Feasibility Study for Multimodal Image-Based Assessment of Patient-Specific Intracranial Arteriovenous Malformation Hemodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Image Data
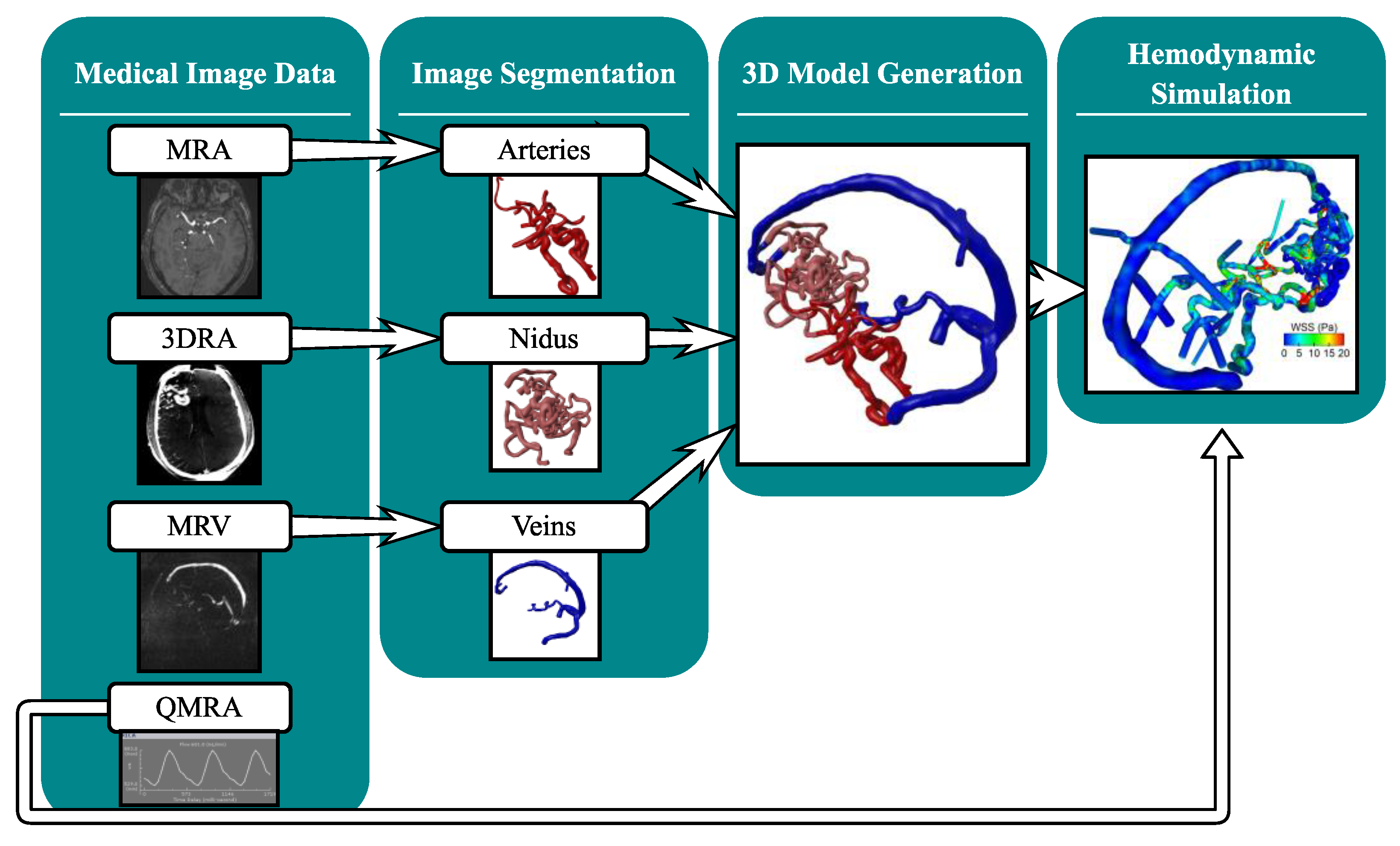
2.2. Multimodal Image Segmentation and 3D Model Generation
2.3. Hemodynamic Simulation
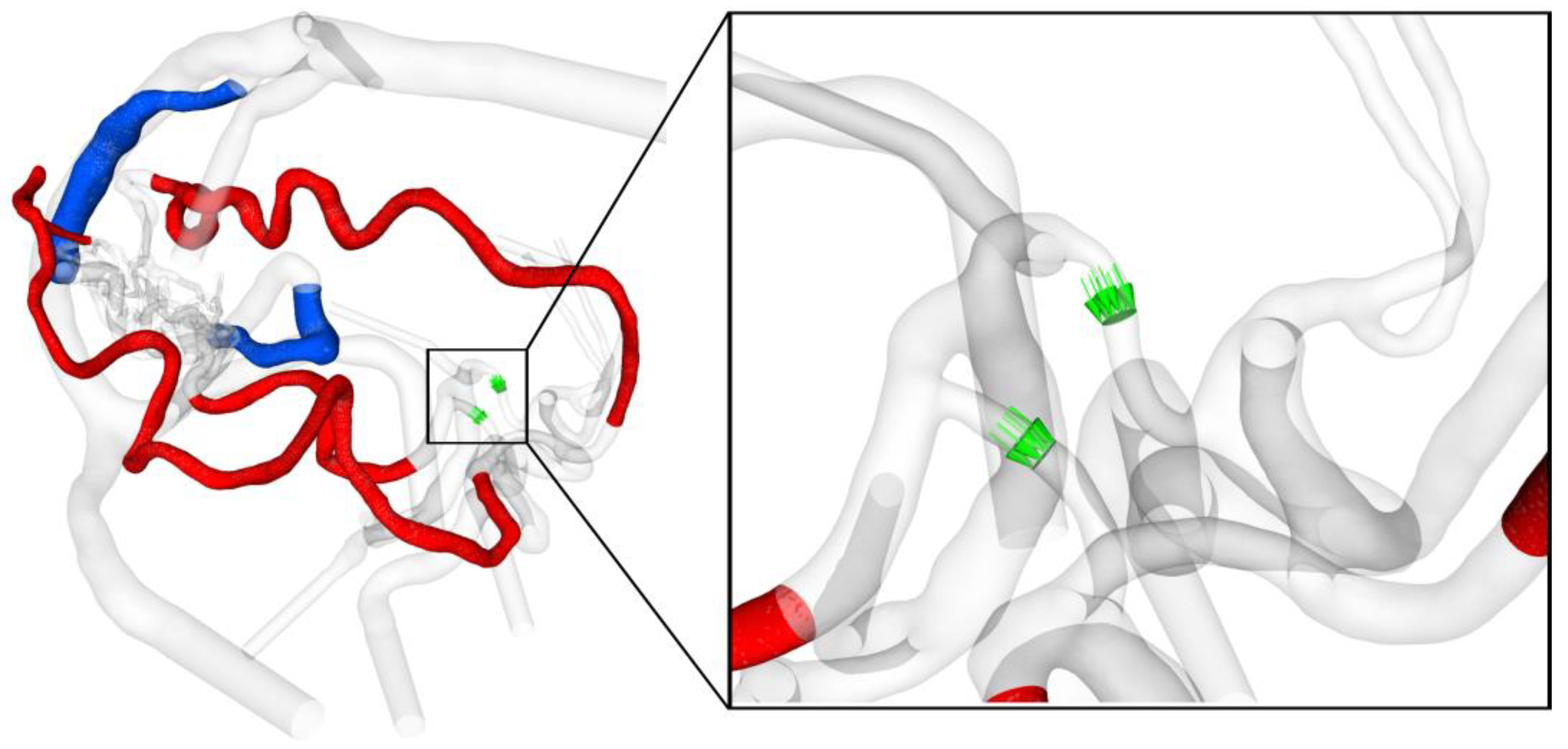
2.4. Analysis of Hemodynamic Parameter
3. Results
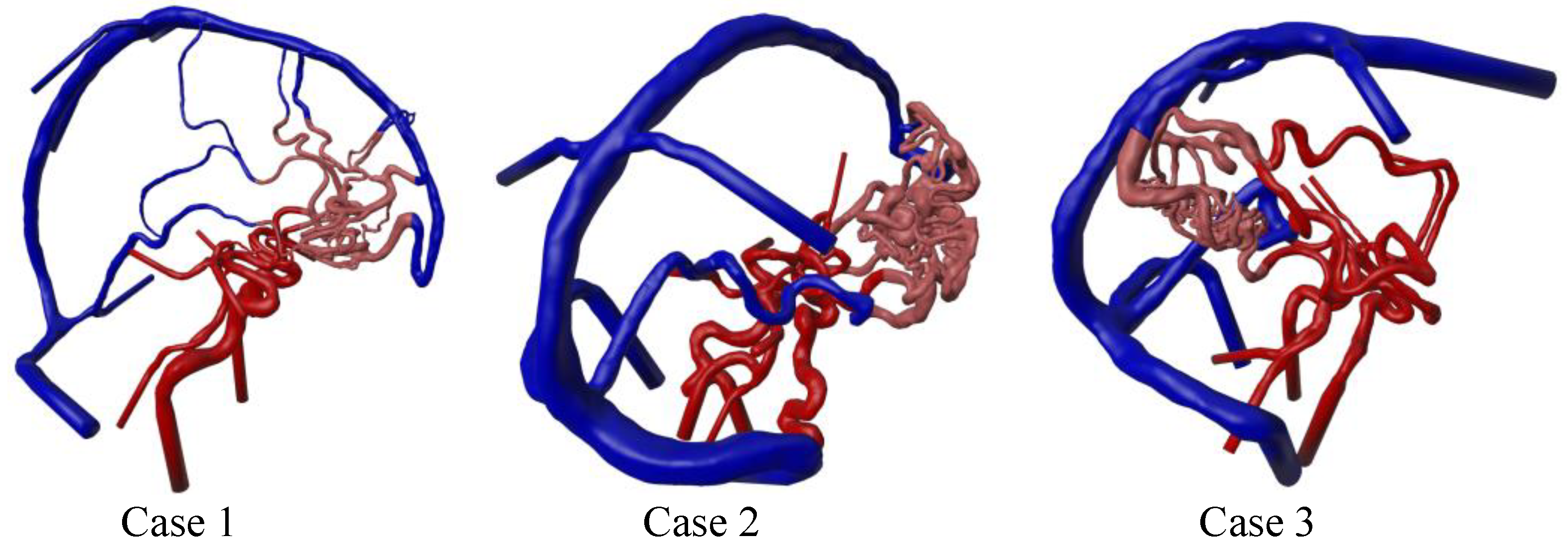
3.1. Three-Dimensional Modeling Results
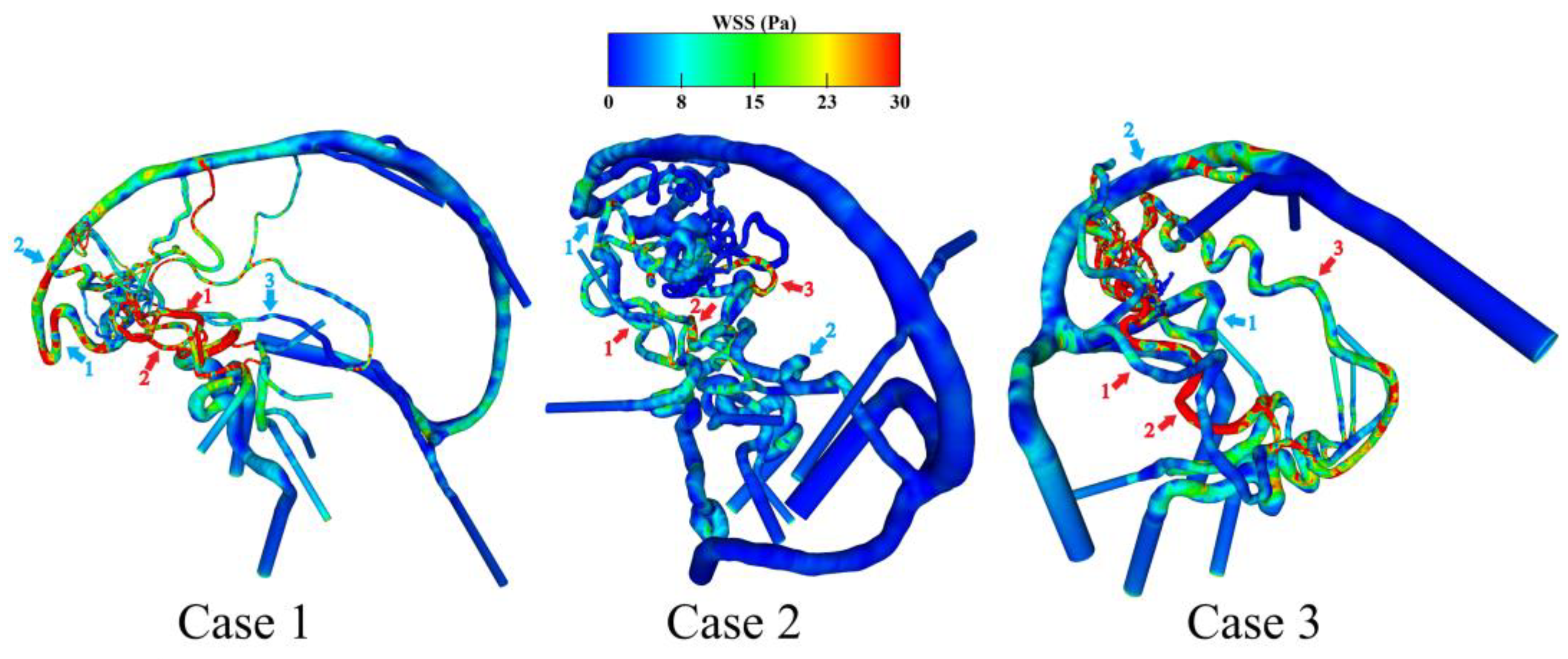
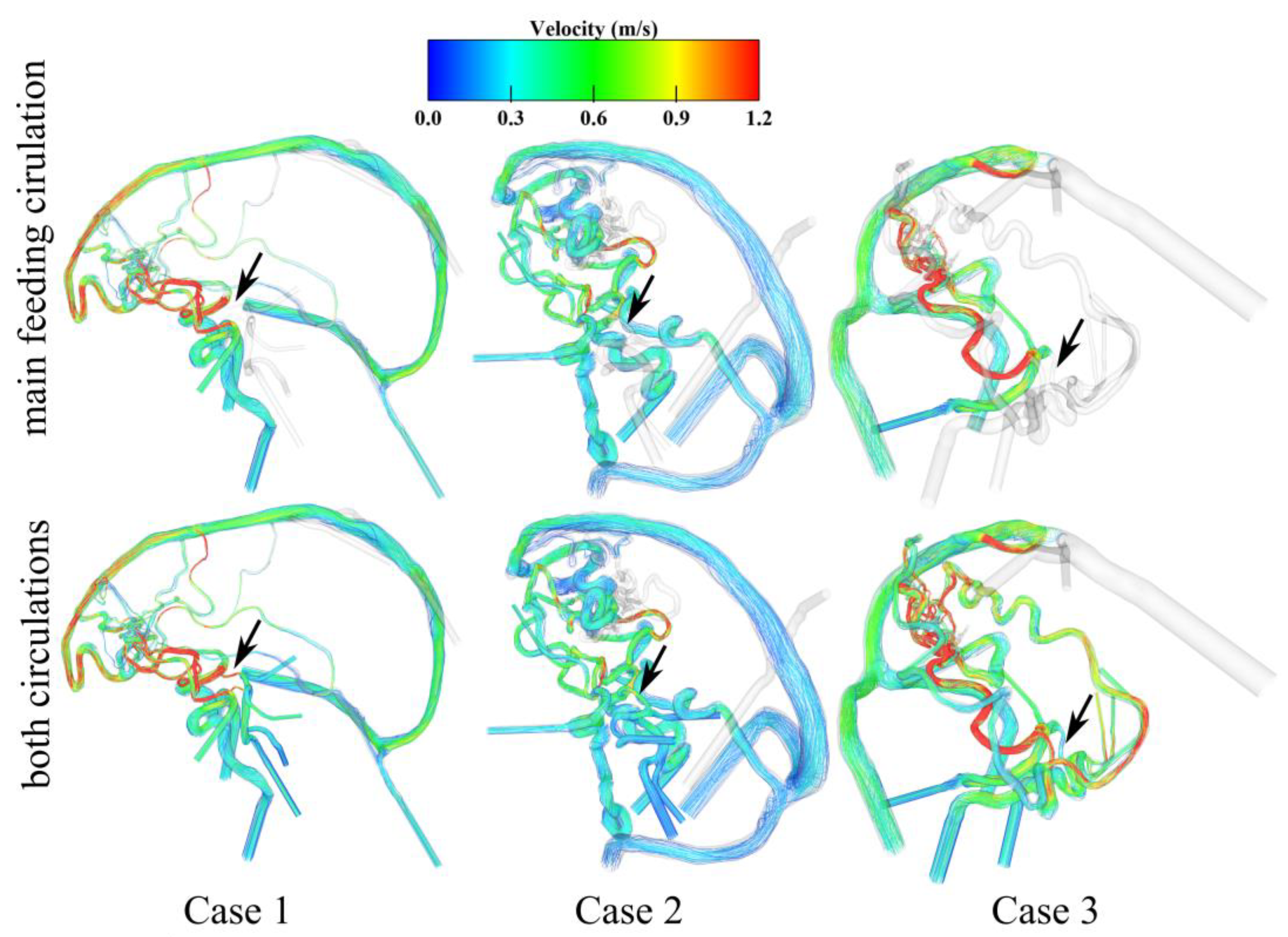
3.2. Hemodynamic Investigation of Shear-Related Phenomena
3.3. Analysis of the AVM-Related Blood-Drawing Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3DRA | 3D rotational angiography |
| AVM | arteriovenous malformations |
| DICOM | Digital imaging and communications in medicine |
| CFD | computational fluid dynamics |
| LPcom | Left posterior communicating artery |
| MRA | magnetic resonance angiography |
| MRV | magnetic resonance venography |
| NOVA | non-invasive optimized vessel analysis |
| QMRA | phase-contrast quantitative magnetic resonance imaging |
| RPcom | Right posterior communicating artery |
| WSS | wall shear stress |
References
- Choi, J.H.; Mast, H.; Sciacca, R.R.; Hartmann, A.; Khaw, A.V.; Mohr, J.P.; Sacco, R.L.; Stapf, C. Clinical outcome after first and recurrent hemorrhage in patients with untreated brain arteriovenous malformation. Stroke 2006, 37, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Asif, K.; Leschke, J.; Lazzaro, M.A. Cerebral arteriovenous malformation diagnosis and management. Semin. Neurol. 2013, 33, 468–475. [Google Scholar] [CrossRef]
- Ozpinar, A.; Mendez, G.; Abla, A.A. Epidemiology, genetics, pathophysiology, and prognostic classifications of cerebral arteriovenous malformations. Handb. Clin. Neurol. 2017, 143, 5–13. [Google Scholar] [PubMed]
- Can, A.; Gross, B.A.; Du, R. The natural history of cerebral arteriovenous malformations. Handb. Clin. Neurol. 2017, 143, 15–24. [Google Scholar]
- Ajiboye, N.; Chalouhi, N.; Starke, R.M.; Zanaty, M.; Bell, R. Cerebral arteriovenous malformations: Evaluation and management. Sci. World J. 2014, 2014, 649036. [Google Scholar] [CrossRef]
- Sheikh, M.A.A.; Shuib, A.S.; Mohyi, M.H.H. A review of hemodynamic parameters in cerebral aneurysm. Interdiscip. Neurosurg. 2020, 22, 100716. [Google Scholar] [CrossRef]
- Levitt, M.R.; McGah, P.M.; Aliseda, A.; Mourad, P.D.; Nerva, J.D.; Vaidya, S.S.; Morton, R.P.; Ghodke, B.V.; Kim, L.J. Cerebral aneurysms treated with flow-diverting stents: Computational models with intravascular blood flow measurements. Am. J. Neuroradiol. 2014, 35, 143–148. [Google Scholar] [CrossRef]
- Stahl, J.; Marsh, L.M.M.; Thormann, M.; Ding, A.; Saalfeld, S.; Behme, D.; Berg, P. Assessment of the flow-diverter efficacy for intracranial aneurysm treatment considering pre- and post-interventional hemodynamics. Comput. Biol. Med. 2023, 156, 106720. [Google Scholar] [CrossRef]
- Gaidzik, F.; Korte, J.; Saalfeld, S.; Janiga, G.; Berg, P. Image-based hemodynamic simulations for intracranial aneurysms: The impact of complex vasculature. Int. J. Comput. Assist. Radiol. Surg. 2024, 19, 687–697. [Google Scholar] [CrossRef]
- Lylyk, P.; Lylyk, I.; Bleise, C.; Scrivano, E.; Lylyk, P.N.; Beneduce, B.; Heilman, C.B.; Malek, A.M. First-in-human endovascular treatment of hydrocephalus with a miniature biomimetic transdural shunt. J. Neurointerv. Surg. 2022, 14, 495–499. [Google Scholar] [CrossRef]
- Steinman, D.A.; Gounis, M.J.; Levitt, M.R. You’re so vein, you probably think this model’s about you: Opportunities and challenges for computational fluid dynamics in cerebral venous disease. J. Neurointerv. Surg. 2023, 15, 621–622. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Fick, T.; Esposito, G.; Germans, M.; Regli, L.; van Doormaal, T. Segmentation techniques of brain arteriovenous malformations for 3D visualization: A systematic review. La Radiol. Medica 2022, 127, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.S.; Do, H.M.; Wintermark, M.; Massoud, T.F. Large-scale ensemble simulations of biomathematical brain arteriovenous malformation models using graphics processing unit computation. Comput. Biol. Med. 2019, 113, 103416. [Google Scholar] [CrossRef]
- Franzetti, G.; Bonfanti, M.; Tanade, C.; Lim, C.S.; Tsui, J.; Hamilton, G.; Díaz-Zuccarini, V.; Balabani, S. A Computational Framework for Pre-Interventional Planning of Peripheral Arteriovenous Malformations. Cardiovasc. Eng. Technol. 2022, 13, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Ullman, H.; Ali, F.; Berg, P.; Ooi, Y.C.; Tateshima, S.; Colby, G.P.; Komuro, Y.; Hu, P.; Khatibi, K.; et al. In Vitro Modeling of Human Brain Arteriovenous Malformation for Endovascular Simulation and Flow Analysis. World Neurosurg. 2020, 141, e873–e879. [Google Scholar] [CrossRef]
- Berg, P.; Voß, S.; Saalfeld, S.; Janiga, G.; Bergersen, A.W.; Valen-Sendstad, K.; Bruening, J.; Goubergrits, L.; Spuler, A.; Cancelliere, N.M.; et al. Multiple Aneurysms AnaTomy CHallenge 2018 (MATCH): Phase I: Segmentation. Cardiovasc. Eng. Technol. 2018, 9, 565–581. [Google Scholar] [CrossRef]
- Pereira, V.M.; Brina, O.; Marcos Gonzales, A.; Narata, A.P.; Bijlenga, P.; Schaller, K.; Lovblad, K.O.; Ouared, R. Evaluation of the influence of inlet boundary conditions on computational fluid dynamics for intracranial aneurysms: A virtual experiment. J. Biomech. 2013, 46, 1531–1539. [Google Scholar] [CrossRef]
- Hodis, S.; Kargar, S.; Kallmes, D.F.; Dragomir-Daescu, D. Artery length sensitivity in patient-specific cerebral aneurysm simulations. Am. J. Neuroradiol. 2015, 36, 737–743. [Google Scholar] [CrossRef]
- Berg, P.; Saalfeld, S.; Voß, S.; Beuing, O.; Janiga, G. A review on the reliability of hemodynamic modeling in intracranial aneurysms: Why computational fluid dynamics alone cannot solve the equation. Neurosurg. Focus 2019, 47, E15. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, R.; Lian, J.; Luo, F.; Han, H.; Deng, X. Flow Dynamics of Cerebral Bridging Veins Entering Superior Sagittal Sinus by Color-Coded Duplex Sonography. J. Med. Imaging Health Inform. 2017, 7, 862–866. [Google Scholar] [CrossRef]
- Zhang, C.; Chau, N.; Ho, H. Patient-Specific Blood Flow Analysis for Cerebral Arteriovenous Malformation Based on Digital Subtraction Angiography Images. Front. Bioeng. Biotechnol. 2020, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Tutino, V.M.; Snyder, K.V.; Meng, H. CFD: Computational fluid dynamics or confounding factor dissemination? The role of hemodynamics in intracranial aneurysm rupture risk assessment. Am. J. Neuroradiol. 2014, 35, 1849–1857. [Google Scholar]
- Cebral, J.R.; Mut, F.; Weir, J.; Putman, C.M. Association of hemodynamic characteristics and cerebral aneurysm rupture. Am. J. Neuroradiol. 2011, 32, 264–270. [Google Scholar] [CrossRef]
- Ye, X.; Wang, L.; Li, M.-T.; Chen, X.-L.; Wang, H.; Ma, L.; Wang, R.; Zhang, Y.; Cao, Y.; Zhao, Y.-L.; et al. Hemodynamic changes in superficial arteriovenous malformation surgery measured by intraoperative ICG fluorescence videoangiography with FLOW 800 software. Chin. Neurosurg. J. 2020, 6, 29. [Google Scholar] [CrossRef]
- Chang, W.; Loecher, M.W.; Wu, Y.; Niemann, D.B.; Ciske, B.; Aagaard-Kienitz, B.; Kecskemeti, S.; Johnson, K.M.; Wieben, O.; Mistretta, C.; et al. Hemodynamic Changes in Patients with Arteriovenous Malformations Assessed Using High-Resolution 3D Radial Phase-Contrast MR Angiography. Am. J. Neuroradiol. 2012, 33, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Stahl, J.; McGuire, L.S.; Rizko, M.; Saalfeld, S.; Berg, P.; Alaraj, A. Are hemodynamics responsible for inflammatory changes in venous vessel walls? A quantitative study of wall-enhancing intracranial arteriovenous malformation draining veins. J. Neurosurg. 2024, 141, 333–342. [Google Scholar]
- Li, G.; Wang, G.; Yu, J.; Hou, K.; Yu, J. Regression of a symptomatic varix after transarterial embolization of a brain arteriovenous malformation: A case report and literature review. Medicine 2019, 98, e18418. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, X.; Zhang, X.; Ding, G.; Ge, L.; Wang, S. Computational modeling and simulation for endovascular embolization of cerebral arteriovenous malformations with liquid embolic agents. Acta Mech. Sin. 2024, 40, 623042. [Google Scholar] [CrossRef]
- Koyanagi, M.; Mosimann, P.J.; Nordmeyer, H.; Heddier, M.; Krause, J.; Narata, A.-P.; Serwi, A.E.; Stracke, C.P.; Chapot, R. The transvenous retrograde pressure cooker technique for the curative embolization of high-grade brain arteriovenous malformations. J. Neurointerv. Surg. 2021, 13, 637–641. [Google Scholar] [CrossRef]
- Ammirati, M. Augmented reality in brain tumor surgery using the microscope focal point as the virtual pointer. Acta Neurochir. 2022, 164, 1. [Google Scholar] [CrossRef]
- Montemurro, N.; Condino, S.; Carbone, M.; Cattari, N.; D’Amato, R.; Cutolo, F.; Ferrari, V. Brain Tumor and Augmented Reality: New Technologies for the Future. Int. J. Environ. Res. Public Health 2022, 19, 6347. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.M.; Lylyk, P.; Cancelliere, N.; Lylyk, P.N.; Lylyk, I.; Anagnostakou, V.; Bleise, C.; Nishi, H.; Epshtein, M.; King, R.M.; et al. Volumetric microscopy of cerebral arteries with a miniaturized optical coherence tomography imaging probe. Sci. Transl. Med. 2024, 16, eadl4497. [Google Scholar] [CrossRef]
- Morales, H.G.; Larrabide, I.; Geers, A.J.; Aguilar, M.L.; Frangi, A.F. Newtonian and non-Newtonian blood flow in coiled cerebral aneurysms. J. Biomech. 2013, 46, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.O.; Steinman, D.A.; Valen-Sendstad, K. Non-Newtonian versus numerical rheology: Practical impact of shear-thinning on the prediction of stable and unstable flows in intracranial aneurysms. Int. J. Numer. Methods Biomed. Eng. 2017, 33, e2836. [Google Scholar] [CrossRef] [PubMed]
- Voß, S.; Niemann, U.; Saalfeld, S.; Janiga, G.; Berg, P. Impact of workflow variability on image-based intracranial aneurysm hemodynamics. Comput. Biol. Med. 2025, 190, 110018. [Google Scholar] [CrossRef]
- Arzani, A. Accounting for residence-time in blood rheology models: Do we really need non-Newtonian blood flow modelling in large arteries? J. R. Soc. Interface 2018, 15, 20180486. [Google Scholar] [CrossRef]


| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Nidus location | right frontal | right frontal | right occipital |
| Number of feeding arteries | 2 | 3 | 3 |
| Number of draining veins | 3 | 2 | 2 |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| WSS feeding artery 1 in Pa | 52.6 | 7.7 | 5.1 |
| WSS feeding artery 2 in Pa | 17.0 | 7.1 | 46.2 |
| WSS feeding artery 3 in Pa | n/a | 12.8 | 12.5 |
| Mean value (standard dev.) in Pa | 34.8 (17.8) | 9.2 (2.6) | 21.3 (17.9) |
| WSS draining vein 1 in Pa | 19.0 | 2.6 | 9.5 |
| WSS draining vein 2 in Pa | 15.5 | 3.3 | 9.5 |
| WSS draining vein 3 in Pa | 3.4 | n/a | n/a |
| Mean value (standard dev.) in Pa | 12.6 (6.7) | 2.9 (0.3) | 9.5 (0.01) |
| Rel. dev. of feeding arteries to draining veins | 63.7% | 68.2% | 55.5% |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| RPcom volume flow rate in mL/min | 70.8 | 122.4 | −255.5 |
| LPcom volume flow rate in mL/min | 40.2 | n/a | −40.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stahl, J.; McGuire, L.S.; Abou-Mrad, T.; Saalfeld, S.; Behme, D.; Alaraj, A.; Berg, P. Feasibility Study for Multimodal Image-Based Assessment of Patient-Specific Intracranial Arteriovenous Malformation Hemodynamics. J. Clin. Med. 2025, 14, 2638. https://doi.org/10.3390/jcm14082638
Stahl J, McGuire LS, Abou-Mrad T, Saalfeld S, Behme D, Alaraj A, Berg P. Feasibility Study for Multimodal Image-Based Assessment of Patient-Specific Intracranial Arteriovenous Malformation Hemodynamics. Journal of Clinical Medicine. 2025; 14(8):2638. https://doi.org/10.3390/jcm14082638
Chicago/Turabian StyleStahl, Janneck, Laura Stone McGuire, Tatiana Abou-Mrad, Sylvia Saalfeld, Daniel Behme, Ali Alaraj, and Philipp Berg. 2025. "Feasibility Study for Multimodal Image-Based Assessment of Patient-Specific Intracranial Arteriovenous Malformation Hemodynamics" Journal of Clinical Medicine 14, no. 8: 2638. https://doi.org/10.3390/jcm14082638
APA StyleStahl, J., McGuire, L. S., Abou-Mrad, T., Saalfeld, S., Behme, D., Alaraj, A., & Berg, P. (2025). Feasibility Study for Multimodal Image-Based Assessment of Patient-Specific Intracranial Arteriovenous Malformation Hemodynamics. Journal of Clinical Medicine, 14(8), 2638. https://doi.org/10.3390/jcm14082638






