- Xu, L.-G.; Han, S.; Liu, Y.; Wang, H.-W.; Yang, Y.-R.; Qiu, F.; Peng, W.-L.; Tang, L.-G. Timing, conditions, and complications of post-operative conception and pregnancy in female renal transplant recipients. Cell Biochem. Biophys. 2011, 61, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Di Loreto, P.; Martino, F.; Chiaramonte, S.; Dissegna, D.; Ronco, C.; Marchesoni, D.; Catapano, P.; Romano, G.; Montanaro, D. Pregnancy after kidney transplantation: Two transplantation centers—Vicenza–udine experience. Transplant. Proc. 2010, 42, 1158–1161. [Google Scholar] [CrossRef]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Shah, S.; Ginat, D.T. Calcineurin Inhibitors. In Neuroimaging Pharmacopoeia, 2nd ed.; Springer: Cham, Switzerland, 2023; pp. 173–183. [Google Scholar] [CrossRef]
- Gow, P.J.; Ghabrial, H.; Smallwood, R.A.; Morgan, D.J.; Ching, M.S. Neonatal hepatic drug elimination. Pharmacol. Toxicol. 2001, 88, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cyclosporine. Drugs and Lactation Database (LactMed®). September 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK501683/ (accessed on 14 February 2025).
- van der Woude, C.; Ardizzone, S.; Bengtson, M.; Fiorino, G.; Fraser, G.; Katsanos, K.; Kolacek, S.; Juillerat, P.; Mulders, A.; Pedersen, N.; et al. The Second European Evidenced-Based Consensus on Reproduction and Pregnancy in Inflammatory Bowel Disease. J. Crohn’s Colitis 2015, 9, 107–124. [Google Scholar] [CrossRef]
- Thiagarajan, K.-F.; Arakali, S.R.; Mealey, K.J.; Cardonick, E.H.; Gaughan, W.J.; Davison, J.M.; Moritz, M.J.; Armenti, V.T. Safety considerations: Breastfeeding after transplant. Prog. Transplant. 2013, 23, 137–146. [Google Scholar] [CrossRef]
- Türkmen, M.A.; Kavukçu, S.; Sarıoǧlu, S.; Soylu, A.; Akhunlar, H.; Yılmaz, O.; Güven, H. Effects of lactational cyclosporine A use on rat pups. Pediatr. Transplant. 2006, 10, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Cheok, M.; Krynetskaia, N.; Thorn, C.; Stocco, G.; Hebert, J.M.; McLeod, H.; Weinshilboum, R.M.; Relling, M.V.; Evans, W.E.; et al. Thiopurine pathway. Pharmacogenet. Genom. 2010, 20, 573–574. [Google Scholar] [CrossRef]
- Van Os, E.C.; Zins, B.J.; Sandborn, W.J.; Mays, D.C.; Tremaine, W.J.; Mahoney, D.W.; Zinsmeister, A.R.; Lipsky, J.J. Azathioprine pharmacokinetics after intravenous, oral, delayed release oral and rectal foam administration. Gut 1996, 39, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Jhaj, R. A case report on azathioprine-induced euprolactinemic galactorrhea. Indian J. Pharmacol. 2021, 53, 234–235. [Google Scholar] [CrossRef]
- Uygur-Bayramiçli, O.; Aydin, D.; Ak, Ö.; Karadayi, N. Hyperprolactinemia caused by azathioprine. J. Clin. Gastroenterol. 2003, 36, 79–80. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Maxwell, C.; Hendel, J. IBD medications during pregnancy and lactation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 116–127. [Google Scholar] [CrossRef]
- Constantinescu, S.; Pai, A.; Coscia, L.A.; Davison, J.M.; Moritz, M.J.; Armenti, V.T. Breast-feeding after transplantation. Best Pr. Res. Clin. Obstet. Gynaecol. 2014, 28, 1163–1173. [Google Scholar] [CrossRef]
- Flint, J.; Panchal, S.; Hurrell, A.; van de Venne, M.; Gayed, M.; Schreiber, K.; Arthanari, S.; Cunningham, J.; Flanders, L.; Moore, L.; et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding—Part I: Standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology 2016, 55, 1693–1697. [Google Scholar] [CrossRef]
- Lewis, G.J.; A Lamont, C.; A Lee, H.; Slapak, M. Successful pregnancy in a renal transplant recipient taking cyclosporin A. BMJ 1983, 286, 603. [Google Scholar] [CrossRef]
- Flechner, S.M.; Katz, A.R.; Rogers, A.; Van Buren, C.; Kahan, B.D. The presence of cyclosporine in body tissues and fluids during pregnancy. Am. J. Kidney Dis. 1985, 5, 60–63. [Google Scholar] [CrossRef]
- Moretti, M.; Ito, S.; Koren, G. Therapeutic drug monitoring in the lactating patient. Reprod. Toxicol. 1995, 9, 580–581. [Google Scholar] [CrossRef]
- Thiru, Y.; Bateman, D.N.; Coulthard, M.G. Drug points: Successful breast feeding while mother was taking cyclosporin. BMJ 1997, 315, 463. [Google Scholar] [CrossRef]
- Nyberg, G.; Haljamäe, U.; Frisenette-Fich, C.; Wennergren, M.; Kjellmer, I. Breast-feeding during treatment with cyclosporine. Transplantation 1998, 65, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, K.D.; Easterling, T.; Davis, C.; Bond, E.F. Breast-feeding by a cyclosporine-treated mother. Obstet. Gynecol. 2001, 97, 816–818. [Google Scholar] [CrossRef]
- Moretti, M.E.; Sgro, M.; Johnson, D.W.; Sauve, R.S.; Woolgar, M.J.; Taddio, A.; Verjee, Z.; Giesbrecht, E.; Koren, G.; Ito, S. Cyclosporine excretion into breast milk. Transplantation 2003, 75, 2144–2146. [Google Scholar] [CrossRef] [PubMed]
- Osadchy, A.; Koren, G. Cyclosporine and lactation: When the mother is willing to breastfeed. Ther. Drug Monit. 2011, 33, 147–148. [Google Scholar] [CrossRef]
- Adam, M. Cyclosporine and lactation. Nephrology 2011, 16, 249. [Google Scholar] [CrossRef]
- Lahiff, C.; Moss, A.C. Cyclosporine in the management of severe ulcerative colitis while breast-feeding. Inflamm. Bowel Dis. 2011, 17, E78. [Google Scholar] [CrossRef]
- Mazzuoccolo, L.D.; Andrada, R.; Pellerano, G.; Neglia, V.; Abeldaño, A. Levels of cyclosporine in breast milk and passage into the circulation of the infant of a mother with psoriasis. Int. J. Dermatol. 2014, 53, 355–356. [Google Scholar] [CrossRef]
- Fiocchi, R.; D’elia, E.; Vittori, C.; Sebastiani, R.; Strobelt, N.; Eleftheriou, G.; Introna, M.; Freddi, C.; Crippa, A. First Report of a Successful Pregnancy in an Everolimus-Treated Heart-Transplanted Patient: Neonatal Disappearance of Immunosuppressive Drugs. Am. J. Transplant. 2016, 16, 1319–1322. [Google Scholar] [CrossRef]
- Kociszewska-Najman, B.; Mazanowska, N.; Borek-Dzięcioł, B.; Pączek, L.; Samborowska, E.; Szpotańska-Sikorska, M.; Pietrzak, B.; Dadlez, M.; Wielgoś, M. Low Content of Cyclosporine A and Its Metabolites in the Colostrum of Post-Transplant Mothers. Nutrients 2020, 12, 2713. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, C.; Wang, H.; An, Y. Breastfeeding by a mother taking cyclosporine for nephrotic syndrome. Int. Breastfeed. J. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- E French, A.; Soldin, S.J.; Soldin, O.P.; Koren, G. Milk Transfer and Neonatal Safety of Tacrolimus. Ann. Pharmacother. 2003, 37, 815–818. [Google Scholar] [CrossRef]
- Gardiner, S.J.; Begg, E.J. Breastfeeding during tacrolimus therapy. Obstet. Gynecol. 2006, 107 Pt 2, 453–455. [Google Scholar] [CrossRef]
- Jain, A.; Venkataramanan, R.; Fung, J.J.; Gartner, J.C.; Lever, J.; Balan, V.; Warty, V.; Starzl, T.E. Pregnancy after liver transplantation under tacrolimus. Transplantation 1997, 64, 559–565. [Google Scholar] [CrossRef]
- Gouraud, A.; Bernard, N.; Millaret, A.; Bruel, M.; Paret, N.; Descotes, J.; Vial, T. Follow-up of tacrolimus breastfed babies. Transplantation 2012, 94, e38–e40. [Google Scholar] [CrossRef]
- Gomez-Lobo, V.; Landy, H.J.; Matsumoto, C.; Fishbein, T.M. Pregnancy in an intestinal transplant recipient. Obstet. Gynecol. 2012, 120, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Bramham, K.; Chusney, G.; Lee, J.; Lightstone, L.; Nelson-Piercy, C. Breastfeeding and tacrolimus: Serial monitoring in breast-fed and bottle-fed infants. Clin. J. Am. Soc. Nephrol. 2013, 8, 563–567. [Google Scholar] [CrossRef]
- Zheng, S.; Easterling, T.R.; Hays, K.; Umans, J.G.; Miodovnik, M.; Clark, S.; Calamia, J.C.; Thummel, K.E.; Shen, D.D.; Davis, C.L.; et al. Tacrolimus placental transfer at delivery and neonatal exposure through breast milk. Br. J. Clin. Pharmacol. 2013, 76, 988–996. [Google Scholar] [CrossRef]
- Kociszewska-Najman, B.; Mazanowska, N.; Pietrzak, B.; Paczek, L.; Szpotanska-Sikorska, M.; Schreiber-Zamora, J.; Hryniewiecka, E.; Zochowska, D.; Samborowska, E.; Dadlez, M.; et al. Low Transfer of Tacrolimus and Its Metabolites into Colostrum of Graft Recipient Mothers. Nutrients 2018, 10, 267. [Google Scholar] [CrossRef]
- Izumi, Y.; Miyashita, T.; Migita, K. Safety of Tacrolimus Treatment during Pregnancy and Lactation in Systemic Lupus Erythematosus: A Report of Two Patients. Tohoku J. Exp. Med. 2014, 234, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, Y.; Yoshida, S.; Kotani, T.; Nakamura, E.; Kimura, Y.; Fujita, D.; Nagayasu, Y.; Shabana, K.; Makino, S.; Takeuchi, T.; et al. Changes in the blood level, efficacy, and safety of tacrolimus in pregnancy and the lactation period in patients with systemic lupus erythematosus. Lupus 2018, 27, 2245–2252. [Google Scholar] [CrossRef]
- Akamine, Y.; Fujiyama, N.; Kagaya, H.; Saito, M.; Miura, H.; Terada, Y.; Takahashi, T.; Satoh, S.; Miura, M. Tacrolimus concentrations after renal transplantation in a mother-neonate dyad: Maternal, neonatal and breast milk measurements. J. Clin. Pharm. Ther. 2021, 46, 1800–1803. [Google Scholar] [CrossRef]
- Kuczaj, A.; Danel, A.; Warwas, S.; Przybyłowski, P.; Śliwka, J.; Pawlak, S.; Trzcińska, I.; Hrapkowicz, T. Toxic Milk- Should We Still be Afraid of Breastfeeding While on Tacrolimus Therapy: A Case Study. J. Heart Lung Transplant. 2024, 43, S631. [Google Scholar] [CrossRef]
- Kociszewska-Najman, B.; Szpotańska-Sikorska, M.; Mazanowska, N.; Pączek, L.; Samborowska, E.; Dadlez, M.; Wielgoś, M.; Pietrzak, B. Transfer of Everolimus into Colostrum of a Kidney Transplant Mother. Ann. Transplant. 2017, 22, 755–758. [Google Scholar] [CrossRef]
- Coulam, C.B.; Moyer, T.P.; Jiang, N.S.; Zincke, H. Breast-feeding after renal transplantation. Transpl. Proc. 1982, 14, 605–609. [Google Scholar]
- Grekas, D.M.; Vasiliou, S.S.; Lazarides, A.N. Immunosuppressive therapy and breast-feeding after renal transplantation. Nephron 1984, 37, 68. [Google Scholar] [CrossRef]
- Gardiner, S.J.; Gearry, R.B.; Roberts, R.L.; Zhang, M.; Barclay, M.L.; Begg, E.J. Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br. J. Clin. Pharmacol. 2006, 62, 453–456. [Google Scholar] [CrossRef] [PubMed]
- E Moretti, M.; Verjee, Z.; Ito, S.; Koren, G. Breast-feeding during maternal use of azathioprine. Ann. Pharmacother. 2006, 40, 2269–2272. [Google Scholar] [CrossRef]
- Sau, A.; Clarke, S.; Bass, J.; Kaiser, A.; Marinaki, A.; Nelson-Piercy, C. Azathioprine and breastfeeding—Is it safe? BJOG 2007, 114, 498–501. [Google Scholar] [CrossRef]
- Christensen, L.A.; Dahlerup, J.F.; Nielsen, M.J.; Fallingborg, J.F.; Schmiegelow, K. Azathioprine treatment during lactation. Aliment. Pharmacol. Ther. 2008, 28, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Zelinkova, Z.; De Boer, I.P.; Van Dijke, M.J.; Kuipers, E.J.; Van Der Woude, C.J. Azathioprine treatment during lactation. Aliment. Pharmacol. Ther. 2009, 30, 90–91. [Google Scholar] [CrossRef]
- Angelberger, S.; Reinisch, W.; Messerschmidt, A.; Miehsler, W.; Novacek, G.; Vogelsang, H.; Dejaco, C. Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J. Crohn’s Colitis 2011, 5, 95–100. [Google Scholar] [CrossRef]
- de Meij, T.G.J.; Jharap, B.; Kneepkens, C.M.F.; van Bodegraven, A.A.; de Boer, N.K.H.; Dutch Initiative on Crohn and Colitis. Long-term follow-up of children exposed intrauterine to maternal thiopurine therapy during pregnancy in females with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Katz, F.H.; Duncan, B.R. Entry of Prednisone into Human Milk. N. Engl. J. Med. 1975, 293, 1154. [Google Scholar] [CrossRef] [PubMed]
- Sagraves, R.; Kaiser, D.; Sharpe, G.L. Prednisone and prednisolone concentrations in the milk of a lactating mother. Drug Intell Clin. Pharm. 1981, 15, 484. Available online: https://archive.org/details/sim_annals-of-pharmacotherapy_1981_15_index/mode/2up (accessed on 20 February 2025).
- Öst, L.; Wettrell, G.; Björkhem, I.; Rane, A. Prednisolone excretion in human milk. J. Pediatr. 1985, 106, 1008–1011. [Google Scholar] [CrossRef]
- A Greenberger, P.; Odeh, Y.K.; Frederiksen, M.C.; Atkinson, A.J. Pharmacokinetics of prednisolone transfer to breast milk. Clin. Pharmacol. Ther. 1993, 53, 324–328. [Google Scholar] [CrossRef]
- Ryu, R.J.; Easterling, T.R.; Caritis, S.N.; Venkataramanan, R.; Umans, J.G.; Ahmed, M.S.; Clark, S.; Kantrowitz-Gordon, I.; Hays, K.; Bs, B.B.; et al. Prednisone Pharmacokinetics During Pregnancy and Lactation. J. Clin. Pharmacol. 2018, 58, 1223–1232. [Google Scholar] [CrossRef]
- Klintmalm, G.B.; Gunby, R.T. Successful Pregnancy in a Liver Transplant Recipient on Belatacept. Liver Transplant. 2020, 26, 1193–1194. [Google Scholar] [CrossRef]
- Coscia, L.R.; Cohen, D.; Dube, G.K.; Hofmann, R.M.; Moritz, M.J.; Gattis, S.P.D.; Basu, A.M. Outcomes With Belatacept Exposure During Pregnancy in Kidney Transplant Recipients: A Case Series. Transplantation 2023, 107, 2047–2054. [Google Scholar] [CrossRef]
- Cuadrado-Payán, E.; Diekmann, F.; Cucchiari, D. Medical Aspects of mTOR Inhibition in Kidney Transplantation. Int. J. Mol. Sci. 2022, 23, 7707. [Google Scholar] [CrossRef]
- McKinzie, C.J.; Casale, J.P.; Guerci, J.C.; Prom, A.; Doligalski, C.T. Outcomes of Children with Fetal and Lactation Immunosuppression Exposure Born to Female Transplant Recipients. Pediatr. Drugs 2022, 24, 483–497. [Google Scholar] [CrossRef]
- Prednisone. Drugs and Lactation Database (LactMed®). April 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK501077/ (accessed on 15 February 2025).
- Hossen, M.; Ma, Y.; Yin, Z.; Xia, Y.; Du, J.; Huang, J.Y.; Huang, J.J.; Zou, L.; Ye, Z.; Huang, Z. Current understanding of CTLA-4: From mechanism to autoimmune diseases. Front. Immunol. 2023, 14, 1198365. [Google Scholar] [CrossRef]
- Archdeacon, P.; Dixon, C.; Belen, O.; Albrecht, R.; Meyer, J. Summary of the US FDA approval of belatacept. Am. J. Transplant. 2012, 12, 554–562. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nulojix (accessed on 2 March 2025).
- El Hennawy, H.M.; Safar, O.; Al Faifi, A.S.; El Nazer, W.; Kamal, A.; Mahedy, A.; Zaitoun, M.; Fahmy, A.E.; Wageh, A. Belatacept rescue therapy of CNI-induced nephrotoxicity, meta-analysis. Transplant. Rev. 2021, 35, 100653. [Google Scholar] [CrossRef]
- Cockfield, S.M.; Wilson, S.; Campbell, P.M.; Cantarovich, M.; Gangji, A.; Houde, I.; Jevnikar, A.M.; Keough-Ryan, T.M.; Monroy-Cuadros, F.-M.; Nickerson, P.W.; et al. Comparison of the effects of standard vs low-dose prolonged-release tacrolimus with or without ACEi/ARB on the histology and function of renal allografts. Am. J. Transplant. 2019, 19, 1730–1744. [Google Scholar] [CrossRef]
- Rodriguez-Ramirez, S.; Al Jurdi, A.; Konvalinka, A.; Riella, L.V. Antibody-mediated rejection: Prevention, monitoring and treatment dilemmas. Curr. Opin. Organ Transplant. 2022, 27, 405–414. [Google Scholar] [CrossRef]
- Wind, M.; Gaasbeek, A.; Oosten, L.; Rabelink, T.; van Lith, J.; Sueters, M.; Teng, Y. Therapeutic plasma exchange in pregnancy: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 29–36. [Google Scholar] [CrossRef]
- Agarwal, K.A.; Pavlakis, M. Sexuality, Contraception, and Pregnancy in Kidney Transplantation. Kidney Med. 2021, 3, 837–847. [Google Scholar] [CrossRef]
| Group | Drug | Blocking Mechanisms | |
|---|---|---|---|
| Immunophilin-binding treatments | Calcineurin inhibitors (CNIs) | Ciclosporin | Binds to cyclophilin, forming a complex that inhibits calcineurin, resulting in reduced cytokine production and diminished T-cell proliferation |
| Tacrolimus | Binds to FK506-binding protein 12, forming a complex that inhibits calcineurin, thereby reducing cytokine production and T-cell proliferation | ||
| mTOR inhibitors | Everolimus | Bind to FK506-binding protein 12, which, in turn, hinders mTOR, leading to a reduction in cytokine-induced T-cell proliferation | |
| Sirolimus | |||
| Co-stimulation blockers | Cytotoxic T-lymphocyte-associated protein 4 (CTLA4)-Ig | Belatacept | Blocks co-stimulation of T-cell activity by CD28 |
| Antimetabolites | Thiopurine | Azathioprine | Blocks purine production, leading to a decrease in T-cell growth |
| Corticoids | Glucocorticoids | Prednisone | Decrease the levels of circulating lymphocytes, monocytes, and eosinophils and suppress the production of cytokines |
| Prednisolone | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Casado, G.; Alonso-Titos, J.; Gonzalez-Mesa, E.; Ortega-Gomez, A. Compatibility of Post-Kidney Transplant Immunosuppression Therapy with Lactation. J. Clin. Med. 2025, 14, 2364. https://doi.org/10.3390/jcm14072364
Gomez-Casado G, Alonso-Titos J, Gonzalez-Mesa E, Ortega-Gomez A. Compatibility of Post-Kidney Transplant Immunosuppression Therapy with Lactation. Journal of Clinical Medicine. 2025; 14(7):2364. https://doi.org/10.3390/jcm14072364
Chicago/Turabian StyleGomez-Casado, Gema, Juana Alonso-Titos, Ernesto Gonzalez-Mesa, and Almudena Ortega-Gomez. 2025. "Compatibility of Post-Kidney Transplant Immunosuppression Therapy with Lactation" Journal of Clinical Medicine 14, no. 7: 2364. https://doi.org/10.3390/jcm14072364
APA StyleGomez-Casado, G., Alonso-Titos, J., Gonzalez-Mesa, E., & Ortega-Gomez, A. (2025). Compatibility of Post-Kidney Transplant Immunosuppression Therapy with Lactation. Journal of Clinical Medicine, 14(7), 2364. https://doi.org/10.3390/jcm14072364







