Effect of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) on Left Ventricular Mechanics in Patients Without Overt Cardiac Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection and Data Extraction
2.4. Risk-of-Bias Assessment
2.5. Statistical Analysis
3. Results
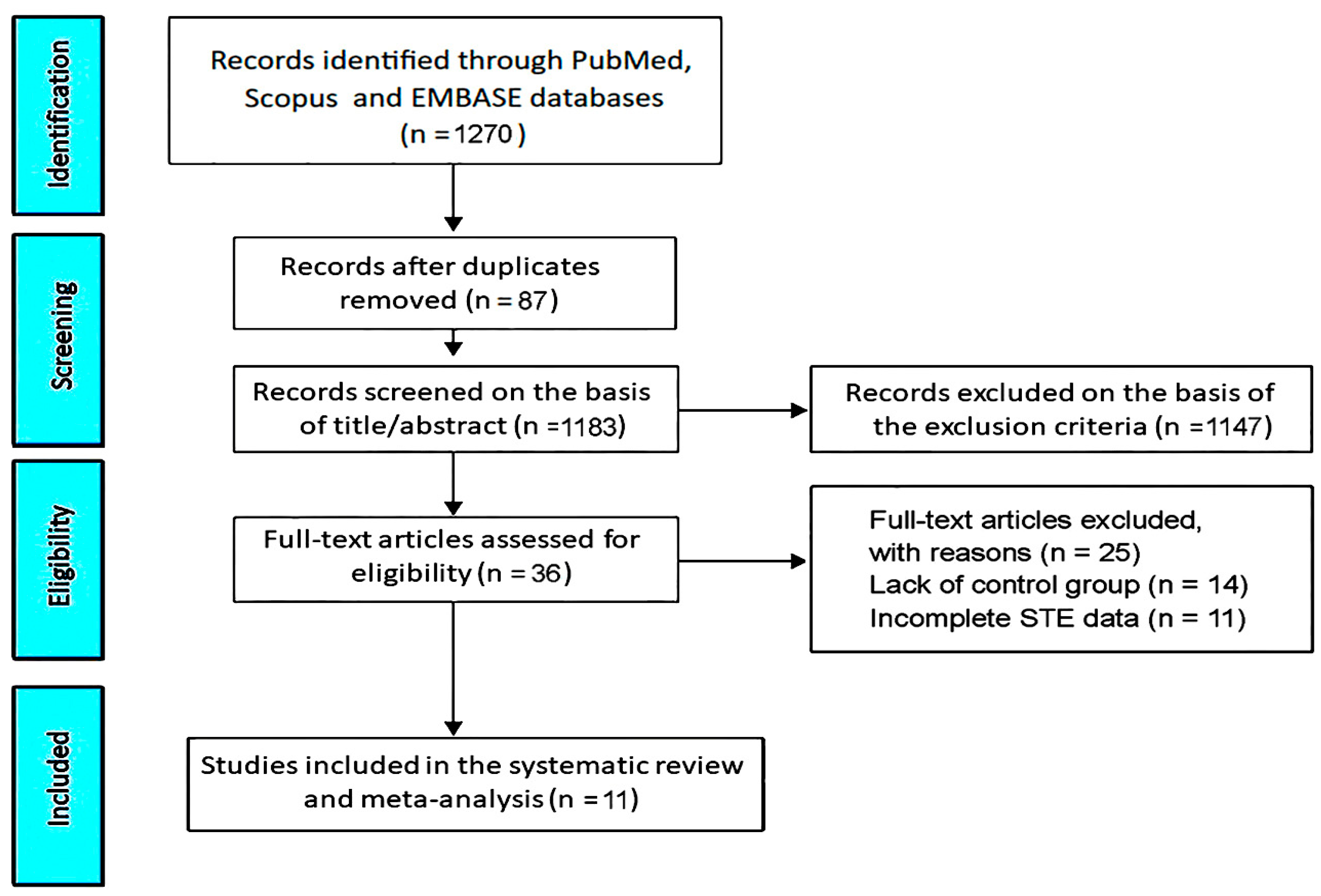
3.1. Study Selection
3.2. Clinical Findings
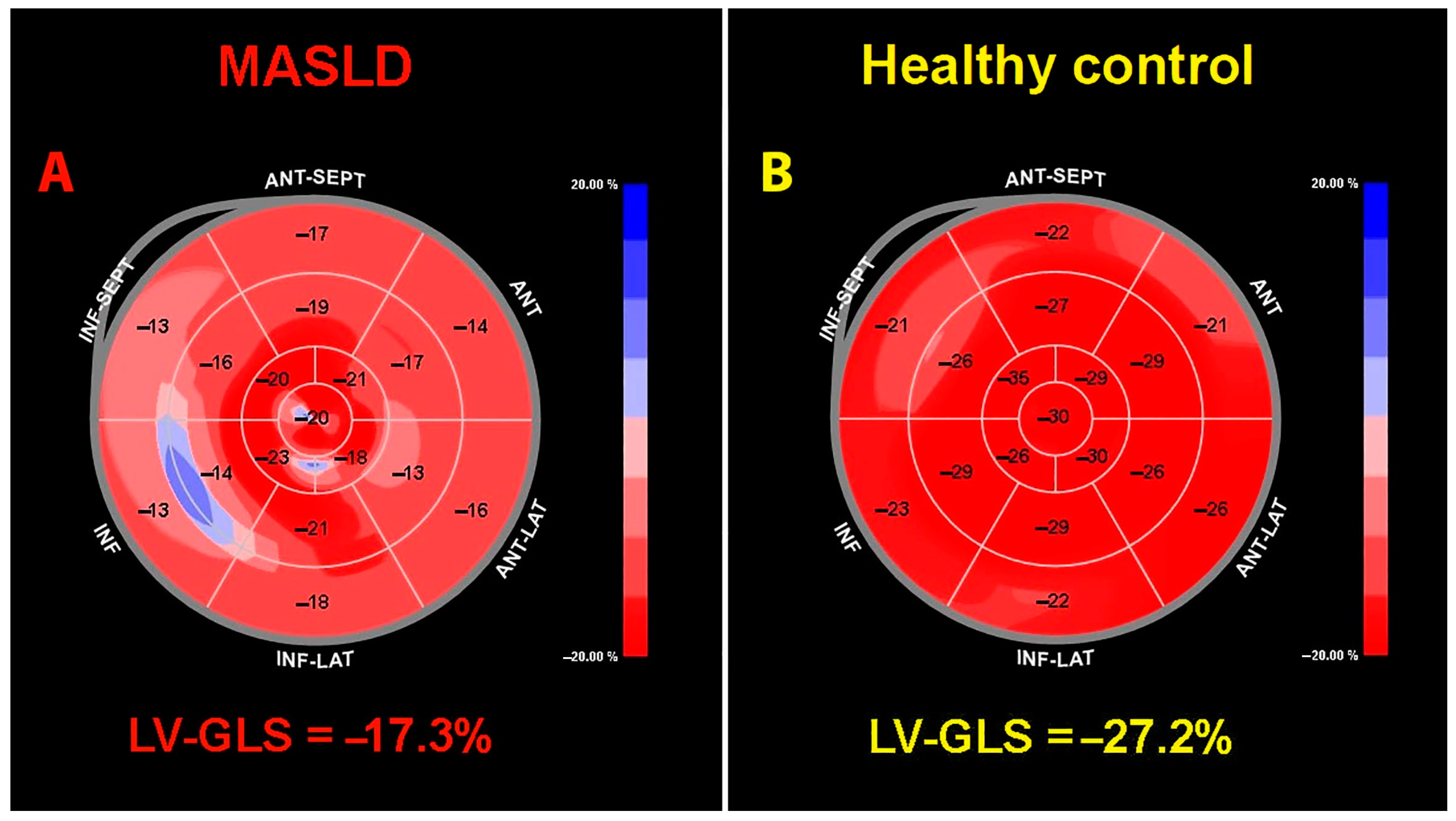
3.3. Conventional Echocardiography and Deformation Imaging Findings
3.4. NIH Quality Rating
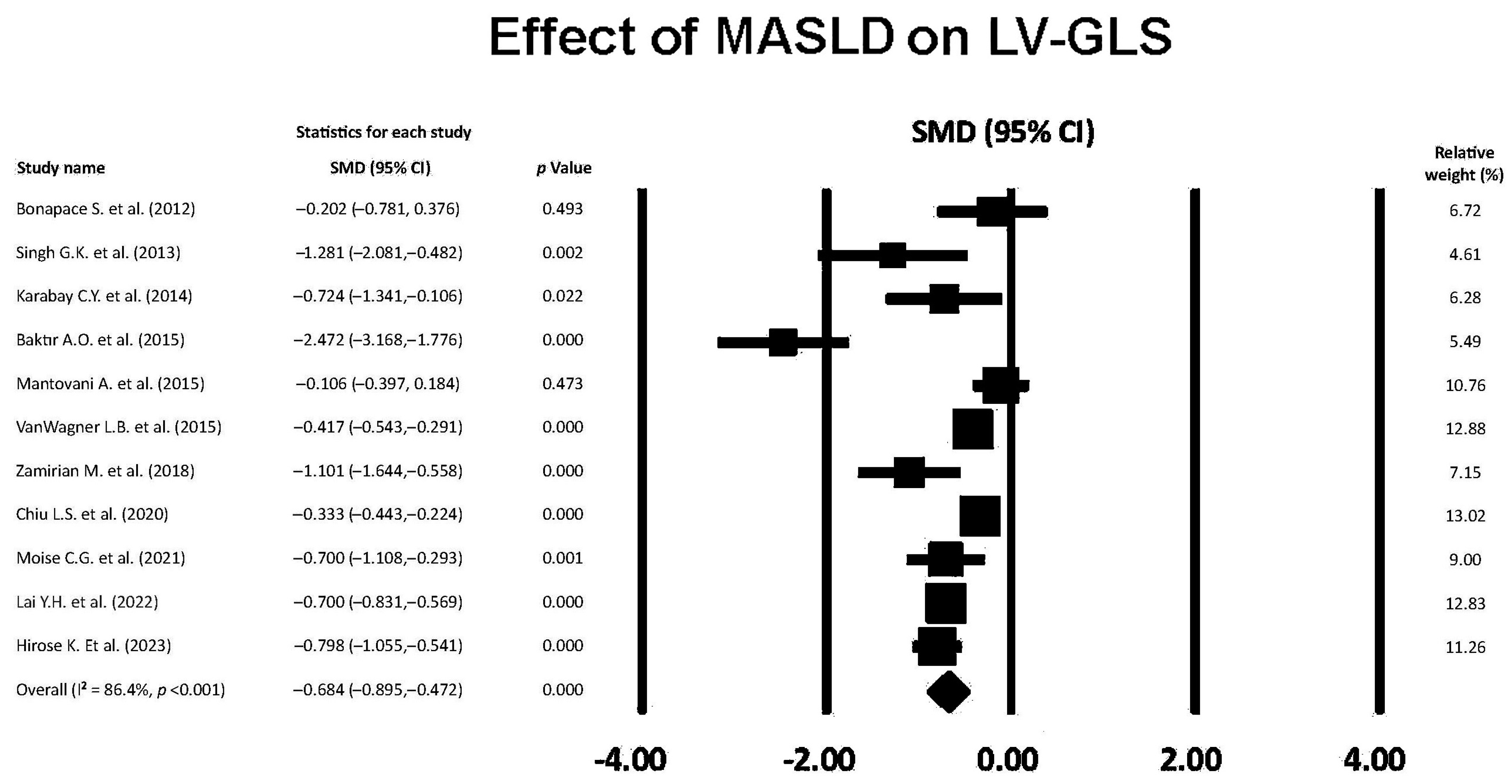
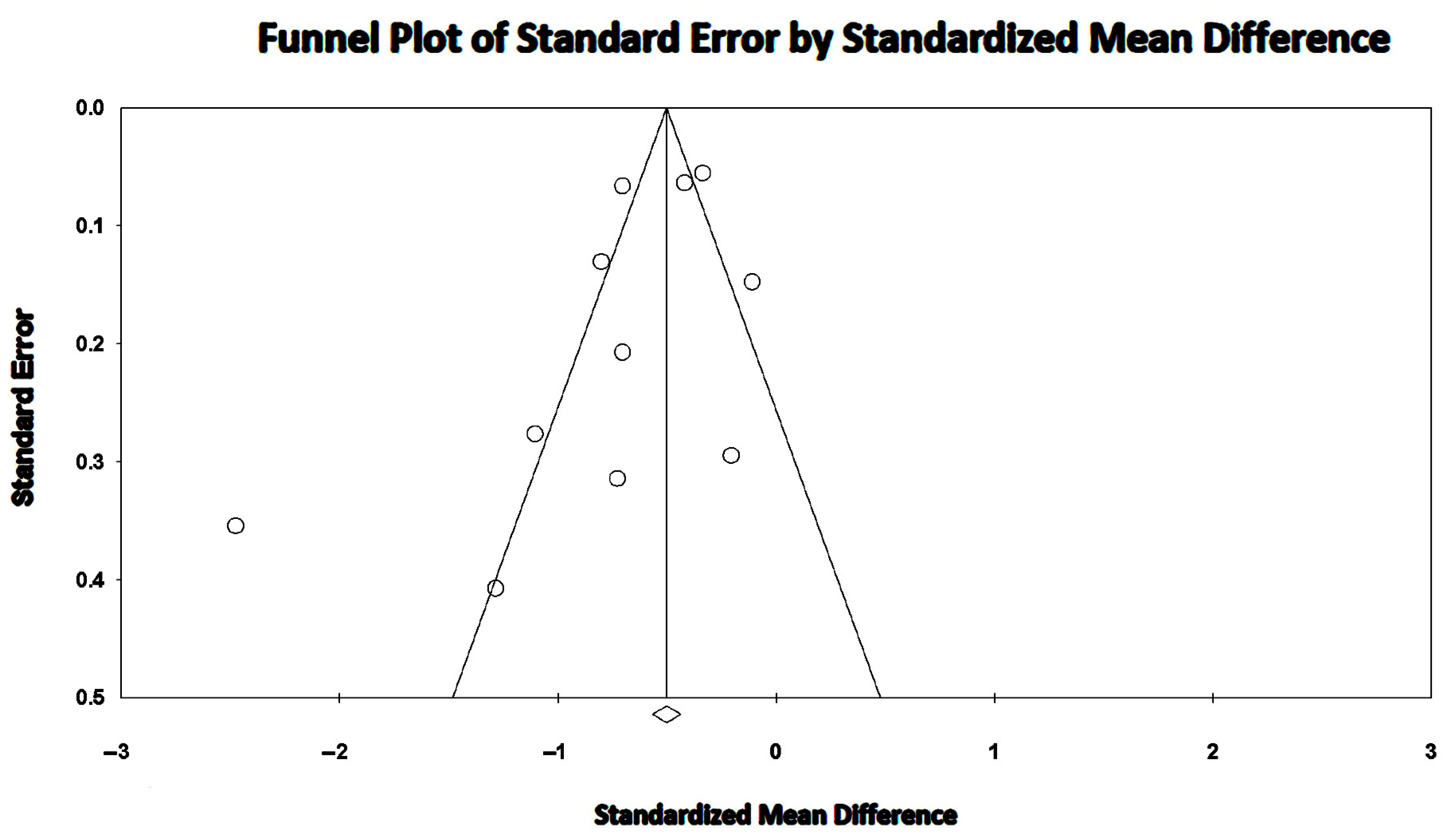
3.5. Effect of MASLD on LV-GLS
3.6. Effect of MASLD on LV-GLSRs
3.7. Effect of MASLD on LV-GLSRe
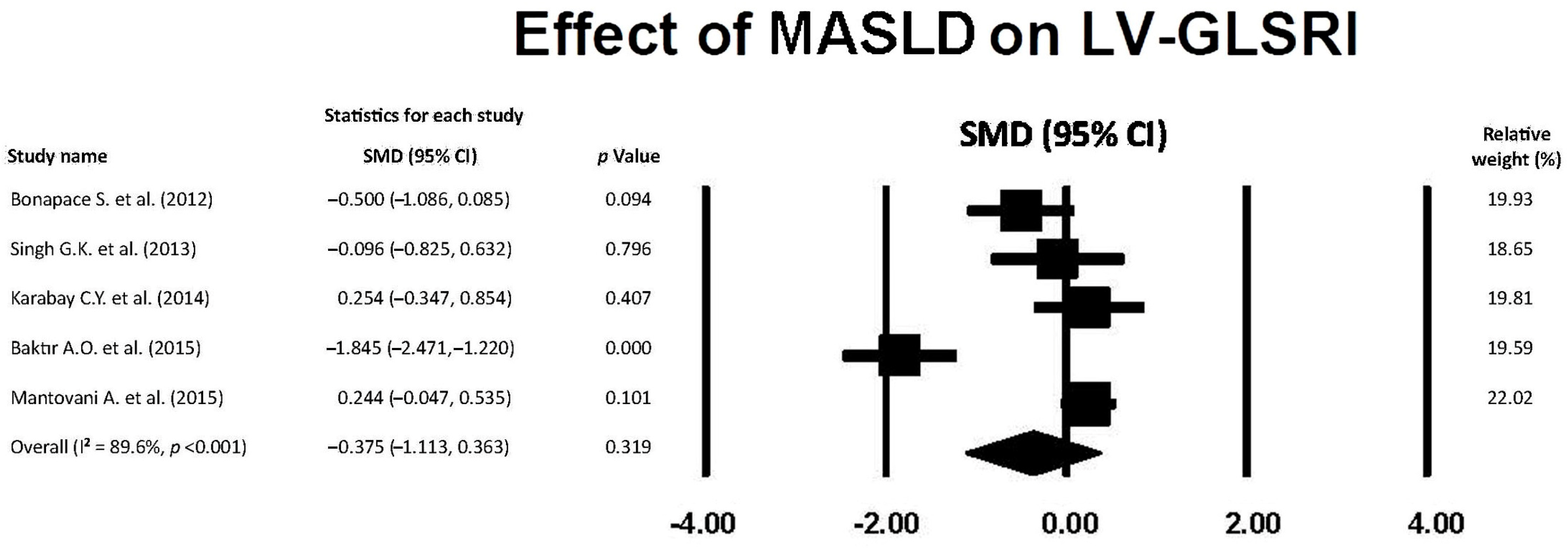
3.8. Effect of MASLD on LV-GLSRl
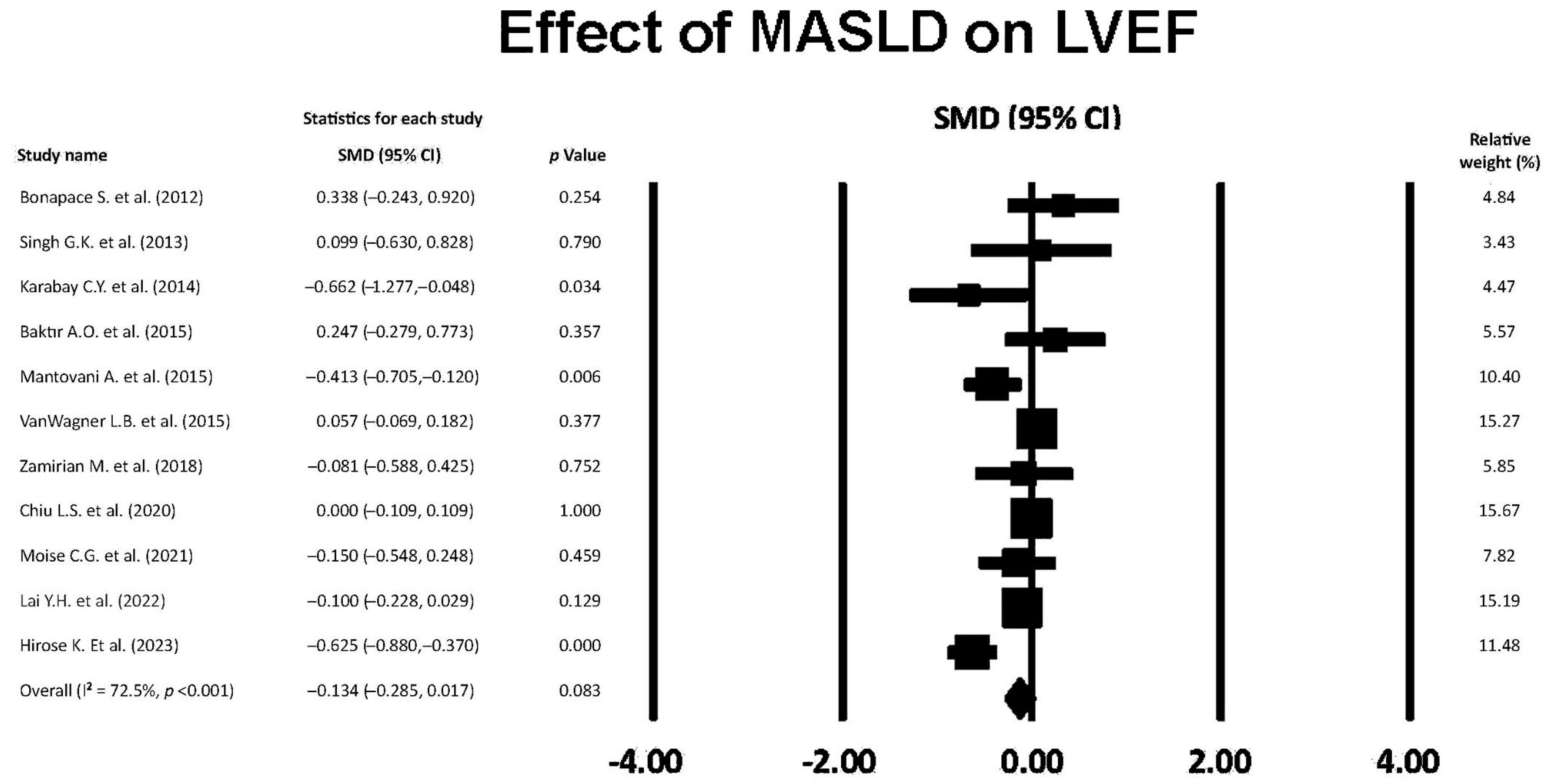
3.9. Effect of MASLD on LVEF
4. Discussion
4.1. Main Findings
4.2. LV Remodeling and LV Diastolic Dysfunction
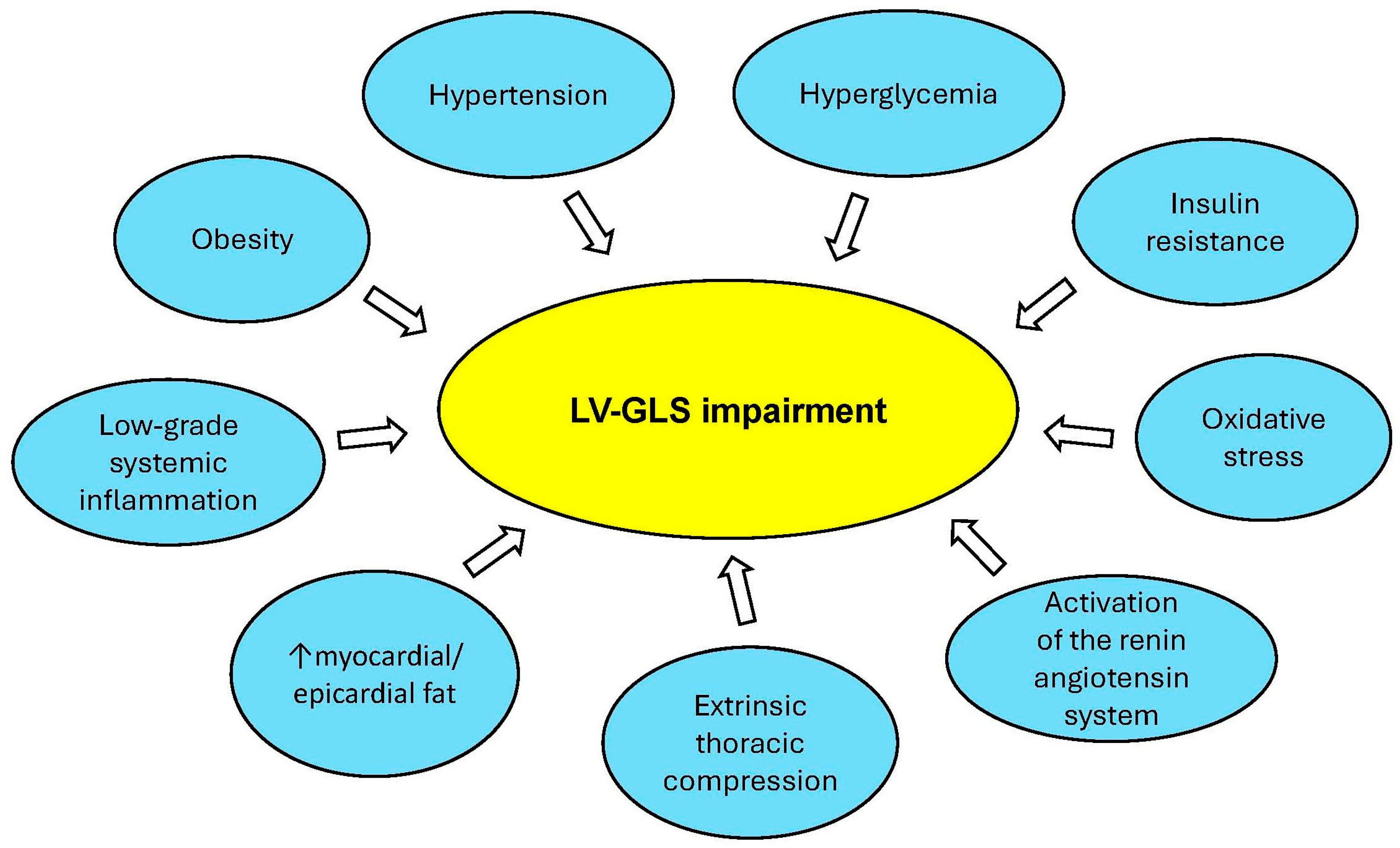
4.3. Subclinical LV Systolic Dysfunction
4.4. Implications for Clinical Practice
4.5. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Zhang, X.; Goh, G.B.; Chan, W.K.; Wong, G.L.; Fan, J.G.; Seto, W.K.; Huang, Y.H.; Lin, H.C.; Lee, I.C.; Lee, H.W.; et al. Unhealthy lifestyle habits and physical inactivity among Asian patients with non-alcoholic fatty liver disease. Liver Int. 2020, 40, 2719–2731. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature 2019, 569, 260–264. [Google Scholar] [CrossRef]
- Lin, H.; Yip, T.C.; Zhang, X.; Li, G.; Tse, Y.K.; Hui, V.W.; Liang, L.Y.; Lai, J.C.; Chan, S.L.; Chan, H.L.; et al. Age and the relative importance of liver-related deaths in nonalcoholic fatty liver disease. Hepatology 2023, 77, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, H.A.; Kim, E.J.; Kim, H.Y.; Kim, H.C.; Ahn, S.H.; Lee, H.; Kim, S.U. Metabolic dysfunction-associated steatotic liver disease and risk of cardiovascular disease. Gut 2024, 73, 533–540. [Google Scholar] [CrossRef]
- Roh, J.H.; Park, J.H.; Lee, H.; Yoon, Y.H.; Kim, M.; Kim, Y.G.; Park, G.M.; Lee, J.H.; Seong, I.W. Higher fatty liver index is associated with increased risk of new onset heart failure in healthy adults: A nationwide population-based study in Korea. BMC Cardiovasc. Disord. 2020, 20, 204. [Google Scholar] [CrossRef]
- Fudim, M.; Zhong, L.; Patel, K.V.; Khera, R.; Abdelmalek, M.F.; Diehl, A.M.; McGarrah, R.W.; Molinger, J.; Moylan, C.A.; Rao, V.N.; et al. Nonalcoholic Fatty Liver Disease and Risk of Heart Failure Among Medicare Beneficiaries. J. Am. Heart Assoc. 2021, 10, e021654. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Benfari, G.; Bonapace, S.; Simon, T.G.; Targher, G. Risk of Heart Failure in Patients With Nonalcoholic Fatty Liver Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 180–191. [Google Scholar] [CrossRef]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Biering-Sørensen, T.; Biering-Sørensen, S.R.; Olsen, F.J.; Sengeløv, M.; Jørgensen, P.G.; Mogelvang, R.; Shah, A.M.; Jensen, J.S. Global Longitudinal Strain by Echocardiography Predicts Long-Term Risk of Cardiovascular Morbidity and Mortality in a Low-Risk General Population: The Copenhagen City Heart Study. Circ. Cardiovasc. Imaging. 2017, 10, e005521. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Albini, A.; Fossile, E.; Pessi, M.A.; Nicolosi, G.L.; Lombardo, M.; Anzà, C.; Ambrosio, G. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc. Toxicol. 2020, 20, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Nakanishi, K.; Daimon, M.; Hirose, K.; Ishiwata, J.; Kaneko, H.; Nakao, T.; Mizuno, Y.; Morita, H.; Di Tullio, M.R.; et al. Aortic valve sclerosis and subclinical LV dysfunction in the general population with normal LV geometry. Eur. J. Prev. Cardiol. 2022, 30, 454–460. [Google Scholar] [CrossRef]
- Lisi, M.; Cameli, M.; Mandoli, G.E.; Pastore, M.C.; Righini, F.M.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; Mondillo, S.; Henein, M.Y. Detection of myocardial fibrosis by speckle-tracking echocardiography: From prediction to clinical applications. Heart Fail. Rev. 2022, 27, 1857–1867. [Google Scholar] [CrossRef]
- Kasner, M.; Sinning, D.; Escher, F.; Lassner, D.; Kühl, U.; Schultheiss, H.P.; Tschöpe, C. The utility of speckle tracking imaging in the diagnostic of acute myocarditis; as proven by endomyocardial biopsy. Int. J. Cardiol. 2013, 168, 3023–3024. [Google Scholar] [CrossRef]
- Ávila-Vanzzini, N.; Fritche-Salazar, J.F.; Vázquez-Castro, N.M.; Rivera-Lara, P.; Pérez-Méndez, O.; Martínez-Herrera, H.; Gómez-Sánchez, M.; Aranda-Frausto, A.; Herrera-Bello, H.; Luna-Luna, M.; et al. Echocardiographic and Histologic Correlations in Patients with Severe Aortic Stenosis: Influence of Overweight and Obesity. J. Cardiovasc. Ultrasound 2016, 24, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Open Med. 2009, 3, e123–e130. [Google Scholar]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 1–11. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Bonapace, S.; Perseghin, G.; Molon, G.; Canali, G.; Bertolini, L.; Zoppini, G.; Barbieri, E.; Targher, G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care 2012, 35, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Vitola, B.E.; Holland, M.R.; Sekarski, T.; Patterson, B.W.; Magkos, F.; Klein, S. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J. Pediatr. 2013, 162, 1160–1168.e1. [Google Scholar] [CrossRef]
- Karabay, C.Y.; Kocabay, G.; Kalayci, A.; Colak, Y.; Oduncu, V.; Akgun, T.; Kalkan, S.; Guler, A.; Kirma, C. Impaired left ventricular mechanics in nonalcoholic fatty liver disease: A speckle-tracking echocardiography study. Eur. J. Gastroenterol. Hepatol. 2014, 26, 325–331. [Google Scholar] [CrossRef]
- Baktır, A.O.; Şarlı, B.; Altekin, R.E.; Karaman, A.; Arınç, H.; Sağlam, H.; Doğan, Y.; Erden, A.; Karaman, H. Non alcoholic steatohepatitis is associated with subclinical impairment in left ventricular function measured by speckle tracking echocardiography. Anatol. J. Cardiol. 2015, 15, 137–142. [Google Scholar] [CrossRef]
- Mantovani, A.; Pernigo, M.; Bergamini, C.; Bonapace, S.; Lipari, P.; Pichiri, I.; Bertolini, L.; Valbusa, F.; Barbieri, E.; Zoppini, G.; et al. Nonalcoholic Fatty Liver Disease Is Independently Associated with Early Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. PLoS ONE 2015, 10, e0135329. [Google Scholar] [CrossRef] [PubMed]
- VanWagner, L.B.; Wilcox, J.E.; Colangelo, L.A.; Lloyd-Jones, D.M.; Carr, J.J.; Lima, J.A.; Lewis, C.E.; Rinella, M.E.; Shah, S.J. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: A population-based study. Hepatology 2015, 62, 773–783. [Google Scholar] [CrossRef]
- Zamirian, M.; Samiee, E.; Moaref, A.; Abtahi, F.; Tahamtan, M. Assessment of Subclinical Myocardial Changes in Non-Alcoholic Fatty Liver Disease: A Case-Control Study Using Speckle Tracking Echocardiography. Iran. J. Med. Sci. 2018, 43, 466–472. [Google Scholar]
- Chiu, L.S.; Pedley, A.; Massaro, J.M.; Benjamin, E.J.; Mitchell, G.F.; McManus, D.D.; Aragam, J.; Vasan, R.S.; Cheng, S.; Long, M.T. The association of non-alcoholic fatty liver disease and cardiac structure and function-Framingham Heart Study. Liver Int. 2020, 40, 2445–2454. [Google Scholar] [CrossRef]
- Moise, C.G.; Donoiu, I.; Târtea, G.C.; Mirea, O.; Rogoveanu, I. Contribution of Modern Echocardiographic Techniques in the Detection of Subclinical Heart Dysfunction in Young Adults with Non-Alcoholic Fatty Liver Disease. Curr. Health Sci. J. 2021, 47, 275–283. [Google Scholar] [CrossRef]
- Lai, Y.H.; Su, C.H.; Hung, T.C.; Yun, C.H.; Tsai, C.T.; Yeh, H.I.; Hung, C.L. Association of Non-Alcoholic Fatty Liver Disease and Hepatic Fibrosis with Epicardial Adipose Tissue Volume and Atrial Deformation Mechanics in a Large Asian Population Free from Clinical Heart Failure. Diagnostics 2022, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Nakanishi, K.; Di Tullio, M.R.; Homma, S.; Sawada, N.; Yoshida, Y.; Hirokawa, M.; Koyama, K.; Kimura, K.; Nakao, T.; et al. Association between non-alcoholic fatty liver disease and subclinical left ventricular dysfunction in the general population. Eur. Heart J. Open 2023, 3, oead108. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Kocabay, G.; Muraru, D.; Peluso, D.; Cucchini, U.; Mihaila, S.; Padayattil-Jose, S.; Gentian, D.; Iliceto, S.; Vinereanu, D.; Badano, L.P. Normal left ventricular mechanics by two-dimensional speckle-tracking echocardiography. Reference values in healthy adults. Rev. Esp. Cardiol. 2014, 67, 651–658. [Google Scholar] [CrossRef]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification; diastolic function; and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Goland, S.; Shimoni, S.; Zornitzki, T.; Knobler, H.; Azoulai, O.; Lutaty, G.; Melzer, E.; Orr, A.; Caspi, A.; Malnick, S. Cardiac abnormalities as a new manifestation of nonalcoholic fatty liver disease: Echocardiographic and tissue Doppler imaging assessment. J. Clin. Gastroenterol. 2006, 40, 949–955. [Google Scholar] [CrossRef]
- Fotbolcu, H.; Yakar, T.; Duman, D.; Karaahmet, T.; Tigen, K.; Cevik, C.; Kurtoglu, U.; Dindar, I. Impairment of the left ventricular systolic and diastolic function in patients with non-alcoholic fatty liver disease. Cardiol. J. 2010, 17, 457–463. [Google Scholar]
- Hallsworth, K.; Hollingsworth, K.G.; Thoma, C.; Jakovljevic, D.; MacGowan, G.A.; Anstee, Q.M.; Taylor, R.; Day, C.P.; Trenell, M.I. Cardiac structure and function are altered in adults with non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 757–762. [Google Scholar] [CrossRef]
- Alp, H.; Karaarslan, S.; Selver Eklioğlu, B.; Atabek, M.E.; Altın, H.; Baysal, T. Association between nonalcoholic fatty liver disease and cardiovascular risk in obese children and adolescents. Can. J. Cardiol. 2013, 29, 1118–1125. [Google Scholar] [CrossRef]
- Sert, A.; Aypar, E.; Pirgon, O.; Yilmaz, H.; Odabas, D.; Tolu, I. Left ventricular function by echocardiography; tissue Doppler imaging; and carotid intima-media thickness in obese adolescents with nonalcoholic fatty liver disease. Am. J. Cardiol. 2013, 112, 436–443. [Google Scholar] [CrossRef]
- Petta, S.; Argano, C.; Colomba, D.; Cammà, C.; Di Marco, V.; Cabibi, D.; Tuttolomondo, A.; Marchesini, G.; Pinto, A.; Licata, G.; et al. Epicardial fat; cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: Association with the severity of liver disease. J. Hepatol. 2015, 62, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, L.; Di Martino, M.; De Merulis, A.; Bezzi, M.; Osborn, J.F.; Catalano, C.; Chiesa, C. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology 2014, 59, 461–470. [Google Scholar] [CrossRef]
- Suto, M.; Tanaka, H.; Mochizuki, Y.; Mukai, J.; Takada, H.; Soga, F.; Dokuni, K.; Hatani, Y.; Hatazawa, K.; Matsuzoe, H.; et al. Impact of overweight on left ventricular function in type 2 diabetes mellitus. Cardiovasc. Diabetol. 2017, 16, 145. [Google Scholar] [CrossRef]
- Kishi, S.; Armstrong, A.C.; Gidding, S.S.; Colangelo, L.A.; Venkatesh, B.A.; Jacobs, D.R., Jr.; Carr, J.J.; Terry, J.G.; Liu, K.; Goff, D.C., Jr.; et al. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: The CARDIA study (Coronary Artery Risk Development in Young Adults). JACC Heart Fail. 2014, 2, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.L.; Lim, W.H.; Seo, J.B.; Kim, S.H.; Zo, J.H.; Kim, M.A. Subclinical alterations in left ventricular structure and function according to obesity and metabolic health status. PLoS ONE 2019, 14, e0222118. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.C.; Delgado, V.; Bertini, M.; van der Meer, R.W.; Rijzewijk, L.J.; Hooi Ewe, S.; Siebelink, H.M.; Smit, J.W.; Diamant, M.; Romijn, J.A.; et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 2010, 122, 2538–2544. [Google Scholar] [CrossRef]
- Lim, S.; Meigs, J.B. Ectopic fat and cardiometabolic and vascular risk. Int. J. Cardiol. 2013, 169, 166–176. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. Ectopic fat; insulin resistance; and nonalcoholic fatty liver disease: Implications for cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1155–1161. [Google Scholar] [CrossRef]
- Ballestri, S.; Lonardo, A.; Bonapace, S.; Byrne, C.D.; Loria, P.; Targher, G. Risk of cardiovascular; cardiac and arrhythmic complications in patients with non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1724–1745. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Vita, T.; Murphy, D.J.; Osborne, M.T.; Bajaj, N.S.; Keraliya, A.; Jacob, S.; Diaz Martinez, A.J.; Nodoushani, A.; Bravo, P.; Hainer, J.; et al. Association between Nonalcoholic Fatty Liver Disease at CT and Coronary Microvascular Dysfunction at Myocardial Perfusion PET/CT. Radiology 2019, 291, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Park, J.H. Role of strain echocardiography in patients with hypertension. Clin. Hypertens. 2022, 28, 6. [Google Scholar] [CrossRef]
- Jędrzejewska, I.; Król, W.; Światowiec, A.; Wilczewska, A.; Grzywanowska-Łaniewska, I.; Dłużniewski, M.; Braksator, W. Left and right ventricular systolic function impairment in type 1 diabetic young adults assessed by 2D speckle tracking echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Takeuchi, M.; Nishikage, T.; Lang, R.M.; Otsuji, Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two-dimensional speckle tracking echocardiography: Correlation with diabetic duration. Eur. J. Echocardiogr. 2009, 10, 926–932. [Google Scholar] [CrossRef]
- Hirose, K.; Nakanishi, K.; Daimon, M.; Sawada, N.; Yoshida, Y.; Iwama, K.; Yamamoto, Y.; Ishiwata, J.; Hirokawa, M.; Koyama, K.; et al. Impact of insulin resistance on subclinical left ventricular dysfunction in normal weight and overweight/obese japanese subjects in a general community. Cardiovasc. Diabetol. 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, J.; Ašanin, M.; Krljanac, G.; Lalić, N.M.; Jotić, A.; Stanković, S.; Rajković, N.; Stošić, L.; Rasulić, I.; Milin, J.; et al. Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: An analysis based on two-dimensional speckle tracking echocardiography. Cardiovasc. Diabetol. 2019, 18, 68. [Google Scholar] [CrossRef]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef]
- Chinali, M.; Devereux, R.B.; Howard, B.V.; Roman, M.J.; Bella, J.N.; Liu, J.E.; Resnick, H.E.; Lee, E.T.; Best, L.G.; de Simone, G. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study). Am. J. Cardiol. 2004, 93, 40–44. [Google Scholar] [CrossRef]
- Dutta, K.; Podolin, D.A.; Davidson, M.B.; Davidoff, A.J. Cardiomyocyte dysfunction in sucrose-fed rats is associated with insulin resistance. Diabetes 2001, 50, 1186–1192. [Google Scholar] [CrossRef]
- Dei Cas, A.; Khan, S.S.; Butler, J.; Mentz, R.J.; Bonow, R.O.; Avogaro, A.; Tschoepe, D.; Doehner, W.; Greene, S.J.; Senni, M.; et al. Impact of diabetes on epidemiology; treatment; and outcomes of patients with heart failure. JACC Heart Fail. 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Ho, J.E.; McCabe, E.L.; Wang, T.J.; Larson, M.G.; Levy, D.; Tsao, C.; Aragam, J.; Mitchell, G.F.; Benjamin, E.J.; Vasan, R.S.; et al. Cardiometabolic Traits and Systolic Mechanics in the Community. Circ. Heart Fail. 2017, 10, e003536. [Google Scholar] [CrossRef]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, M.N.; Kim, S.; Shim, W.J. Serum Aldosterone Is Related to Left Ventricular Geometry and Function in Young Adults with Never-Treated Primary Hypertension. J. Clin. Med. 2019, 8, 1045. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Trevisan, R.; Granato, A.; Zompatori, M.; Lombardo, M. Modified Haller index validation and correlation with left ventricular strain in a cohort of subjects with obesity and without overt heart disease. Intern. Emerg. Med. 2022, 17, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anzà, C. A New modified anthropometric haller index obtained without radiological exposure. Int. J. Cardiovasc. Imaging. 2018, 34, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Grenne, B.; Eek, C.; Sjøli, B.; Dahlslett, T.; Uchto, M.; Hol, P.K.; Skulstad, H.; Smiseth, O.A.; Edvardsen, T.; Brunvand, H. Acute coronary occlusion in non-ST-elevation acute coronary syndrome: Outcome and early identification by strain echocardiography. Heart 2010, 96, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Phelan, D.; Thavendiranathan, P.; Popovic, Z.; Collier, P.; Griffin, B.; Thomas, J.D.; Marwick, T.H. Application of a parametric display of two-dimensional speckle-tracking longitudinal strain to improve the etiologic diagnosis of mild to moderate left ventricular hypertrophy. J. Am. Soc. Echocardiogr. 2014, 27, 888–895. [Google Scholar] [CrossRef]
- Ternacle, J.; Bodez, D.; Guellich, A.; Audureau, E.; Rappeneau, S.; Lim, P.; Radu, C.; Guendouz, S.; Couetil, J.P.; Benhaiem, N.; et al. Causes and Consequences of Longitudinal LV Dysfunction Assessed by 2D Strain Echocardiography in Cardiac Amyloidosis. JACC Cardiovasc. Imaging 2016, 9, 126–138. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Bordoni, T.; Naselli, A.; Nicolosi, G.L.; Grasso, E.; Bianchi, S.; Ferrulli, A.; Lombardo, M.; Ambrosio, G. Influence of gestational diabetes mellitus on subclinical myocardial dysfunction during pregnancy: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 17–24. [Google Scholar] [CrossRef]
- Otterstad, J.E.; Froeland, G.; St John Sutton, M.; Holme, I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur. Heart J. 1997, 18, 507–513. [Google Scholar] [CrossRef]
- Konstam, M.A.; Abboud, F.M. Ejection Fraction: Misunderstood and Overrated (Changing the Paradigm in Categorizing Heart Failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef]
- Sade, L.E.; Joshi, S.S.; Cameli, M.; Cosyns, B.; Delgado, V.; Donal, E.; Edvardsen, T.; Carvalho, R.F.; Manka, R.; Podlesnikar, T.; et al. Current clinical use of speckle-tracking strain imaging: Insights from a worldwide survey from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2023, 24, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Cerini, F.; Cerrone, A.; Argiento, L.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M.; Rumi, M.G.; Viganò, M. Liver stiffness measurement identifies subclinical myocardial dysfunction in non-advanced non-alcoholic fatty liver disease patients without overt heart disease. Intern. Emerg. Med. 2022, 17, 1425–1438. [Google Scholar] [CrossRef]
- Whitsett, M.; VanWagner, L.B. Physical activity as a treatment of non-alcoholic fatty liver disease: A systematic review. World J. Hepatol. 2015, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology; clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wong, G.L.; Yip, T.C.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.; Lin, H.; Li, G.L.; Lai, J.C.; Chan, H.L.; et al. Angiotensin-converting enzyme inhibitors prevent liver-related events in nonalcoholic fatty liver disease. Hepatology 2022, 76, 469–482. [Google Scholar] [CrossRef]
- Zhou, X.D.; Kim, S.U.; Yip, T.C.; Petta, S.; Nakajima, A.; Tsochatzis, E.; Boursier, J.; Bugianesi, E.; Hagström, H.; Chan, W.K.; et al. Long-term liver-related outcomes and liver stiffness progression of statin usage in steatotic liver disease. Gut 2024, 73, 1883–1892. [Google Scholar] [CrossRef]
- Kahl, S.; Gancheva, S.; Straßburger, K.; Herder, C.; Machann, J.; Katsuyama, H.; Kabisch, S.; Henkel, E.; Kopf, S.; Lagerpusch, M.; et al. Empagliflozin Effectively Lowers Liver Fat Content in Well-Controlled Type 2 Diabetes: A Randomized; Double-Blind; Phase 4; Placebo-Controlled Trial. Diabetes Care 2020, 43, 298–305. [Google Scholar] [CrossRef]
- Spengler, E.K.; Loomba, R. Recommendations for Diagnosis; Referral for Liver Biopsy; and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clin. Proc. 2015, 90, 1233–1246. [Google Scholar] [CrossRef]
- Mehta, S.R.; Thomas, E.L.; Bell, J.D.; Johnston, D.G.; Taylor-Robinson, S.D. Non-invasive means of measuring hepatic fat content. World J. Gastroenterol. 2008, 14, 3476–3483. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef] [PubMed]
- Negishi, T.; Negishi, K.; Thavendiranathan, P.; Cho, G.Y.; Popescu, B.A.; Vinereanu, D.; Kurosawa, K.; Penicka, M.; Marwick, T.H.; SUCCOUR Investigators. Effect of Experience and Training on the Concordance and Precision of Strain Measurements. JACC Cardiovasc. Imaging 2017, 10, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Rösner, A.; Barbosa, D.; Aarsæther, E.; Kjønås, D.; Schirmer, H.; D’hooge, J. The influence of frame rate on two-dimensional speckle-tracking strain measurements: A study on silico-simulated models and images recorded in patients. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Bonanomi, A.; Rigamonti, E.; Lombardo, M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol. Angiol. 2024, 72, 111–124. [Google Scholar] [CrossRef]


| Study Name and Country | Number of Patients | Mean Age (yrs) | Males (%) | Study Design | Main Echocardiographic Findings in MASLD Patients vs. Healthy Controls |
|---|---|---|---|---|---|
| Bonapace, S. et al. (2012) [22], Italy | MASLD = 32 Controls = 18 | MASLD = 64.8 Controls = 63 | MASLD = 78.1 Controls = 72.2 | Prospective | ↔LVMi, LVEF, LAVi ↔E/A ratio, ↑E/e’ ratio ↔LV-GLS, GLSR in systole |
| Singh, G.K. et al. (2013) [23], USA | MASLD = 15 Controls = 14 | MASLD = 15 Controls = 15 | MASLD = 60 Controls = 53.3 | Prospective | ↑RWT, LVMi ↔LVEF ↓LV-GLS, GLSR in systole |
| Karabay, C.Y. et al. (2014) [24], Turkey | MASLD = 22 Controls = 21 | MASLD = 42.8 Controls = 40.5 | MASLD = 56.5 Controls = 57.1 | Prospective | ↑RWT, LVMi ↔LVEF, ↓E/A ratio, ↑E/e’ ratio ↓LV-GLS, GLSR in systole |
| Baktır, A.O. et al. (2015) [25], Turkey | MASLD = 28 Controls = 28 | MASLD = 41.6 Controls = 41.2 | MASLD = 57.1 Controls = 57.1 | Prospective | ↑RWT ↔LVEF, LAVi, E/A ratio, E/e’ ratio ↓LV-GLS, LV-GRS, ↔LV-GCS |
| Mantovani, A. et al. (2015) [26], Italy | MASLD = 158 Controls = 64 | MASLD = 68.6 Controls = 66.9 | MASLD = 63.9 Controls = 85.9 | Prospective | ↔LVMi, ↓LVEF, ↑LAVi ↔E/A ratio, ↑E/e’ ratio ↔LV-GLS, GLSR in systole |
| VanWagner, L.B. et al. (2015) [27], USA | MASLD = 271 Controls = 2442 | MASLD = 50.5 Controls = 50.1 | MASLD = 54.6 Controls = 39.7 | Prospective | ↑RWT, LVMi, LAVi ↔LVEF, ↓E/A ratio, ↑E/e’ ratio ↓LV-GLS, ↔LV-GCS |
| Zamirian, M. et al. (2018) [28], Iran | MASLD = 30 Controls = 30 | MASLD = 38.4 Controls = 36.9 | MASLD = 53.3 Controls = 50 | Prospective | ↓LV-EDD, LV-ESD ↔LVEF, E/A ratio, ↑E/e’ ratio ↓LV-GLS |
| Chiu, L.S. et al. (2020) [29], USA | MASLD = 384 Controls = 1972 | MASLD = 53 Controls = 52 | MASLD = 53.9 Controls = 47 | Prospective | ↑RWT, LVMi, ↓E/A ratio, ↑E/e’ ratio ↔LV-EDD, LV-ESD, LVEF ↓LV-GLS |
| Moise, C.G. et al. (2021) [30], Romania | MASLD = 35 Controls = 80 | MASLD = 38 Controls = 29 | MASLD = 57.1 Controls = 63.7 | Prospective | ↑LV-EDD, LV-ESD, RWT, LVMi ↔LVEF ↓LV-GLS, ↔LV-GCS |
| Lai, Y.H. et al. (2022) [31], Taiwan | MASLD = 302 Controls = 1019 | MASLD = 56.4 Controls = 46.3 | MASLD = 74.8 Controls = 50.7 | Retrospective | ↑RWT, LVMi, LAVi, LV-EDV ↔LVEF, ↓E/A ratio, ↑E/e’ ratio ↓LV-GLS, LASr, ↑LA stiffness |
| Hirose, K. et al. (2023) [32], Japan | MASLD = 71 Controls = 410 | MASLD = 56 Controls = 57 | MASLD = 94.4 Controls = 63.2 | Prospective | ↑LV-EDD, LV-ESD, RWT, LVMi ↓E/A ratio, ↔E/e’ ratio, LAVi ↓LV-GLS |
| Number of Studies for Parameters Assessed (%) | Sample Size MASLD vs. Controls | MASLD | Controls | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (yrs) | 11 (100) | 1348 vs. 6098 | 47.7 (15–68.6) | 45.3 (15–66.9) | <0.05 |
| Males (%) | 11 (100) | 1348 vs. 6098 | 63.9 (53.3–94.4) | 58.2 (39.7–85.9) | <0.05 |
| Anthropometrics | |||||
| BSA (m2) | 3 (27.3) | 690 vs. 4494 | 2.13 (2–2.3) | 1.90 (1.8–2) | <0.05 |
| BMI (Kg/m2) | 11 (100) | 1348 vs. 6098 | 30.3 (25.8–37) | 25.5 (20–29.7) | <0.05 |
| WC | 5 (45.5) | 698. 3910 | 101.2 (91.1–111.8) | 89.3 (77.9–100) | <0.05 |
| Cardiovascular risk factors | |||||
| Hypertension (%) | 6 (54.5) | 1218 vs. 5925 | 56.8 (30.8–81.6) | 37.6 (8–73.4) | <0.05 |
| Smoking (%) | 7 (63.6) | 968 vs. 4957 | 26.5 (10–46.5) | 26.2 (10.7–50) | NS |
| Type 2 diabetes (%) | 7 (63.6) | 1253 vs. 5925 | 43.4 (0–100) | 37.2 (1.7–100) | <0.05 |
| Dyslipidemia (%) | 3 (27.3) | 644 vs. 3871 | 39.3 (11.9–56.7) | 29.2 (4.2–50.2) | <0.05 |
| Obesity (%) | 2 (18.2) | 293 vs. 2463 | 74.1 (68.2–80.1) | 32.3 (23.8–40.9) | <0.05 |
| Hemodynamics (%) | |||||
| Heart rate | 4 (36.4) | 240 vs. 176 | 76.6 (72.2–83) | 77.1 (74.6–80) | NS |
| SBP (mmHg) | 10 (90.9) | 1318 vs. 6068 | 128.7 (120.7–143.9) | 121.9 (109–139.7) | <0.05 |
| DBP (mmHg) | 10 (90.9) | 1318 vs. 6068 | 79.4 (75–83.2) | 74.2 (68–81) | <0.05 |
| Biochemical parameters | |||||
| AST (U/L) | 7 (63.6) | 628 vs. 1574 | 31.5 (23–45.2) | 23.8 (20–33) | <0.05 |
| ALT (U/L) | 7 (63.6) | 628 vs. 1574 | 37.7 (24–66.1) | 22.9 (15–33.4) | <0.05 |
| GGT (U/L) | 5 (45.5) | 591 vs. 1539 | 49.1 (34–71) | 26.7 (19–39.2) | <0.05 |
| Fasting glucose (mg/dL) | 8 (72.7) | 1261 vs. 5967 | 117.8 (91–154.8) | 106.8 (90–149.4) | <0.05 |
| HbA1C (%) | 5 (45.5) | 834 vs. 3953 | 6.7 (6.1–7.5) | 6.1 (5.5–7) | <0.05 |
| Fasting insulin (U/L) | 3 (27.3) | 588 vs. 3475 | 22.6 (10.9–36) | 8.3 (6.6–10.3) | <0.05 |
| HOMA-IR | 5 (45.5) | 638 vs. 3524 | 5.3 (3.2–7.8) | 1.8 (0.96–2.6) | <0.05 |
| eGFR (mL/min/1.73 m2) | 3 (27.3) | 644 vs. 3871 | 85.8 (74–97.9) | 87.6 (75–96) | <0.05 |
| Total cholesterol (mg/dL) | 9 (81.8) | 1283 vs. 5988 | 194.7 (165–233.4) | 183.9 (127–200.9) | <0.05 |
| HDL-cholesterol (mg/dL) | 9 (81.8) | 1283 vs. 5988 | 46.9 (36–51.5) | 53.2 (45–66) | <0.05 |
| LDL-cholesterol (mg/dL) | 9 (81.8) | 1283 vs. 5988 | 120 (98.3–149) | 109.9 (69–129.8) | <0.05 |
| Triglycerides (mg/dL) | 9 (81.8) | 1283 vs. 5988 | 161.8 (120.5–217) | 112.5 (64–167.3) | <0.05 |
| CRP (mg/dL) | 4 (36.4) | 666 vs. 3892 | 2.1 (0.08–5.1) | 1.3 (0.04–3.2) | <0.05 |
| Current medical treatment | |||||
| ACE-i/ARBs (%) | 4 (36.4) | 645 vs. 2464 | 52.1 (21.1–77) | 42.1 (14.4–68) | <0.05 |
| CCB (%) | 4 (36.4) | 645 vs. 2464 | 34.9 (23.9–47) | 24.4 (10.2–45) | <0.05 |
| BB (%) | 2 (18.2) | 542 vs. 2036 | 28.4 (21.5–35.4) | 13.3 (7.8–18.9) | <0.05 |
| Diuretics (%) | 2 (18.2) | 190 vs. 82 | 27.5 (16–39) | 18.8 (11–26.6) | <0.05 |
| Statins (%) | 4 (36.4) | 645 vs. 2464 | 42.5 (25.3–74.1) | 41.2 (17.3–79.9) | NS |
| Oral hypoglycemic agents (%) | 3 (27.3) | 261 vs. 492 | 49.4 (15.5–81.6) | 45.1 (5.1–70.3) | NS |
| Insulin (%) | 2 (18.2) | 190 vs. 82 | 34.4 (33–35.8) | 31.8 (23–40.6) | <0.05 |
| Echocardiographic Parameters | Number of Studies for Parameters Assessed (%) | Sample Size MASLD vs. Controls | MASLD | Controls | p Value |
|---|---|---|---|---|---|
| TTE parameters | |||||
| IVS thickness (mm) | 5 (45.5) | 417 vs. 1178 | 9.7 (8.6–11) | 8.5 (8.2–8.8) | <0.05 |
| LV-PW thickness (mm) | 4 (36.4) | 387 vs. 1148 | 10 (9.5–10.6) | 8.4 (8.1–8.7) | <0.05 |
| LV-EDD (mm) | 6 (54.5) | 570 vs. 2541 | 46.6 (41.9–49.1) | 46.3 (44–49) | <0.05 |
| RWT | 8 (72.7) | 1128 vs. 5986 | 0.42 (0.35–0.62) | 0.39 (0.29–0.58) | <0.05 |
| LVMi (g/m2) | 9 (81.8) | 1296 vs. 6040 | 87.7 (69.2–112.8) | 78.9 (60–107.9) | <0.05 |
| LV-EDV (mL) | 7 (63.6) | 1212 vs. 5625 | 100.3 (80.7–115.8) | 97 (72.4–115.9) | <0.05 |
| LV-ESV (mL) | 6 (54.5) | 910 vs. 4606 | 38.6 (22.4–46.6) | 37.3 (24–42.9) | <0.05 |
| LVEF (%) | 11 (100) | 1348 vs. 6098 | 62.3 (56.7–73.7) | 62.9 (57.1–71.3) | <0.05 |
| LAVi (mL/m2) | 6 (54.5) | 862 vs. 3981 | 26.5 (19.6–35.6) | 24.2 (18.8–26.7) | <0.05 |
| E/A ratio | 9 (81.8) | 1298 vs. 6004 | 0.96 (0.8–1.21) | 1.13 (0.68–1.4) | <0.05 |
| E/e’ ratio | 9 (81.8) | 1298 vs. 6004 | 8.4 (6.9–10) | 6.7 (5.6–8.4) | <0.05 |
| STE indices | |||||
| LV-GLS (%) | 11 (100) | 1348 vs. 6098 | 17.2 (7.7–19.9) | 19.1 (14.8–23.7) | <0.05 |
| LV-GLSRs (s−1) | 5 (45.5) | 255 vs. 145 | 1.0 (0.9–1.1) | 1.2 (1–1.7) | <0.05 |
| LV-GLSRe (s−1) | 5 (45.5) | 255 vs. 145 | 1.1 (0.8–1.3) | 1.4 (0.9–2.3) | <0.05 |
| LV-GLSRl (s−1) | 5 (45.5) | 255 vs. 145 | 0.9 0.5–1.2) | 1.0 (0.5–1.5) | <0.05 |
| LV-GCS (%) | 3 (27.3) | 334 vs. 2550 | 19.8 (15–23.6) | 19.2 (15.4–23.3) | <0.05 |
| LV-GRS (%) | 1 (9.1) | 28 vs. 28 | 41.1 (25.1–57.1) | 57.2 (43.2–71.2) | <0.05 |
| LASr (%) | 1 (9.1) | 302 vs. 1019 | 34 (26–42) | 40.2 (32.8–47.6) | <0.05 |
| NIH Quality Assessment Tool of Case-Control Studies Criteria Met | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Quality |
| Bonapace, S. et al. [22] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | Yes | Yes | 10 (Good) |
| Singh, G.K. et al. [23] | Yes | Yes | No | NS | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 7 (Fair) |
| Karabay, C.Y. et al. [24] | Yes | Yes | No | NS | Yes | Yes | NS | Yes | Yes | Yes | Yes | No | 8 (Fair) |
| Baktır, A.O. et al. [25] | Yes | Yes | No | NS | Yes | Yes | NS | Yes | Yes | Yes | Yes | No | 8 (Fair) |
| Mantovani, A. et al. [26] | Yes | Yes | No | NS | Yes | Yes | NS | Yes | Yes | Yes | Yes | Yes | 9 (Good) |
| VanWagner, L.B. et al. [27] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NS | Yes | 10 (Good) |
| Zamirian, M. et al. [28] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 11 (Good) |
| Chiu, L.S. et al. [29] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 (Good) |
| Moise, C.G. et al. [30] | Yes | Yes | No | NS | Yes | Yes | NS | Yes | Yes | Yes | NS | No | 7 (Fair) |
| Lai, Y.H. et al. [31] | Yes | Yes | No | Yes | Yes | Yes | NS | Yes | Yes | Yes | Yes | No | 9 (Good) |
| Hirose, K. et al. [32] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 10 (Good) |
| Moderators | Coefficient | Standard Error | 95%CI Lower | 95%CI Upper | p-Value |
|---|---|---|---|---|---|
| Age | 0.0116 | 0.0496 | −0.0856 | 0.1087 | 0.81 |
| Male sex | −0.0026 | 0.0162 | −0.0344 | 0.0292 | 0.87 |
| BMI | 0.0688 | 0.1493 | −0.2238 | 0.3615 | 0.65 |
| SBP | 0.0007 | 0.0104 | −0.0197 | 0.0211 | 0.94 |
| FPG | 0.0387 | 0.0341 | −0.0282 | 0.1056 | 0.26 |
| Total cholesterol | −0.0093 | 0.0162 | −0.0409 | 0.0224 | 0.57 |
| Anti-hypertensive therapy | −0.0376 | 0.0374 | −0.1109 | 0.0356 | 0.31 |
| Ultrasound system: non-GE | −0.2826 | 1.1742 | −2.5839 | 2.0188 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonaglioni, A.; Cerini, F.; Fagiani, V.; Nicolosi, G.L.; Rumi, M.G.; Lombardo, M.; Muti, P. Effect of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) on Left Ventricular Mechanics in Patients Without Overt Cardiac Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 2690. https://doi.org/10.3390/jcm14082690
Sonaglioni A, Cerini F, Fagiani V, Nicolosi GL, Rumi MG, Lombardo M, Muti P. Effect of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) on Left Ventricular Mechanics in Patients Without Overt Cardiac Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2025; 14(8):2690. https://doi.org/10.3390/jcm14082690
Chicago/Turabian StyleSonaglioni, Andrea, Federica Cerini, Valeria Fagiani, Gian Luigi Nicolosi, Maria Grazia Rumi, Michele Lombardo, and Paola Muti. 2025. "Effect of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) on Left Ventricular Mechanics in Patients Without Overt Cardiac Disease: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 14, no. 8: 2690. https://doi.org/10.3390/jcm14082690
APA StyleSonaglioni, A., Cerini, F., Fagiani, V., Nicolosi, G. L., Rumi, M. G., Lombardo, M., & Muti, P. (2025). Effect of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) on Left Ventricular Mechanics in Patients Without Overt Cardiac Disease: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 14(8), 2690. https://doi.org/10.3390/jcm14082690








