Carotid Resistance and Pulsatility: Non-Invasive Markers for Diabetes Mellitus-Related Vascular Diseases
Abstract
:1. Introduction
2. Materials and Methods
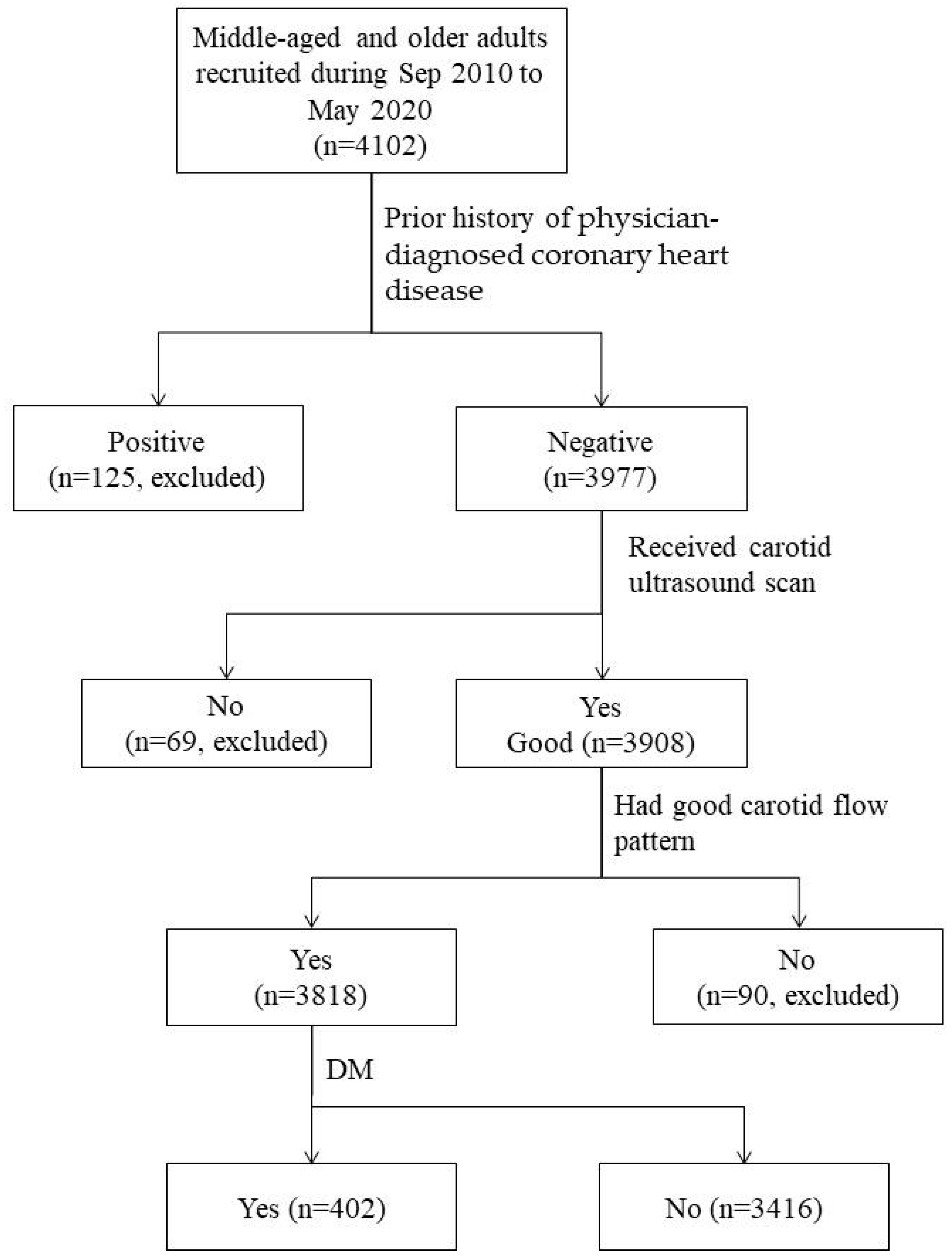
2.1. Study Subjects
2.2. Anthropometric and Biochemical Measurements
2.3. Ultrasonographic Measurements of Carotid Blood Flow
| PI= | PSV-EDV | , | RI= | PSV-EDV | . |
| MFV | MFV |
2.4. Statistical Analyses
3. Results
3.1. Baseline Anthropometric and Biochemical Measurements Between DM Cases and Non-DM Controls
3.2. Comparisons of Carotid RIs and PIs Between DM Cases and Non-DM Controls
3.3. Association Analyses for Carotid RIs and PIs with the Presence of DM
3.4. Analyses for the Added Predictability of Carotid RIs and PIs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | diabetes mellitus |
| PI | pulsatility index |
| RI | resistance index |
| CCA | common carotid arteries |
| ICA | internal carotid arteries |
| ECA | external carotid arteries |
| OR | odds ratio |
| CI | confidence interval |
| SD | standard deviation |
| DALY | disability-adjusted life year |
| PWV | pulse wave velocity |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| TCHO | total cholesterol |
| FTG | fasting triglyceride |
| FPG | fasting plasma glucose |
| PSV | peak-systolic velocity |
| EDV | end-diastolic velocity |
| MFV | time-weighted maximum flow velocity |
| AUROC | area under the receiver operating characteristic curve |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [PubMed]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [PubMed]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar]
- Wang, Y.; O’Neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: A systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 136. [Google Scholar]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as a risk factor for stroke in women compared with men: A systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014, 383, 1973–1980. [Google Scholar]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. Neurol. 2021, 20, 795–820. [Google Scholar]
- Chase-Vilchez, A.Z.; Chan, I.H.Y.; Peters, S.A.E.; Woodward, M. Diabetes as a risk factor for incident peripheral arterial disease in women compared to men: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2020, 19, 151. [Google Scholar]
- Chan, J.C.N.; Lim, L.L.; Wareham, N.J.; Shaw, J.E.; Orchard, T.J.; Zhang, P.; Lau, E.S.H.; Eliasson, B.; Kong, A.P.S.; Ezzati, M.; et al. The Lancet Commission on diabetes: Using data to transform diabetes care and patient lives. Lancet 2021, 396, 2019–2082. [Google Scholar]
- Spracklen, C.N.; Horikoshi, M.; Kim, Y.J.; Lin, K.; Bragg, F.; Moon, S.; Suzuki, K.; Tam, C.H.T.; Tabara, Y.; Kwak, S.-H.; et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature 2020, 582, 240–245. [Google Scholar]
- Avolio, A.P.; Kuznetsova, T.; Heyndrickx, G.R.; Kerkhof, P.L.M.; Li, J.K. Arterial flow, pulse pressure and pulse wave velocity in men and women at various ages. Adv. Exp. Med. Biol. 2018, 1065, 153–168. [Google Scholar]
- Kosmopoulos, M.; Chiriacò, M.; Stamatelopoulos, K.; Tsioufis, C.; Masci, P.G.; Kontogiannis, C.; Mengozzi, A.; Pugliese, N.R.; Taddei, S.; Virdis, A.; et al. The relationship between telomere length and putative markers of vascular ageing: A systematic review and meta-analysis. Mech. Ageing Dev. 2022, 201, 111604. [Google Scholar] [PubMed]
- Prenner, S.B.; Chirinos, J.A. Arterial stiffness in diabetes mellitus. Atherosclerosis 2015, 238, 370–379. [Google Scholar] [PubMed]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar]
- van Sloten, T.T.; Sedaghat, S.; Laurent, S.; London, G.M.; Pannier, B.; Ikram, M.A.; Kavousi, M.; Mattace-Raso, F.; Franco, O.H.; Boutouyrie, P.; et al. Carotid stiffness is associated with incident stroke: A systematic review and individual participant data meta-analysis. J. Am. Coll. Cardiol. 2015, 66, 2116–2125. [Google Scholar]
- Yang, Y.; Ren, X.; Sha, Z.; Li, P.; Xiao, M.; Chen, S.; Wang, L.; Cui, L. Clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: An up-to-date meta-analysis. Med. Sci. Monit. 2015, 21, 2467–2475. [Google Scholar]
- Yiu, K.H.; Zhao, C.T.; Chen, Y.; Siu, C.W.; Chan, Y.H.; Lau, K.K.; Liu, S.; Lau, C.-P.; Tse, H.-F. Association of subclinical myocardial injury with arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2013, 12, 94. [Google Scholar]
- Li, Y.; Tian, X.X.; Liu, T.; Wang, R.T. Association between whole blood viscosity and arterial stiffness in patients with type 2 diabetes mellitus. Endocrine 2015, 49, 148–154. [Google Scholar]
- Lal, A.; Barry, M.A.T.; Mitchell, P.; Thiagalingam, A. ECG-gated retinal vessel calibre as a novel measure of aberrant pulsatile retinal flow in diabetes mellitus: A cross-sectional study. J. Diabetes Metab. Disord. 2024, 23, 1887–1898. [Google Scholar]
- Loimaala, A.; Groundstroem, K.; Majahalme, S.; Nenonen, A.; Vuori, I. Impaired myocardial function in newly onset type 2 diabetes associates with arterial stiffness. Eur. J. Echocardiogr. 2006, 7, 341–347. [Google Scholar]
- Yoo, H.J.; Hwang, S.Y.; Hong, H.C.; Choi, H.Y.; Yang, S.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Choi, K.M.; Choi, D.S.; et al. Association of circulating omentin-1 level with arterial stiffness and carotid plaque in type 2 diabetes. Cardiovasc. Diabetol. 2011, 10, 103. [Google Scholar]
- Climie, R.E.; Gallo, A.; Picone, D.S.; Di Lascio, N.; van Sloten, T.T.; Guala, A.; Mayer, C.C.; Hametner, B.; Bruno, R.M. Measuring the interaction between the macro-and micro-vasculature. Front. Cardiovasc. Med. 2019, 6, 169. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Cheng, H.M.; Bai, C.H.; Yeh, W.T.; Chen, J.R.; Pan, W.H. Blood pressure, carotid flow pulsatility, and the risk of stroke: A community-based study. Stroke 2016, 47, 2262–2268. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.K.; Pego, P.; Mazzucco, S.; Li, L.; Howard, D.P.; Küker, W.; Rothwell, P.M. Age and sex-specific associations of carotid pulsatility with small vessel disease burden in transient ischemic attack and ischemic stroke. Int. J. Stroke 2018, 13, 832–839. [Google Scholar] [CrossRef] [PubMed]
- Yoo, I.H.; Kim, J.M.; Han, S.H.; Ryu, J.; Jung, K.H.; Park, K.Y. Increased pulsatility index of the basilar artery is a risk factor for neurological deterioration after stroke: A case control study. ClinHypertens 2022, 28, 27. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Sohn, Y.H.; Baik, J.S.; Kim, G.W.; Kim, J.S. Arterial pulsatility as an index of cerebral microangiopathy in diabetes. Stroke 2000, 31, 1111–1115. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, M.H.; Lee, K.Y.; Kim, C.S.; Kim, H.J.; Nam, J.S.; Ahn, C.W.; Cha, B.S.; Lee, E.J.; Kil Lim, S.; et al. Cerebral arterial pulsatility and insulin resistance in type 2 diabetic patients. Diabetes Res. Clin. Pract. 2008, 79, 237–242. [Google Scholar] [CrossRef]
- Zou, C.; Jiao, Y.; Li, X.; Wang, P.; Zheng, J.; Zhao, Y.; Boodhun, D.; Hu, Z.; Zheng, C. Differences between healthy adults and patients with type 2 diabetes mellitus in reactivity of toe microcirculation by ultrasound combined with a warm bath test. Medicine 2017, 96, e7035. [Google Scholar] [CrossRef]
- Nealon, R.S.; Howe, P.R.; Jansen, L.; Garg, M.; Wong, R.H. Impaired cerebrovascular responsiveness and cognitive performance in adults with type 2 diabetes. J. Diabetes Complicat. 2017, 31, 462–467. [Google Scholar] [CrossRef]
- Wu, T.W.; Chou, C.L.; Cheng, C.F.; Lu, S.X.; Wang, L.Y. Prevalences of diabetes mellitus and carotid atherosclerosis and their relationships in middle-aged adults and elders: A community-based study. J. Formos. Med. Assoc. 2022, 121, 1133–1140. [Google Scholar] [CrossRef]
- Chou, C.L.; Liu, C.C.; Wu, T.W.; Cheng, C.F.; Lu, S.X.; Wu, Y.J.; Wang, L.-Y. Predictability of cardiovascular risk scores for carotid atherosclerosis in community-dwelling middle-aged and elderly adults. J. Clin. Med. 2024, 13, 2563. [Google Scholar] [CrossRef]
- Wu, T.W.; Wu, Y.J.; Chou, C.L.; Cheng, C.F.; Lu, S.X.; Wang, L.Y. Hemodynamic parameters and diabetes mellitus in community-dwelling middle-aged adults and elders: A community-based study. Sci. Rep. 2024, 14, 12032. [Google Scholar]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to measure arterial stiffness in humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Okumura, K.; Aso, Y. High serum pentosidine concentrations are associated with increased arterial stiffness and thickness in patients with type 2 diabetes. Metabolism 2005, 54, 345–350. [Google Scholar] [PubMed]
- Zhang, L.; Yin, J.K.; Duan, Y.Y.; Liu, X.; Xu, L.; Wang, J.; Yang, Y.-L.; Yuan, L.-J.; Cao, T.-S. Evaluation of carotid artery elasticity changes in patients with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 39. [Google Scholar]
- Cohen, J.B.; Mitchell, G.F.; Gill, D.; Burgess, S.; Rahman, M.; Hanff, T.C.; Ramachandran, V.S.; Mutalik, K.M.; Townsend, R.R.; Chirinos, J.A. Arterial stiffness and diabetes risk in Framingham Heart Study and UK Biobank. Circ. Res. 2022, 131, 545–554. [Google Scholar]
- Kitagawa, T.; Mitsumura, H.; Sato, T.; Takatsu, H.; Komatsu, T.; Sakuta, K.; Iguchi, Y. Relation between severity of cerebral small vessel disease and pulsatility index of internal carotid artery in small vessel occlusion. Clin. Neurol. Neurosurg. 2024, 237, 108127. [Google Scholar]
- Sasaki, N.; Maeda, R.; Ozono, R.; Nakano, Y.; Higashi, Y. Association of flow parameters and diameter in the common carotid artery with impaired glucose metabolism. J. Atheroscler Thromb. 2022, 29, 654–666. [Google Scholar] [CrossRef]
| Variables | Non-DM Controls (n = 3416) | DM Patients (n = 402) | p-Values | ||
|---|---|---|---|---|---|
| Continuous variables | Mean | SD | Mean | SD | |
| Age (years) | 55.5 | 8.9 | 60.0 | 8.4 | <0.0001 |
| BMI (kg/m2) | 24.3 | 3.4 | 26.1 | 3.8 | <0.0001 |
| Waist circumference (cm) | 84.8 | 10.0 | 90.3 | 9.8 | <0.0001 |
| Hip circumference (cm) | 96.2 | 7.1 | 98.0 | 7.7 | <0.0001 |
| WHR (%) | 88.1 | 7.1 | 92.1 | 6.6 | <0.0001 |
| SBP (mmHg) | 125.7 | 18.6 | 131.4 | 17.1 | <0.0001 |
| DBP (mmHg) | 76.0 | 12.6 | 78.2 | 12.1 | 0.0012 |
| TCHO (mg/dL) | 206.0 | 37.6 | 196.9 | 43.7 | <0.0001 |
| LDL (mg/dL) | 122.2 | 32.1 | 115.6 | 34.7 | 0.0003 |
| HDL (mg/dL) | 56.5 | 15.0 | 49.1 | 12.6 | <0.0001 |
| LDL-/HDL-C ratio | 2.31 | 0.84 | 2.48 | 0.89 | 0.0001 |
| Log (FTG) | 4.57 | 0.55 | 4.86 | 0.58 | <0.0001 |
| Categorical variables | n | % | n | % | |
| Female sex | 2259 | 66.0 | 222 | 55.2 | <0.0001 |
| Schooling years ≥ 12 years | 1308 | 38.3 | 111 | 27.6 | <0.0001 |
| Hypertension | 715 | 20.9 | 175 | 43.5 | <0.0001 |
| Current cigarette smoker | 709 | 20.8 | 120 | 29.9 | <0.0001 |
| Current alcohol drinker | 472 | 13.9 | 57 | 13.2 | 0.86 |
| Non-DM Controls (n = 3416) | DM Patients (n = 402) | Difference Between DM Cases and Non-DM Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude | Age–Sex-Adjusted | |||||||
| Indicator | Mean | SD | Mean | SD | Mean | (95% CI) | Mean | (95% CI) |
| CCA | ||||||||
| RI | 0.72 | 0.05 | 0.74 | 0.05 | 0.020 *** | (0.015~0.026) | 0.013 *** | (0.008~0.018) |
| PI | 1.52 | 0.32 | 1.62 | 0.34 | 0.103 *** | (0.070~0.136) | 0.065 *** | (0.034~0.096) |
| ICA | ||||||||
| RI | 0.60 | 0.06 | 0.62 | 0.06 | 0.020 *** | (0.013~0.026) | 0.013 *** | (0.006~0.019) |
| PI | 1.03 | 0.23 | 1.09 | 0.23 | 0.059 *** | (0.035~0.083) | 0.038 ** | (0.014~0.062) |
| ECA | ||||||||
| RI | 0.80 | 0.06 | 0.82 | 0.06 | 0.017 *** | (0.011~0.024) | 0.014 *** | (0.007~0.020) |
| PI | 1.99 | 0.48 | 2.15 | 0.54 | 0.163 *** | (0.113~0.214) | 0.137 *** | (0.087~0.186) |
| Indicators (per SD) | Age–Sex-Adjusted | Multivariable-Adjusted 1 | Added AUROC (%) 2 | |||
|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | Δ AUROC | (95% CI) | |
| CCA | ||||||
| RI | 1.33 *** | (1.18~1.50) | 1.25 ** | (1.11~1.42) | 0.26 | (−0.32~0.83) |
| PI | 1.23 *** | (1.11~1.37) | 1.15 * | (1.02~1.29) | 0.10 | (−0.24~0.45) |
| ICA | ||||||
| RI | 1.22 ** | (1.10~1.36) | 1.22 ** | (1.09~1.37) | 0.24 | (−0.27~0.76) |
| PI | 1.16 ** | (1.05~1.28) | 1.14 * | (1.02~1.26) | 0.08 | (−0.23~0.38) |
| ECA | ||||||
| RI | 1.25 *** | (1.11~1.40) | 1.36 *** | (1.21~1.54) | 0.85 * | (0.11~1.59) |
| PI | 1.29 *** | (1.16~1.42) | 1.30 *** | (1.17~1.45) | 0.69 * | (0.01~1.37) |
| Base Model | Base + ECA RI | Base + ECA PI | ||||
|---|---|---|---|---|---|---|
| Variable | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) |
| Age (per 10 years) | 1.50 *** | (1.32~1.70) | 1.41 *** | (1.24~1.60) | 1.45 *** | (1.28~1.64) |
| Sex (M/F) | 0.89 | (0.67~1.17) | 0.78 | (0.59~1.03) | 0.75 * | (0.57~1.00) |
| Schooling years < 12 years (Y/N) | 1.39 * | (1.09~1.78) | 1.38 * | (1.08~1.76) | 1.40 * | (1.10~1.80) |
| Cigarette smoking (Y/N) | 1.35 * | (1.01~1.81) | 1.42 * | (1.06~1.90) | 1.39 * | (1.04~1.87) |
| Hypertension (Y/N) | 1.50 ** | (1.18~1.90) | 1.46 ** | (1.15~1.85) | 1.44 ** | (1.14~1.84) |
| BMI (per 1.0 SD) | 1.25 ** | (1.11~1.40) | 1.24 ** | (1.10~1.39) | 1.22 ** | (1.08~1.37) |
| WHR (per 1.0 SD) | 1.27 ** | (1.12~1.45) | 1.33 *** | (1.17~1.52) | 1.30 *** | (1.14~1.49) |
| TCHO (per 1.0 SD) | 0.79 ** | (0.70~0.90) | 0.78 ** | (0.69~0.89) | 0.79 ** | (0.69~0.90) |
| HDL-C (per 1.0 SD) | 0.83 * | (0.70~0.98) | 0.84 * | (0.71~0.99) | 0.83 * | (0.70~0.98) |
| Log (FTG) (per 1.0) | 1.88 *** | (1.47~2.40) | 1.95 *** | (1.53~2.50) | 1.92 *** | (1.51~2.46) |
| ECA RI (per 1.0 SD) | - | 1.36 *** | (1.21~1.54) | - | ||
| ECA PI (per 1.0 SD) | - | - | 1.30 *** | (1.17~1.45) | ||
| AUROC (%) | 75.1 | (72.7~77.5) | 75.9 | (73.6~78.3) | 75.8 | (73.4~78.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-C.; Chou, C.-L.; Chen, C.-F.; Cheng, C.-F.; Lu, S.-X.; Wu, Y.-J.; Wu, T.-W.; Wang, L.-Y. Carotid Resistance and Pulsatility: Non-Invasive Markers for Diabetes Mellitus-Related Vascular Diseases. J. Clin. Med. 2025, 14, 2523. https://doi.org/10.3390/jcm14072523
Liu C-C, Chou C-L, Chen C-F, Cheng C-F, Lu S-X, Wu Y-J, Wu T-W, Wang L-Y. Carotid Resistance and Pulsatility: Non-Invasive Markers for Diabetes Mellitus-Related Vascular Diseases. Journal of Clinical Medicine. 2025; 14(7):2523. https://doi.org/10.3390/jcm14072523
Chicago/Turabian StyleLiu, Chun-Chieh, Chao-Liang Chou, Chuen-Fei Chen, Chun-Fang Cheng, Shu-Xin Lu, Yih-Jer Wu, Tzu-Wei Wu, and Li-Yu Wang. 2025. "Carotid Resistance and Pulsatility: Non-Invasive Markers for Diabetes Mellitus-Related Vascular Diseases" Journal of Clinical Medicine 14, no. 7: 2523. https://doi.org/10.3390/jcm14072523
APA StyleLiu, C.-C., Chou, C.-L., Chen, C.-F., Cheng, C.-F., Lu, S.-X., Wu, Y.-J., Wu, T.-W., & Wang, L.-Y. (2025). Carotid Resistance and Pulsatility: Non-Invasive Markers for Diabetes Mellitus-Related Vascular Diseases. Journal of Clinical Medicine, 14(7), 2523. https://doi.org/10.3390/jcm14072523







