Prognostic Role of Electrocardiographic Alternans in Ischemic Heart Disease
Abstract
1. Introduction
2. Materials and Methods
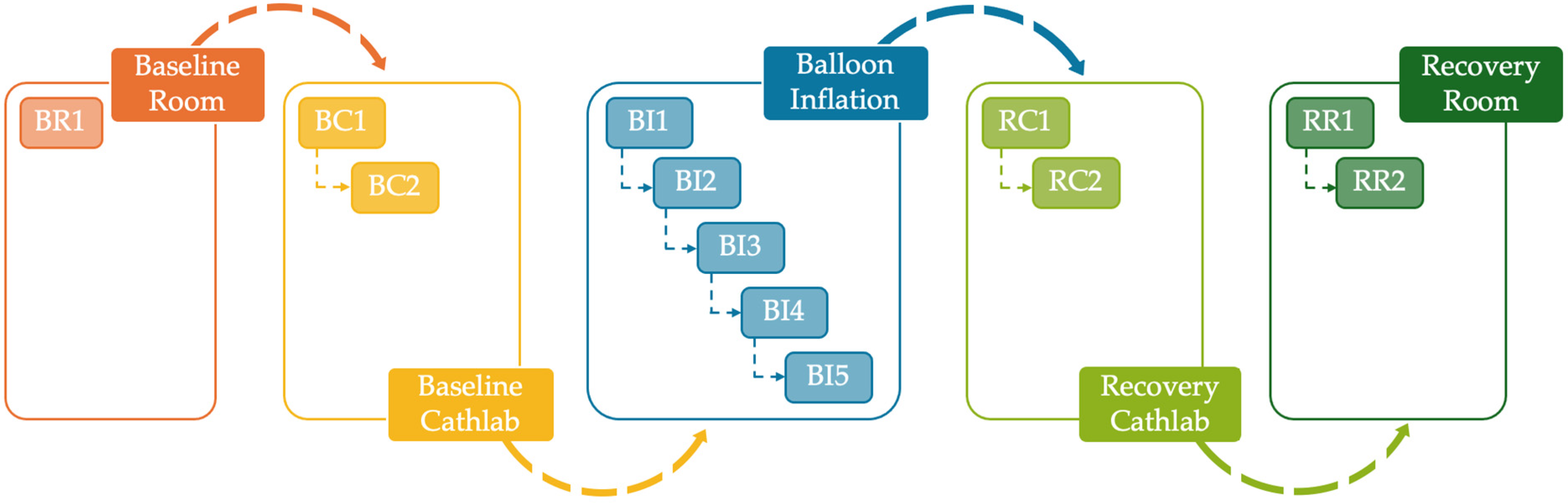
2.1. Study Population Description
- Less than 35%, 10%, 2%, and 1% of patients had two, three, four and five ECG acquisitions during balloon inflation, respectively;
- More than 70% of patients had the ECG acquisition in the baseline room;
- More than 99% and 10% of patients had one and two ECG acquisitions in the baseline cathlab, respectively;
- About 90% and 2% of patients had one and two ECG acquisitions in the recovery cathlab, respectively;
- More than 90% and 2% of patients had one and two ECG acquisitions in the recovery room, respectively.
2.2. Recursive Extraction of Electrocardiographic Windows and Preprocessing
2.3. Identification and Synchronization of Electrocardiogram Sections
2.4. Electrocardiographic Alternans Detection Through the Correlation Method
2.5. Statistics
2.5.1. Single-Subject Level
- The PWA, QRSA, and TWA local amplitudes (µV), computed as the median PWA, QRSA, and TWA amplitudes over all heartbeats;
- The PWA, QRSA, and TWA local magnitudes (µV·beat), computed as the median product of PWA, QRSA, and TWA amplitudes over the heartbeats with an amplitude greater than 0 µV, and their amount (which accounts for the duration of the ECGA episode, and expressed as number of heartbeats).
2.5.2. Multi-Subject Level
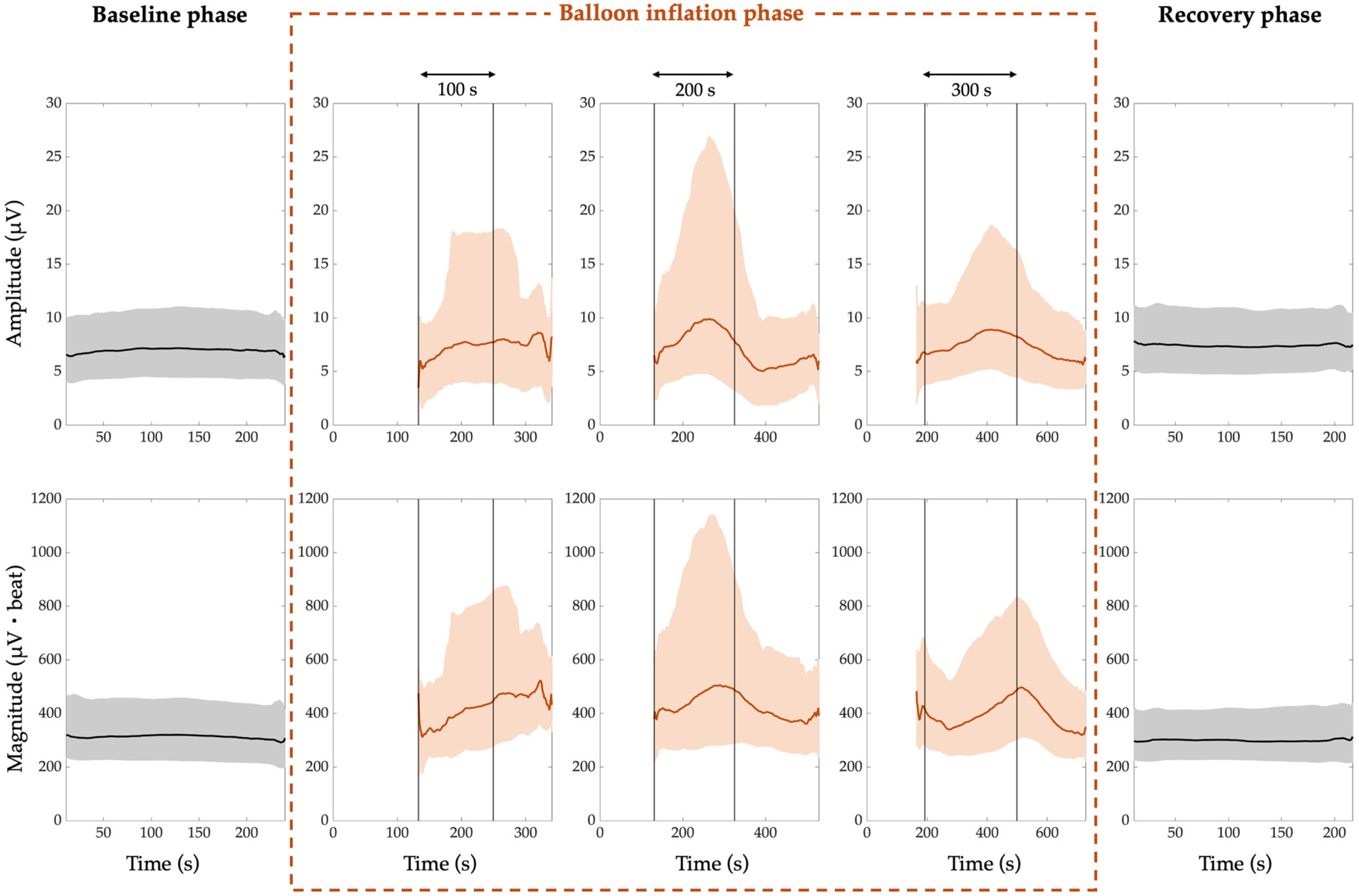
3. Results
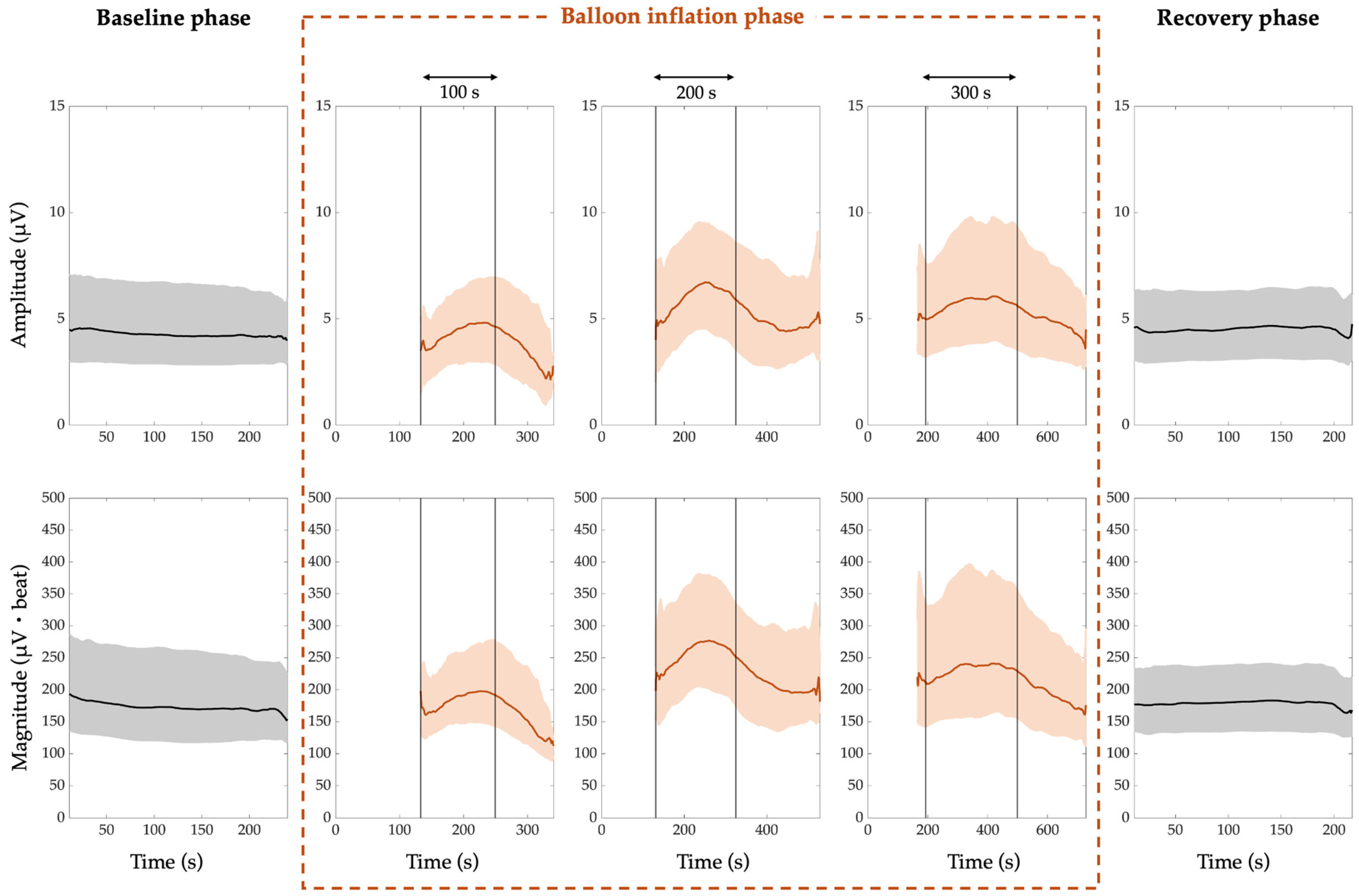
- The median PWA, QRSA, and TWA amplitude trend is around 4 µV, 5 µV, and 7 µV, respectively;
- The median PWA, QRSA, and TWA magnitude trend is around 169 µV·beat, 396 µV·beat and 310 µV·beat, respectively.
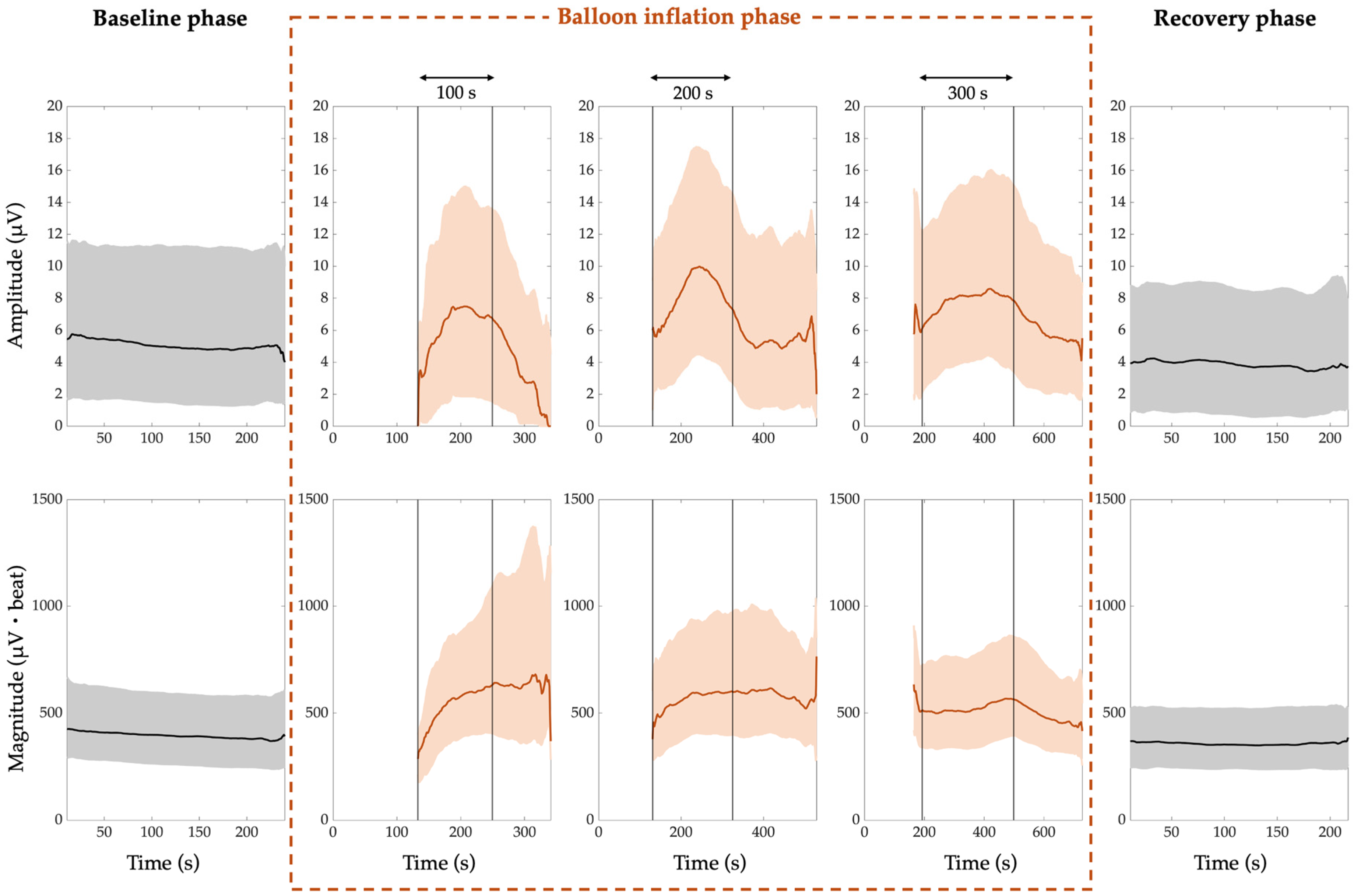
- The median PWA, QRSA, and TWA amplitude trend is around 5 µV, 4 µV, and 8 µV, respectively;
- The median PWA, QRSA, and TWA magnitude trend is around 182 µV·beat, 360 µV·beat and 296 µV·beat, respectively.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APD | Action Potential Duration |
| CM | Correlation Method |
| ECG | Electrocardiogram |
| ECGA | Electrocardiographic Alternans |
| HRT | Heart-Rate Turbulence |
| HRV | Heart-Rate Variability |
| PTCA | Percutaneous Transluminal Coronary Angioplasty |
| PWA | P-Wave Alternans |
| QRSA | QRS-complex Alternans |
| SCD | Sudden Cardiac Death |
| TWA | T-Wave Alternans |
References
- Bhandari, B.; Quintanilla Rodriguez, B.S.; Masood, W. Ischemic Cardiomyopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Gräni, C.; Benz, D.C.; Gupta, S.; Windecker, S.; Kwong, R.Y. Sudden Cardiac Death in Ischemic Heart Disease: From Imaging Arrhythmogenic Substrate to Guiding Therapies. JACC Cardiovasc. Imaging 2020, 13, 2223–2238. [Google Scholar] [CrossRef] [PubMed]
- Arsenos, P.; Gatzoulis, K.A.; Tsiachris, D.; Dilaveris, P.; Sideris, S.; Sotiropoulos, I.; Archontakis, S.; Antoniou, C.-K.; Kordalis, A.; Skiadas, I.; et al. Arrhythmic Risk Stratification in Ischemic, Non-Ischemic and Hypertrophic Cardiomyopathy: A Two-Step Multifactorial, Electrophysiology Study Inclusive Approach. World J. Cardiol. 2022, 14, 139–151. [Google Scholar] [CrossRef] [PubMed]
- García, J.; Wagner, G.; Sömmo, L.; Lander, P.; Laguna, P. Identification of the Occluded Artery in Patients with Myocardial Ischemia Induced by Prolonged Percutaneous Transluminal Coronary Angioplasty Using Traditional vs Transformed ECG-Based Indexes. Comput. Biomed. Res. 1999, 32, 470–482. [Google Scholar] [CrossRef]
- Qu, Z.; Weiss, J.N. Cardiac Alternans: From Bedside to Bench and Back. Circ. Res. 2023, 132, 127–149. [Google Scholar] [CrossRef]
- Duca, Ș.-T.; Tudorancea, I.; Haba, M.Ú.C.; Costache, A.-D.; Șerban, I.-L.; Pavăl, D.R.; Loghin, C.; Costache-Enache, I.-I. Enhancing Comprehensive Assessments in Chronic Heart Failure Caused by Ischemic Heart Disease: The Diagnostic Utility of Holter ECG Parameters. Med. Lith. 2024, 60, 1315. [Google Scholar] [CrossRef] [PubMed]
- Marcantoni, I.; Sbrollini, A.; Morettini, M.; Swenne, C.A.; Burattini, L. Enhanced Adaptive Matched Filter for Automated Identification and Measurement of Electrocardiographic Alternans. Biomed. Signal Process. Control 2021, 68, 102619. [Google Scholar] [CrossRef]
- Kaufmann, D.K.; Raczak, G.; Szwoch, M.; Wabich, E.; Świątczak, M.; Daniłowicz-Szymanowicz, L. Baroreflex Sensitivity but Not Microvolt T-Wave Alternans Can Predict Major Adverse Cardiac Events in Ischemic Heart Failure. Cardiol. J. 2022, 29, 1004–1012. [Google Scholar] [CrossRef]
- Cetin, M.; Ornek, E.; Bastug, S.; Kara, M.; Deveci, B.; Korkmaz, A.; Ozeke, O.; Cay, S.; Ozcan, F.; Topaloglu, S.; et al. An Incessant Tachycardia with Alternating QRS Complexes: What Is the Mechanism? J. Innov. Card. Rhythm. Manag. 2022, 13, 4900–4904. [Google Scholar] [CrossRef]
- Vandenberk, B.; Floré, V.; Röver, C.; Vos, M.A.; Dunnink, A.; Leftheriotis, D.; Friede, T.; Flevari, P.; Zabel, M.; Willems, R. Repeating Noninvasive Risk Stratification Improves Prediction of Outcome in ICD Patients. Ann. Noninvasive Electrocardiol. 2020, 25, e12794. [Google Scholar] [CrossRef]
- Rivolta, M.W.; Martinez, J.P.; Sassi, R.; Laguna, P. Spatial Correlation between Myocyte’s Repolarization Times and Their Alternans Drives T-Wave Alternans on the ECG. IEEE J. Biomed. Health Inform. 2022, 26, 5372–5383. [Google Scholar] [CrossRef]
- Elmberg, V.; Almer, J.; Pahlm, O.; Wagner, G.S.; Engblom, H.; Ringborn, M. A 12-Lead ECG-Method for Quantifying Ischemia-Induced QRS Prolongation to Estimate the Severity of the Acute Myocardial Event. J. Electrocardiol. 2016, 49, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Laguna, P.; Sörnmo, L. The STAFF III ECG Database and Its Significance for Methodological Development and Evaluation. J. Electrocardiol. 2014, 47, 408–417. [Google Scholar] [CrossRef]
- Warren, S.G.; Wagner, G.S. The STAFF Studies of the First 5minutes of Percutaneous Coronary Angioplasty Balloon Occlusion in Man. J. Electrocardiol. 2014, 47, 402–407. [Google Scholar] [CrossRef]
- Burattini, L.; Zareba, W.; Moss, A.J. Correlation Method for Detection of Transient T-Wave Alternans in Digital Holter ECG Recordings. Ann. Noninvasive Electrocardiol. 1999, 4, 416–424. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Nearing, B.D.; Verrier, R.L. Modified Moving Average Analysis of T-Wave Alternans to Predict Ventricular Fibrillation with High Accuracy. J. Appl. Physiol. 2002, 92, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.S.; Jackson, L.E.; Smith, J.M.; Garan, H.; Ruskin, J.N.; Cohen, R.J. Electrical Alternans and Vulnerability to Ventricular Arrhythmias. N. Engl. J. Med. 1994, 330, 235–241. [Google Scholar] [CrossRef]
- Burattini, L.; Bini, S.; Burattini, R. Correlation Method versus Enhanced Modified Moving Average Method for Automatic Detection of T-Wave Alternans. Comput. Methods Programs Biomed. 2010, 98, 94–102. [Google Scholar] [CrossRef]
- Burattini, L.; Bini, S.; Burattini, R. Comparative Analysis of Methods for Automatic Detection and Quantification of Microvolt T-Wave Alternans. Med. Eng. Phys. 2009, 31, 1290–1298. [Google Scholar] [CrossRef]
- Martínez, J.P.; Olmos, S. Methodological Principles of T Wave Alternans Analysis: A Unified Framework. IEEE Trans. Biomed. Eng. 2005, 52, 599–613. [Google Scholar] [CrossRef]
- Burattini, L.; Man, S.; Burattini, R.; Swenne, C.A. Comparison of Standard versus Orthogonal ECG Leads for T-Wave Alternans Identification. Ann. Noninvasive Electrocardiol. 2012, 17, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Inamura, Y.; Nishizaki, M.; Shimizu, M.; Fujii, H.; Yamawake, N.; Suzuki, M.; Sakurada, H.; Hiraoka, M.; Isobe, M. Early Repolarization and Positive T-Wave Alternans as Risk Markers for Life-Threatening Arrhythmias in Patients with Vasospastic Angina. Int. J. Cardiol. 2015, 196, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.P.; Martín-Yebra, A.; Monasterio, V.; Demidova, M.; Platonov, P.; Laguna, P. Time Course and Spatial Distribution of T Wave Alternans Induced by Coronary Artery Occlusion in Pigs. In Proceedings of the 2011 Computing in Cardiology, Hangzhou, China, 18–21 September 2011; IEEE: New York, NY, USA, 2011; Volume 38, pp. 669–672. [Google Scholar]
- Ueda, N.; Zipes, D.P.; Wu, J. Coronary Occlusion and Reperfusion Promote Early Afterdepolarizations and Ventricular Tachycardia in a Canine Tissue Model of Type 3 Long QT Syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H607–H612. [Google Scholar] [CrossRef]
- Bernikova, O.G.; Tsvetkova, A.S.; Gonotkov, M.A.; Ovechkin, A.O.; Demidova, M.M.; Azarov, J.E.; Platonov, P.G. Prolonged Repolarization in the Early Phase of Ischemia Is Associated with Ventricular Fibrillation Development in a Porcine Model. Front. Physiol. 2023, 14, 1035032. [Google Scholar] [CrossRef]
- Burton, F. Dispersion of Ventricular Repolarization and Refractory Period. Cardiovasc. Res. 2001, 50, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Oknińska, M.; Mączewski, M.; Mackiewicz, U. Ventricular Arrhythmias in Acute Myocardial Ischaemia—Focus on the Ageing and Sex. Ageing Res. Rev. 2022, 81, 101722. [Google Scholar] [CrossRef]
- Merchant, F.M.; Sayadi, O.; Sohn, K.; Weiss, E.H.; Puppala, D.; Doddamani, R.; Singh, J.P.; Heist, E.K.; Owen, C.; Kulkarni, K.; et al. Real-Time Closed-Loop Suppression of Repolarization Alternans Reduces Arrhythmia Susceptibility In Vivo. Circ. Arrhythm. Electrophysiol. 2020, 13, E008186. [Google Scholar] [CrossRef]
- Taggart, P.; Yellon, D.M. Preconditioning and Arrhythmias. Circulation 2002, 106, 2999–3001. [Google Scholar] [CrossRef]
| Section 1 | Onset 2 | Offset 2 |
|---|---|---|
| P section |
| |
| QRS section | Qon = R − 0.05 s | J = R + 0.05 s |
| T section |
|
|
| ECGA | Baseline | Balloon Inflation | |||||
|---|---|---|---|---|---|---|---|
| Form | Feature | 1st Minute | 2nd Minute | 3rd Minute | 4th Minute | 5th Minute | |
| PWA | A 1 (µV) | 4[3;7] | 5*[4;7] | 6*[4;9] | 6*[4;9] | 6*[4;10] | 6*[4;11] |
| M 2 (µV·beat) | 169[126;250] | 208*[154;328] | 237*[160;382] | 239*[161;389] | 234*[171;398] | 240*[172;385] | |
| QRSA | A 1 (µV) | 5[2;9] | 6[3;11] | 8*,†[4;14] | 8*[5;15] | 9*[5;15] | 9*[5;13] |
| M 2 (µV·beat) | 396[258;528] | 511*[350;756] | 523*[355;720] | 508*[356;756] | 573*[366;805] | 552*[437;790] | |
| TWA | A 1 (µV) | 7[5;9] | 7[5;10] | 7[5;11] | 9*,§[6;13] | 9*[6;18] | 9*[6;18] |
| M 2 (µV·beat) | 310[234;404] | 376*[264;558] | 328*[260;486] | 396*[271;613] | 416*[278;724] | 460*[270;724] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcantoni, I.; Iammarino, E.; Dell’Orletta, A.; Burattini, L. Prognostic Role of Electrocardiographic Alternans in Ischemic Heart Disease. J. Clin. Med. 2025, 14, 2620. https://doi.org/10.3390/jcm14082620
Marcantoni I, Iammarino E, Dell’Orletta A, Burattini L. Prognostic Role of Electrocardiographic Alternans in Ischemic Heart Disease. Journal of Clinical Medicine. 2025; 14(8):2620. https://doi.org/10.3390/jcm14082620
Chicago/Turabian StyleMarcantoni, Ilaria, Erica Iammarino, Alessandro Dell’Orletta, and Laura Burattini. 2025. "Prognostic Role of Electrocardiographic Alternans in Ischemic Heart Disease" Journal of Clinical Medicine 14, no. 8: 2620. https://doi.org/10.3390/jcm14082620
APA StyleMarcantoni, I., Iammarino, E., Dell’Orletta, A., & Burattini, L. (2025). Prognostic Role of Electrocardiographic Alternans in Ischemic Heart Disease. Journal of Clinical Medicine, 14(8), 2620. https://doi.org/10.3390/jcm14082620









