Global Prevalence of Mitral Regurgitation: A Systematic Review and Meta-Analysis of Population-Based Studies
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
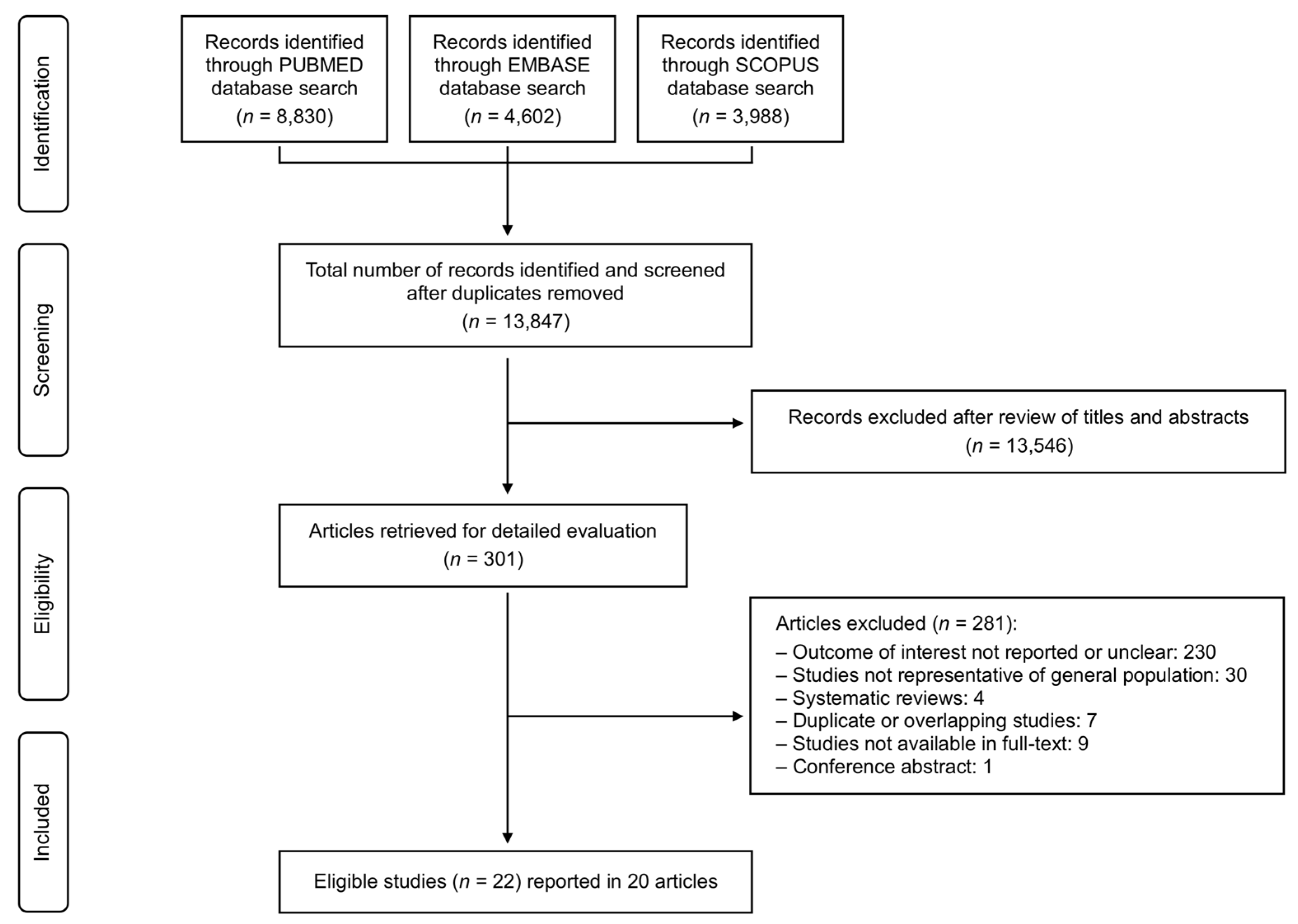
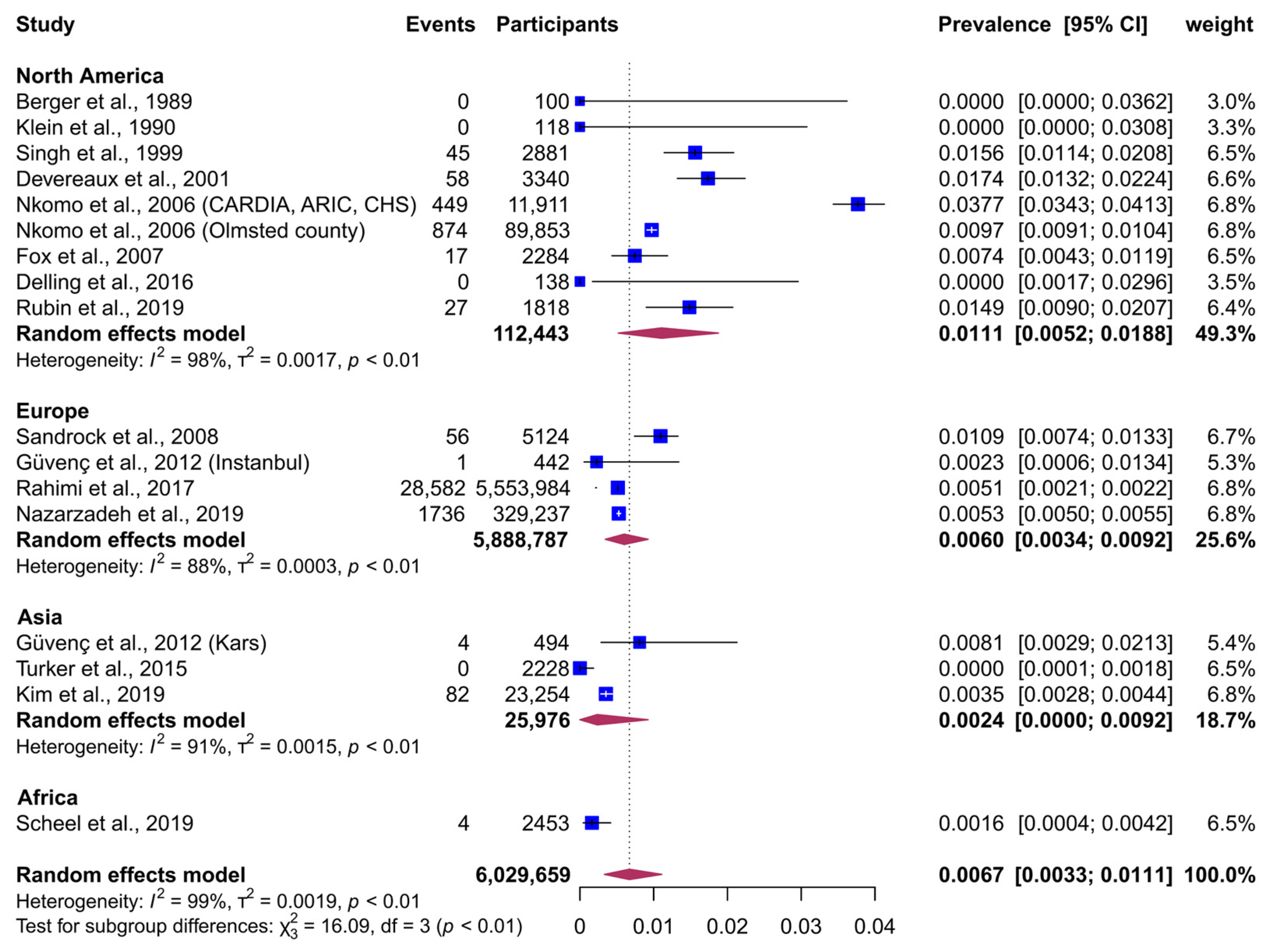
3. Results
Data Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| ICD | International Classification of Diseases |
| MR | Mitral Regurgitation |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| UK | United Kingdom |
References
- Dziadzko, V.; Clavel, M.A.; Dziadzko, M.; Medina-Inojosa, J.R.; Michelena, H.; Maalouf, J.; Nkomo, V.; Thapa, P.; Enriquez-Sarano, M. Outcome and undertreatment of mitral regurgitation: A community cohort study. Lancet 2018, 391, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Enriquez-Sarano, M.; Akins, C.W.; Vahanian, A. Mitral regurgitation. Lancet 2009, 373, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: Executive summary. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Enriquez-Sarano, M.; Avierinos, J.F.; Messika-Zeitoun, D.; Detaint, D.; Capps, M.; Nkomo, V.; Scott, C.; Schaff, H.V.; Tajik, A.J. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N. Engl. J. Med. 2005, 352, 875–883. [Google Scholar] [CrossRef]
- Del Forno, B.; De Bonis, M.; Agricola, E.; Melillo, F.; Schiavi, D.; Castiglioni, A.; Montorfano, M.; Alfieri, O. Mitral valve regurgitation: A disease with a wide spectrum of therapeutic options. Nat. Rev. Cardiol. 2020, 17, 807–827. [Google Scholar] [CrossRef]
- von Kappelgaard, L.; Gislason, G.; Davidsen, M.; Zwisler, A.D.; Juel, K. Temporal trends and socioeconomic differences in the incidence of left-sided valvular heart disease in Denmark. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 608–615. [Google Scholar] [CrossRef]
- d’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef]
- Rahimi, K.; Mohseni, H.; Otto, C.M.; Conrad, N.; Tran, J.; Nazarzadeh, M.; Woodward, M.; Dwyer, T.; MacMahon, S. Elevated blood pressure and risk of mitral regurgitation: A longitudinal cohort study of 5.5 million United Kingdom adults. PLoS Med. 2017, 14, e1002404. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Kraft, C.D.; Levine, R.A.; Nihoyannopoulos, P.; Otto, C.M.; Quinones, M.A.; Rakowski, H.; et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. J. Am. Soc. Echocardiogr. 2003, 16, 777–802. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.L.; Badano, L.; Zamorano, J.L.; et al. Recommendations for the assessment of valvular regurgitation. Part 2: Mitral and tricuspid regurgitation (native and prosthetic valves). Eur. Heart J. Cardiovasc. Imaging 2013, 14, 612–621. [Google Scholar]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L.; Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the assessment of valvular regurgitation. Part 1: Aortic and pulmonary regurgitation (native and prosthetic valves). Eur. Heart J. Cardiovasc Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Munn, Z.; Moola, S.; Lisy, K.; Riitano, D.; Tufanaru, C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int. J. Evid.-Based Healthcare 2015, 13, 147–153. [Google Scholar] [CrossRef]
- Freeman, M.F.; Tukey, J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- Miller, J.J. The inverse of the Freeman-Tukey double arcsine transformation. Am. Stat. 1978, 32, 138. [Google Scholar]
- Cochran, W.G. The comparison of percentages in matched samples. Biometrika 1950, 37, 256–266. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Hecht, S.R.; Van Tosh, A.; Lingam, U. Pulsed and continuous wave Doppler echocardiographic assessment of valvular regurgitation in normal subjects. J. Am. Coll. Cardiol. 1989, 13, 1540–1545. [Google Scholar] [CrossRef]
- Klein, A.L.; Burstow, D.J.; Tajik, A.J.; Zachariah, P.K.; Taliercio, C.P.; Taylor, C.L.; Bailey, K.R.; Seward, J.B. Age-related prevalence of valvular regurgitation in normal subjects: A comprehensive color flow examination of 118 volunteers. J. Am. Soc. Echocardiogr. 1990, 3, 54–63. [Google Scholar] [CrossRef]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902. [Google Scholar] [CrossRef]
- Devereux, R.B.; Jones, E.C.; Roman, M.J.; Howard, B.V.; Fabsitz, R.R.; Liu, J.E.; Palmieri, V.; Welty, T.K.; Lee, E.T. Prevalence and correlates of mitral valve prolapse in a population-based sample of American Indians: The Strong Heart Study. Am. J. Med. 2001, 111, 679–685. [Google Scholar] [CrossRef]
- Fox, E.R.; Wilson, R.S.; Penman, A.D.; King, J.J.; Towery, J.G.; Butler, K.R.; McMullan, M.R.; Skelton, T.N.; Mosley, T.H.; Taylor, H.A. Epidemiology of pure valvular regurgitation in the large middle-aged African American cohort of the Atherosclerosis Risk in Communities study. Am. Heart J. 2007, 154, 1229–1234. [Google Scholar] [CrossRef]
- Sandrock, M.; Schmidt-Trucksäss, A.; Schmitz, D.; Niess, A.; Dickhuth, H.H. Influence of physiologic cardiac hypertrophy on the prevalence of heart valve regurgitation. J. Ultrasound Med. 2008, 27, 85–93. [Google Scholar] [CrossRef]
- van Bemmel, T.; Delgado, V.; Bax, J.J.; Gussekloo, J.; Blauw, G.J.; Westendorp, R.G.; Holman, E.R. Impact of valvular heart disease on activities of daily living of nonagenarians: The Leiden 85-plus study a population based study. BMC Geriatr. 2010, 10, 17. [Google Scholar] [CrossRef]
- Güvenç, T.S.; Canga, Y.; Karabağ, Y.; Ozen, K.; Balcı, B. Prevalence of mitral valve prolapse in residents living at moderately high altitude. Wilderness Environ. Med. 2012, 23, 300–306. [Google Scholar] [CrossRef]
- Vaes, B.; Rezzoug, N.; Pasquet, A.; Wallemacq, P.; Van Pottelbergh, G.; Matheï, C.; Vanoverschelde, J.L.; Degryse, J. The prevalence of cardiac dysfunction and the correlation with poor functioning among the very elderly. Int. J. Cardiol. 2012, 155, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, F.; Collerton, J.; Kingston, A.; Kenny, A.; Davies, K.; Jagger, C.; Robinson, L.; Kirkwood, T.B.; Keavney, B. Prevalence of left ventricular dysfunction in a UK community sample of very old people: The Newcastle 85+ study. Heart 2012, 98, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Turker, Y.; Turker, Y.; Baltaci, D.; Basar, C.; Akkaya, M.; Ozhan, H.; Melen Investigators. The prevalence and clinical characteristics of mitral valve prolapse in a large population-based epidemiologic study: The MELEN study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2208–2212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delling, F.N.; Rong, J.; Larson, M.G.; Lehman, B.; Fuller, D.; Osypiuk, E.; Stantchev, P.; Hackman, B.; Manning, W.J.; Benjamin, E.J.; et al. Evolution of mitral valve prolapse: Insights from the Framingham Heart Study. Circulation 2016, 133, 1688–1695. [Google Scholar] [CrossRef]
- Rubin, J.; Aggarwal, S.R.; Swett, K.R.; Kirtane, A.J.; Kodali, S.K.; Nazif, T.M.; Pu, M.; Dadhania, R.; Kaplan, R.C.; Rodriguez, C.J. Burden of valvular heart diseases in hispanic/latino individuals in the United States: The Echocardiographic Study of Latinos. Mayo Clin. Proc. 2019, 94, 1488–1498. [Google Scholar] [CrossRef]
- Kim, M.S.; Cho, S.J.; Park, S.J.; Cho, S.W.; Choi, S.H.; Kim, H.S.; Carriere, K.; Kim, E.K.; Chang, S.A.; Lee, S.C.; et al. Frequency and clinical associating factors of valvular heart disease in asymptomatic Korean adults. Sci. Rep. 2019, 9, 16741. [Google Scholar] [CrossRef]
- Nazarzadeh, M.; Pinho-Gomes, A.C.; Smith Byrne, K.; Canoy, D.; Raimondi, F.; Ayala Solares, J.R.; Otto, C.M.; Rahimi, K. Systolic blood pressure and risk of valvular heart disease: A mendelian randomization study. JAMA Cardiol. 2019, 4, 788–795. [Google Scholar] [CrossRef]
- Scheel, A.; Ssinabulya, I.; Aliku, T.; Bradley-Hewitt, T.; Clauss, A.; Clauss, S.; Crawford, L.; DeWyer, A.; Donofrio, M.T.; Jacobs, M.; et al. Community study to uncover the full spectrum of rheumatic heart disease in Uganda. Heart 2019, 105, 60–66. [Google Scholar] [CrossRef]
- He, S.; Deng, H.; Jiang, J.; Liu, F.; Liao, H.; Xue, Y.; Zheng, M.; Li, H.; Wu, S. The evolving epidemiology of elderly with degenerative valvular heart disease: The Guangzhou (China) Heart Study. Biomed. Res. Int. 2021, 2021, 9982569. [Google Scholar] [CrossRef]
- Yadgir, S.; Johnson, C.O.; Aboyans, V.; Adebayo, O.M.; Adedoyin, R.A.; Afarideh, M.; Alahdab, F.; Alashi, A.; Alipour, V.; Arabloo, J.; et al. Global, regional, and national burden of calcific aortic valve and degenerative mitral valve diseases, 1990-2017. Circulation 2020, 141, 1670–1680. [Google Scholar] [CrossRef]
- Hahn, R.T.; Clavel, M.A.; Mascherbauer, J.; Mick, S.L.; Asgar, A.W.; Douglas, P.S. Sex-related factors in valvular heart disease: JACC focus seminar 5/7. J. Am. Coll. Cardiol. 2022, 79, 1506–1518. [Google Scholar] [CrossRef] [PubMed]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018, 392, 1015–1035. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef]
- Delling, F.N.; Vasan, R.S. Epidemiology and pathophysiology of mitral valve prolapse: New insights into disease progression, genetics, and molecular basis. Circulation 2014, 129, 2158–2170. [Google Scholar] [CrossRef]
- Mata, D.A.; Ramos, M.A.; Bansal, N.; Khan, R.; Guille, C.; Di Angelantonio, E.; Sen, S. Prevalence of depression and depressive symptoms among resident physicians: A systematic review and meta-analysis. JAMA 2015, 314, 2373–2383. [Google Scholar] [CrossRef]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global prevalence and incidence of tinnitus: A systematic review and meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]

| Study | Country | Study Period (yrs) | Sample Size (N.) | Mean Age of Participants (yrs) | Males (%) | Method/Criteria for Identifying MR |
|---|---|---|---|---|---|---|
| Berger et al., 1989 [23] | USA | NR | 100 | 45 | 52 | Echocardiography |
| Klein et al., 1990 [24] | USA | 1987−1988 | 118 | 48 | 44.9 | Echocardiography |
| Singh et al., 1999 [25] | USA | 1991−1995 | 2881 | 54 | 46.4 | Echocardiography |
| Devereux et al., 2001 [26] | USA | 1993−1995 | 3340 | 64.5 | 38 | Echocardiography |
| Nkomo et al., 2006 [3] | USA | CARDIA: 1985−1992; ARIC: 1987−1995; CHS: 1989−1992 | 11,911 | 55.6 | 42.3 | Echocardiography |
| Nkomo et al., 2006 [3] | USA (Olmsted County) | 1990−2000 | 89,853 | 44.8 | 45.5 | Echocardiography |
| Fox et al., 2007 [27] | USA | 1993−1995 | 2284 | 60.0 | 38.2 | Echocardiography |
| Sandrock et al., 2008 [28] | Germany | 2001−2006 | 5124 | 39.5 | 78.9 | Echocardiography |
| van Bemmel et al., 2010 [29] | The Netherlands | 1997−1999 | 81 | 90 | 33 | Echocardiography |
| Güvenç et al., 2012 [30] | Turkey (Istanbul, Europe) | NR | 442 | 50 | 35 | Echocardiography |
| Güvenç et al., 2012 [30] | Turkey (Kars, Asia) | NR | 494 | 51.1 | 46 | Echocardiography |
| Vaes et al., 2012 [31] | Belgium | 2008−2009 | 556 | 84.7 | 37.1 | Echocardiography |
| Yousaf et al., 2012 [32] | United Kingdom | 2007−2009 | 357 | 87.9 | 38 | Echocardiography |
| Turker et al., 2015 [33] | Turkey | 2010 | 2228 | 49 | 36 | Echocardiography |
| d’Arcy et al., 2016 [8] | United Kingdom | 2012 | 2500 | 73 | 48.5 | Echocardiography |
| Delling et al., 2016 [34] | USA | 1996−2008 | 138 | 55 | 37 | Echocardiography |
| Rahimi et al., 2017 [9] | United Kingdom | 1990−2015 | 5,553,984 | 43.8 | 45.3 | ICD-10, with most cases being confirmed by echocardiography |
| Rubin et al., 2019 [35] | USA | 2011−2014 | 1818 | 55.2 | 42.3 | Echocardiography |
| Kim et al., 2019 [36] | South Korea | 2012−2016 | 23,254 | 63.3 | 66 | Echocardiography |
| Nazarzadeh et al., 2019 [37] | United Kingdom | 2006−2010 | 329,237 | 56.9 | 46 | ICD-10 and UK biobank codes, with most cases being confirmed by echocardiography |
| Scheel et al., 2019 [38] | Uganda | NR | 2453 | 20 | 45 | Echocardiography |
| He et al., 2021 [39] | China | 2015−2018 | 3538 | 72 | 38.1 | Echocardiography |
| Variable | N. of Studies | N. of Participants | Prevalence, % (95% CI) | p-Value 2 |
|---|---|---|---|---|
| Continent | ||||
| Africa | 1 | 2453 | 0.16 (0.03−0.37) | 0.001 |
| Asia | 3 | 25,976 | 0.24 (0.00−0.92) | … |
| Europe | 4 | 5,888,787 | 0.60 (0.34−0.92) | … |
| North America | 9 | 112,443 | 1.11 (0.52−1.88) | … |
| Predominant Ethnicity | ||||
| American Indian | 1 | 3340 | 1.74 (1.34−2.25) | <0.001 |
| Asian | 1 | 23,254 | 0.35 (0.26−0.42) | … |
| Black | 2 | 4737 | 0.40 (0.03−1.16) | … |
| Hispanic | 1 | 1818 | 1.49 (1.01−2.18) | … |
| White | 12 | 5,996,510 | 0.62 (0.20−1.21) | … |
| Method/Criteria for Identifying Mitral Regurgitation | 0.176 | |||
| Doppler Echocardiography | 15 | 146.438 | 0.69 (0.31–1.21) | … |
| ICD-10 | 2 | 5,883,221 | 0.52 (0.51–0.52) | … |
| Study Size | 0.228 | |||
| <1000 | 5 | 1292 | 0.22 (0.00–0.65) | … |
| ≥1000 | 12 | 6,028,367 | 0.86 (0.39–1.50) | … |
| Univariable Meta-Regression Analysis | Multivariable Meta-Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | N. of Estimates | β Coefficient | (95% CI) | p-Value | N. of Estimates | β Coefficient | (95% CI) | p-Value |
| Age | 58 | +0.0038 | (+0.0026, +0.0050) | <0.0001 | 50 | +0.0043 | (+0.0029, +0.0057) | <0.0001 |
| Proportion of males | 50 | +0.0023 | (−0.0895, +0.0941) | 0.9605 | 50 | +0.0049 | (−0.0636, +0.0733) | 0.8890 |
| Study year | 58 | −0.0004 | (−0.0036, +0.0028) | 0.8189 | 50 | −0.0011 | (−0.0037, +0.0015) | 0.4201 |
| Females | Males | Overall | ||||
|---|---|---|---|---|---|---|
| Age | Predicted Prevalence, % | (95% CI) | Predicted Prevalence, % | (95% CI) | Predicted Prevalence, % | (95% CI) |
| 30 | 0.00 | (0.00−0.19) | 0.00 | (0.00−0.27) | 0.00 | (0.00−0.11) |
| 40 | 0.06 | (0.00−0.67) | 0.10 | (0.00−0.82) | 0.08 | (0.00−0.50) |
| 50 | 0.59 | (0.07−1.46) | 0.68 | (0.09−1.68) | 0.63 | (0.25−1.16) |
| 60 | 1.50 | (0.65−2.66) | 1.63 | (0.67−2.95) | 1.56 | (1.01−2.22) |
| 70 | 2.77 | (1.50−4.37) | 2.93 | (1.54−4.73) | 2.85 | (1.96−3.90) |
| 80 | 4.38 | (2.54−6.67) | 4.58 | (2.59−7.09) | 4.48 | (3.00−6.23) |
| 90 | 6.32 | (3.71−9.55) | 6.57 | (3.79−10.0) | 6.45 | (4.17−9.16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figlioli, G.; Sticchi, A.; Christodoulou, M.N.; Hadjidemetriou, A.; Amorim Moreira Alves, G.; De Carlo, M.; Praz, F.; Caterina, R.D.; Nikolopoulos, G.K.; Bonovas, S.; et al. Global Prevalence of Mitral Regurgitation: A Systematic Review and Meta-Analysis of Population-Based Studies. J. Clin. Med. 2025, 14, 2749. https://doi.org/10.3390/jcm14082749
Figlioli G, Sticchi A, Christodoulou MN, Hadjidemetriou A, Amorim Moreira Alves G, De Carlo M, Praz F, Caterina RD, Nikolopoulos GK, Bonovas S, et al. Global Prevalence of Mitral Regurgitation: A Systematic Review and Meta-Analysis of Population-Based Studies. Journal of Clinical Medicine. 2025; 14(8):2749. https://doi.org/10.3390/jcm14082749
Chicago/Turabian StyleFiglioli, Gisella, Alessandro Sticchi, Maria Nefeli Christodoulou, Andreas Hadjidemetriou, Gabriel Amorim Moreira Alves, Marco De Carlo, Fabien Praz, Raffaele De Caterina, Georgios K. Nikolopoulos, Stefanos Bonovas, and et al. 2025. "Global Prevalence of Mitral Regurgitation: A Systematic Review and Meta-Analysis of Population-Based Studies" Journal of Clinical Medicine 14, no. 8: 2749. https://doi.org/10.3390/jcm14082749
APA StyleFiglioli, G., Sticchi, A., Christodoulou, M. N., Hadjidemetriou, A., Amorim Moreira Alves, G., De Carlo, M., Praz, F., Caterina, R. D., Nikolopoulos, G. K., Bonovas, S., & Piovani, D. (2025). Global Prevalence of Mitral Regurgitation: A Systematic Review and Meta-Analysis of Population-Based Studies. Journal of Clinical Medicine, 14(8), 2749. https://doi.org/10.3390/jcm14082749











