Effects of Mindfulness and Exercise on Growth Factors, Inflammation, and Stress Markers in Chronic Stroke: The MindFit Project Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Setting
2.2. Recruitment
2.3. Eligibility Criteria
2.4. Randomization and Blinding
2.5. Interventions
2.5.1. General Overview
2.5.2. Mindfulness-Based Stress Reduction
2.5.3. Physical Exercise
2.5.4. Computerized Cognitive Training
2.6. Intervention Adherence and Safety
2.7. Assessments and Outcome Measures
2.7.1. Baseline Measures
2.7.2. Biomarker Outcomes
2.7.3. Behavioral Outcomes
2.8. Statistical Analysis
3. Results
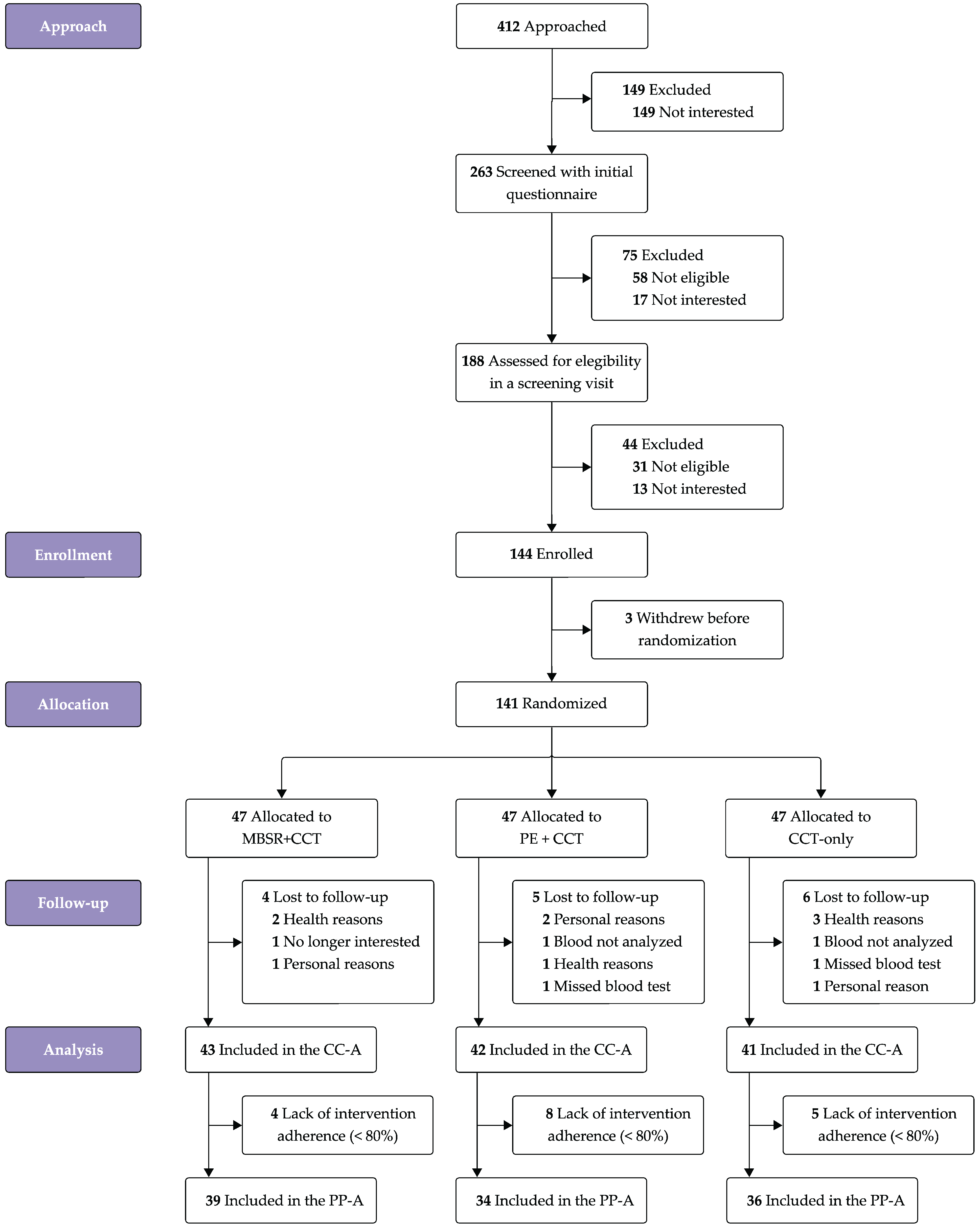
3.1. Participants
3.2. Intervention Adherence and Safety
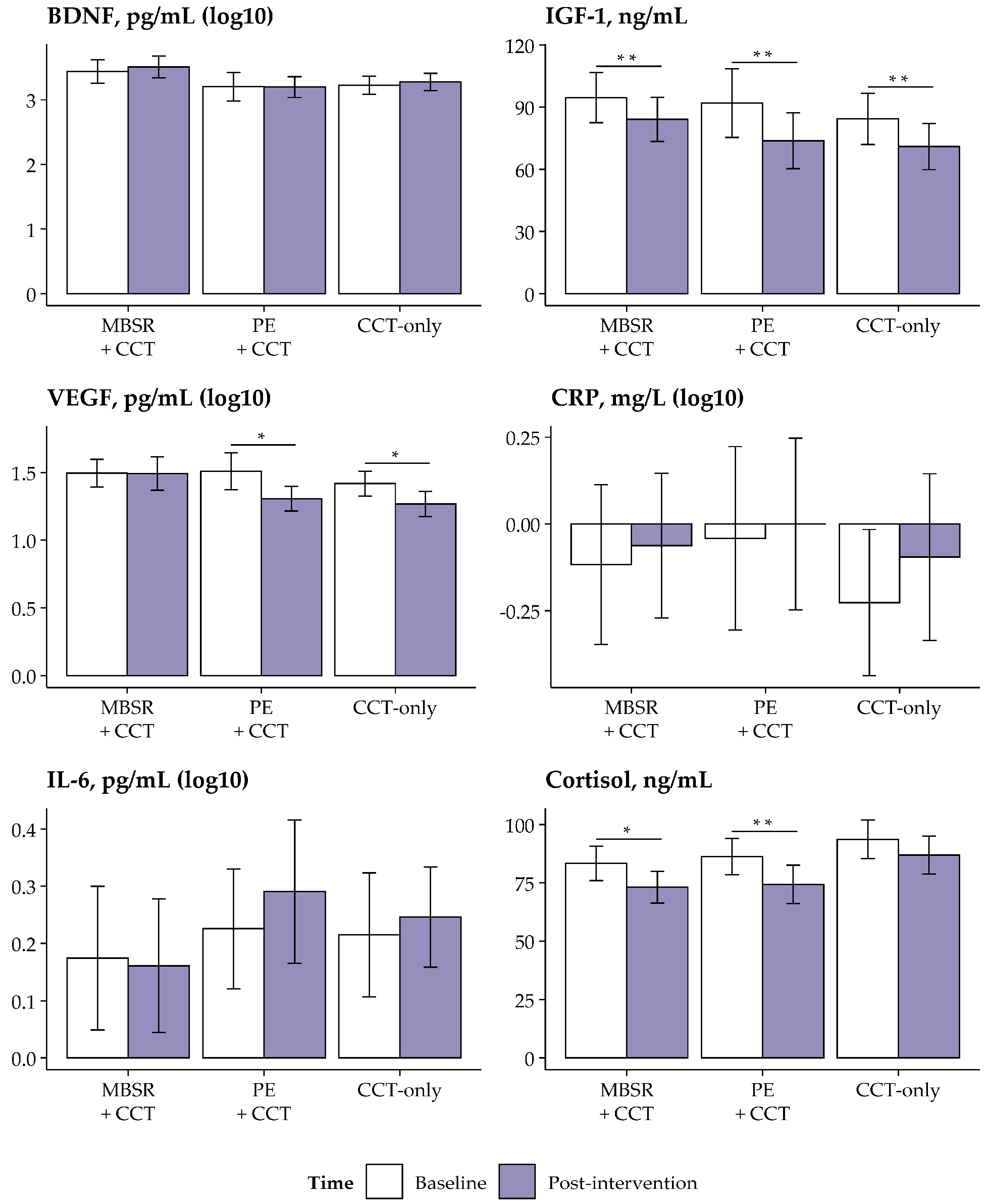
3.3. Between-Group Changes in Biomarkers
3.4. Within-Group Changes in Biomarkers
3.5. Mediation and Correlational Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANCOVA | Analysis of Covariance |
| BDI-II | Beck Depression Inventory-II |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMI | Body Mass Index |
| CCT | Computerized Cognitive Training |
| CRP | C-Reactive Protein |
| FDR | False Discovery Rate |
| HPA | Hypothalamic–Pituitary–Adrenal |
| IGF-1 | Insulin-Like Growth Factor-1 |
| IL-6 | Interleukin-6 |
| LLOQ | Lower Limit of Quantification |
| MBIs | Mindfulness-Based Interventions |
| MBSR | Mindfulness-Based Stress Reduction |
| MedAS | Mediterranean Diet Adherence Screener |
| MMSE | Mini-Mental State Examination |
| mRS | Modified Rankin Scale |
| NIHSS | National Institutes of Health Stroke Scale |
| NGF | Nerve Growth Factor |
| NO | Nitric Oxide |
| PE | Physical Exercise |
| Post-int. | Post-Intervention |
| PSQI | Pittsburg Sleep Quality Index |
| RPE | Rate of Perceived Exertion |
| SDF | Stromal Cell-Derived Factor 1-Alpha (SDF1-α) |
| SFT | Senior Fitness Test |
| VEGF | Vascular Endothelial Growth Factor |
| VREM | Short Version of the Minnesota Leisure Time Physical Activity Questionnaire |
| WAIS-III | Weschler Adult Intelligence Scale-III |
References
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; Abd ElHafeez, S.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- eClinicalMedicine. The Rising Global Burden of Stroke. eClinicalMedicine 2023, 59, 02028. [Google Scholar] [CrossRef]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Wolfe, C.D.A.; Rudd, A.G. Natural History, Predictors and Outcomes of Depression after Stroke: Systematic Review and Meta-Analysis. Br. J. Psychiatry 2013, 202, 14–21. [Google Scholar] [CrossRef] [PubMed]
- El Husseini, N.; Katzan, I.L.; Rost, N.S.; Blake, M.L.; Byun, E.; Pendlebury, S.T.; Aparicio, H.J.; Marquine, M.J.; Gottesman, R.F.; Smith, E.E.; et al. Cognitive Impairment After Ischemic and Hemorrhagic Stroke: A Scientific Statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e272–e291. [Google Scholar] [CrossRef]
- Ferro, J.M.; Santos, A.C. Emotions after Stroke: A Narrative Update. Int. J. Stroke 2020, 15, 256–267. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke Rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Lou, S.; Carstensen, K.; Jørgensen, C.R.; Nielsen, C.P. Stroke Patients’ and Informal Carers’ Experiences with Life after Stroke: An Overview of Qualitative Systematic Reviews. Disabil. Rehabil. 2017, 39, 301–313. [Google Scholar] [CrossRef]
- Mancin, S.; Sguanci, M.; Reggiani, F.; Morenghi, E.; Piredda, M.; De Marinis, M.G. Dysphagia Screening Post-Stroke: Systematic Review. BMJ Support. Palliat. Care 2024, 13, e641–e650. [Google Scholar] [CrossRef]
- Cicerone, K.D.; Goldin, Y.; Ganci, K.; Rosenbaum, A.; Wethe, J.V.; Langenbahn, D.M.; Malec, J.F.; Bergquist, T.F.; Kingsley, K.; Nagele, D.; et al. Evidence-Based Cognitive Rehabilitation: Systematic Review of the Literature From 2009 Through 2014. Arch. Phys. Med. Rehabil. 2019, 100, 1515–1533. [Google Scholar] [CrossRef] [PubMed]
- Solana, J.; Cáceres, C.; García-Molina, A.; Opisso, E.; Roig, T.; Tormos, J.M.; Gómez, E.J. Improving Brain Injury Cognitive Rehabilitation by Personalized Telerehabilitation Services: Guttmann Neuropersonal Trainer. IEEE J. Biomed. Health 2015, 19, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Mingming, Y.; Bolun, Z.; Zhijian, L.; Yingli, W.; Lanshu, Z. Effectiveness of Computer-Based Training on Post-Stroke Cognitive Rehabilitation: A Systematic Review and Meta-Analysis. Neuropsychol. Rehabil. 2022, 32, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Liu, F.; Lin, S.; Guo, J.; Chen, X.; Chen, S.; Yu, L.; Lin, R. The Effects of Computer-Assisted Cognitive Rehabilitation on Cognitive Impairment after Stroke: A Systematic Review and Meta-Analysis. J. Clin. Nurs. 2022, 31, 1136–1148. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, H.; Li, G.; Xu, C.; Wu, Y.; Li, H. Efficacy of Computerized Cognitive Training on Improving Cognitive Functions of Stroke Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Nurs. Pract. 2022, 28, e12966. [Google Scholar] [CrossRef]
- Castells-Sánchez, A.; Roig-Coll, F.; Dacosta-Aguayo, R.; Lamonja-Vicente, N.; Torán-Monserrat, P.; Pera, G.; García-Molina, A.; Tormos, J.M.; Montero-Alía, P.; Heras-Tébar, A.; et al. Molecular and Brain Volume Changes Following Aerobic Exercise, Cognitive and Combined Training in Physically Inactive Healthy Late-Middle-Aged Adults: The Projecte Moviment Randomized Controlled Trial. Front. Hum. Neurosci. 2022, 16, 854175. [Google Scholar] [CrossRef]
- Gates, N.; Rutjes, A.; Di Nisio, M.; Karim, S.; Chong, L.; March, E.; Martínez, G.; Vernooij, R. Computerised Cognitive Training for 12 or More Weeks for Maintaining Cognitive Function in Cognitively Healthy People in Late Life. Cochrane Database Syst. Rev. 2020, 2, CD012277. [Google Scholar] [CrossRef]
- Lin, Z.; Tao, J.; Gao, Y.; Yin, D.; Chen, A.; Chen, L. Analysis of Central Mechanism of Cognitive Training on Cognitive Impairment after Stroke: Resting-State Functional Magnetic Resonance Imaging Study. J. Int. Med. Res. 2014, 42, 659–668. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Ye, H.; Tao, J.; Huang, J.; Gao, Y.; Lin, Z.; Chen, L. Effect of Integrated Cognitive Therapy on Hippocampal Functional Connectivity Patterns in Stroke Patients with Cognitive Dysfunction: A Resting-State FMRI Study. Evid. Based Complement. Altern. Med. 2014, 2014, 962304. [Google Scholar] [CrossRef]
- Nyberg, C.K.; Nordvik, J.E.; Becker, F.; Rohani, D.A.; Sederevicius, D.; Fjell, A.M.; Walhovd, K.B. A Longitudinal Study of Computerized Cognitive Training in Stroke Patients—Effects on Cognitive Function and White Matter. Top. Stroke Rehabil. 2018, 25, 241–247. [Google Scholar] [CrossRef]
- Valenzuela, M.; Sachdev, P. Can Cognitive Exercise Prevent the Onset of Dementia? Systematic Review of Randomized Clinical Trials with Longitudinal Follow-Up. Am. J. Geriatr. Psychiatry 2009, 17, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Damirchi, A.; Hosseini, F.; Babaei, P. Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. Am. J. Alzheimers Dis. Other Demen. 2018, 33, 20–29. [Google Scholar] [CrossRef]
- Nicastri, C.M.; McFeeley, B.M.; Simon, S.S.; Ledreux, A.; Håkansson, K.; Granholm, A.-C.; Mohammed, A.H.; Daffner, K.R. BDNF Mediates Improvement in Cognitive Performance after Computerized Cognitive Training in Healthy Older Adults. Alzheimers Dement. 2022, 8, e12337. [Google Scholar] [CrossRef]
- Ploughman, M.; Eskes, G.A.; Kelly, L.P.; Kirkland, M.C.; Devasahayam, A.J.; Wallack, E.M.; Abraha, B.; Hasan, S.M.M.; Downer, M.B.; Keeler, L.; et al. Synergistic Benefits of Combined Aerobic and Cognitive Training on Fluid Intelligence and the Role of IGF-1 in Chronic Stroke. Neurorehabil. Neural Repair 2019, 33, 199–212. [Google Scholar] [CrossRef]
- Livingston-Thomas, J.; Nelson, P.; Karthikeyan, S.; Antonescu, S.; Jeffers, M.S.; Marzolini, S.; Corbett, D. Exercise and Environmental Enrichment as Enablers of Task-Specific Neuroplasticity and Stroke Recovery. Neurotherapeutics 2016, 13, 395–402. [Google Scholar] [CrossRef]
- Han, P.-P.; Han, Y.; Shen, X.-Y.; Gao, Z.-K.; Bi, X. Enriched Environment-Induced Neuroplasticity in Ischemic Stroke and Its Underlying Mechanisms. Front. Cell. Neurosci. 2023, 17, 1210361. [Google Scholar] [CrossRef]
- Bunketorp-Käll, L.; Lundgren-Nilsson, Å.; Samuelsson, H.; Pekny, T.; Blomvé, K.; Pekna, M.; Pekny, M.; Blomstrand, C.; Nilsson, M. Long-Term Improvements After Multimodal Rehabilitation in Late Phase After Stroke. Stroke 2017, 48, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.T.; Paz, L.V.; Wieck, A.; Mestriner, R.G.; de Miranda Monteiro, V.A.C.; Xavier, L.L. Environmental Enrichment in Stroke Research: An Update. Transl. Stroke Res. 2024, 15, 339–351. [Google Scholar] [CrossRef]
- O’ Donoghue, M.; Boland, P.; Taylor, S.; Hennessy, E.; Murphy, E.; Leahy, S.; McManus, J.; Lisiecka, D.; Purtill, H.; Galvin, R.; et al. OptiCogs: Feasibility of a Multicomponent Intervention to Rehabilitate People with Cognitive Impairment Post-Stroke. Pilot Feasibility Stud. 2023, 9, 178. [Google Scholar] [CrossRef]
- Mak, T.C.T.; Wong, T.W.L.; Ng, S.S.M. The Use of Mindfulness-Based Interventions in Stroke Rehabilitation: A Scoping Review. Rehabil. Psychol. 2023, 68, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Geng, Y.; Li, M.; Ye, J.; Liu, Z. Effectiveness of Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy on Depression in Poststroke Patients-A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Psychosom. Res. 2022, 163, 111071. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Sasaki, J.E.; Zeng, N.; Wang, C.; Sun, L. A Systematic Review with Meta-Analysis of Mindful Exercises on Rehabilitative Outcomes Among Poststroke Patients. Arch. Phys. Med. Rehabil. 2018, 99, 2355–2364. [Google Scholar] [CrossRef]
- Eng, J.J.; Reime, B. Exercise for Depressive Symptoms in Stroke Patients: A Systematic Review and Meta-Analysis. Clin. Rehabil. 2014, 28, 731–739. [Google Scholar] [CrossRef]
- Li, G.; Tao, X.; Lei, B.; Hou, X.; Yang, X.; Wang, L.; Zhang, S.; Lv, Y.; Wang, T.; Yu, L. Effects of Exercise on Post-Stroke Cognitive Function: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Top. Stroke Rehabil. 2024, 31, 645–666. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, L.E.; Waiwood, A.M.; Cumming, T.B.; Marsland, A.L.; Bernhardt, J.; Erickson, K.I. Effects of Physical Activity on Poststroke Cognitive Function. Stroke 2017, 48, 3093–3100. [Google Scholar] [CrossRef]
- Saunders, D.; Sanderson, M.; Hayes, S.; Johnson, L.; Kramer, S.; Carter, D.; Jarvis, H.; Brazzelli, M.; Mead, G. Physical Fitness Training for Stroke Patients. Cochrane Database Syst. Rev. 2020, 3, CD003316. [Google Scholar] [CrossRef]
- Khalil, M.H. The BDNF-Interactive Model for Sustainable Hippocampal Neurogenesis in Humans: Synergistic Effects of Environmentally-Mediated Physical Activity, Cognitive Stimulation, and Mindfulness. Int. J. Mol. Sci. 2024, 25, 12924. [Google Scholar] [CrossRef]
- Creswell, J.D.; Lindsay, E.K.; Villalba, D.K.; Chin, B. Mindfulness Training and Physical Health: Mechanisms and Outcomes. Psychosom. Med. 2019, 81, 224–232. [Google Scholar] [CrossRef]
- Leistner, C.; Menke, A. Chapter 4—Hypothalamic–Pituitary–Adrenal Axis and Stress. In Handbook of Clinical Neurology; Lanzenberger, R., Kranz, G.S., Savic, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 175, pp. 55–64. ISBN 0072-9752. [Google Scholar]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef]
- Black, D.S.; Slavich, G.M. Mindfulness Meditation and the Immune System: A Systematic Review of Randomized Controlled Trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef]
- Dunn, T.J.; Dimolareva, M. The Effect of Mindfulness-Based Interventions on Immunity-Related Biomarkers: A Comprehensive Meta-Analysis of Randomised Controlled Trials. Clin. Psychol. Rev. 2022, 92, 102124. [Google Scholar] [CrossRef] [PubMed]
- Grasmann, J.; Almenräder, F.; Voracek, M.; Tran, U.S. Only Small Effects of Mindfulness-Based Interventions on Biomarker Levels of Inflammation and Stress: A Preregistered Systematic Review and Two Three-Level Meta-Analyses. Int. J. Mol. Sci. 2023, 24, 4445. [Google Scholar] [CrossRef]
- Koncz, A.; Demetrovics, Z.; Takacs, Z.K. Meditation Interventions Efficiently Reduce Cortisol Levels of At-Risk Samples: A Meta-Analysis. Health Psychol. Rev. 2021, 15, 56–84. [Google Scholar] [CrossRef] [PubMed]
- Matiz, A.; Scaggiante, B.; Conversano, C.; Gemignani, A.; Pascoletti, G.; Fabbro, F.; Crescentini, C. The Effect of Mindfulness-Based Interventions on Biomarkers in Cancer Patients and Survivors: A Systematic Review. Stress Health 2024, 40, e3375. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, M.C.; Thompson, D.R.; Jenkins, Z.M.; Ski, C.F. Mindfulness Mediates the Physiological Markers of Stress: Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2017, 95, 156–178. [Google Scholar] [CrossRef]
- Reive, C. The Biological Measurements of Mindfulness-Based Stress Reduction: A Systematic Review. Explore 2019, 15, 295–307. [Google Scholar] [CrossRef]
- Sanada, K.; Montero-Marin, J.; Alda Díez, M.; Salas-Valero, M.; Pérez-Yus, M.C.; Morillo, H.; Demarzo, M.M.P.; García-Toro, M.; García-Campayo, J. Effects of Mindfulness-Based Interventions on Salivary Cortisol in Healthy Adults: A Meta-Analytical Review. Front. Psychol. 2016, 7, 471. [Google Scholar] [CrossRef]
- Sanada, K.; Alda Díez, M.; Salas Valero, M.; Pérez-Yus, M.C.; Demarzo, M.M.P.; Montero-Marín, J.; García-Toro, M.; García-Campayo, J. Effects of Mindfulness-Based Interventions on Biomarkers in Healthy and Cancer Populations: A Systematic Review. BMC Complement. Altern. Med. 2017, 17, 125. [Google Scholar] [CrossRef]
- Sanada, K.; Montero-Marin, J.; Barceló-Soler, A.; Ikuse, D.; Ota, M.; Hirata, A.; Yoshizawa, A.; Hatanaka, R.; Valero, M.S.; Demarzo, M.; et al. Effects of Mindfulness-Based Interventions on Biomarkers and Low-Grade Inflammation in Patients with Psychiatric Disorders: A Meta-Analytic Review. Int. J. Mol. Sci. 2020, 21, 2484. [Google Scholar] [CrossRef]
- Gomutbutra, P.; Yingchankul, N.; Chattipakorn, N.; Chattipakorn, S.; Srisurapanont, M. The Effect of Mindfulness-Based Intervention on Brain-Derived Neurotrophic Factor (BDNF): A Systematic Review and Meta-Analysis of Controlled Trials. Front. Psychol. 2020, 11, 2209. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.-A. Exercise Builds Brain Health: Key Roles of Growth Factor Cascades and Inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Lu, Y.; Bu, F.-Q.; Wang, F.; Liu, L.; Zhang, S.; Wang, G.; Hu, X.-Y. Recent Advances on the Molecular Mechanisms of Exercise-Induced Improvements of Cognitive Dysfunction. Transl. Neurodegener. 2023, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Boa Sorte Silva, N.C.; Barha, C.K.; Erickson, K.I.; Kramer, A.F.; Liu-Ambrose, T. Physical Exercise, Cognition, and Brain Health in Aging. Trends Neurosci. 2024, 47, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef]
- Dadkhah, M.; Saadat, M.; Ghorbanpour, A.M.; Moradikor, N. Experimental and Clinical Evidence of Physical Exercise on BDNF and Cognitive Function: A Comprehensive Review from Molecular Basis to Therapy. Brain Behav. Immun. Integr. 2023, 3, 100017. [Google Scholar] [CrossRef]
- De Las Heras, B.; Rodrigues, L.; Cristini, J.; Moncion, K.; Ploughman, M.; Tang, A.; Fung, J.; Roig, M. Measuring Neuroplasticity in Response to Cardiovascular Exercise in People with Stroke: A Critical Perspective. Neurorehabil. Neural Repair 2024, 38, 303–321. [Google Scholar] [CrossRef]
- El-Tamawy, M.S.; Abd-Allah, F.; Ahmed, S.M.; Darwish, M.H.; Khalifa, H.A. Aerobic Exercises Enhance Cognitive Functions and Brain Derived Neurotrophic Factor in Ischemic Stroke Patients. NeuroRehabilitation 2014, 34, 209–213. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Fu, T.-C.; Huang, S.-C.; Chen, C.P.-C.; Wang, J.-S. Increased Serum Brain-Derived Neurotrophic Factor with High-Intensity Interval Training in Stroke Patients: A Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2021, 64, 101385. [Google Scholar] [CrossRef]
- Kim, T.-G.; Bae, S.-H.; Kim, K.-Y. Effects of Dual-Task Training with Different Intensity of Aerobic Exercise on Cognitive Function and Neurotrophic Factors in Chronic Stroke Patients. Res. J. Pharm. Technol. 2019, 12, 693–698. [Google Scholar] [CrossRef]
- Steen Krawcyk, R.; Vinther, A.; Petersen, N.C.; Faber, J.; Iversen, H.K.; Christensen, T.; Lambertsen, K.L.; Rehman, S.; Klausen, T.W.; Rostrup, E.; et al. Effect of Home-Based High-Intensity Interval Training in Patients with Lacunar Stroke: A Randomized Controlled Trial. Front. Neurol. 2019, 10, 664. [Google Scholar] [CrossRef]
- Valkenborghs, S.R.; van Vliet, P.; Nilsson, M.; Zalewska, K.; Visser, M.M.; Erickson, K.I.; Callister, R. Aerobic Exercise and Consecutive Task-Specific Training (AExaCTT) for Upper Limb Recovery after Stroke: A Randomized Controlled Pilot Study. Physiother. Res. Int. 2019, 24, e1775. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, S.K.; Ironside, D.D.; Johnson, L.; Kuys, S.S.; Thompson-Butel, A.G. Effect of Exercise on Brain-Derived Neurotrophic Factor in Stroke Survivors: A Systematic Review and Meta-Analysis. Stroke 2022, 53, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-C.; Tsai, H.-H.; Fu, T.-C.; Wang, J.-S. Exercise Training Enhances Platelet Mitochondrial Bioenergetics in Stroke Patients: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2186. [Google Scholar] [CrossRef]
- Kirzinger, B.; Stroux, A.; Rackoll, T.; Endres, M.; Flöel, A.; Ebinger, M.; Nave, A.H. Elevated Serum Inflammatory Markers in Subacute Stroke Are Associated with Clinical Outcome but Not Modified by Aerobic Fitness Training: Results of the Randomized Controlled PHYS-STROKE Trial. Front. Neurol. 2021, 12, 713018. [Google Scholar] [CrossRef]
- Serra, M.C.; Hafer-Macko, C.E.; Robbins, R.; O’Connor, J.C.; Ryan, A.S. Randomization to Treadmill Training Improves Physical and Metabolic Health in Association with Declines in Oxidative Stress in Stroke. Arch. Phys. Med. Rehabil. 2022, 103, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Amorós-Aguilar, L.; Rodríguez-Quiroga, E.; Sánchez-Santolaya, S.; Coll-Andreu, M. Effects of Combined Interventions with Aerobic Physical Exercise and Cognitive Training on Cognitive Function in Stroke Patients: A Systematic Review. Brain Sci. 2021, 11, 473. [Google Scholar] [CrossRef]
- Bermudo-Gallaguet, A.; Ariza, M.; Dacosta-Aguayo, R.; Agudelo, D.; Camins-Vila, N.; Boldó, M.; Carrera, Ò.; Vidal, S.; Ferrer-Uris, B.; Busquets, A.; et al. Effects and Mechanisms of Mindfulness Training and Physical Exercise on Cognition, Emotional Wellbeing, and Brain Outcomes in Chronic Stroke Patients: Study Protocol of the MindFit Project Randomized Controlled Trial. Front. Aging Neurosci. 2022, 14, 936077. [Google Scholar] [CrossRef]
- Bermudo-Gallaguet, A.; Ariza, M.; Agudelo, D.; Camins-Vila, N.; Boldó, M.; Ferrer-Uris, B.; Busquets, A.; Pera, G.; Cáceres, C.; Gomis, M.; et al. Effects of Mindfulness and Physical Exercise on Cognition and Emotion in Chronic Stroke Patients: The MindFit Project Randomized Clinical Trial. manuscript in preparation.
- Bermudo-Gallaguet, A.; Bielsa-Pascual, J.; García-Sierra, R.; Feijoo-Cid, M.; Arreciado Marañon, A.; Ariza, M.; Agudelo, D.; Camins-Vila, N.; Boldó, M.; Durà Mata, M.J.; et al. Understanding and Enhancing Post-Stroke Recovery: Insights from a Nested Qualitative Study within the MindFit Project Randomized Clinical Trial. Complement. Ther. Med. 2024, 87, 103100. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Montaner, J.; Alvarez-Sabín, J. NIH Stroke Scale and Its Adaptation to Spanish. Neurologia 2006, 21, 192–202. [Google Scholar] [PubMed]
- Blesa, R.; Pujol, M.; Aguilar, M.; Santacruz, P.; Bertran-Serra, I.; Hernández, G.; Sol, J.M.; Peña-Casanova, J. Clinical Validity of the ‘Mini-Mental State’ for Spanish Speaking Communities. Neuropsychologia 2001, 39, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Kabat-Zinn, J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness; Delacorte Press: New York, NY, USA, 1990; ISBN 0-385-29897-8. [Google Scholar]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical Activity and Exercise Recommendations for Stroke Survivors. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef]
- Borg, G.A.V. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Liguori, G.; Feito, Y.; Fountaine, C.J.; Roy, B. ACSM’s Guidelines for Exercise Testing and Prescription, 11th ed.; American College of Sports Medicine’s guidelines for exercise testing and prescription; Wolters Kluwer: Philadelphia, PA, USA, 2021; ISBN 978-1-975150-22-8. [Google Scholar]
- González, N.; Bilbao, A.; Forjaz, M.J.; Ayala, A.; Orive, M.; Garcia-Gutierrez, S.; Hayas, C.L.; Quintana, J.M.; OFF (Older Falls Fracture)-IRYSS group. Psychometric Characteristics of the Spanish Version of the Barthel Index. Aging Clin. Exp. Res. 2018, 30, 489–497. [Google Scholar] [CrossRef]
- Fernández Sanz, A.; Ruíz Serrano, J.; Tejada Meza, H.; Marta Moreno, J. Validation of the Spanish-Language Version of the Simplified Modified Rankin Scale Telephone Questionnaire. Neurol. (Engl. Ed.) 2022, 37, 271–276. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Goodglass, H.; Kaplan, E.; Barresi, B. Evaluación de La Afasia y de Trastornos Relacionados, 3rd ed.; Editorial Médica Panamericana: Madrid, Spain, 2005; ISBN 84-7903-785-7. [Google Scholar]
- Schmid, M. Rey Auditory Verbal Learning Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Rey, A. REY. Test de Copia de Una Figura Compleja, 9th ed.; TEA Ediciones: Madrid, Spain, 2009; ISBN 84-7174-962-9. [Google Scholar]
- Golden, C.J. STROOP. Test de Colores y Palabras, 3rd ed.; TEA Ediciones: Madrid, Spain, 2001; ISBN 84-7174-654-9. [Google Scholar]
- Tombaugh, T.N. Trail Making Test A and B: Normative Data Stratified by Age and Education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Peña-Casanova, J.; Quiñones-Úbeda, S.; Gramunt-Fombuena, N.; Quintana-Aparicio, M.; Aguilar, M.; Badenes, D.; Cerulla, N.; Molinuevo, J.L.; Ruiz, E.; Robles, A.; et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Verbal Fluency Tests. Arch. Clin. Neuropsychol. 2009, 24, 395–411. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS III. Escala de Inteligencia de Wechsler Para Adultos-III, 2nd, rev. ed.; TEA Ediciones: Madrid, Spain, 2001; ISBN 84-7174-695-6. [Google Scholar]
- Sanz, J.; Perdigón, A.L.; Vázquez, C. The Spanish Adaptation of Beck’s Depression Inventory-Ll (BDI-II): 2. Psychometric Properties in the General Population. Clin. Salud 2003, 14, 249–280. [Google Scholar]
- Bados, A.; Solanas, A.; Andrés, R. Psycometric Properties of the Spanish Version of Depression, Anxiety and Stress Scales (DASS). Psicothema 2005, 17, 679–683. [Google Scholar]
- Soler, J.; Tejedor, R.; Feliu-Soler, A.; Pascual, J.C.; Cebolla, A.; Soriano, J.; Alvarez, E.; Perez, V. Psychometric Proprieties of Spanish Version of Mindful Attention Awareness Scale (MAAS). Actas Esp. Psiquiatr. 2012, 40, 19–26. [Google Scholar]
- Aguado, J.; Luciano, J.V.; Cebolla, A.; Serrano-Blanco, A.; Soler, J.; García-Campayo, J. Bifactor Analysis and Construct Validity of the Five Facet Mindfulness Questionnaire (FFMQ) in Non-Clinical Spanish Samples. Front. Psychol. 2015, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, J.C. Senior Fitness Test Manual, 2nd ed.; Human Kinetics: Champaign, IL, USA, 2013; ISBN 978-1-4504-1118-9. [Google Scholar]
- Thompson, C.; Porter Starr, K.N.; Kemp, E.C.; Chan, J.; Jackson, E.; Phun, J. Feasibility of Virtually Delivering Functional Fitness Assessments and a Fitness Training Program in Community-Dwelling Older Adults. Int. J. Environ. Res. Public Health 2023, 20, 5996. [Google Scholar] [CrossRef]
- Yilmaz, E.; Akinci, B.; Utku, G.; Erdinc, E.; Atmaca, I.; Gurlek, E. An Online Functional Assessment Experience in Individuals over 65+ during COVID 19 Pandemics: Physiotherapist Opinion & Participant Opinion. Eur. J. Cardiovasc. Nurs. 2021, 20, zvab060.140. [Google Scholar] [CrossRef]
- Ruiz Comellas, A.; Pera, G.; Baena Díez, J.M.; Mundet Tudurí, X.; Alzamora Sas, T.; Elosua, R.; Torán Monserrat, P.; Heras, A.; Forés Raurell, R.; Fusté Gamisans, M.; et al. Validación de Una Versión Reducida En Español Del Cuestionario de Actividad Física En El Tiempo Libre de Minnesota (VREM). Rev. Esp. Salud Publica 2012, 86, 495–508. [Google Scholar]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef] [PubMed]
- Royuela Rico, A.; Macías Fernández, J.A. Propiedades Clinimetricas de La Versión Castellana Del Cuestionario de Pittsburgh. Vigilia-Sueño 1997, 9, 81–94. [Google Scholar]
- Herbers, J.; Miller, R.; Walther, A.; Schindler, L.; Schmidt, K.; Gao, W.; Rupprecht, F. How to Deal with Non-Detectable and Outlying Values in Biomarker Research: Best Practices and Recommendations for Univariate Imputation Approaches. Compr. Psychoneuroendocrinol. 2021, 7, 100052. [Google Scholar] [CrossRef]
- Rupprecht, F. Lnormimp. Available online: https://github.com/nx10/lnormimp-r (accessed on 14 February 2025).
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Soft 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Robitzsch, A.; Grund, S.; Henke, T. miceadds: Some Additional Multiple Imputation Functions, Especially for ‘Mice’. Available online: https://CRAN.R-project.org/package=miceadds (accessed on 14 February 2025).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R Package for Assessment, Comparison, and Testing of Statistical Models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Stat. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. False Discovery Rate–Adjusted Multiple Confidence Intervals for Selected Parameters. J. Am. Stat. Assoc. 2005, 100, 71–81. [Google Scholar] [CrossRef]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach, 3rd ed.; Methodology in the social sciences; The Guilford Press: New York, NY, USA, 2022; ISBN 978-1-4625-4905-4. [Google Scholar]
- O’Rourke, H.P.; MacKinnon, D.P. Reasons for Testing Mediation in the Absence of an Intervention Effect: A Research Imperative in Prevention and Intervention Research. J. Stud. Alcohol Drugs 2018, 79, 171–181. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-8058-0283-2. [Google Scholar]
- Richardson, J.T.E. Eta Squared and Partial Eta Squared as Measures of Effect Size in Educational Research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Lange, C.; Storkebaum, E.; de Almodóvar, C.R.; Dewerchin, M.; Carmeliet, P. Vascular Endothelial Growth Factor: A Neurovascular Target in Neurological Diseases. Nat. Rev. Neurol. 2016, 12, 439–454. [Google Scholar] [CrossRef]
- Matsuo, R.; Ago, T.; Kamouchi, M.; Kuroda, J.; Kuwashiro, T.; Hata, J.; Sugimori, H.; Fukuda, K.; Gotoh, S.; Makihara, N.; et al. Clinical Significance of Plasma VEGF Value in Ischemic Stroke—Research for Biomarkers in Ischemic Stroke (REBIOS) Study. BMC Neurol. 2013, 13, 32. [Google Scholar] [CrossRef]
- Meesters, A.; den Bosch-Meevissen, Y.M.C.I.; Weijzen, C.A.H.; Buurman, W.A.; Losen, M.; Schepers, J.; Thissen, M.R.T.M.; Alberts, H.J.E.M.; Schalkwijk, C.G.; Peters, M.L. The Effect of Mindfulness-Based Stress Reduction on Wound Healing: A Preliminary Study. J. Behav. Med. 2018, 41, 385–397. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Sher, L.D.; Geddie, H.; Olivier, L.; Cairns, M.; Truter, N.; Beselaar, L.; Essop, M.F. Chronic Stress and Endothelial Dysfunction: Mechanisms, Experimental Challenges, and the Way Ahead. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H488–H506. [Google Scholar] [CrossRef]
- Toda, N.; Nakanishi-Toda, M. How Mental Stress Affects Endothelial Function. Pflug. Arch. Eur. J. Physiol. 2011, 462, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Tsurumi, Y.; Murohara, T.; Krasinski, K.; Chen, D.; Witzenbichler, B.; Kearney, M.; Couffinhal, T.; Isner, J.M. Reciprocal Relation between VEGF and NO in the Regulation of Endothelial Integrity. Nat. Med. 1997, 3, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Heine, V.M.; Zareno, J.; Maslam, S.; Joëls, M.; Lucassen, P.J. Chronic Stress in the Adult Dentate Gyrus Reduces Cell Proliferation near the Vasculature and VEGF and Flk-1 Protein Expression. Eur. J. Neurosci. 2005, 21, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wei, S.; Wei, X.; Wang, J.; Zhang, Y.; Qiao, M.; Wu, J. Anger Emotional Stress Influences VEGF/VEGFR2 and Its Induced PI3K/AKT/mTOR Signaling Pathway. Neural Plast. 2016, 2016, 4129015. [Google Scholar] [CrossRef]
- Nindl, B.C.; Pierce, J.R. Insulin-Like Growth Factor I as a Biomarker of Health, Fitness, and Training Status. Med. Sci. Sports Exerc. 2010, 42, 39–49. [Google Scholar] [CrossRef]
- Rai, M.; Demontis, F. Muscle-to-Brain Signaling Via Myokines and Myometabolites. Brain Plast. 2022, 8, 43–63. [Google Scholar] [CrossRef]
- de Las Heras, B.; Rodrigues, L.; Cristini, J.; Weiss, M.; Prats-Puig, A.; Roig, M. Does the Brain-Derived Neurotrophic Factor Val66Met Polymorphism Modulate the Effects of Physical Activity and Exercise on Cognition? Neuroscientist 2022, 28, 69–86. [Google Scholar] [CrossRef]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.; Sun, B.; Tandon, N.N. Brain-Derived Neurotrophic Factor Is Stored in Human Platelets and Released by Agonist Stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef]
- Webb, N.J.A.; Bottomley, M.J.; Watson, C.J.; Brenchley, P.E.C. Vascular Endothelial Growth Factor (VEGF) Is Released from Platelets during Blood Clotting: Implications for Measurement of Circulating VEGF Levels in Clinical Disease. Clin. Sci. 1998, 94, 395–404. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Jia, Y.; Zhang, Y.; Meng, D. Effects of Cortisol on Cognitive and Emotional Disorders after Stroke: A Scoping Review. Heliyon 2024, 10, e40278. [Google Scholar] [CrossRef]
- Juster, R.-P.; McEwen, B.S.; Lupien, S.J. Allostatic Load Biomarkers of Chronic Stress and Impact on Health and Cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 14 February 2025).
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 Levels in Healthy Individuals: A Meta-Analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.H.; Vahdatpour, C.; Sanfeliu, A.; Tropea, D. The Role of Insulin-Like Growth Factor 1 (IGF-1) in Brain Development, Maturation and Neuroplasticity. Neuroscience 2016, 325, 89–99. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age between 18–80 years old | Cognitive impairment (MMSE ≤ 23) |
| Diagnosis of ischemic or hemorrhagic stroke | Severe aphasia (>2 in item 9 in NIHSS) |
| Stroke diagnosis between 3–60 months ago | Severe sensory problems |
| Consent from a physician to engage in PE | Diagnosis of transient ischemic attack |
| Fluency in Spanish or Catalan | Other neurological conditions |
| Severe pre-stroke psychiatric disorder | |
| History of alcohol or substance abuse |
| Week | MindFit MBSR | Standard MBSR [76] |
|---|---|---|
| 1 | Orientation Session | Orientation Session + Session 1 |
| 2 | Session 1 | Session 2 |
| 3 | Session 2 | Session 3 |
| 4 | Session 3 | Session 4 |
| 5 | No session | Session 5 |
| 6 | Session 4 | Session 6 + All-day session |
| 7 | Session 5 | Session 7 |
| 8 | Session 6 | Session 8 |
| 9 | All-day Session | - |
| 10 | No session | - |
| 11 | Session 7 | - |
| 12 | Session 8 | - |
| Type of Session | Sess/wk | Min/sess | Brief Description |
|---|---|---|---|
| Synchronous | |||
| Strength, agility, and balance training | 2 | 60 | Sessions included two balance and agility exercises, followed by six strength exercises alternating upper and lower limbs. Sets/reps per exercise: 2 × 10 → 3 × 15 Intensity: Borg RPE scale 12–14/20 (constant across weeks) |
| Aerobic training | 1 | 45 | Low-impact cardiovascular exercises Intensity progression:
|
| Autonomous | |||
| Walking training | 2 | 45 | Walking sessions conducted around participants’ homes Intensity progression:
|
| Variable | MBSR+CCT (n = 39) | PE+CCT (n = 34) | CCT-Only (n = 36) | p-Value |
| Age, y | 55.46 (10.74) | 58.80 (11.59) | 58.67 (8.57) | 0.291 |
| Sex, n (%) | 0.424 | |||
| Male | 19 (48.70) | 20 (58.80) | 23 (63.89) | |
| Female | 20 (51.30) | 14 (41.20) | 13 (36.11) | |
| Education, y | 12.54 (3.02) | 13.09 (4.00) | 11.58 (3.38) | 0.170 |
| Number of strokes | 1.05 (0.22) | 1.12 (0.48) | 1.14 (0.35) | 0.356 |
| Time since the stroke, mo | 33.96 (21.66) | 26.72 (18.91) | 21.06 (16.53) | 0.017 |
| Type of stroke, n (%) | 0.645 | |||
| Ischemic stroke | 24 (61.50) | 26 (76.50) | 24 (66.67) | |
| Intracerebral hemorrhage | 11 (28.20) | 7 (20.60) | 10 (27.78) | |
| Subarachnoid hemorrhage | 4 (10.30) | 1 (2.90) | 2 (5.56) | |
| Circulation, n (%) | 0.219 | |||
| Anterior | 30 (76.92) | 21 (61.76) | 25 (69.44) | |
| Posterior | 9 (23.08) | 13 (38.24) | 9 (25.00) | |
| Unknown | 0 (0.00) | 0 (0.00) | 2 (5.56) | |
| Brain side affected, n (%) | 0.119 | |||
| Right | 19 (48.72) | 8 (23.53) | 17 (47.22) | |
| Left | 15 (38.46) | 23 (67.65) | 17 (47.22) | |
| Bilateral | 4 (10.26) | 3 (8.82) | 2 (5.56) | |
| Unknown | 1 (2.56) | 0 (0.00) | 0 (0.00) | |
| National Institutes of Health Stroke Scale (0–42) | 2.87 (3.33) | 2.24 (2.52) | 3.11 (3.39) | 0.483 |
| Modified Rankin Scale (0–6) | 2.26 (1.04) | 2.06 (0.89) | 2.47 (1.11) | 0.297 |
| Barthel Index (0–100) | 91.54 (17.48) | 95.00 (10.66) | 92.50 (14.22) | 0.585 |
| Mini-Mental State Examination (0–30) | 28.46 (1.71) | 28.85 (1.23) | 28.56 (1.23) | 0.545 |
| WAIS-III - Vocabulary subtest (0–66) | 46.69 (11.03) | 46.47 (11.28) | 43.69 (8.87) | 0.398 |
| Beck Depression Inventory-II (0–63) | 18.36 (10.09) | 11.47 (7.08) | 16.53 (9.84) | 0.009 |
| Body mass index, kg/m2 | 27.16 (4.88) | 27.12 (5.63) | 27.89 (4.58) | 0.768 |
| Metabolic syndrome, n (%) | 0.369 | |||
| Presence | 18 (46.15) | 21 (61.76) | 21 (58.33) | |
| Absence | 21 (53.85) | 13 (38.24) | 15 (41.67) |
| Biomarker | Group | Difference Score (T1 − T0) | Omnibus Test | Post Hoc Comparison | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n * | M (SD) | Madj (SE) † | F | p | padj ‡ | ηp2 | Contrast | MD (95% CI) § | p | padj § | d | ||
| Growth factors | |||||||||||||
| BDNF, pg/mL (log10) | MBSR+CCT (G1) | 39 | 0.07 (0.42) | 0.11 (0.06) | 1.69 | 0.190 | 0.228 | 0.03 | |||||
| PE+CCT (G2) | 34 | −0.01 (0.45) | −0.04 (0.06) | ||||||||||
| CCT−only (G3) | 36 | 0.05 (0.31) | 0.04 (0.06) | ||||||||||
| IGF-1, ng/mL | MBSR+CCT (G1) | 39 | −10.48 (17.91) | −8.80 (2.96) | 2.55 | 0.083 | 0.166 | 0.05 | |||||
| PE+CCT (G2) | 34 | −18.21 (24.89) | −18.39 (3.11) | ||||||||||
| CCT−only (G3) | 36 | −13.32 (21.02) | −14.98 (3.09) | ||||||||||
| VEGF, pg/mL (log10) | MBSR+CCT (G1) | 39 | 0.00 (0.45) | 0.02 (0.05) | 5.31 | 0.006 | 0.038 | 0.10 | G1 vs. G3 | 0.22 (0.04, 0.41) | 0.004 | 0.013 | 0.71 |
| PE+CCT (G2) | 34 | −0.20 (0.37) | −0.18 (0.05) | G2 vs. G3 | 0.02 (−0.17, 0.21) | 0.790 | 1.000 | 0.07 | |||||
| CCT−only (G3) | 36 | −0.15 (0.31) | −0.20 (0.05) | G1 vs. G2 | 0.20 (0.02, 0.39) | 0.008 | 0.025 | 0.65 | |||||
| Inflammatory markers | |||||||||||||
| CRP, mg/L (log10) | MBSR+CCT (G1) | 39 | 0.05 (0.86) | −0.02 (0.10) | 0.69 | 0.506 | 0.506 | 0.01 | |||||
| PE+CCT (G2) | 33 | 0.04 (0.50) | 0.11 (0.11) | ||||||||||
| CCT−only (G3) | 34 | 0.13 (0.76) | 0.15 (0.11) | ||||||||||
| IL-6, pg/mL (log10) | MBSR+CCT (G1) | 39 | −0.01 (0.37) | −0.04 (0.04) | 1.91 | 0.154 | 0.228 | 0.04 | |||||
| PE+CCT (G2) | 34 | 0.06 (0.21) | 0.07 (0.04) | ||||||||||
| CCT−only (G3) | 36 | 0.03 (0.23) | 0.05 (0.04) | ||||||||||
| Stress markers | |||||||||||||
| Cortisol, ng/mL | MBSR+CCT (G1) | 39 | −10.25 (22.62) | −13.60 (3.37) | 2.89 | 0.060 | 0.166 | 0.05 | |||||
| PE+CCT (G2) | 34 | −11.88 (19.36) | −12.52 (3.53) | ||||||||||
| CCT−only (G3) | 36 | −6.81 (27.39) | −2.59 (3.52) | ||||||||||
| Biomarker | Group | n * | Baseline (T0) M (SD) | Post-int. (T1) M (SD) | Difference Score (T1 − T0) | t | p | padj † | d | |
|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | 95% CI † | |||||||||
| Growth factors | ||||||||||
| BDNF, pg/mL (log10) | MBSR+CCT | 39 | 3.44 (0.57) | 3.51 (0.54) | 0.07 (0.42) | −0.10, 0.23 | 1.02 | 0.316 | 0.493 | 0.16 |
| PE+CCT | 34 | 3.20 (0.65) | 3.20 (0.48) | −0.01 (0.45) | −0.19, 0.18 | −0.09 | 0.930 | 0.975 | −0.02 | |
| CCT-only | 36 | 3.22 (0.43) | 3.28 (0.41) | 0.05 (0.31) | −0.08, 0.18 | 0.99 | 0.329 | 0.493 | 0.17 | |
| IGF-1, ng/mL | MBSR+CCT | 39 | 94.58 (38.62) | 84.10 (34.07) | −10.48 (17.91) | −17.48, −3.49 | −3.66 | <0.001 | 0.005 | −0.59 |
| PE+CCT | 34 | 91.96 (49.19) | 73.75 (40.17) | −18.21 (24.89) | −28.70, −7.73 | −4.27 | <0.001 | 0.003 | −0.73 | |
| CCT-only | 36 | 84.32 (37.76) | 71.00 (34.21) | −13.32 (21.02) | −21.91, −4.74 | −3.80 | <0.001 | 0.005 | −0.63 | |
| VEGF, pg/mL (log10) | MBSR+CCT | 39 | 1.49 (0.33) | 1.49 (0.39) | 0.00 (0.45) | −0.18, 0.18 | −0.03 | 0.975 | 0.975 | −0.01 |
| PE+CCT | 34 | 1.51 (0.40) | 1.31 (0.27) | −0.20 (0.37) | −0.36, −0.05 | −3.17 | 0.003 | 0.012 | −0.54 | |
| CCT-only | 36 | 1.42 (0.28) | 1.27 (0.29) | −0.15 (0.31) | −0.28, −0.02 | −2.91 | 0.006 | 0.019 | −0.49 | |
| Inflammatory markers | ||||||||||
| CRP, mg/L (log10) | MBSR+CCT | 39 | −0.12 (0.73) | −0.06 (0.66) | 0.05 (0.86) | −0.28, 0.39 | 0.40 | 0.692 | 0.831 | 0.06 |
| PE+CCT | 33 | −0.04 (0.78) | 0.00 (0.72) | 0.04 (0.50) | −0.17, 0.26 | 0.47 | 0.639 | 0.821 | 0.08 | |
| CCT-only | 34 | −0.23 (0.63) | −0.10 (0.71) | 0.13 (0.76) | −0.19, 0.45 | 1.01 | 0.320 | 0.493 | 0.17 | |
| IL-6, pg/mL (log10) | MBSR+CCT | 39 | 0.17 (0.40) | 0.16 (0.37) | −0.01 (0.37) | −0.16, 0.13 | −0.22 | 0.824 | 0.926 | −0.04 |
| PE+CCT | 34 | 0.23 (0.31) | 0.29 (0.37) | 0.06 (0.21) | −0.02, 0.15 | 1.84 | 0.074 | 0.167 | 0.32 | |
| CCT-only | 36 | 0.21 (0.33) | 0.25 (0.27) | 0.03 (0.23) | −0.06, 0.12 | 0.82 | 0.416 | 0.576 | 0.14 | |
| Stress markers | ||||||||||
| Cortisol, ng/mL | MBSR+CCT | 39 | 83.36 (23.50) | 73.11 (21.62) | −10.25 (22.62) | −19.09, −1.41 | −2.83 | 0.007 | 0.019 | −0.45 |
| PE+CCT | 34 | 86.22 (23.05) | 74.33 (24.65) | −11.88 (19.36) | −20.04, −3.73 | −3.58 | 0.001 | 0.005 | −0.61 | |
| CCT-only | 36 | 93.67 (25.28) | 86.85 (24.94) | −6.81 (27.39) | −17.99, 4.37 | −1.49 | 0.145 | 0.289 | −0.25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermudo-Gallaguet, A.; Ariza, M.; Agudelo, D.; Camins-Vila, N.; Boldó, M.; Peters, S.; Sawicka, A.K.; Dacosta-Aguayo, R.; Soriano-Raya, J.J.; Via, M.; et al. Effects of Mindfulness and Exercise on Growth Factors, Inflammation, and Stress Markers in Chronic Stroke: The MindFit Project Randomized Clinical Trial. J. Clin. Med. 2025, 14, 2580. https://doi.org/10.3390/jcm14082580
Bermudo-Gallaguet A, Ariza M, Agudelo D, Camins-Vila N, Boldó M, Peters S, Sawicka AK, Dacosta-Aguayo R, Soriano-Raya JJ, Via M, et al. Effects of Mindfulness and Exercise on Growth Factors, Inflammation, and Stress Markers in Chronic Stroke: The MindFit Project Randomized Clinical Trial. Journal of Clinical Medicine. 2025; 14(8):2580. https://doi.org/10.3390/jcm14082580
Chicago/Turabian StyleBermudo-Gallaguet, Adrià, Mar Ariza, Daniela Agudelo, Neus Camins-Vila, Maria Boldó, Sarah Peters, Angelika Katarzyna Sawicka, Rosalia Dacosta-Aguayo, Juan José Soriano-Raya, Marc Via, and et al. 2025. "Effects of Mindfulness and Exercise on Growth Factors, Inflammation, and Stress Markers in Chronic Stroke: The MindFit Project Randomized Clinical Trial" Journal of Clinical Medicine 14, no. 8: 2580. https://doi.org/10.3390/jcm14082580
APA StyleBermudo-Gallaguet, A., Ariza, M., Agudelo, D., Camins-Vila, N., Boldó, M., Peters, S., Sawicka, A. K., Dacosta-Aguayo, R., Soriano-Raya, J. J., Via, M., Clemente, I. C., García-Molina, A., Durà Mata, M. J., Torán-Monserrat, P., Erickson, K. I., & Mataró, M. (2025). Effects of Mindfulness and Exercise on Growth Factors, Inflammation, and Stress Markers in Chronic Stroke: The MindFit Project Randomized Clinical Trial. Journal of Clinical Medicine, 14(8), 2580. https://doi.org/10.3390/jcm14082580










