Loading Pressure Induced by 4 mm Implants on the Inferior Alveolar Nerve: A 3D Finite Element Analysis Model
Abstract
1. Introduction
2. Materials and Methods
3. Results
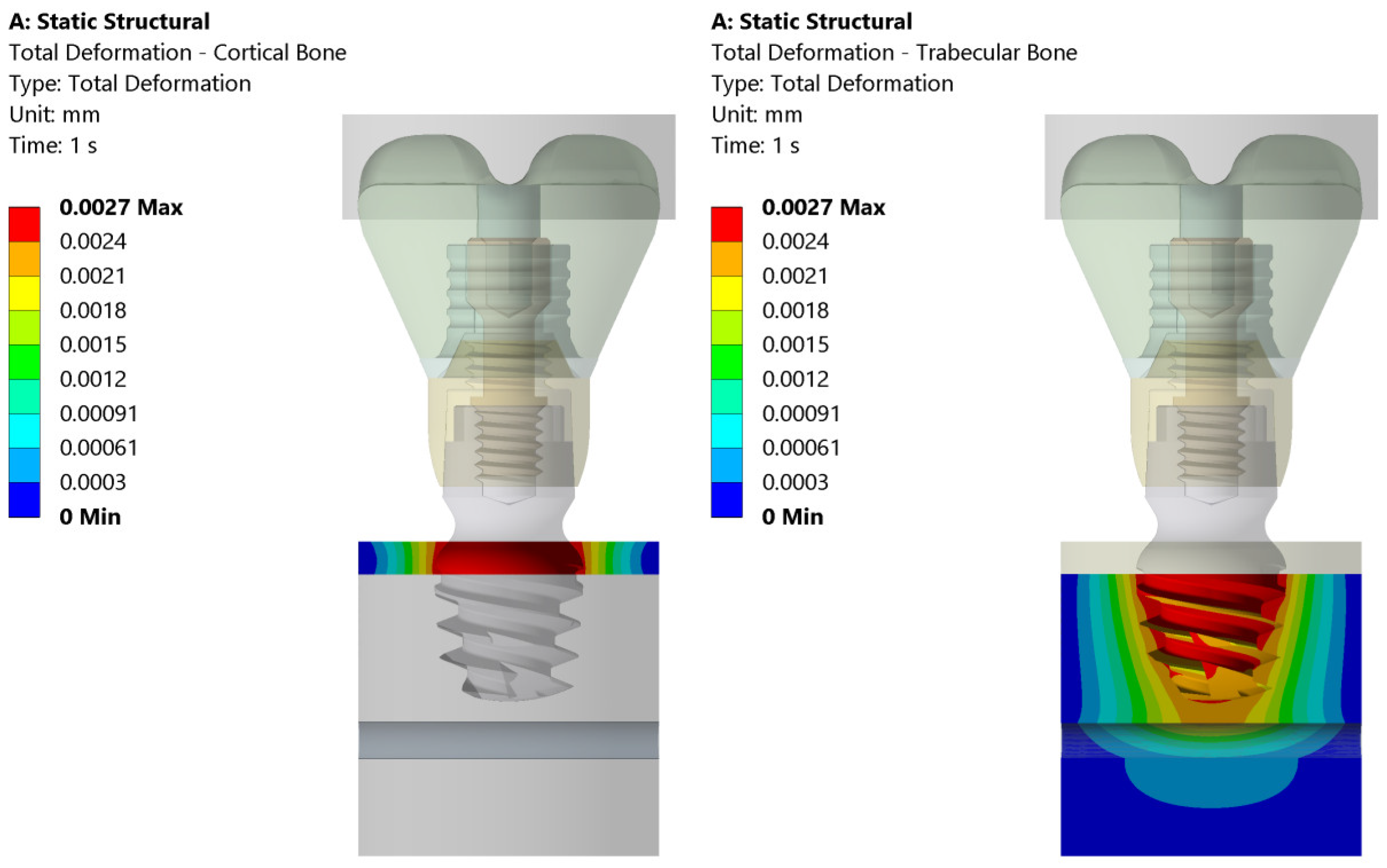
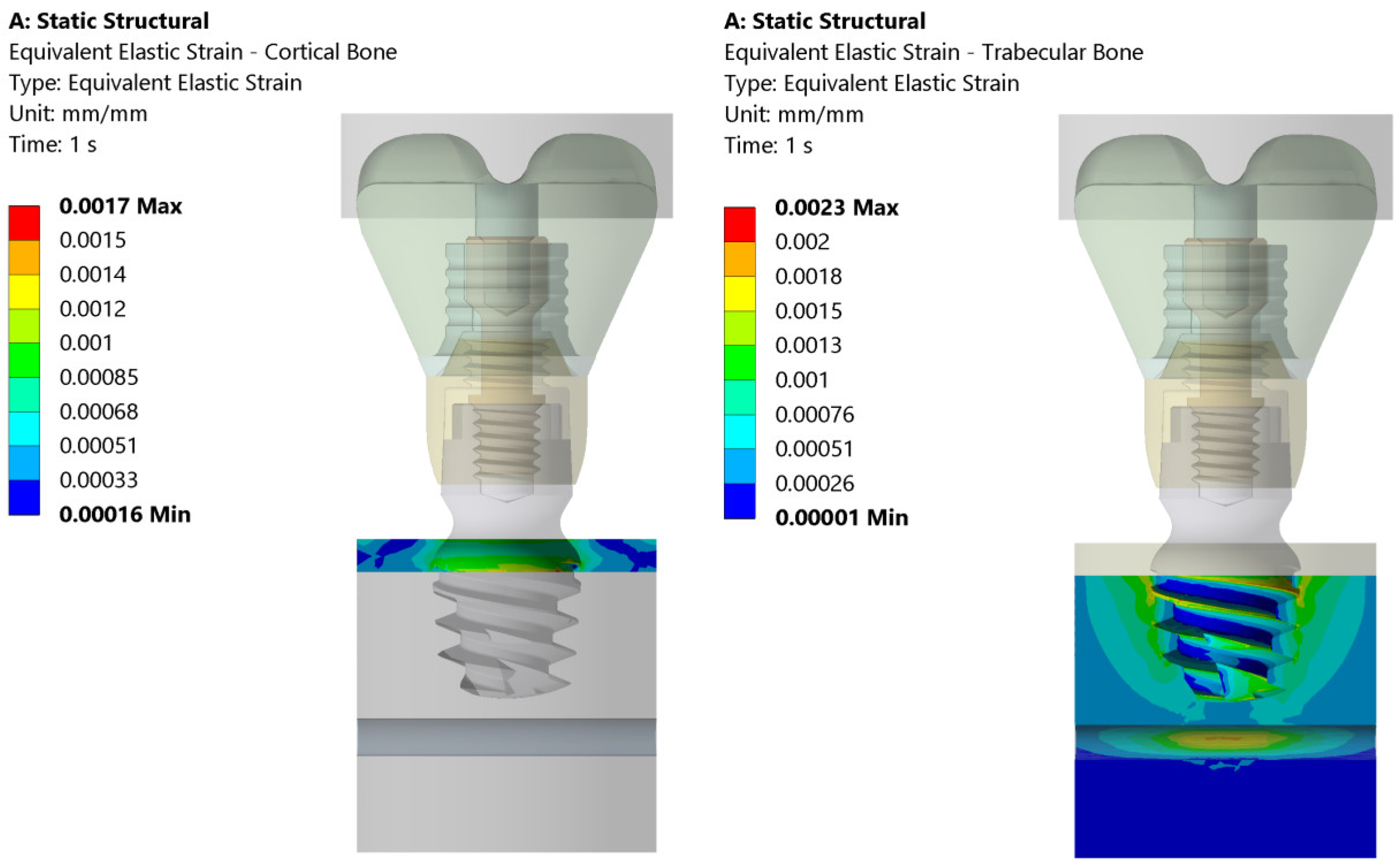
3.1. Stress and Strain Deformation
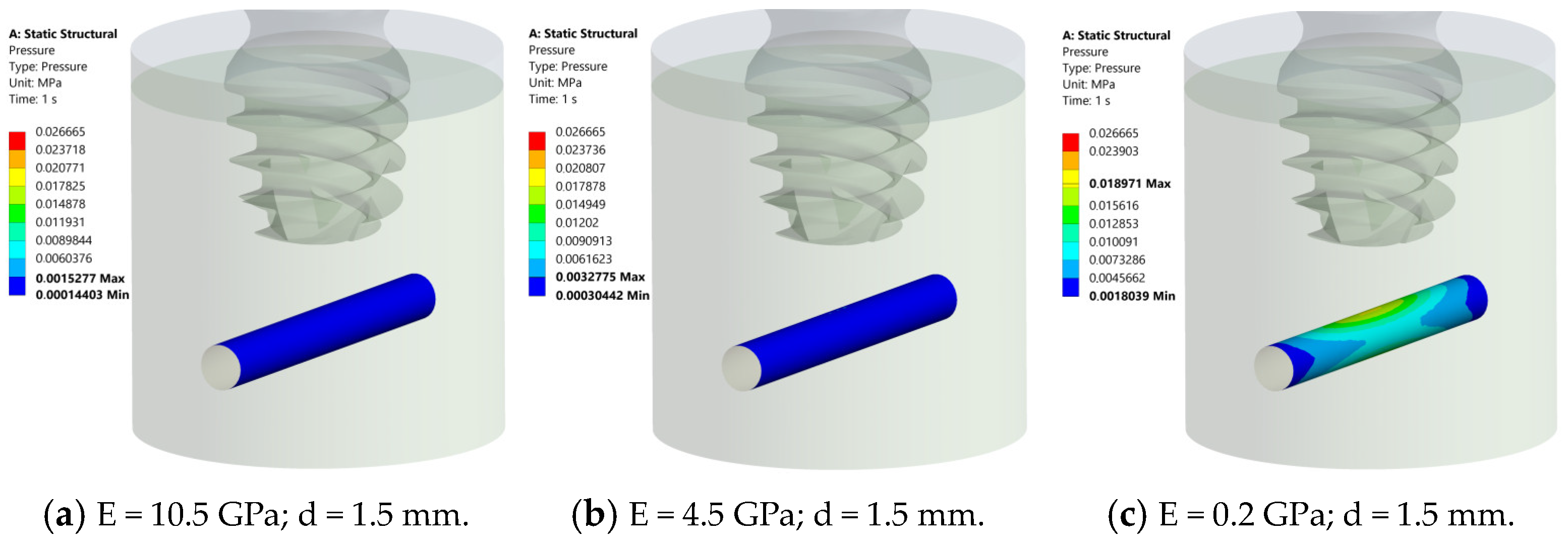
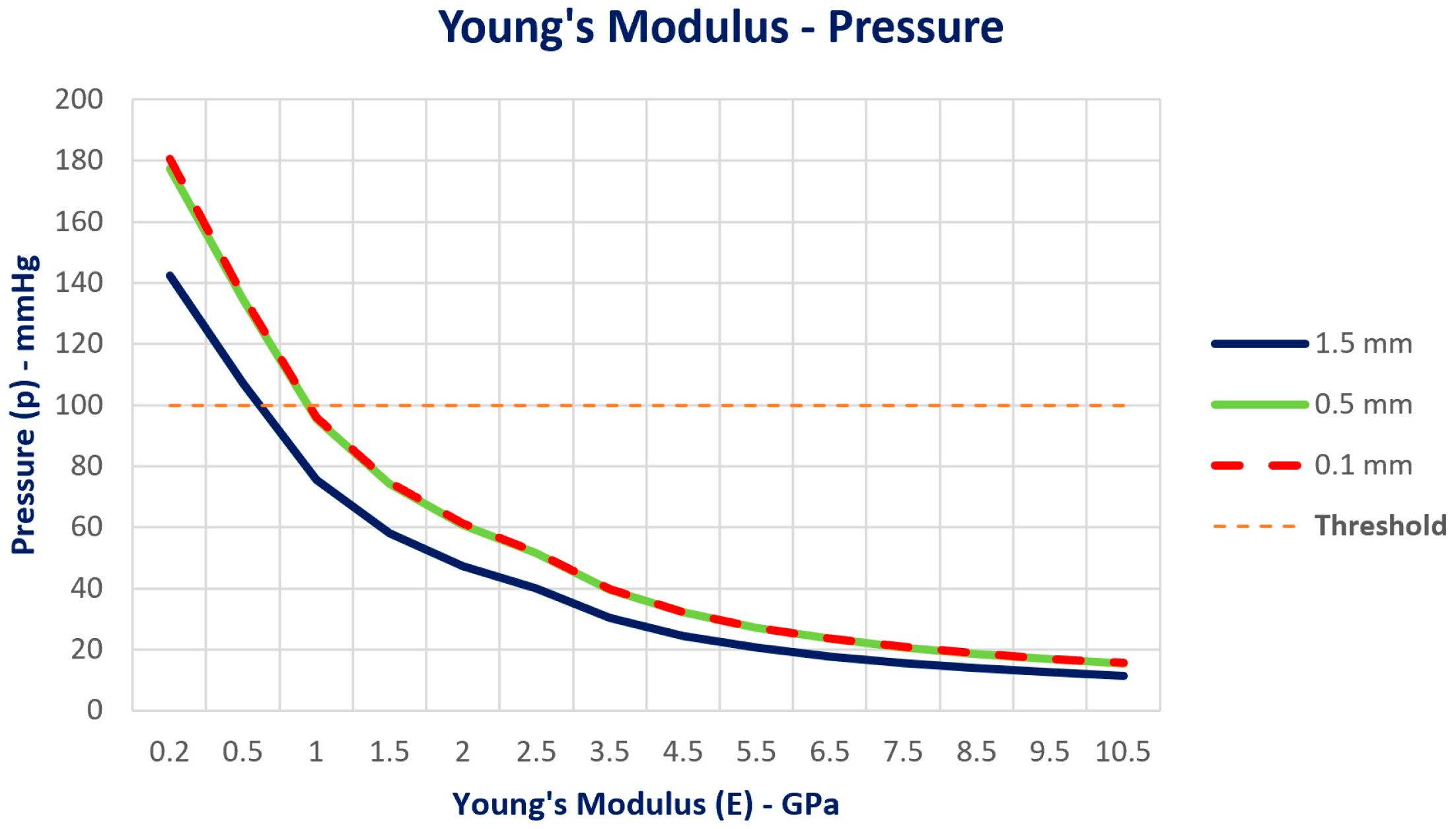
3.2. Pressure Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toledano-Serrabona, J.; Sánchez-Garcés, M.Á.; Sánchez-Torres, A.; Gay-Escoda, C. Alveolar distraction osteogenesis for dental implant treatments of the vertical bone atrophy: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e70–e75. [Google Scholar] [CrossRef] [PubMed]
- Yun, K.I.; Choi, H.; Wright, R.F.; Ahn, H.S.; Chang, B.M.; Kim, H.J. Efficacy of Alveolar Vertical Distraction Osteogenesis and Autogenous Bone Grafting for Dental Implants: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Urban, I.; Monje, A.; Kunrath, M.F.; Dahlin, C. Guided bone regeneration in implant dentistry: Basic principle, progress over 35 years, and recent research activities. Periodontol. 2000 2023, 93, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Kermanshah, H.; Keshtkar, A.; Hassani, A.; Bitaraf, T. Comparing short implants to standard dental implants: A systematic review and meta-analysis of randomized controlled trials with extended follow-up. Evid.-Based Dent. 2023, 24, 192–193. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants—A Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 167–184. [Google Scholar]
- Pistilli, R.; Simion, M.; Barausse, C.; Gasparro, R.; Pistilli, V.; Bellini, P.; Felice, P. Guided Bone Regeneration with Nonresorbable Membranes in the Rehabilitation of Partially Edentulous Atrophic Arches: A Retrospective Study on 122 Implants with a 3- to 7-Year Follow-Up. Int. J. Periodontics Restor. Dent. 2020, 40, 685–692. [Google Scholar] [CrossRef]
- Sáenz-Ravello, G.; Ossandón-Zúñiga, B.; Muñoz-Meza, V.; Mora-Ferraro, D.; Baeza, M.; Fan, S.; Sagheb, K.; Schiegnitz, E.; Díaz, L. Short implants compared to regular dental implants after bone augmentation in the atrophic posterior mandible: Umbrella review and meta-analysis of success outcomes. Int. J. Implant. Dent. 2023, 9, 18. [Google Scholar] [CrossRef]
- Jung, R.E.; Al-Nawas, B.; Araujo, M.; Avila-Ortiz, G.; Barter, S.; Brodala, N.; Chappuis, V.; Chen, B.; De Souza, A.; Almeida, R.F.; et al. Group 1 ITI Consensus Report: The influence of implant length and design and medications on clinical and patient-reported outcomes. Clin. Oral Implants Res. 2018, 29 (Suppl. S16), 69–77. [Google Scholar]
- Esposito, M.; Buti, J.; Barausse, C.; Gasparro, R.; Sammartino, G.; Felice, P. Short implants versus longer implants in vertically augmented atrophic mandibles: A systematic review of randomised controlled trials with a 5-year post-loading follow-up. Int. J. Oral Implantol. 2019, 12, 267–280. [Google Scholar]
- Nisand, D.; Picard, N.; Rocchietta, I. Short implants compared to implants in vertically augmented bone: A systematic review. Clin. Oral Implants Res. 2015, 26 (Suppl. S11), 170–179. [Google Scholar] [CrossRef]
- Terheyden, H.; Meijer, G.J.; Raghoebar, G.M. Vertical bone augmentation and regular implants versus short implants in the vertically deficient posterior mandible: A systematic review and meta-analysis of randomized studies. Int. J. Oral Maxillofac. Surg. 2021, 50, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Barausse, C.; Ravidà, A.; Bonifazi, L.; Pistilli, R.; Saleh, M.H.A.; Gasparro, R.; Sammartino, G.; Wang, H.L.; Felice, P. Extra-short (4-mm) implants placed after regenerative failures in the posterior atrophic mandible: A retrospective study. Int. J. Oral Implantol. 2023, 16, 31–38. [Google Scholar]
- Romanos, G.E. Severe atrophy of the posterior mandible and inferior alveolar nerve transposition. Int. J. Periodontics Restor. Dent. 2021, 41, e199–e204. [Google Scholar]
- Haeberle, C.B.; Abreu, A.; Metzler, K.; Robles-Moreno, M. Complications associated with rehabilitation of a unilateral partially edentulous mandible with inferior alveolar nerve transposition and implant placement: A clinical report. J. Prosthodont. 2021, 30, 285–289. [Google Scholar]
- Ellies, L.G.; Hawker, P.B. The prevalence of altered sensation associated with implant surgery. Int. J. Oral Maxillofac. Implants. 1993, 8, 674–679. [Google Scholar]
- Khawaja, N.; Renton, T. Case studies on implant removal influencing the resolution of inferior alveolar nerve injury. Br. Dent. J. 2009, 206, 365–370. [Google Scholar]
- Alhassani, A.A.; AlGhamdi, A.S. Inferior alveolar nerve injury in implant dentistry: Diagnosis, causes, prevention, and management. J. Oral Implantol. 2010, 36, 401–407. [Google Scholar]
- Sammartino, G.; Marenzi, G.; Citarella, R.; Ciccarelli, R.; Wang, H.L. Analysis of the occlusal stress transmitted to the inferior alveolar nerve by an osseointegrated threaded fixture. J. Periodontol. 2008, 79, 1735–1744. [Google Scholar]
- Sammartino, G.; Wang, H.L.; Citarella, R.; Lepore, M.; Marenzi, G. Analysis of occlusal stresses transmitted to the inferior alveolar nerve by multiple threaded implants. J. Periodontol. 2013, 84, 1655–1661. [Google Scholar]
- Gümrükçü, Z.; Korkmaz, Y.T. Influence of implant number, length, and tilting degree on stress distribution in atrophic maxilla: A finite element study. Med. Biol. Eng. Comput. 2018, 56, 979–989. [Google Scholar] [CrossRef]
- Sammartino, G.; Prados-Frutos, J.C.; Riccitiello, F.; Felice, P.; Cerone, V.; Gasparro, R.; Wang, H.L. The relevance of the use of radiographic planning in order to avoid complications in mandibular implantology: A retrospective study. Biomed. Res. Int. 2016, 2016, 8175284. [Google Scholar]
- Ausiello, P.; Ciaramella, S.; De Benedictis, A.; Lanzotti, A.; Tribst, J.P.M.; Watts, D.C. The use of different adhesive filling material and mass combinations to restore class II cavities under loading and shrinkage effects: A 3D-FEA. Comput. Methods Biomech. Biomed. Engin. 2021, 24, 485–495. [Google Scholar] [PubMed]
- Prati, C.; Tribst, J.P.M.; Piva, A.M.O.D.; Zamparini, F.; Ausiello, P. 3D finite element analysis of rotary instruments in root canal dentine with different elastic moduli. Appl. Sci. 2021, 11, 2547. [Google Scholar] [CrossRef]
- Cipollina, A.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Tumedei, M.; Romasco, T.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite element analysis (FEA) of a premaxillary device: A new type of subperiosteal implant to treat severe atrophy of the maxilla. Biomimetics 2023, 8, 336. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, L.; Wang, Y.; Chu, Y.; Lu, Y. Finite element analysis in the dental sciences: A bibliometric and a visual study. Int. Dent. J. 2024, 25, 855–867. [Google Scholar]
- Ausiello, P.; Di Lauro, A.E.; Tribst, J.P.M.; Watts, D.C. Stress distribution in resin-based CAD-CAM implant-supported crowns. Dent. Mater. 2023, 39, 114–122. [Google Scholar]
- Talreja, K.S.; Rodrigues, S.J.; Pai, U.Y.; Shetty, T.; Saldanha, S.; Mahesh, M.; Hegde, P.; Shenoy, S.B.; Naik, N.; Mukherjee, S.; et al. A nonlinear three-dimensional finite element analysis of stress distribution and microstrain evaluation in short dental implants with three different implant-abutment connections in single and splinted conditions in the posterior mandible. Int. J. Dent. 2023, 2023, 8851098. [Google Scholar]
- Huang, Z.L.; Shi, J.Y.; Zhang, X.; Gu, Y.X.; Lai, H.C. The influence of the shock-absorbing restorative materials on the stress distributions of short dental implant rehabilitations. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 24–34. [Google Scholar]
- Ausiello, P.; Tribst, J.P.M.; Ventre, M.; Salvati, E.; di Lauro, A.E.; Martorelli, M.; Lanzotti, A.; Watts, D.C. The role of cortical zone level and prosthetic platform angle in dental implant mechanical response: A 3D finite element analysis. Dent. Mater. 2021, 37, 1688–1697. [Google Scholar] [CrossRef]
- Garavelli, C.; Curreli, C.; Palanca, M.; Aldieri, A.; Cristofolini, L.; Viceconti, M. Experimental validation of a subject-specific finite element model of lumbar spine segment using digital image correlation. PLoS ONE 2022, 17, e0272529. [Google Scholar] [CrossRef]
- Zannoni, C.; Mantovani, R.; Viceconti, M. Material properties assignment to finite element models of bone structures: A new method. Med. Eng. Phys. 1998, 20, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Schileo, E.; Dall’Ara, E.; Taddei, F.; Malandrino, A.; Schotkamp, T.; Baleani, M.; Viceconti, M. An accurate estimation of bone density improves the accuracy of subject-specific finite element models. J. Biomech. 2008, 41, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Tadde, F.; Schileo, E.; Helgason, B.; Cristofolini, L.; Viceconti, M. The material mapping strategy influences the accuracy of CT-based finite element models of bones: An evaluation against experimental measurements. Med. Eng. Phys. 2007, 29, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular Bone Modulus-Density Relationships Depend on Anatomic Site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Lee, S.T.; Chen, J.F. Percutaneous trigeminal ganglion balloon compression for treatment of trigeminal neuralgia—Part I: Pressure recordings. Surg. Neurol. 2003, 59, 63–66, discussion 66–67. [Google Scholar] [CrossRef]
- Faverani, L.P.; Barão, V.A.; Ramalho-Ferreira, G.; Delben, J.A.; Ferreira, M.B.; Garcia Júnior, I.R.; Assunção, W.G. The influence of bone quality on the biomechanical behavior of full-arch implant-supported fixed prostheses. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 37, 164–170. [Google Scholar] [CrossRef]
- Kitagawa, T.; Tanimoto, Y.; Nemoto, K.; Aida, M. Influence of cortical bone quality on stress distribution in bone around dental implant. Dent. Mater. J. 2005, 24, 219–224. [Google Scholar] [CrossRef]
- Chuang, C.L.; Lai, C.L.; Huang, A.C.; Su, P.H.; Chu, L.P.; Hsieh, K.C.; Lu, H.K. Comparison of whole body bone mineral density measurements between dual energy X-ray absorptiometry and novel bioelectrical impedance analysis. Sci. Rep. 2024, 14, 29127. [Google Scholar] [CrossRef]
- Calvo-Gallego, J.L.; Gutiérrez-Millán, F.; Ojeda, J.; Pérez, M.Á.; Martínez-Reina, J. The correlation between bone density and mechanical variables in bone remodelling models: Insights from a case study corresponding to the femur of a healthy adult. Mathematics 2022, 10, 3367. [Google Scholar] [CrossRef]
- Helgason, B.; Perilli, E.; Schileo, E.; Taddei, F.; Brynjólfsson, S.; Viceconti, M. Mathematical relationships between bone density and mechanical properties: A literature review. Clin. Biomech. 2008, 23, 135–146. [Google Scholar] [CrossRef]
- Morgan, E.F.; Yeh, O.C.; Chang, W.C.; Keaveny, T.M. Nonlinear behavior of trabecular bone at small strains. J. Biomech. Eng. 2001, 123, 1–9. [Google Scholar] [PubMed]
- Bertl, K.; Subotic, M.; Heimel, P.; Schwarze, U.Y.; Tangl, S.; Ulm, C. Morphometric characteristics of cortical and trabecular bone in atrophic edentulous mandibles. Clin. Oral Implant. Res. 2015, 26, 780–787. [Google Scholar]
- Ataman-Duruel, E.T.; Beycioğlu, Z.; Yılmaz, D.; Goyushov, S.; Çimen, T.; Duruel, O.; Yılmaz, H.G.; Tözüm, T.F. Evaluation of cortical thicknesses and bone density values of mandibular canal borders and coronal site of alveolar crest. J. Oral Maxillofac. Res. 2023, 14, e4. [Google Scholar]
- Aboelfadl, A.; Keilig, L.; Ebeid, K.; Ahmed, M.A.M.; Nouh, I.; Refaie, A.; Bourauel, C. Biomechanical behavior of implant-retained prostheses in the posterior maxilla using different materials: A finite element study. BMC Oral Health 2024, 24, 455. [Google Scholar]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Barausse, C.; Pistilli, R.; Bonifazi, L.; Tayeb, S.; Pellegrino, G.; Ravidà, A.; Felice, P. Four-mm-short implants in the rehabilitation of posterior atrophic jaws: A retrospective study on 212 patients with a mean follow-up of 8.02 years. Clin. Oral Implant. Res. 2024, 35, 1607–1615. [Google Scholar] [CrossRef]
- Esposito, M.; Barausse, C.; Pistilli, R.; Checchi, V.; Diazzi, M.; Gatto, M.R.; Felice, P. Posterior jaws rehabilitated with partial prostheses supported by 4.0 x 4.0 mm or by longer implants: Four-month post-loading data from a randomised controlled trial. Eur. J. Oral Implantol. 2015, 8, 221–230. [Google Scholar]
- Ravidà, A.; Serroni, M.; Borgnakke, W.S.; Romandini, M.; Wang, I.I.; Arena, C.; Annunziata, M.; Cecoro, G.; Saleh, M.H.A. Short (≤6 mm) compared with ≥10-mm dental implants in different clinical scenarios: A systematic review of randomized clinical trials with meta-analysis, trial sequential analysis and quality of evidence grading. J. Clin. Periodontol. 2024, 51, 936–965. [Google Scholar]
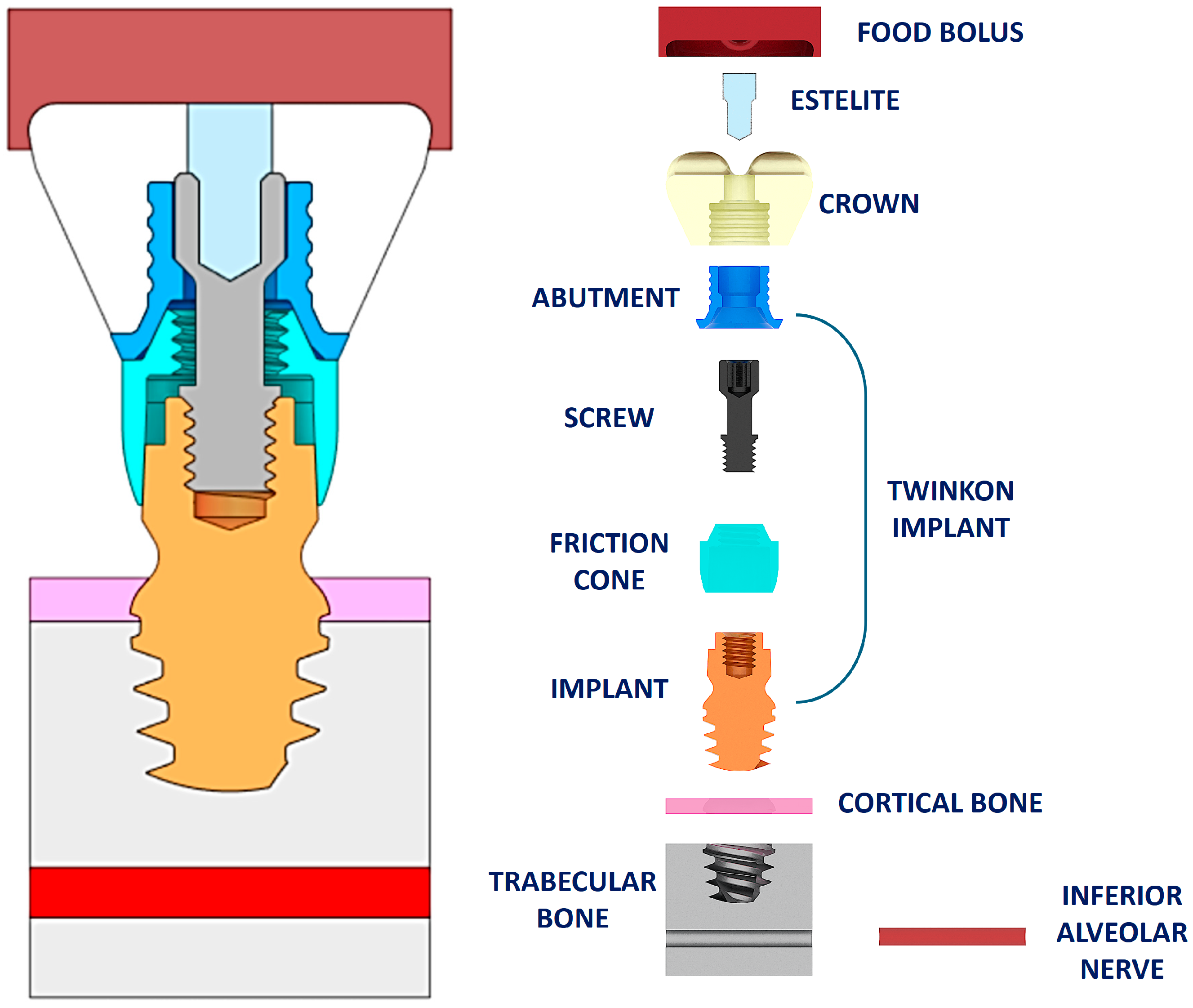
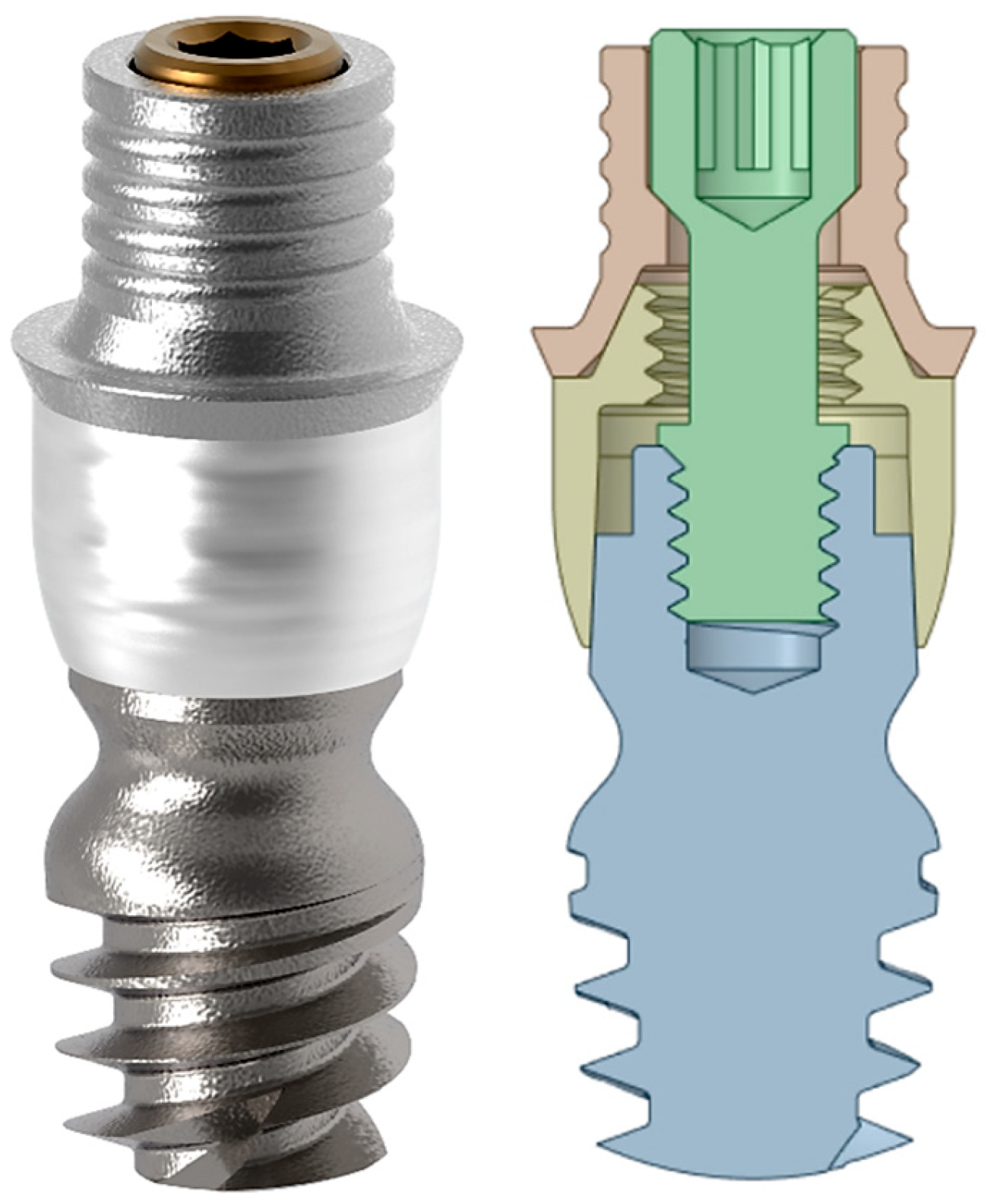
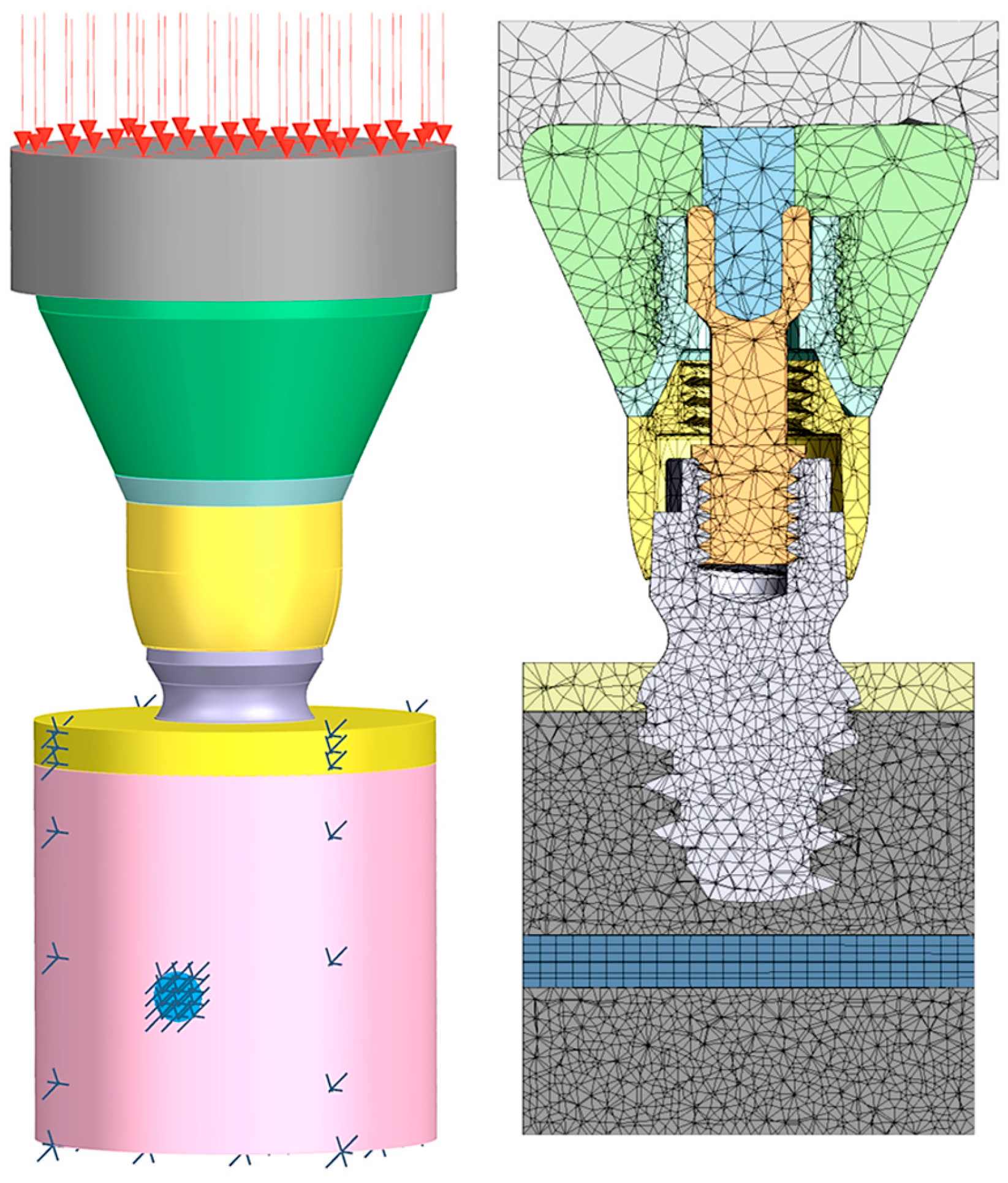
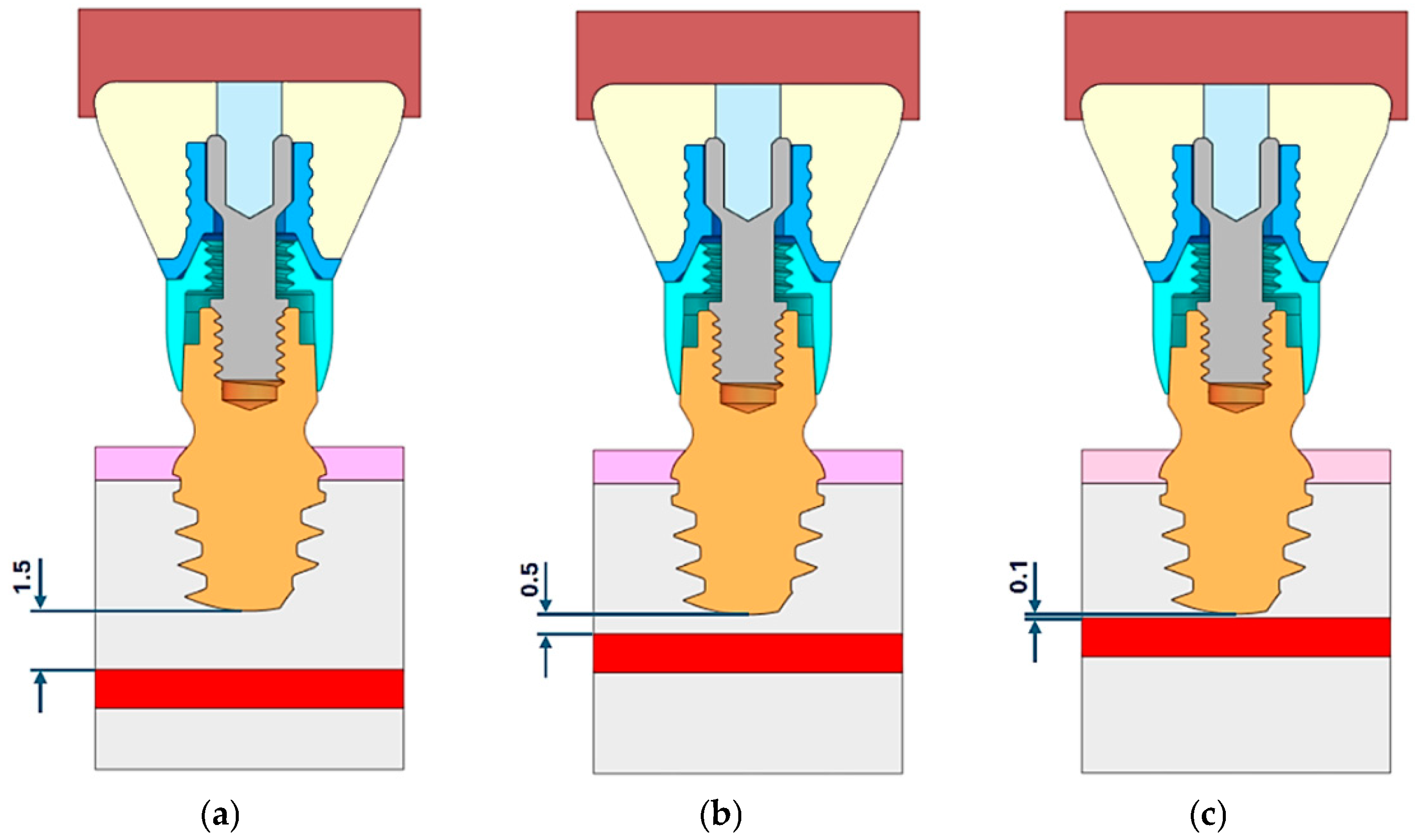




| Structure | Young’s Modulus | Poisson’s Ratio | |
|---|---|---|---|
| 1 | Bolus | 3.4 GPa | 0.10 |
| 2 | Cortical Bone | 13.7 GPa | 0.30 |
| 3 | Trabecular Bone | 4.5 GPa | 0.30 |
| 4 | Estelite P-Block (Composite Resin) | 13.8 GPa | 0.30 |
| 5 | Ti-6al-4v | 113 GPa | 0.35 |
| 6 | Inferior Alveolar Nerve | 0.0013 GPa | 0.40 |
| 7 | Zirconia (Crown) | 200 GPa | 0.30 |
| HU | ρQCT | ρASH | E (GPa) | |
|---|---|---|---|---|
| 1 | 1250 | 1.048 | 0.998 | 10.5 |
| 2 | 1175 | 0.984 | 0.942 | 9.5 |
| 3 | 1100 | 0.920 | 0.886 | 8.5 |
| 4 | 1000 | 0.835 | 0.812 | 7.5 |
| 5 | 900 | 0.750 | 0.737 | 6.5 |
| 6 | 800 | 0.665 | 0.662 | 5.5 |
| 7 | 700 | 0.580 | 0.587 | 4.5 |
| 8 | 575 | 0.473 | 0.494 | 3.5 |
| 9 | 450 | 0.367 | 0.401 | 2.5 |
| 10 | 375 | 0.303 | 0.345 | 2.0 |
| 11 | 300 | 0.239 | 0.289 | 1.5 |
| 12 | 210 | 0.162 | 0.221 | 1.0 |
| 13 | 105 | 0.073 | 0.143 | 0.5 |
| 14 | 20 | 0.001 | 0.080 | 0.2 |
| 1.5 mm | 0.5 mm | 0.1 mm | |
|---|---|---|---|
| E = 0.2 GPa | p: 0.018 MPa | p: 0.023 MPa | p: 0.024 MPa |
| E = 4.5 GPa | p: 0.003 MPa | p: 0.004 MPa | p: 0.004 MPa |
| E = 10.5 GPa | p: 0.001 MPa | p: 0.002 MPa | p: 0.002 MPa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasparro, R.; Renno, F.; De Vita, S.; Lanzotti, A.; Martorelli, M.; Penta, F.; Sammartino, G.; Ausiello, P. Loading Pressure Induced by 4 mm Implants on the Inferior Alveolar Nerve: A 3D Finite Element Analysis Model. J. Clin. Med. 2025, 14, 2535. https://doi.org/10.3390/jcm14072535
Gasparro R, Renno F, De Vita S, Lanzotti A, Martorelli M, Penta F, Sammartino G, Ausiello P. Loading Pressure Induced by 4 mm Implants on the Inferior Alveolar Nerve: A 3D Finite Element Analysis Model. Journal of Clinical Medicine. 2025; 14(7):2535. https://doi.org/10.3390/jcm14072535
Chicago/Turabian StyleGasparro, Roberta, Fabrizio Renno, Simone De Vita, Antonio Lanzotti, Massimo Martorelli, Francesco Penta, Gilberto Sammartino, and Pietro Ausiello. 2025. "Loading Pressure Induced by 4 mm Implants on the Inferior Alveolar Nerve: A 3D Finite Element Analysis Model" Journal of Clinical Medicine 14, no. 7: 2535. https://doi.org/10.3390/jcm14072535
APA StyleGasparro, R., Renno, F., De Vita, S., Lanzotti, A., Martorelli, M., Penta, F., Sammartino, G., & Ausiello, P. (2025). Loading Pressure Induced by 4 mm Implants on the Inferior Alveolar Nerve: A 3D Finite Element Analysis Model. Journal of Clinical Medicine, 14(7), 2535. https://doi.org/10.3390/jcm14072535











