Rehabilitation with and Without Robot and Allied Digital Technologies (RADTs) in Stroke Patients: A Study Protocol for a Multicentre Randomised Controlled Trial on the Effectiveness, Acceptability, Usability, and Economic-Organisational Sustainability of RADTs from Subacute to Chronic Phase (STROKEFIT4)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.1.1. Primary Objective
2.1.2. Secondary Objectives
- To demonstrate the superiority of rehabilitative treatment integrated with RADTs compared to traditional rehabilitation treatment in the recovery of activities of daily living, should non-inferiority be demonstrated;
- To compare the improvements between the two groups in all targeted domains (upper limb, lower limb, balance, cognitive functions), and in accordance with the International Classification of Functioning, Disability, and Health (ICF) [26];
- To analyse the neurophysiological parameters and factors involved in neuroplasticity processes;
- To compare the time pattern of manual dexterity and walking performance recovery in the two groups;
- To assess the effects of the rehabilitation treatment in terms of daily life activities and quality of life through medium-term follow-up;
- To evaluate the acceptability and usability of the rehabilitative treatment integrated with RADTs for patients, their families, and healthcare practitioners;
- To create a model capable of predicting the effectiveness of robotic and technological treatment in post-stroke patients;
- To assess the economic sustainability of the rehabilitative treatment integrated with RADTs for the patient, payer, and society through the creation of a model for the assessment and prediction of cost-effectiveness and cost–utility. Additionally, a budget impact analysis will be performed from the perspective of the national healthcare system. To deal with uncertainty related to the values of the model parameters, each analysis will be accompanied by multiparametric sensitivity analyses.
2.2. Study Design
2.3. Study Setting
- 1.
- Don Carlo Gnocchi Foundation ONLUS, with the following six centres:
- Roma (RM), Centro Santa Maria della Provvidenza;
- Milano (MI), IRCCS Santa Maria Nascente;
- Sant’Angelo dei Lombardi (AV), Polo Specialistico Riabilitativo;
- Salerno (SA), Centro Santa Maria al Mare;
- Acerenza (PZ), Centro “Gala”;
- Tricarico (MT), Polo specialistico riabilitativo.
- 2.
- IRCCS Mondino Foundation, one centre: Pavia (PV);
- 3.
- IRCCS Scientific Clinical Institutes Maugeri, with the following five centres:
- IRCCS Bari (BA);
- IRCCS Telese (BN);
- IRCCS Milano (MI);
- IRCCS Pavia (PV);
- IRCCS Montescano (PV).
- 4.
- IRCCS Ospedale Policlinico San Martino, one centre: Genova (GE).
2.4. Study Population
2.4.1. Inclusion Criteria
- First-ever diagnosis of ischaemic or haemorrhagic stroke confirmed by computed tomography or magnetic resonance imaging;
- Age 18 years and over;
- Time since stroke equal to or less than 6 months;
- Mild to severe impairment of the upper limb (motor section of the Fugl–Meyer Assessment of Upper Extremity [29] ≤ 58) and/or mild to severe impairment of the lower limb (score on the Functional Ambulation Categories scale [30] ≤ 4) and/or mild to severe impairment of balance (Berg Balance Scale [31] ≤ 45);
- Clinical stability allowing transfer to the gym and execution of the planned treatments.
2.4.2. Exclusion Criteria
- Clinical instability;
- Behavioural/cognitive disorders preventing adequate patient compliance with both traditional and robotic rehabilitation treatment (severe cognitive deficit, Montreal Cognitive Assessment [32] < 10);
- Rigidity or hypertonia (Modified Ashworth Scale [33] = 4) in the plegic/paretic limb;
- Serious uncorrectable visual impairments preventing the patient from performing treatment with technological and/or robotic devices;
- Pregnant women;
- Refusal to sign the informed consent.
2.5. Recruitment
2.6. Baseline Assessment
2.6.1. Demographic and Clinical Characteristics
- Demographics (Age, Gender, Handedness, Height, Weight, BMI, Smoker, Years of education);
- Current rehabilitation setting;
- Past medical history;
- Acute event data;
- Medications taken;
- Previous rehabilitation settings (ad hoc questionnaire carried out before recruitment);
- Comorbidities (Cumulative Illness Rating Scale—severity index (CIRS-SI) and Cumulative Illness Rating Scale—comorbidity index (CIRS-CI) [40]);
- Social situation (Blaylock Risk Assessment Screening Score [41]);
2.6.2. Genetic Analyses
- Whole blood samples will be collected at T0 (2 × 4 mL purple cap tubes, EDTA) and frozen (−20 °C or −80 °C) until shipment/analysis;
- DNA extraction of whole blood samples will be performed with a research extraction kit (Zymo Research, Irvine, CA, USA);
- The BDNF rs6265 genotyping will be performed using a polymerase chain reaction (PCR) combined with restriction enzyme digestion (HpyCH4IV enzyme). The electrophoresis resolution of fragments will detect, for each patient, the presence of a valine (Val) to methionine (Met) substitution at codon 66 (Val66Met). Patients will be then identified as “non-carrier” of substitution (homozygous GG) and “carrier” of A substitution (Met protein replacement at codon 66, as heterozygous AG or homozygous AA).
2.7. Randomisation
2.8. Interventions—Common Characteristics
- 1.
- Sensorimotor Functions
- a
- Neuro-musculoskeletal and movement-related functions:
- i.
- Mobility and stability of joint functions in one or more joints of the upper limb, lower limb, and spine;
- ii.
- Muscle tone and strength;
- iii.
- Movement functions.
- b
- Sensory and pain functions;
- c
- Exercise tolerance function (physical endurance, aerobic capacity, and fatigue resistance).
- 2.
- Specific Mental Functions (attention, memory, perceptual functions, higher-level cognitive functions, language and calculation mental functions, self- and time-experience);
- 3.
- Activities and Participation: self-care and daily life activities (washing, grooming, dressing, eating, etc.).
2.8.1. Rehabilitation of Sensorimotor Functions—Neuro-Musculoskeletal and Movement-Related Functions
- Passive, active, and active-assisted exercises involving the three major joints of the limb in their degrees of freedom, exercises for maintaining reciprocal joint relationships, exercises facilitating scapula and carpal bone movement;
- Muscle tone control exercises, incremental muscle recruitment exercises, and muscle endurance exercises;
- Coordination exercises of voluntary movements, spasticity inhibition exercises, management exercises of muscle stiffness. by muscle and joint stretching.
- Passive, active, and active-assisted exercises involving the three major joints of the limb in their degrees of freedom, exercises for maintaining reciprocal joint relationships, exercises facilitating pelvic and tarsal bone movement;
- Muscle tone control exercises, incremental muscle recruitment exercises, and muscle endurance exercises;
- Control of reactions (postural, body straightening, body adjustment, balance, support, and fall defence), coordination exercises of voluntary movements, spasticity inhibition exercises, management exercises of muscle stiffness by muscle and joint stretching, and gait training.
2.8.2. Rehabilitation of Sensorimotor Functions—Sensory Function
- Targeted proprioceptive exercises for the proprioceptive and kinaesthetic functions of the plegic/paretic upper and lower limbs;
- Proprioception exercises related to sitting and standing positions, static and dynamic balance exercises (in terms of displacements, directional changes, and speed in monopodalic and bipodalic conditions);
- Reduction and control of pain sensation.
2.8.3. Rehabilitation of Sensorimotor Functions—Exercise Tolerance Functions
- General physical endurance exercises;
- Gradual, progressive, and prolonged aerobic exercises;
- Fatigue control and management at varying effort levels.
2.8.4. Rehabilitation of Specific Mental Functions
- Attention training exercises (e.g., visual search exercises, barrage);
- Memory training exercises (e.g., repetition and delayed recall of word lists);
- Recognition and interpretation exercises of sensory stimuli;
- Exercises for higher-level cognitive functions (e.g., decision-making processes, planning, problem-solving, etc.);
- Exercises for language-specific mental functions (recognition and use of signs, symbols, and other language components);
- Exercises for specific mental functions of simple mathematical calculations and complex mathematical operations;
- Exercises for awareness of one’s identity in the reality of one’s environment and time.
2.8.5. Rehabilitation of Activities and Participation, Self-Care, and Daily Life Activities
- Exercises of learning and application of learned knowledge and problem-solving;
- Execution of single or multiple tasks and routine organisation exercises;
- Mobility (postural passages; transfers; object transportation and movement; task-oriented and manipulation exercises; walking and moving in different places and using devices);
- Activities aimed at self-care, washing and drying, dressing, and eating;
- Performance of domestic and daily activities;
- Reintegration into social life.
2.9. Specific Interventions
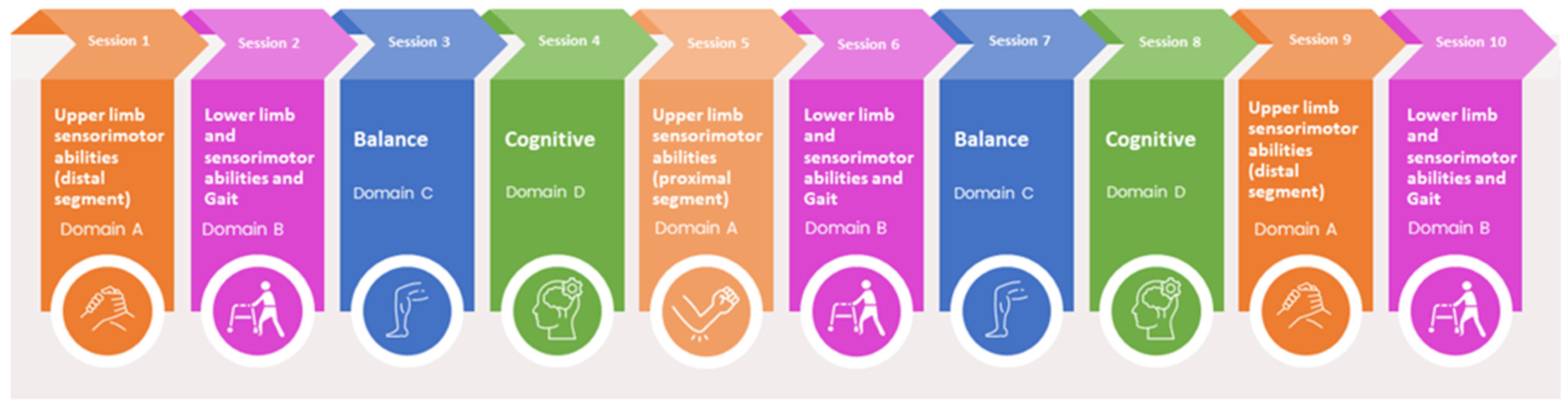
2.9.1. Experimental Group: Integrated Treatment with Robotic and Technological Devices
- (A)
- Upper limb sensorimotor abilities;
- (B)
- Lower limb sensorimotor abilities and gait;
- (C)
- Balance;
- (D)
- Cognitive abilities.
- Planar end-effector robots for shoulder-elbow rehabilitation, or exoskeletons or electromechanical systems for shoulder, elbow, and wrist rehabilitation;
- End-effector robots or exoskeletons for the hand;
- Sensor-based devices for comprehensive upper limb treatment.
- End-effector robots or exoskeletons (overground and non-overground) for the lower limb;
- Treadmills with body-weight support systems.
- Robotic and digital stabilometric platforms;
- Sensor-based systems.
2.9.2. Control Group: Traditional Treatment
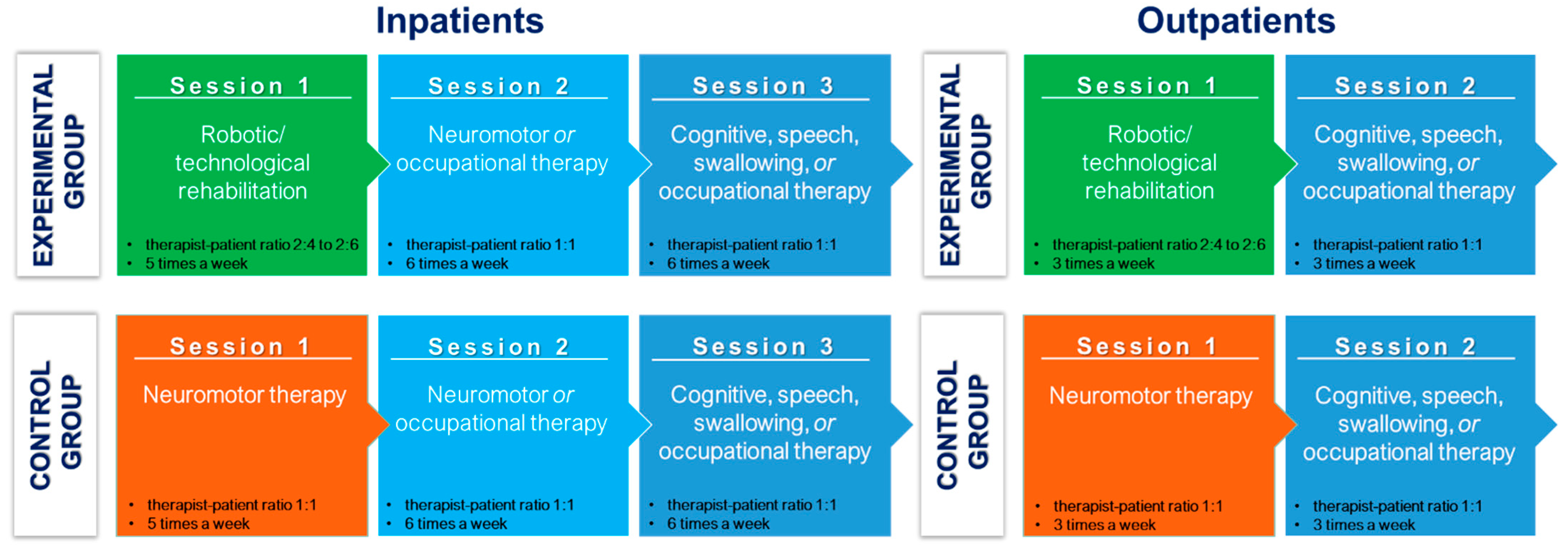
2.10. Intervention Schedule
- Five times a week for 5 weeks, for inpatients;
- Three times a week for 8.3 weeks, for outpatients.
2.11. Intervention Adherence
2.12. Outcome Assessments
2.12.1. Primary Endpoint
2.12.2. Secondary Endpoint
- 1.
- Treatment effects, measured by clinical scales at the beginning (T0) and end of the treatment (T1)
- Body function
- Participation
- a.
- EQ-5D-5L [62].
- 2.
- Treatment Effects—Neurophysiology
- 3.
- Treatment Effects—Biochemical Analysis
- 4.
- Time pattern of recovery during rehabilitation
- 5.
- Follow-up
- Ad hoc questionnaire on the rehabilitation activity performed (type of rehabilitation setting, number of sessions, frequency, duration, type of treatment, etc.);
- Modified Barthel Index;
- EQ-5D-5L.
2.13. Costs, Sustainability, and Acceptability Assessments
2.13.1. Questionnaire for Patients
- Acceptability and usability
- a.
- Technology Acceptance Model [69]: after 5 sessions, after 15 sessions, at T1;
- b.
- Ad hoc questionnaire for needs and available solutions and perception of their relative complexity: at T0 and T1;
- Satisfaction (ad hoc questionnaire): at T0 and T1;
- Confidence and knowledge of ICT technologies (ad hoc questionnaire): at T0;
- Costs (ad hoc questionnaire): at T0, after 15 sessions, at T1;
- Quality of life (EQ-5D-5L [62]): at T0, after 15 sessions, at T1.
2.13.2. Questionnaires for Operators
- Confidence and knowledge of ICT technologies: at the beginning of the study;
- Quality of work (Work-Related Quality Of Life scale, [70]): at the beginning of the study, 6 months after, and 12 months after;
- Technology Acceptance Model [69]: at the beginning of the study, 6 months after, and 12 months after;
- Ad hoc questionnaire for needs and available solutions and perception of their relative complexity: at the beginning of the study, 6 months after, and 12 months after;
- Satisfaction (ad hoc questionnaire): at the beginning of the study, 6 months after, and 12 months after.
2.14. Patient’s Involvement in the Study Design
2.15. Sample Size
- The non-inferiority of robotic treatment compared to traditional treatment;
- A non-inferiority margin of 5 points (about half of the MCID);
- A statistical power of 80%;
- A bilateral 95% confidence interval;
- A standard deviation of the primary outcome of 20 points.
2.16. Blinding
2.17. Study Withdrawal
2.18. Statistical Analysis
2.18.1. Primary Analysis
2.18.2. Secondary Analyses
2.19. Safety Evaluation
2.20. Ethics and Dissemination
2.21. Patients’ Enrolment Monitoring
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
The StrokeFit4 study group
Conflicts of Interest
Abbreviations
| RADTs | Robot and Allied Digital Technologies |
| HTA | Health Technology Assessment |
| Fit4MedRob | Fit for Medical Robotics |
| CIRS | Cumulative Illness Rating Scale |
| ICF | International Classification of Functioning, Disability, and Health |
| BDNF | brain-derived neurotrophic factor |
| qEEG | quantitative electroencephalography |
| CUA | cost–utility analysis |
| ICUR | incremental cost–utility ratio |
| ITT | Intention-To-Treat |
| PP | Per-Protocol |
| CI | confidence interval |
References
- Prange, G.B.; Jannink, M.J.A.; Groothuis-Oudshoorn, C.G.M.; Hermens, H.J.; IJzerman, M.J. Systematic Review of the Effect of Robot-Aided Therapy on Recovery of the Hemiparetic Arm after Stroke. JRRD 2006, 43, 171. [Google Scholar] [CrossRef] [PubMed]
- Riener, R. Rehabilitation Robotics. FNT Robot. 2012, 3, 1–137. [Google Scholar] [CrossRef]
- Laut, J.; Porfiri, M.; Raghavan, P. The Present and Future of Robotic Technology in Rehabilitation. Curr. Phys. Med. Rehabil. Rep. 2016, 4, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.V.G.; Roby-Brami, A. Augmented Feedback, Virtual Reality and Robotics for Designing New Rehabilitation Methods. In Rethinking Physical and Rehabilitation Medicine: New Technologies Induce New Learning Strategies; Didier, J.-P., Bigand, E., Eds.; Springer: Paris, France, 2010; pp. 223–245. ISBN 978-2-8178-0034-9. [Google Scholar]
- Germanotta, M.; Cruciani, A.; Pecchioli, C.; Loreti, S.; Spedicato, A.; Meotti, M.; Mosca, R.; Speranza, G.; Cecchi, F.; Giannarelli, G.; et al. Reliability, Validity and Discriminant Ability of the Instrumental Indices Provided by a Novel Planar Robotic Device for Upper Limb Rehabilitation. J. NeuroEng. Rehabil. 2018, 15, 39. [Google Scholar] [CrossRef]
- Germanotta, M.; Gower, V.; Papadopoulou, D.; Cruciani, A.; Pecchioli, C.; Mosca, R.; Speranza, G.; Falsini, C.; Cecchi, F.; Vannetti, F.; et al. Reliability, Validity and Discriminant Ability of a Robotic Device for Finger Training in Patients with Subacute Stroke. J. NeuroEng. Rehabil. 2020, 17, 1. [Google Scholar] [CrossRef]
- Camardella, C.; Cappiello, G.; Curto, Z.; Germanotta, M.; Aprile, I.; Mazzoleni, S.; Scoglio, A.; Frisoli, A. A Random Tree Forest Decision Support System to Personalize Upper Extremity Robot-Assisted Rehabilitation in Stroke: A Pilot Study. IEEE Int. Conf. Rehabil. Robot. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Pavan, A.; Fasano, A.; Cortellini, L.; Lattanzi, S.; Papadopoulou, D.; Insalaco, S.; Germanotta, M.; Aprile, I. Implementation of a Robot-Mediated Upper Limb Rehabilitation Protocol for a Customized Treatment after Stroke: A Retrospective Analysis. NeuroRehabil. 2024, 54, 411–420. [Google Scholar] [CrossRef]
- Aprile, I.; Germanotta, M.; Cruciani, A.; Loreti, S.; Pecchioli, C.; Cecchi, F.; Montesano, A.; Galeri, S.; Diverio, M.; Falsini, C.; et al. Upper Limb Robotic Rehabilitation After Stroke: A Multicenter, Randomized Clinical Trial. J. Neurol. Phys. Ther. 2020, 44, 3–14. [Google Scholar] [CrossRef]
- Rodgers, H.; Bosomworth, H.; Krebs, H.I.; Van Wijck, F.; Howel, D.; Wilson, N.; Aird, L.; Alvarado, N.; Andole, S.; Cohen, D.L.; et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): A Multicentre Randomised Controlled Trial. Lancet 2019, 394, 51–62. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, H.; Zhang, J.; Yang, S.; Cai, S. Robot-Assisted Therapy for Upper Extremity Motor Impairment After Stroke: A Systematic Review and Meta-Analysis. Phys. Ther. 2021, 101, pzab010. [Google Scholar] [CrossRef]
- Yang, X.; Shi, X.; Xue, X.; Deng, Z. Efficacy of Robot-Assisted Training on Rehabilitation of Upper Limb Function in Patients With Stroke: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2023, 104, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and Robot-Assisted Arm Training for Improving Activities of Daily Living, Arm Function, and Arm Muscle Strength after Stroke. Cochrane Database Syst. Rev. 2018, 2018, CD006876. [Google Scholar] [CrossRef] [PubMed]
- Aprile, I.; Guardati, G.; Cipollini, V.; Papadopoulou, D.; Mastrorosa, A.; Castelli, L.; Monteleone, S.; Redolfi, A.; Galeri, S.; Germanotta, M. Robotic Rehabilitation: An Opportunity to Improve Cognitive Functions in Subjects With Stroke. An Explorative Study. Front. Neurol. 2020, 11, 588285. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; Selb, M.; Kiekens, C.; Todhunter-Brown, A.; Arienti, C.; Stucki, G.; Meyer, T.; 3rd Cochrane Rehabilitation Methodology Meeting participants. Rehabilitation Definition for Research Purposes. A Global Stakeholders’ Initiative by Cochrane Rehabilitation. Eur. J. Phys. Rehabil. Med. 2022, 58, 333–341. [Google Scholar] [CrossRef]
- Santoro, M.; Siotto, M.; Germanotta, M.; Bray, E.; Mastrorosa, A.; Galli, C.; Papadopoulou, D.; Aprile, I. Bdnf Rs6265 Polymorphism and Its Methylation in Patients with Stroke Undergoing Rehabilitation. Int. J. Mol. Sci. 2020, 21, 8438. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Shaka, Z.; Momtazmanesh, S.; Ajdari, A.; Rezaei, N. Circulating Brain-Derived Neurotrophic Factor as a Potential Biomarker in Stroke: A Systematic Review and Meta-Analysis. J. Transl. Med. 2022, 20, 126. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Stokowska, A.; Tatlisumak, T.; Jood, K.; Pekna, M. Neurofilament Light Chain (NfL) in Blood—A Biomarker Predicting Unfavourable Outcome in the Acute Phase and Improvement in the Late Phase after Stroke. Cells 2021, 10, 1537. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cui, Y.; Roberts, C.; Lu, M.; Wilhelmsson, U.; Pekny, M.; Chopp, M. Beneficial Effects of Gfap/Vimentin Reactive Astrocytes for Axonal Remodeling and Motor Behavioral Recovery in Mice after Stroke. Glia 2014, 62, 2022–2033. [Google Scholar] [CrossRef]
- Huppertz, V.; Guida, S.; Holdoway, A.; Strilciuc, S.; Baijens, L.; Schols, J.M.G.A.; Van Helvoort, A.; Lansink, M.; Muresanu, D.F. Impaired Nutritional Condition After Stroke From the Hyperacute to the Chronic Phase: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 12, 780080. [Google Scholar] [CrossRef]
- Turchetti, G.; Spadoni, E.; Geisler, E.E. Health Technology Assessment. Evaluation of Biomedical Innovative Technologies. IEEE Eng. Med. Biol. Mag. 2010, 29, 70–76. [Google Scholar] [CrossRef]
- Flynn, N.; Froude, E.; Cooke, D.; Dennis, J.; Kuys, S. The Sustainability of Upper Limb Robotic Therapy for Stroke Survivors in an Inpatient Rehabilitation Setting. Disabil. Rehabil. 2022, 44, 7522–7527. [Google Scholar] [CrossRef] [PubMed]
- Turchetti, G.; Vitiello, N.; Trieste, L.; Romiti, S.; Geisler, E.; Micera, S. Why Effectiveness of Robot-Mediated Neurorehabilitation Does Not Necessarily Influence Its Adoption. IEEE Rev. Biomed. Eng. 2014, 7, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, A.; Salvini, P.; Pagliai, T.; Morachioli, A.; Acerbi, G.; Trieste, L.; Cavallo, F.; Turchetti, G.; Dario, P. On Robots and Insurance. Int. J. Soc. Robot. 2016, 8, 381–391. [Google Scholar] [CrossRef]
- Ford, I.; Norrie, J. Pragmatic Trials. N. Engl. J. Med. 2016, 375, 454–463. [Google Scholar] [CrossRef]
- World Health Organization. IFC: International Classification of Functioning, Disability and Health; World Health Organization: Geneva, Switzerland, 2001.
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef]
- Bernhardt, J.; Hayward, K.S.; Kwakkel, G.; Ward, N.S.; Wolf, S.L.; Borschmann, K.; Krakauer, J.W.; Boyd, L.A.; Carmichael, S.T.; Corbett, D.; et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil. Neural Repair. 2017, 31, 793–799. [Google Scholar] [CrossRef]
- Cecchi, F.; Carrabba, C.; Bertolucci, F.; Castagnoli, C.; Falsini, C.; Gnetti, B.; Hochleitner, I.; Lucidi, G.; Martini, M.; Mosca, I.E.; et al. Transcultural Translation and Validation of Fugl–Meyer Assessment to Italian. Disabil. Rehabil. 2021, 43, 3717–3722. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical Gait Assessment in the Neurologically Impaired. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphinee, S.; Williams, J.I. The Balance Scale: Reliability Assessment with Elderly Residents and Patients with an Acute Stroke. Scand. J. Rehabil. Med. 1995, 27, 27–36. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Banks, J.L.; Marotta, C.A. Outcomes Validity and Reliability of the Modified Rankin Scale: Implications for Stroke Clinical Trials: A Literature Review and Synthesis. Stroke 2007, 38, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index Questionnaire (CRIq): A New Instrument for Measuring Cognitive Reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Courneya, K.S.; Bryant, H.E. The Lifetime Total Physical Activity Questionnaire: Development and Reliability. Med. Sci. Sports Exerc. 1998, 30, 266–274. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of Acute Cerebral Infarction: A Clinical Examination Scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Suda, M.; Kawakami, M.; Okuyama, K.; Ishii, R.; Oshima, O.; Hijikata, N.; Nakamura, T.; Oka, A.; Kondo, K.; Liu, M. Validity and Reliability of the Semmes-Weinstein Monofilament Test and the Thumb Localizing Test in Patients with Stroke. Front. Neurol. 2021, 11, 625917. [Google Scholar] [CrossRef]
- Mead, G.; Lynch, J.; Greig, C.; Young, A.; Lewis, S.; Sharpe, M. Evaluation of Fatigue Scales in Stroke Patients. Stroke 2007, 38, 2090–2095. [Google Scholar] [CrossRef]
- Salvi, F.; Miller, M.D.; Grilli, A.; Giorgi, R.; Towers, A.L.; Morichi, V.; Spazzafumo, L.; Mancinelli, L.; Espinosa, E.; Rappelli, A.; et al. A Manual of Guidelines to Score the Modified Cumulative Illness Rating Scale and Its Validation in Acute Hospitalized Elderly Patients. J. Am. Geriatr. Soc. 2008, 56, 1926–1931. [Google Scholar] [CrossRef]
- Blaylock, A.; Cason, C.L. Discharge Planning Predicting Patients’ Needs. J. Gerontol. Nurs. 1992, 18, 5–9. [Google Scholar] [CrossRef]
- McGarvey, S.R.; Morrey, B.F.; Askew, L.J.; An, K.N. Reliability of Isometric Strength Testing. Temporal Factors and Strength Variation. Clin. Orthop. Relat. Res. 1984, 185, 301–305. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salva, A.; Guigoz, Y.; Vellas, B. Screening for Undernutrition in Geriatric Practice: Developing the Short-Form Mini-Nutritional Assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Crary, M.A.; Mann, G.D.C.; Groher, M.E. Initial Psychometric Assessment of a Functional Oral Intake Scale for Dysphagia in Stroke Patients. Arch. Phys. Med. Rehabil. 2005, 86, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G. Beck Depression Inventory–II. Psychol. Assess. 1996. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- McAuley, E.; Duncan, T.; Tammen, V.V. Psychometric Properties of the Intrinsic Motivation Inventory in a Competitive Sport Setting: A Confirmatory Factor Analysis. Res. Q. Exerc. Sport 1989, 60, 48–58. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Hunter, S.M.; Crome, P.; Sim, J.; Donaldson, C.; Pomeroy, V.M. Development of Treatment Schedules for Research: A Structured Review to Identify Methodologies Used and a Worked Example of ‘Mobilisation and Tactile Stimulation’ for Stroke Patients. Physiotherapy 2006, 92, 195–207. [Google Scholar] [CrossRef]
- Donaldson, C.; Tallis, R.C.; Pomeroy, V.M. A Treatment Schedule of Conventional Physical Therapy Provided to Enhance Upper Limb Sensorimotor Recovery after Stroke: Expert Criterion Validity and Intra-Rater Reliability. Physiotherapy 2009, 95, 110–119. [Google Scholar] [CrossRef]
- Gensini, G.F.; Zaninelli, A.; Ricci, S. SPREAD, Stroke Prevention And Educational Awareness Diffusion, VII Edizione, Ictus Cerebrale: Linee Guida Italiane di Prevenzione e Trattamento. Raccomandazioni e Sintesi. Available online: https://siapav.it/pdf/spread202012 (accessed on 14 March 2012).
- Overview|Stroke Rehabilitation in Adults|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/NG236 (accessed on 5 April 2024).
- Norrving, B.; Barrick, J.; Davalos, A.; Dichgans, M.; Cordonnier, C.; Guekht, A.; Kutluk, K.; Mikulik, R.; Wardlaw, J.; Richard, E.; et al. Action Plan for Stroke in Europe 2018-2030. Eur. Stroke J. 2018, 3, 309–336. [Google Scholar] [CrossRef]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better Reporting of Interventions: Template for Intervention Description and Replication (TIDieR) Checklist and Guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Symbol Digit Modalities Test; Western Psychological Services Los Angeles: Los Angeles, CA, USA, 1973. [Google Scholar] [CrossRef]
- Demeurisse, G.; Demol, O.; Robaye, E. Motor Evaluation in Vascular Hemiplegia. Eur. Neurol. 1980, 19, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Downie, W.W.; Leatham, P.A.; Rhind, V.M.; Wright, V.; Branco, J.A.; Anderson, J.A. Studies with Pain Rating Scales. Ann. Rheum. Dis. 1978, 37, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N.; Alchaar, H.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of Pain Syndromes Associated with Nervous or Somatic Lesions and Development of a New Neuropathic Pain Diagnostic Questionnaire (DN4). Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Lyle, R.C. A Performance Test for Assessment of Upper Limb Function in Physical Rehabilitation Treatment and Research. Int. J. Rehabil. Res. 1981, 4, 483–492. [Google Scholar] [CrossRef]
- Steele, B. Timed Walking Tests of Exercise Capacity in Chronic Cardiopulmonary Illness. J. Cardiopulm. Rehabil. 1996, 16, 25–33. [Google Scholar] [CrossRef]
- Feng, Y.-S.; Kohlmann, T.; Janssen, M.F.; Buchholz, I. Psychometric Properties of the EQ-5D-5L: A Systematic Review of the Literature. Qual. Life Res. 2021, 30, 647–673. [Google Scholar] [CrossRef]
- Van Putten, M.J.A.M. The Revised Brain Symmetry Index. Clin. Neurophysiol. 2007, 118, 2362–2367. [Google Scholar] [CrossRef]
- Sheorajpanday, R.V.A.; Nagels, G.; Weeren, A.J.T.M.; Van Putten, M.J.A.M.; De Deyn, P.P. Reproducibility and Clinical Relevance of Quantitative EEG Parameters in Cerebral Ischemia: A Basic Approach. Clin. Neurophysiol. 2009, 120, 845–855. [Google Scholar] [CrossRef]
- Claassen, J.; Hirsch, L.J.; Kreiter, K.T.; Du, E.Y.; Sander Connolly, E.; Emerson, R.G.; Mayer, S.A. Quantitative Continuous EEG for Detecting Delayed Cerebral Ischemia in Patients with Poor-Grade Subarachnoid Hemorrhage. Clin. Neurophysiol. 2004, 115, 2699–2710. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult Norms for the Box and Block Test of Manual Dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.J. Refining the Ten-Metre Walking Test for Use with Neurologically Impaired People. Physiotherapy 2002, 88, 386–397. [Google Scholar] [CrossRef]
- Finch, A.P.; Meregaglia, M.; Ciani, O.; Roudijk, B.; Jommi, C. An EQ-5D-5L Value Set for Italy Using Videoconferencing Interviews and Feasibility of a New Mode of Administration. Soc. Sci. Med. 2022, 292, 114519. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, V.; Bala, H. Technology Acceptance Model 3 and a Research Agenda on Interventions. Decis. Sci. 2008, 39, 273–315. [Google Scholar] [CrossRef]
- Van Laar, D.; Edwards, J.A.; Easton, S. The Work-Related Quality of Life Scale for Healthcare Workers. J. Adv. Nurs. 2007, 60, 325–333. [Google Scholar] [CrossRef]
- Shima, N.; Miyamoto, K.; Shibata, M.; Nakashima, T.; Kaneko, M.; Shibata, N.; Shima, Y.; Kato, S.; W-PICS investigators. Activities of Daily Living Status and Psychiatric Symptoms after Discharge from an Intensive Care Unit: A Single-center 12-month Longitudinal Prospective Study. Acute Med. Surg. 2020, 7, e557. [Google Scholar] [CrossRef]
- Andrew, M.K.; MacDonald, S.; Godin, J.; McElhaney, J.E.; LeBlanc, J.; Hatchette, T.F.; Bowie, W.; Katz, K.; McGeer, A.; Semret, M.; et al. Persistent Functional Decline Following Hospitalization with Influenza or Acute Respiratory Illness. J. Am. Geriatr. Soc. 2021, 69, 696–703. [Google Scholar] [CrossRef]
- Hsieh, Y.-W.; Wang, C.-H.; Wu, S.-C.; Chen, P.-C.; Sheu, C.-F.; Hsieh, C.-L. Establishing the Minimal Clinically Important Difference of the Barthel Index in Stroke Patients. Neurorehabil. Neural Repair. 2007, 21, 233–238. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- White, I.R.; Horton, N.J.; Carpenter, J.; Statistics, R.I.M.A.S.; Pocock, S.J. Strategy for Intention to Treat Analysis in Randomised Trials with Missing Outcome Data. BMJ 2011, 342, d40. [Google Scholar] [CrossRef]
- White, I.R.; Carpenter, J.; Horton, N.J. Including All Individuals Is Not Enough: Lessons for Intention-to-Treat Analysis. Clin. Trials 2012, 9, 396–407. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]

| Study Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-Allocation | Follow-Up | ||||||||||||
| Timepoint | T0 | Ts5 | Ts10 | Ts15 | Ts20 | T1 | T2.1 | T2.2 | T2.3 | T2.4 | T2.5 | T2.6 | |||
| Screening assessment | Informed consent | X | |||||||||||||
| Contact details | X | ||||||||||||||
| Demography | X | ||||||||||||||
| Stroke details | X | ||||||||||||||
| Fugl–Meyer Assessment of Upper Extremity | X | ||||||||||||||
| Functional Ambulation Categories | X | ||||||||||||||
| Berg Balance Scale | X | ||||||||||||||
| Randomisation | X | ||||||||||||||
| Baseline assessment | Premorbid lifestyle
| X | |||||||||||||
Clinical picture
| X | ||||||||||||||
Previous rehabilitation settings
| X | ||||||||||||||
Comorbidities
| X | ||||||||||||||
Social situation
| X | ||||||||||||||
Nutritional status
| X | ||||||||||||||
Psychological clinical picture
| X | ||||||||||||||
| X | ||||||||||||||
Genetic analysis
| X | ||||||||||||||
| Interventions | Integrated treatment with RADTs |  | |||||||||||||
| Traditional treatment |  | ||||||||||||||
| Outcomes assessment | Primary outcome | ||||||||||||||
| X | X | |||||||||||||
| Secondary outcomes | |||||||||||||||
Clinical scales
| X | X | |||||||||||||
| X | X | |||||||||||||
Neurophysiology
| X | X | |||||||||||||
Biochemical analysis
| X | X | |||||||||||||
Recovery rate
| X | X | X | X | X | X | |||||||||
Follow-up
| X | X | X | X | X | X | |||||||||
| Acceptability and usability of technologies | |||||||||||||||
| X | X | X | ||||||||||||
| X | X | X | ||||||||||||
| X | ||||||||||||||
| X | X | |||||||||||||
| X | X | |||||||||||||
Costs assessments
| X | X | X | ||||||||||||
Rehabilitation sessions performed
| X | ||||||||||||||
Safety evaluation
|  | ||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aprile, I.G.; Germanotta, M.; Fasano, A.; Siotto, M.; Mauro, M.C.; Pavan, A.; Nicora, G.; Sgandurra, G.; Malovini, A.; Oreni, L.; et al. Rehabilitation with and Without Robot and Allied Digital Technologies (RADTs) in Stroke Patients: A Study Protocol for a Multicentre Randomised Controlled Trial on the Effectiveness, Acceptability, Usability, and Economic-Organisational Sustainability of RADTs from Subacute to Chronic Phase (STROKEFIT4). J. Clin. Med. 2025, 14, 2692. https://doi.org/10.3390/jcm14082692
Aprile IG, Germanotta M, Fasano A, Siotto M, Mauro MC, Pavan A, Nicora G, Sgandurra G, Malovini A, Oreni L, et al. Rehabilitation with and Without Robot and Allied Digital Technologies (RADTs) in Stroke Patients: A Study Protocol for a Multicentre Randomised Controlled Trial on the Effectiveness, Acceptability, Usability, and Economic-Organisational Sustainability of RADTs from Subacute to Chronic Phase (STROKEFIT4). Journal of Clinical Medicine. 2025; 14(8):2692. https://doi.org/10.3390/jcm14082692
Chicago/Turabian StyleAprile, Irene Giovanna, Marco Germanotta, Alessio Fasano, Mariacristina Siotto, Maria Cristina Mauro, Arianna Pavan, Giovanna Nicora, Giuseppina Sgandurra, Alberto Malovini, Letizia Oreni, and et al. 2025. "Rehabilitation with and Without Robot and Allied Digital Technologies (RADTs) in Stroke Patients: A Study Protocol for a Multicentre Randomised Controlled Trial on the Effectiveness, Acceptability, Usability, and Economic-Organisational Sustainability of RADTs from Subacute to Chronic Phase (STROKEFIT4)" Journal of Clinical Medicine 14, no. 8: 2692. https://doi.org/10.3390/jcm14082692
APA StyleAprile, I. G., Germanotta, M., Fasano, A., Siotto, M., Mauro, M. C., Pavan, A., Nicora, G., Sgandurra, G., Malovini, A., Oreni, L., Dubbini, N., Parimbelli, E., Comandè, G., Lunetta, C., Fiore, P., De Icco, R., Trompetto, C., Trieste, L., Turchetti, G., ... Messa, C., on behalf of the STROKEFIT4 Study Group. (2025). Rehabilitation with and Without Robot and Allied Digital Technologies (RADTs) in Stroke Patients: A Study Protocol for a Multicentre Randomised Controlled Trial on the Effectiveness, Acceptability, Usability, and Economic-Organisational Sustainability of RADTs from Subacute to Chronic Phase (STROKEFIT4). Journal of Clinical Medicine, 14(8), 2692. https://doi.org/10.3390/jcm14082692










