Evaluation of PTSD-Induced Alterations in Bone Biomechanics and the Protective Potential of CE-123 in a Wistar Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fear Conditioning
2.3. Treatment
2.4. Growth Plate Morphology
2.5. Bone Analysis
2.6. Statistical Analysis
3. Results
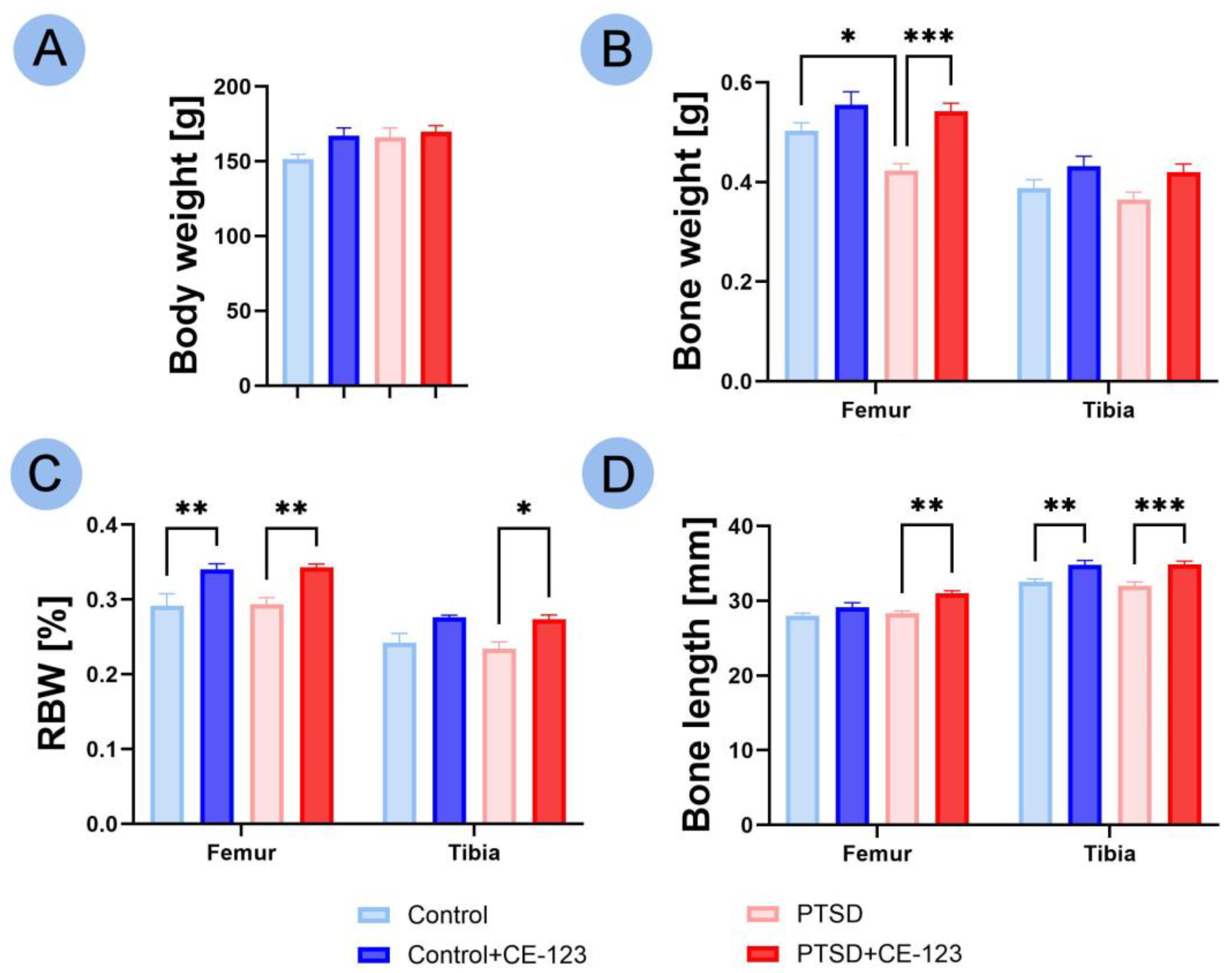
3.1. Animal and Bone Characteristics
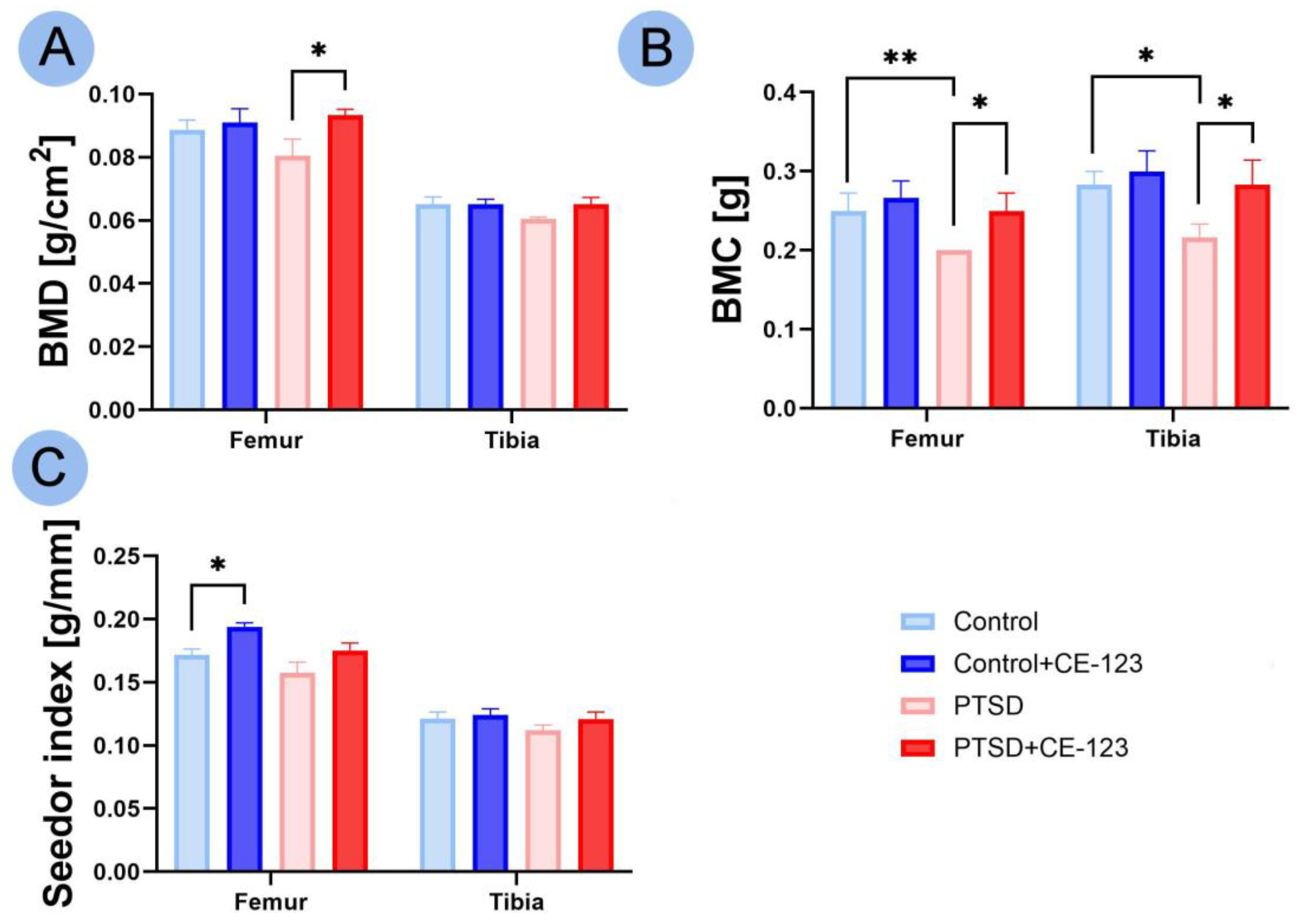
3.2. Bone Properties
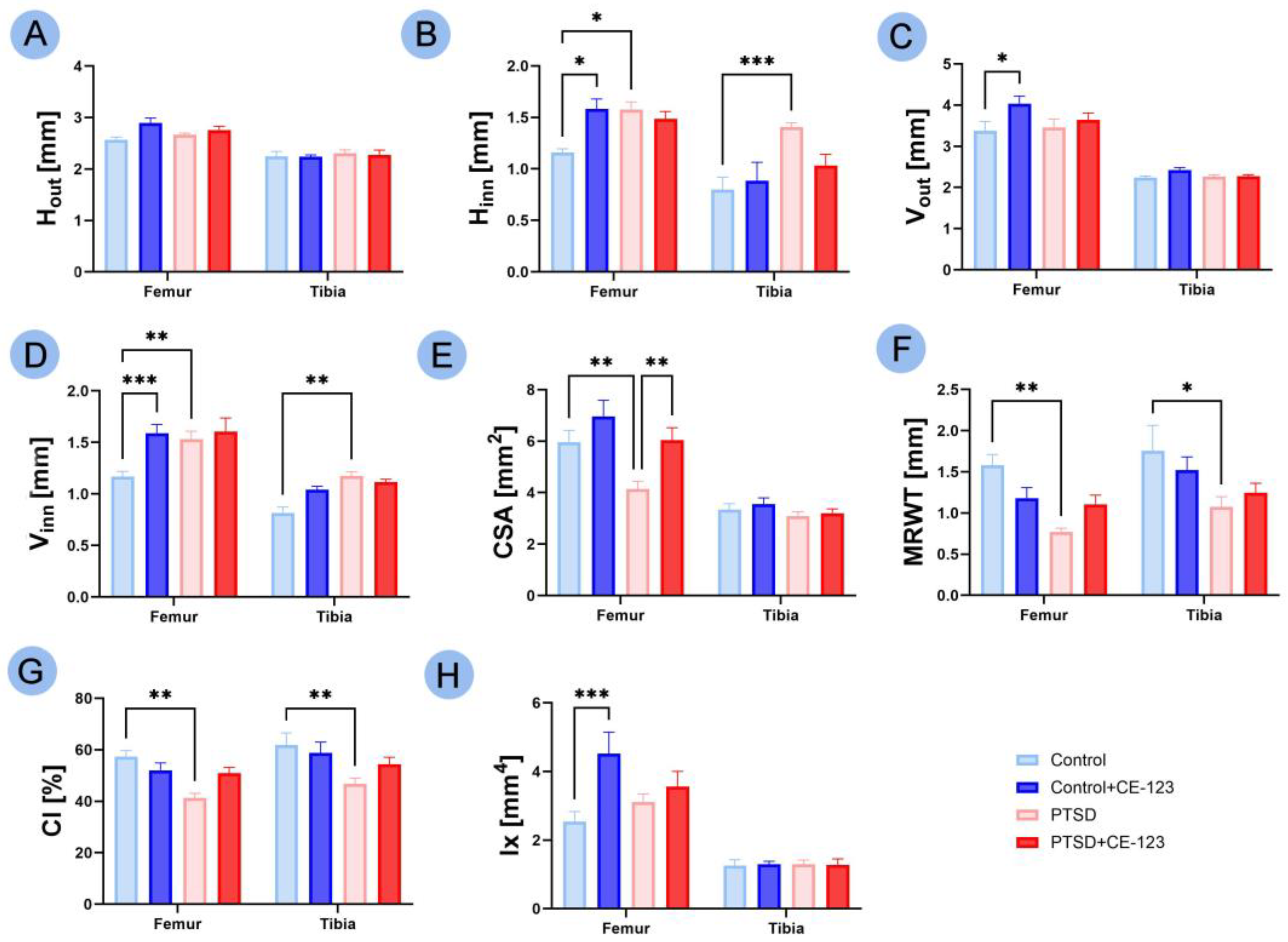
3.3. Geometrical Properties
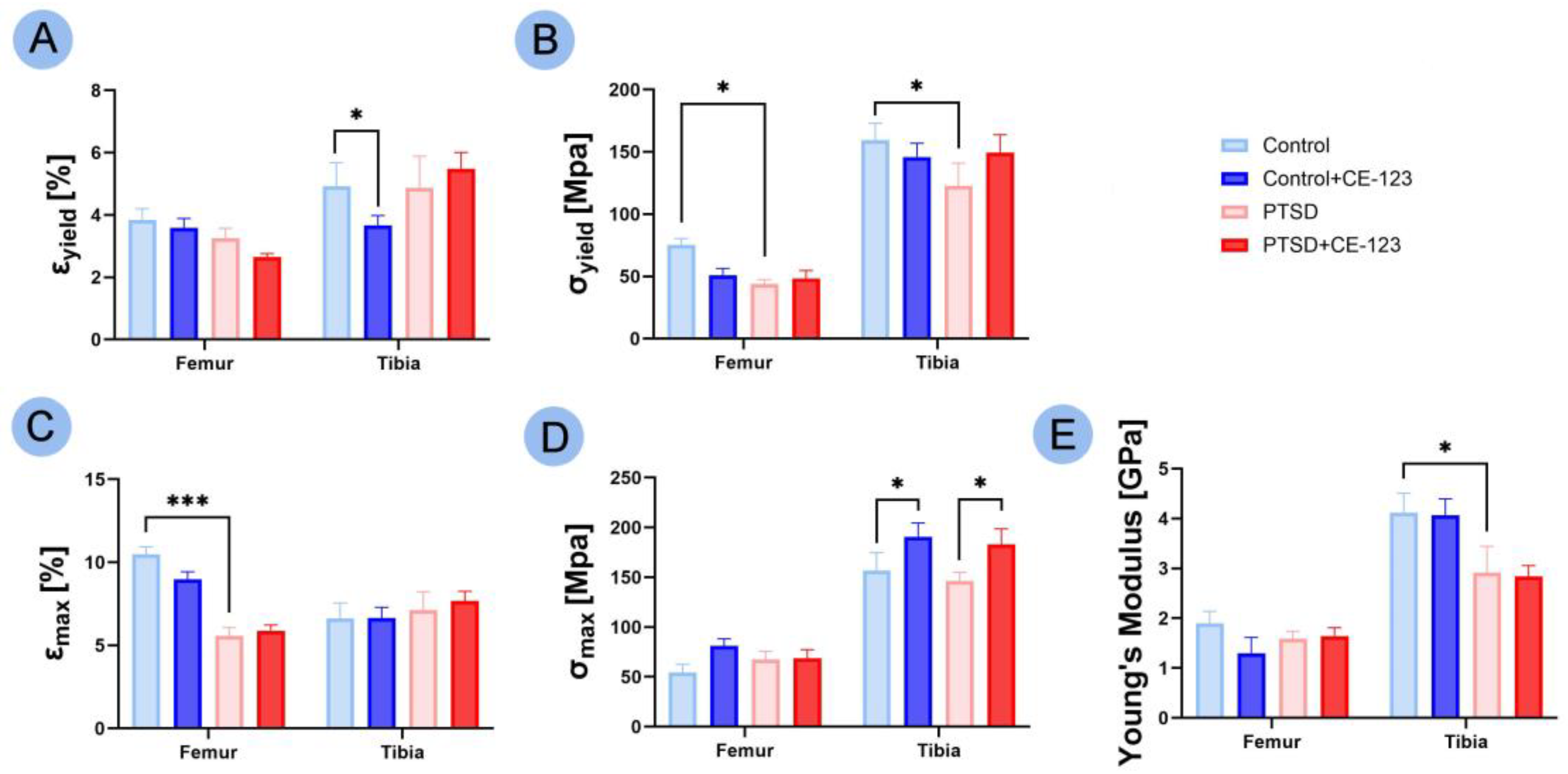
3.4. Mechanical Properties
3.5. Bone Material Properties
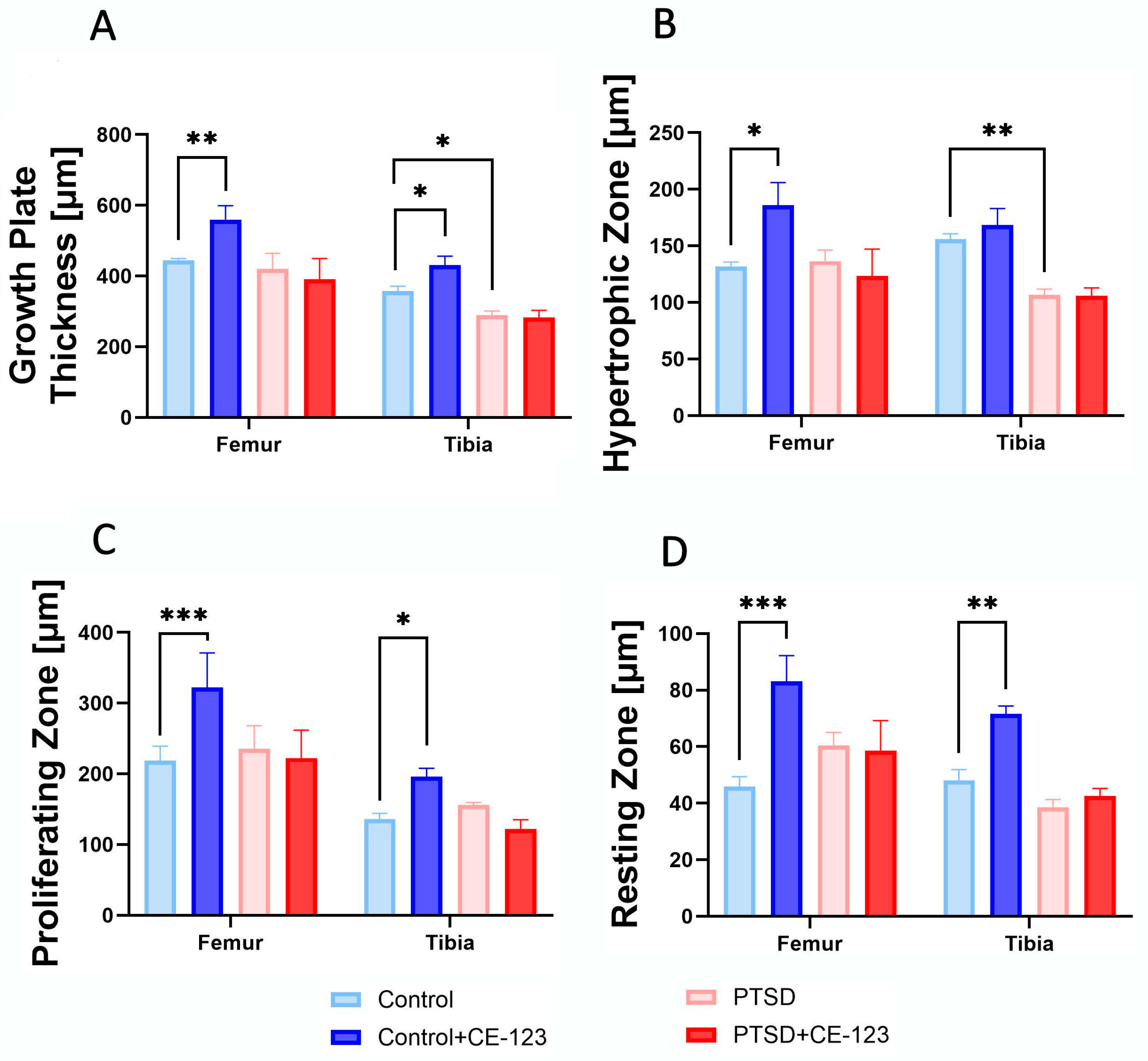
3.6. Growth Plate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTSD | Post-traumatic stress disorder |
| HPA | Hypothalamic–pituitary–adrenal |
| CUMS | Chronic unpredictable mild stress |
| DAT | Dopamine transporter |
| CS | The conditioned stimulus |
| BMD | Bone mineral density |
| BMC | Bone mineral content |
| Fyield | Yield force |
| Fmax | Breaking force |
| Wyield | Elastic work |
| Wmax | Breaking work |
| Hout | Transversal outer diameter |
| Hinn | Transversal inner diameter |
| Vout | Cranial–caudal outer diameter |
| Vinn | Cranial–caudal inner diameter |
| CSA | Cross-sectional area |
| MRWT | Mean relative wall thickness |
| CI | Cortical index |
| Ix | Moment of inertia |
| Ɛyield | Yield strain |
| Ɛmax | Breaking strain |
| σyield | Yield stress |
| σmax | Breaking stress |
| RBW | Relative Bone Weight |
| GH | Growth hormone |
References
- Messman, T.L.; LaPlena, N.; Wilensky, S. Trauma-Related Disorders and Posttraumatic Stress Disorder. In Encyclopedia of Mental Health, 3rd ed.; Friedman, H.S., Markey, C.H., Eds.; Academic Press: Oxford, UK, 2023; pp. 501–510. ISBN 978-0-323-91498-7. [Google Scholar]
- Shalev, A.Y. Posttraumatic Stress Disorder and Stress-Related Disorders. Psychiatr. Clin. N. Am. 2009, 32, 687–704. [Google Scholar] [CrossRef]
- Chen, X. The Causes and Effects of Post-Traumatic Stress Disorder. SHS Web Conf. 2023, 157, 04029. [Google Scholar] [CrossRef]
- Palmer, S.J. The Complex Neurology That Links Physical Health to Post-Traumatic Stress Disorder. Br. J. Neurosci. Nuesing 2023, 19, 37–39. [Google Scholar] [CrossRef]
- Da Costa Silva, L.; Laisney, M.; Charretier, L.; Eustache, F.; Quinette, P. Memory alterations in post-traumatic stress disorder. Biol. Aujourdhui 2023, 217, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Govindula, A.; Ranadive, N.; Nampoothiri, M.; Rao, C.M.; Arora, D.; Mudgal, J. Emphasizing the Crosstalk Between Inflammatory and Neural Signaling in Post-Traumatic Stress Disorder (PTSD). J. Neuroimmune Pharmacol. 2023, 18, 248–266. [Google Scholar] [CrossRef]
- Pivac, N.; Vuic, B.; Sagud, M.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Konjevod, M.; Tudor, L.; Svob Strac, D.; Uzun, S.; Kozumplik, O.; et al. PTSD, Immune System, and Inflammation. In Neuroinflammation, Gut-Brain Axis and Immunity in Neuropsychiatric Disorders; Kim, Y.-K., Ed.; Springer Nature: Singapore, 2023; pp. 225–262. ISBN 978-981-19-7376-5. [Google Scholar]
- Milligan Armstrong, A.; Porter, T.; Quek, H.; White, A.; Haynes, J.; Jackaman, C.; Villemagne, V.; Munyard, K.; Laws, S.M.; Verdile, G.; et al. Chronic Stress and Alzheimer’s Disease: The Interplay between the Hypothalamic–Pituitary–Adrenal Axis, Genetics and Microglia. Biol. Rev. 2021, 96, 2209–2228. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Huang, J.; Xiang, X.; Li, R.; Zhai, Y.; Lin, S.; Liu, W. Psychological Stress Disturbs Bone Metabolism via miR-335-3p/Fos Signaling in Osteoclast. Elife 2025, 13, RP95944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Ye, X. Pdk3’s Role in RANKL-Induced Osteoclast Differentiation: Insights from a Bone Marrow Macrophage Model. PeerJ 2024, 12, e18222. [Google Scholar] [CrossRef]
- Sidles, S.J.; Kelly, R.R.; Kelly, K.D.; Hathaway-Schrader, J.D.; Khoo, S.K.; Jones, J.A.; Cray, J.J.; LaRue, A.C. Inescapable Foot Shock Induces a PTSD-like Phenotype and Negatively Impacts Adult Murine Bone. Dis. Model. Mech. 2024, 17, dmm050044. [Google Scholar] [CrossRef]
- Yu, H.; Watt, H.; Kesavan, C.; Johnson, P.J.; Wergedal, J.E.; Mohan, S. Lasting Consequences of Traumatic Events on Behavioral and Skeletal Parameters in a Mouse Model for Post-Traumatic Stress Disorder (PTSD). PLoS ONE 2012, 7, e42684. [Google Scholar] [CrossRef]
- Benchenane, K. Les modèles animaux du traumatisme et du trouble de stress post-traumatique. Biol. Aujourd’hui 2023, 217, 89–101. [Google Scholar] [CrossRef]
- Lubec, J.; Hussein, A.M.; Kalaba, P.; Feyissa, D.D.; Arias-Sandoval, E.; Cybulska-Klosowicz, A.; Bezu, M.; Stojanovic, T.; Korz, V.; Malikovic, J.; et al. Low-Affinity/High-Selectivity Dopamine Transport Inhibition Sufficient to Rescue Cognitive Functions in the Aging Rat. Biomolecules 2023, 13, 467. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Davis, G.L.; Areal, L.B.; Rabil, M.J.; Tran, V.; Mayer, F.P.; Blakely, R.D. Male DAT Val559 Mice Exhibit Compulsive Behavior under Devalued Reward Conditions Accompanied by Cellular and Pharmacological Changes. Cells 2022, 11, 4059. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Surowka, P.; Marszalek-Grabska, M.; Kalaba, P.; Dragacevic, V.; Kotlinska, P.; Filip, M.; Lubec, G.; Kotlinska, J.H. Novel Dopamine Transporter Inhibitor, CE-123, Ameliorates Spatial Memory Deficits Induced by Maternal Separation in Adolescent Rats: Impact of Sex. Int. J. Mol. Sci. 2022, 23, 10718. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, C.; Ge, X.; Lin, Y. Enhancing Osteogenic Potential: Controlled Release of Dopamine D1 Receptor Agonist SKF38393 Compared to Free Administration. Biomedicines 2024, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Y.; Xie, N.; Sun, X.-D.; Nice, E.C.; Liou, Y.-C.; Huang, C.; Zhu, H.; Shen, Z. Insights and Implications of Sexual Dimorphism in Osteoporosis. Bone Res. 2024, 12, 8. [Google Scholar] [CrossRef]
- Lguensat, A.; Boudjafad, Z.; Giorla, E.; Bennis, M.; Baunez, C.; Garcia, R.; Ba-M’hamed, S. Repeated Ethanol Exposure Following Avoidance Conditioning Impairs Avoidance Extinction and Modifies Conditioning-Associated Prefrontal Dendritic Changes in a Mouse Model of Post-Traumatic Stress Disorder. Eur. J. Neurosci. 2021, 54, 7710–7732. [Google Scholar] [CrossRef]
- Grochecki, P.; Smaga, I.; Wydra, K.; Marszalek-Grabska, M.; Slowik, T.; Kedzierska, E.; Listos, J.; Gibula-Tarlowska, E.; Filip, M.; Kotlinska, J.H. Impact of Mephedrone on Fear Memory in Adolescent Rats: Involvement of Matrix Metalloproteinase-9 (MMP-9) and N-Methyl-D-Aspartate (NMDA) Receptor. Int. J. Mol. Sci. 2023, 24, 1941. [Google Scholar] [CrossRef]
- Osiak-Wicha, C.; Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Andres, K.; Schwarz, T.; Świetlicki, M.; Mielnik-Błaszczak, M.; Arciszewski, M.B. Developmental Changes in Tibia and Humerus of Goose: Morphometric, Densitometric, and Mechanical Analysis. Animal 2023, 17, 100960. [Google Scholar] [CrossRef]
- Kelly, R.R.; McDonald, L.T.; Jensen, N.R.; Sidles, S.J.; LaRue, A.C. Impacts of Psychological Stress on Osteoporosis: Clinical Implications and Treatment Interactions. Front. Psychiatry 2019, 10, 200. [Google Scholar] [CrossRef]
- Steppe, L.; Megafu, M.; Tschaffon-Müller, M.E.A.; Ignatius, A.; Haffner-Luntzer, M. Fracture Healing Research: Recent Insights. Bone Rep. 2023, 19, 101686. [Google Scholar] [CrossRef] [PubMed]
- Cizza, G.; Primma, S.; Coyle, M.; Gourgiotis, L.; Csako, G. Depression and Osteoporosis: A Research Synthesis with Meta-Analysis. Horm. Metab. Res. 2010, 42, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Haffner-Luntzer, M.; Foertsch, S.; Fischer, V.; Prystaz, K.; Tschaffon, M.; Mödinger, Y.; Bahney, C.S.; Marcucio, R.S.; Miclau, T.; Ignatius, A.; et al. Chronic Psychosocial Stress Compromises the Immune Response and Endochondral Ossification during Bone Fracture Healing via β-AR Signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 8615–8622. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-F.; Zhang, Y.; Li, K.; Wang, L.; Ma, Y.-M.; Xiao, W.-L.; Chen, W.-L.; Zhang, J.-F.; Yuan, Q.; Le, N.; et al. Discrimination of Vertebral Fragility Fracture with Lumbar Spine Bone Mineral Density Measured by Quantitative Computed Tomography. J. Orthop. Transl. 2019, 16, 33–39. [Google Scholar] [CrossRef]
- Ma, Z.; Qiu, L.; Li, J.; Wu, Z.; Liang, S.; Zhao, Y.; Yang, J.; Hu, M.; Li, Y. Construction a Novel Osteoporosis Model in Immune-Deficient Mice with Natural Ageing. Biochem. Biophys. Res. Commun. 2024, 735, 150820. [Google Scholar] [CrossRef]
- Kelly, R.R.; Sidles, S.J.; LaRue, A.C. Effects of Neurological Disorders on Bone Health. Front. Psychol. 2020, 11, 612366. [Google Scholar] [CrossRef]
- Wippert, P.-M.; Rector, M.; Kuhn, G.; Wuertz-Kozak, K. Stress and Alterations in Bones: An Interdisciplinary Perspective. Front. Endocrinol. 2017, 8, 96. [Google Scholar] [CrossRef]
- Xavier, A.; Toumi, H.; Lespessailles, E. Animal Model for Glucocorticoid Induced Osteoporosis: A Systematic Review from 2011 to 2021. Int. J. Mol. Sci. 2022, 23, 377. [Google Scholar] [CrossRef]
- Ettinger, M.P. Aging Bone and Osteoporosis: Strategies for Preventing Fractures in the Elderly. Arch. Intern. Med. 2003, 163, 2237–2246. [Google Scholar] [CrossRef]
- Sambataro, S.; Gonzaga, C.M. A Non-Traumatic Stress Fracture of the Tibial Plateau. PM&R 2009, 1, 691–693. [Google Scholar] [CrossRef]
- Jethwa, J.T. Musculoskeletal and Psychological Rehabilitation. Indian J. Orthop. 2023, 57, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, Y.; Tu, S.; Zhou, J.; Wang, Y.; Wei, J.; Zhang, S.; Hou, Y.; Shao, Y.; Ai, H.; et al. Dopamine Promotes Osteogenic Differentiation of PDLSCs by Activating DRD1 and DRD2 during Orthodontic Tooth Movement via ERK1/2 Signaling Pathway. Regen. Ther. 2024, 27, 268–278. [Google Scholar] [CrossRef]
- Pan, S.; Xie, D.; Gao, C.; Xiong, K. Editorial: Characteristic Clinical Immune Phenotypes and Molecular Mechanisms Associated with Inflammatory Diseases. Front. Immunol. 2024, 15, 1384971. [Google Scholar] [CrossRef]
- Basom, R.S.L. Bone, Brain, and Behavior: Examining the Effects of Acetylcholine Within the Neuroskeletal Relationship. Doctoral Dissertation, Kent State University, Kent, OH, USA, 2024. [Google Scholar]
- McMenemy, L.; Behan, F.P.; Kaufmann, J.; Cain, D.; Bennett, A.N.; Boos, C.J.; Fear, N.T.; Cullinan, P.; Bull, A.M.J.; Phillips, A.T.M.; et al. Association Between Combat-Related Traumatic Injury and Skeletal Health: Bone Mineral Density Loss Is Localized and Correlates With Altered Loading in Amputees: The Armed Services Trauma Rehabilitation Outcome (ADVANCE) Study. J. Bone Miner. Res. 2023, 38, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Foertsch, S.; Haffner-Luntzer, M.; Kroner, J.; Gross, F.; Kaiser, K.; Erber, M.; Reber, S.O.; Ignatius, A. Chronic Psychosocial Stress Disturbs Long-Bone Growth in Adolescent Mice. Dis. Model Mech. 2017, 10, 1399–1409. [Google Scholar] [CrossRef]
- Wijani, L.A.A.; Irwanto; I’tishom, R.; Athiyah, A.F.; Rejeki, P.S.; Etika, R. Chronic Unpredictable Mild Stress Impacts On Chondrocyte Apoptosis And Long-Bone Growth In Adolescent Mice. Nat. Vol. Essent. Oil 2022, 9, 165–175. [Google Scholar]
- Su, Y.-W.; Wong, D.S.K.; Fan, J.; Chung, R.; Wang, L.; Chen, Y.; Xian, C.H.; Yao, L.; Wang, L.; Foster, B.K.; et al. Enhanced BMP Signalling Causes Growth Plate Cartilage Dysrepair in Rats. Bone 2021, 145, 115874. [Google Scholar] [CrossRef]
- Bush, P.G.; Hall, A.C.; Macnicol, M.F. New Insights into Function of the Growth Plate: Clinical Observations, Chondrocyte Enlargement And A Possible Role For Membrane Transporters. J. Bone Joint Surg. Br. 2008, 90-B, 1541–1547. [Google Scholar] [CrossRef]
- Mirtz, T.A.; Chandler, J.P.; Eyers, C.M. The Effects of Physical Activity on the Epiphyseal Growth Plates: A Review of the Literature on Normal Physiology and Clinical Implications. J. Clin. Med. Res. 2011, 3, 1–7. [Google Scholar] [CrossRef][Green Version]
- Shahnazari, M.; Dwyer, D.; Chu, V.; Asuncion, F.; Stolina, M.; Ominsky, M.; Kostenuik, P.; Halloran, B. Bone Turnover Markers in Peripheral Blood and Marrow Plasma Reflect Trabecular Bone Loss but Not Endocortical Expansion in Aging Mice. Bone 2012, 50, 628–637. [Google Scholar] [CrossRef]
- Tamaki, H.; Akamine, T.; Goshi, N.; Kurata, H.; Sakou, T. Effects of Exercise Training and Etidronate Treatment on Bone Mineral Density and Trabecular Bone in Ovariectomized Rats. Bone 1998, 23, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Price, J.S.; Lanyon, L.E. Functional Adaptation to Mechanical Loading in Both Cortical and Cancellous Bone Is Controlled Locally and Is Confined to the Loaded Bones. Bone 2010, 46, 314–321. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osiak-Wicha, C.; Kras, K.; Tomaszewska, E.; Muszyński, S.; Grochecki, P.; Kotlińska, J.H.; Słowik, T.; Świetlicki, M.; Arciszewski, K.; Lubec, G.; et al. Evaluation of PTSD-Induced Alterations in Bone Biomechanics and the Protective Potential of CE-123 in a Wistar Rat Model. J. Clin. Med. 2025, 14, 2427. https://doi.org/10.3390/jcm14072427
Osiak-Wicha C, Kras K, Tomaszewska E, Muszyński S, Grochecki P, Kotlińska JH, Słowik T, Świetlicki M, Arciszewski K, Lubec G, et al. Evaluation of PTSD-Induced Alterations in Bone Biomechanics and the Protective Potential of CE-123 in a Wistar Rat Model. Journal of Clinical Medicine. 2025; 14(7):2427. https://doi.org/10.3390/jcm14072427
Chicago/Turabian StyleOsiak-Wicha, Cezary, Katarzyna Kras, Ewa Tomaszewska, Siemowit Muszyński, Paweł Grochecki, Jolanta H. Kotlińska, Tymoteusz Słowik, Michał Świetlicki, Kamil Arciszewski, Gert Lubec, and et al. 2025. "Evaluation of PTSD-Induced Alterations in Bone Biomechanics and the Protective Potential of CE-123 in a Wistar Rat Model" Journal of Clinical Medicine 14, no. 7: 2427. https://doi.org/10.3390/jcm14072427
APA StyleOsiak-Wicha, C., Kras, K., Tomaszewska, E., Muszyński, S., Grochecki, P., Kotlińska, J. H., Słowik, T., Świetlicki, M., Arciszewski, K., Lubec, G., & Arciszewski, M. B. (2025). Evaluation of PTSD-Induced Alterations in Bone Biomechanics and the Protective Potential of CE-123 in a Wistar Rat Model. Journal of Clinical Medicine, 14(7), 2427. https://doi.org/10.3390/jcm14072427






