A Discussion of a Case of Paradoxical Ipsilateral Hemiparesis in a Patient Diagnosed with Pterional Meningioma
Abstract
:1. Introduction
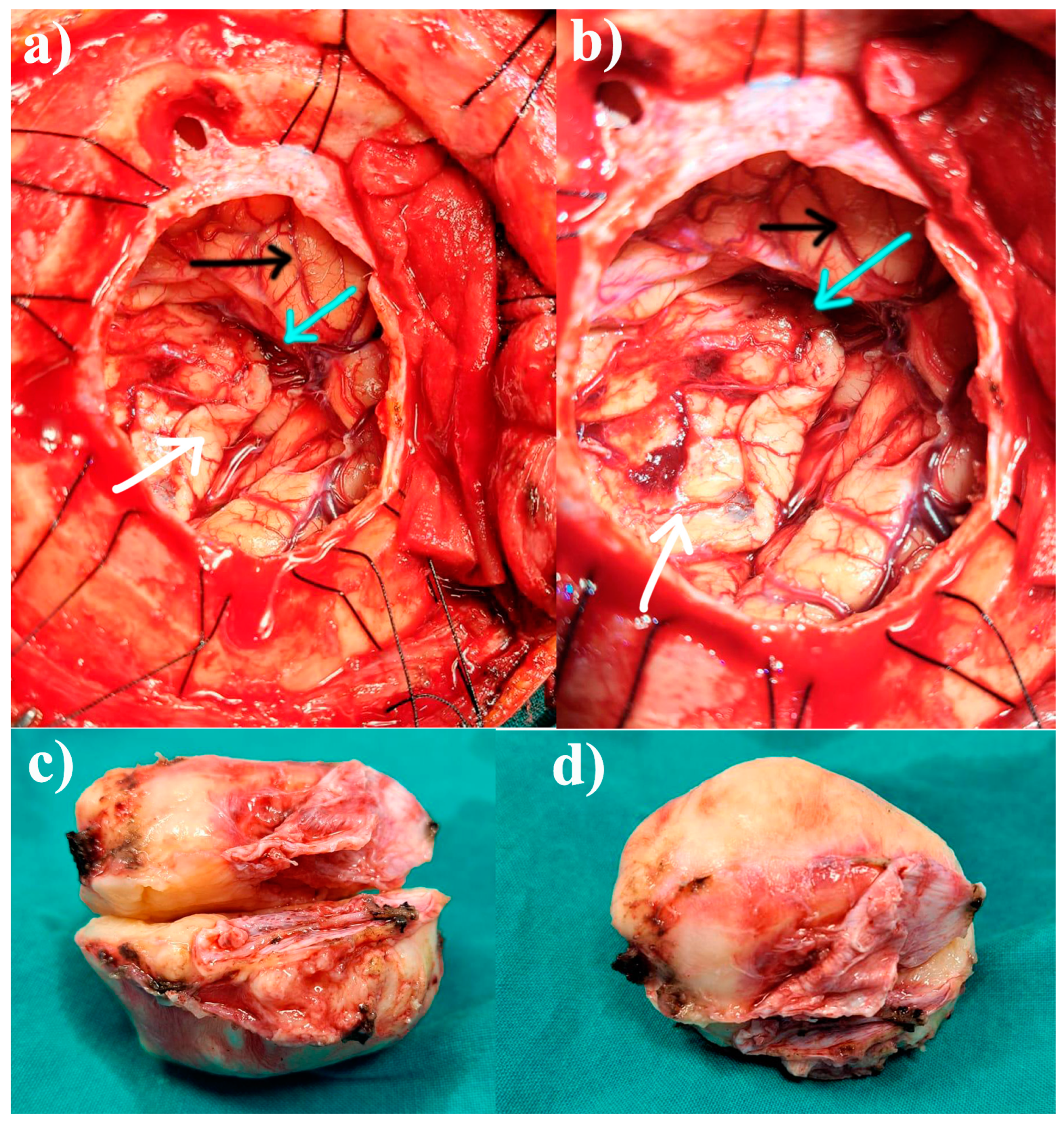
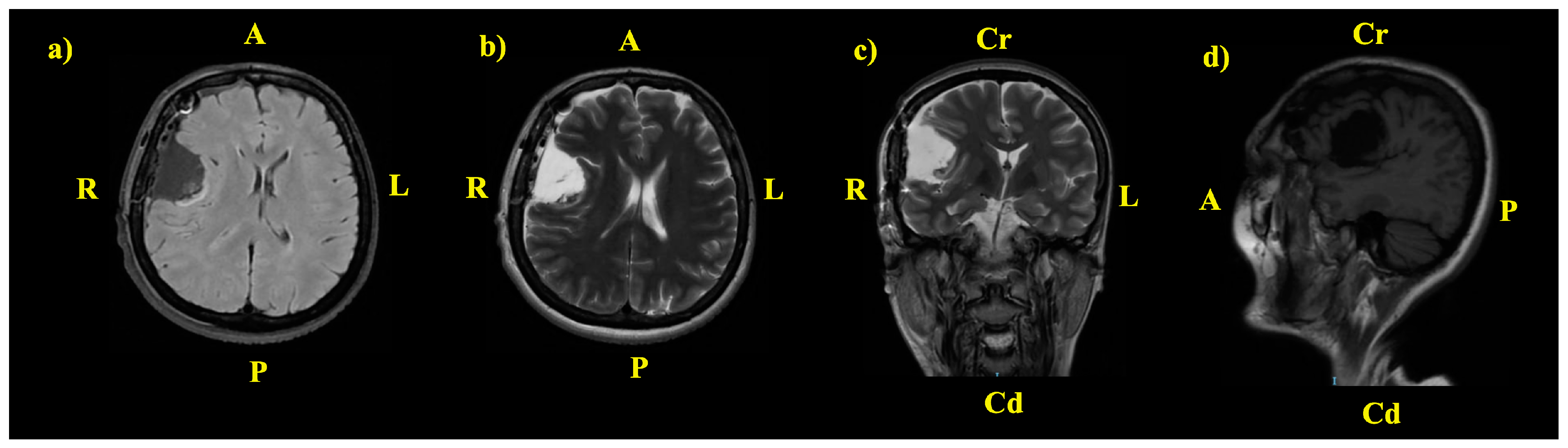
2. The Case: An Intriguing Case Unraveling Clinical and Paraclinical Complexities
3. Discussion
3.1. Pathophysiological Hypotheses
3.1.1. Disorders of Anatomical Decussation
3.1.2. The Concept of Diaschisis
3.1.3. Kernohan-Woltman Notch Phenomenon (KWNP)
3.1.4. The Syndrome of the Third Frontal Convolution
3.2. Discussions of a Rare Encounter: What Is the Possible Cause of IH in Our Case?
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrasco-Moro, R.; Castro-Dufourny, I.; Martínez-San Millán, J.S.; Cabañes-Martínez, L.; Pascual, J.M. Ipsilateral hemiparesis: The forgotten history of this paradoxical neurological sign. Neurosurg. Focus. FOC 2019, 47, E7. [Google Scholar] [CrossRef]
- Nathan, P.W.; Smith, M.C.; Deacon, P. The corticospinal tracts in man. Course and location of fibres at different segmental levels. Brain 1990, 113 Pt 2, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Welniarz, Q.; Dusart, I.; Roze, E. The corticospinal tract: Evolution, development, and human disorders. Dev. Neurobiol. 2017, 77, 810–829. [Google Scholar] [CrossRef]
- Lohia, A.; McKenzie, J. Neuroanatomy, Pyramidal Tract Lesions. [Updated 24 July 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK540976/ (accessed on 1 February 2025).
- Turton, A.J. The Contribution of Reorganised Motor Pathways to Recovery of Arm and Hand Function After Stroke; Open University: Milton Keynes, UK, 1996. [Google Scholar]
- Annavarapu, R.N.; Kathi, S.; Vadla, V.K. Non-invasive imaging modalities to study neurodegenerative diseases of aging brain. J. Chem. Neuroanat. 2019, 95, 54–69. [Google Scholar] [CrossRef]
- Poretti, A.; Boltshauser, E.; Loenneker, T.; Valente, E.M.; Brancati, F.; Il’yasov, K.; Huisman, T.A. Diffusion tensor imaging in Joubert syndrome. AJNR Am. J. Neuroradiol. 2007, 28, 1929–1933. [Google Scholar] [CrossRef]
- Lindsay, K.W.; Bone, I.; Fuller, G. Section II—investigations of the central and peripheral nervous systems. In Neurology and Neurosurgery Illustrated, 5th ed.; Lindsay, K.W., Bone, I., Fuller, G., Eds.; Churchill Livingstone: London, UK, 2010; pp. 33–65. [Google Scholar]
- Vasileva, P.; Hristov, H.; Bussarsky, A.; Tanova, R.; Karakostov, V.; Ferdinandov, D. Transcranial Corticospinal Motor-Evoked Potentials in Cases of Ventral and Ventrolateral Intradural Extramedullary Cervical Spinal Cord Tumors. Medicina 2024, 60, 1488. [Google Scholar] [CrossRef] [PubMed]
- Zedde, M.; Grisendi, I.; Assenza, F.; Napoli, M.; Moratti, C.; Di Cecco, G.; D’Aniello, S.; Valzania, F.; Pascarella, R. Stroke-Induced Secondary Neurodegeneration of the Corticospinal Tract—Time Course and Mechanisms Underlying Signal Changes in Conventional and Advanced Magnetic Resonance Imaging. J. Clin. Med. 2024, 13, 1969. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Matsumura, Y.; Fukuda, T.; Nemoto, Y.; Shirahata, N.; Suzuki, T.; Shakudo, M.; Yawata, S.; Tanaka, S.; Takemoto, K.; et al. MR imaging of Wallerian degeneration in the brainstem: Temporal relationships. AJNR Am. J. Neuroradiol. 1990, 11, 897–902. [Google Scholar]
- Saada, F.; Antonios, N. Existence of Ipsilateral Hemiparesis in Ischemic and Hemorrhagic Stroke: Two Case Reports and Review of the Literature. Eur. Neurol. 2013, 71, 25–31. [Google Scholar] [CrossRef]
- Tan, Z.-R.; Zhang, C.; Tian, F.-F. Spectrum of clinical features and neuroimaging findings in acute cerebral infarction patients with unusual ipsilateral motor impairment—A series of 22 cases. BMC Neurol. 2019, 19, 279. [Google Scholar] [CrossRef]
- Song, Y.-M.; Lee, J.-Y.; Park, J.-M.; Yoon, B.-W.; Roh, J.-K. Ipsilateral Hemiparesis Caused by a Corona Radiata Infarct After a Previous Stroke on the Opposite Side. Arch. Neurol. 2005, 62, 809–811. [Google Scholar] [CrossRef]
- Kajtazi, N.I.; Bafaquh, M.; Rizvi, T.; Sheikh, S.E.; Ghamdi, J.A.; Amoudi, R.A.; Jabbar, A.A.; Shammari, K.A.; Saqqur, M.; Ghamdi, S.A.; et al. Ipsilateral weakness caused by ipsilateral stroke: A case series. J. Stroke Cerebrovasc. Dis. 2023, 32, 107090. [Google Scholar] [CrossRef] [PubMed]
- Jidal, M.; Horache, K.; Fikri, M.; El Kettani, N.; Jiddane, M.; Touarsa, F. A rare case of ispilateral hemiparesis in a patient with uncrossed pyramidal tract shown by tractography. Radiol. Case Rep. 2024, 19, 3512–3516. [Google Scholar] [CrossRef] [PubMed]
- Cane, G.; Mazzeo, L.A.; Vismara, D.; Salmaggi, A. Corticospinal tract decussation alteration: A single case of a healthy woman presenting with left fronto-parietal intraparenchimal haemorrhage showing homolateral symptoms and signs. J. Clin. Images Med. Case Rep. 2022, 3, 2116. [Google Scholar] [CrossRef]
- Sicotte, N.L.; Salamon, G.; Shattuck, D.W.; Hageman, N.; Rüb, U.; Salamon, N.; Drain, A.E.; Demer, J.L.; Engle, E.C.; Alger, J.R.; et al. Diffusion tensor MRI shows abnormal brainstem crossing fibers associated with ROBO3 mutations. Neurology 2006, 67, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.W.; Smith, M.C. Long descending tracts in man. I. Review of present knowledge. Brain 1955, 78, 248–303. [Google Scholar] [CrossRef]
- ten Donkelaar, H.J.; Lammens, M.; Wesseling, P.; Hori, A.; Keyser, A.; Rotteveel, J. Development and malformations of the human pyramidal tract. J. Neurol. 2004, 251, 1429–1442. [Google Scholar] [CrossRef]
- Jones, T.A.; Adkins, D.L. Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection. Physiology 2015, 30, 358–370. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, L. Ipsilateral hemiparesis and contralateral lower limb paresis caused by anterior cerebral artery territory infarct. Neurosciences 2016, 21, 256–259. [Google Scholar] [CrossRef]
- Hosokawa, S.; Tsuji, S.; Uozumi, T.; Matsunaga, K.; Toda, K.; Ota, S. Ipsilateral hemiplegia caused by right internal capsule and thalamic hemorrhage: Demonstration of predominant ipsilateral innervation of motor and sensory systems by MRI, MEP, and SEP. Neurology 1996, 46, 1146–1149. [Google Scholar] [CrossRef]
- Patra, D.P.; Narayan, V.; Savardekar, A.; Dossani, R.H.; Cajavilca, C.; Javalkar, V.; Gonzalez-Toledo, E.; Cuellar, H.H. Acute Supratentorial Ischemic Stroke with Ipsilateral Hemiparesis: Pathomechanism and Management Challenges. World Neurosurg. 2018, 119, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.S.; Obara, K.; Muramatsu, K. Diaschisis. Neurol. Res. 1993, 15, 362–366. [Google Scholar] [CrossRef]
- Finger, S.; Koehler, P.J.; Jagella, C. The Monakow concept of diaschisis: Origins and perspectives. Arch. Neurol. 2004, 61, 283–288. [Google Scholar] [CrossRef]
- Young, G.B. Diaschisis. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press: Oxford, UK, 2014; p. 995. [Google Scholar]
- Carrera, E.; Tononi, G. Diaschisis: Past, present, future. Brain 2014, 137, 2408–2422. [Google Scholar] [CrossRef]
- Garcia, J.O.; Battelli, L.; Plow, E.; Cattaneo, Z.; Vettel, J.; Grossman, E.D. Understanding diaschisis models of attention dysfunction with rTMS. Sci. Rep. 2020, 10, 14890. [Google Scholar] [CrossRef] [PubMed]
- León Ruiz, M.; Rodríguez Sarasa, M.L.; Sanjuán Rodríguez, L.; Benito-León, J.; García-Albea Ristol, E.; Arce Arce, S. Current evidence on transcranial magnetic stimulation and its potential usefulness in post-stroke neurorehabilitation: Opening new doors to the treatment of cerebrovascular disease. Neurología (Engl. Ed.) 2018, 33, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Liu, T.; Li, D.; Tian, X.; Zheng, R.; Ma, Y.; Zheng, S.; Xing, J.; Wang, W.; et al. Application of deep brain stimulation and transcranial magnetic stimulation in stroke neurorestoration: A review. J. Neurorestoratol. 2024, 12, 100120. [Google Scholar] [CrossRef]
- Campo, P.; Garrido, M.I.; Moran, R.J.; Maestú, F.; García-Morales, I.; Gil-Nagel, A.; del Pozo, F.; Dolan, R.J.; Friston, K.J. Remote effects of hippocampal sclerosis on effective connectivity during working memory encoding: A case of connectional diaschisis? Cereb. Cortex 2012, 22, 1225–1236. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Y.; Wang, J.; Hong, W.; Zhao, Z. Research Progress in Diffusion Spectrum Imaging. Brain Sci. 2023, 13, 1497. [Google Scholar] [CrossRef]
- He, B.; Liu, Z. Multimodal functional neuroimaging: Integrating functional MRI and EEG/MEG. IEEE Rev. Biomed. Eng. 2008, 1, 23–40. [Google Scholar] [CrossRef]
- Murhega, R.B.; Balemba Ghislain, M.; Mudekereza, P.S.; Musilimu, S.; Bisimwa, I.; Munguakonkwa Budema, P.; Mubenga, L.E. Kernohan-Woltman notch phenomenon in patient with subdural hematoma and ipsilateral hemiparesis in Bukavu. Clin. Case Rep. 2023, 11, e7643. [Google Scholar] [CrossRef]
- Kernohan, J.W.; Woltman, H.W. Incisura of the crus due to contralateral brain tumor. Arch. Neurol. Psychiatry 1929, 21, 274–287. [Google Scholar] [CrossRef]
- Laaidi, A.; Hmada, S.; Naja, A.; Lakhdar, A. Kernohan Woltman notch phenomenon caused by subdural chronic hematoma: Systematic review and an illustrative case. Ann. Med. Surg. 2022, 79, 104006. [Google Scholar] [CrossRef]
- Yik, J.H.; Seet, R.C.; Pek, C.H.; Lim, E.C. A woman with progressive ataxia and hemiparesis on the right side: Where’s the lesion? CMAJ 2007, 177, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Lyon, R.; Manley, G.T. Transcranial motor evoked potential recording in a case of Kernohan’s notch syndrome: Case report. Neurosurgery 2004, 54, 999–1002; discussion 1002–1003. [Google Scholar] [CrossRef]
- Beucler, N.; Cungi, P.J.; Baucher, G.; Coze, S.; Dagain, A.; Roche, P.H. The Kernohan-Woltman Notch Phenomenon: A Systematic Review of Clinical and Radiologic Presentation, Surgical Management, and Functional Prognosis. J. Korean Neurosurg. Soc. 2022, 65, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.G.; Pyun, S.B. Diffusion tensor tractography in two cases of kernohan-woltman notch phenomenon. Ann. Rehabil. Med. 2013, 37, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Chakravarty, A. The Kinetics of Transtentorial Brain Herniation: Kernohan-Woltman Notch Phenomenon Revisited. Curr. Neurol. Neurosci. Rep. 2023, 23, 571–580. [Google Scholar] [CrossRef]
- Grosset, L.; Dimitrovic, A.; Guillonnet, A.; Tamazyan, R.; Benzakoun, J.; Dusonchet, A.; Chabriat, H.; Oppenheim, C.; Zuber, M.; Calvet, D.; et al. MRI-Proven Incident Ischemia: A New Marker of Disease Progression in Small Vessel Diseases. Stroke 2025, 56, 39–45. [Google Scholar] [CrossRef]
- Lin, Y.; Chen-Lung Chou, A.; Lin, X.; Wu, Z.; Ju, Q.; Li, Y.; Ye, Z.; Zhang, B. A case of Kernohan-Woltman notch phenomenon caused by an epidural hematoma: The diagnostic and prognostic value of PET/CT imaging. BMC Neurol. 2022, 22, 419. [Google Scholar] [CrossRef]
- Zhang, C.H.; DeSouza, R.M.; Kho, J.S.; Vundavalli, S.; Critchley, G. Kernohan-Woltman notch phenomenon: A review article. Br. J. Neurosurg. 2017, 31, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Moro, R.; Martínez-San Millán, J.S.; Pérez-Pérez, M.; Pascual, J.M. Syndrome of the third frontal convolution: Léon Ectors’ legacy on paradoxical ipsilateral hemiparesis. Acta Neurol. Belg. 2024, 124, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Arboix, A.; Martí-Vilalta, J.L. Hemiparesis and Other Types of Motor Weakness; Chapter 1, Stroke Syndromes; Caplan, L.R., van Gijn, J., Eds.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Fujii, Y.; Nakada, T. Cortical reorganization in patients with subcortical hemiparesis: Neural mechanisms of functional recovery and prognostic implication. J. Neurosurg. 2003, 98, 64–73. [Google Scholar] [CrossRef]
- Adler, D.E.; Milhorat, T.H. The tentorial notch: Anatomical variation, morphometric analysis, and classification in 100 human autopsy cases. J. Neurosurg. 2002, 96, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Azar, M.; Fattahi, A.; Tabibkhooei, A.; Taheri, M. A Small Meningioma with Extensive Peritumoral Brain Edema: A Case Report. Iran. J. Med. Sci. 2019, 44, 265–269. [Google Scholar]
- von Bernhardi, R.; Bernhardi, L.E.; Eugenín, J. What Is Neural Plasticity? Adv. Exp. Med. Biol. 2017, 1015, 1–15. [Google Scholar] [CrossRef]
- Sophie Su, Y.; Veeravagu, A.; Grant, G. Chapter 8: Neuroplasticity after Traumatic Brain Injury. In Translational Research in Traumatic Brain Injury (Frontiers in Neuroscience); Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group © 2016 by Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Puderbaugh, M.; Emmady, P.D. Neuroplasticity. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557811/ (accessed on 1 February 2025). [PubMed]




| Neurological Examination | Findings |
|---|---|
| Cranial nerves | Normal |
| Sensation | Normal |
| Speech | Normal |
| Cognition | Normal |
| Comprehension and repetition | Normal |
| Motor examination | MRCS grade 4 for the right upper and lower limbs |
| Deep tendon reflexes | 3+ (brisk response) on the right side |
| Pathologic reflexes | Right positive Babinski sign |
| Other | Headaches; generalized tonic-clonic seizures |
| Inflammatory Marker | Value (Normal Range) | Coagulation Tests/Markers | Value (Normal Range) |
|---|---|---|---|
| ESR | 6 (3–11 mm/h) | APTT | 29.50 (24.3–35 s) |
| WBC | 6.3 (4.8—10.8 × 103/uL) | Fibrinogen | 240.27 (220–496 mg/dL) |
| CRP | 0.309 (<5 mg/L) | PT | 13.100 (11.8–15.1) |
| INR | 1.0178 (0.85–1.15) | ||
| APP | 78.596 (70–140%) |
| Disorders of Anatomical Decussation | Diaschisis | Kernohan-Woltman Notch Phenomenon | The Syndrome of the Third Frontal Convolution |
|---|---|---|---|
| Lesions above the pyramidal decussation produce contralateral weakness, while those below the pyramidal decussation will produce ipsilateral weakness | Recovery is a passive process and can be more efficient in young patients than in older ones | Sudden and massive uncal herniation caused by a supratentorial mass lesion | The presence of intracranial hypertension, seizures +/− contralateral hemiparesis |
| The hemiparesis is disproportionate (predominates on the lower or upper limb) | Regresses in phases correlated to neuroanatomical pathways, starting from the lesional site in the brain | Hemiparesis due to compression of the contralateral peduncle is usually well proportioned on one side of the body | Frontal syndrome: Obtundation; instability; mental symptoms; language impairment |
| Advanced neuroimaging studies, such as DTI and tractography, can provide a certain diagnosis | Reduced activity in regions with diaschisis on advanced neuroimaging studies | Extensive midline shift due to mass effect, resulting in the indentation of the contralateral cerebral crus by the tentorium cerebelli | Radiological syndrome: angiographic engorgement of an anterior collateral artery from the anterior division of the middle meningeal artery, contralateral displacement of the ventricles on ventriculography |
| Partial or complete uncrossed pyramidal tracts on either side at the level of the medulla oblongata could be observed | The signs are prominent in acute injuries and are discreet/absent in chronic and slow-growing lesions | The grade of mass effect, the degree of brainstem displacement, anatomical variations of the tentorial incisura, and the width of the tentorial notch are considered contributing factors | Lateral displacement of the diencephalic-brainstem axis and compression of the contralateral cerebral peduncle against the free tentorial border |
| Independent laterality/motor dominance | Dependent laterality/motor dominance | Independent laterality/motor dominance | Independent laterality/motor dominance |
| The mass effect is unnecessary | The mass effect is unnecessary | The mass effect is necessary | The mass effect is necessary |
| Independent tentorial anthropometry | Independent tentorial anthropometry | Tentorial anthropometry may play a role | Tentorial anthropometry may play a role |
| Motor deficit distribution is dependent on primary lesion topography | Motor deficit distribution is dependent on primary lesion topography | Motor deficit distribution is mostly crural | Brodmann area 8 motor syndrome: Conjugated head rotation and eye deviation towards the lesion, motor discoordination, and pseudo parkinsonism |
| Other NS anomalies frequent | Independent of other NS anomalies | Independent of other NS anomalies | Independent of other NS anomalies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tataranu, L.G. A Discussion of a Case of Paradoxical Ipsilateral Hemiparesis in a Patient Diagnosed with Pterional Meningioma. J. Clin. Med. 2025, 14, 2689. https://doi.org/10.3390/jcm14082689
Tataranu LG. A Discussion of a Case of Paradoxical Ipsilateral Hemiparesis in a Patient Diagnosed with Pterional Meningioma. Journal of Clinical Medicine. 2025; 14(8):2689. https://doi.org/10.3390/jcm14082689
Chicago/Turabian StyleTataranu, Ligia Gabriela. 2025. "A Discussion of a Case of Paradoxical Ipsilateral Hemiparesis in a Patient Diagnosed with Pterional Meningioma" Journal of Clinical Medicine 14, no. 8: 2689. https://doi.org/10.3390/jcm14082689
APA StyleTataranu, L. G. (2025). A Discussion of a Case of Paradoxical Ipsilateral Hemiparesis in a Patient Diagnosed with Pterional Meningioma. Journal of Clinical Medicine, 14(8), 2689. https://doi.org/10.3390/jcm14082689






