Coenzyme Q10 and the Blood–Brain Barrier: An Overview
Abstract
1. Introduction
2. Blood–Brain Barriers
3. Intranasal Drug Delivery
4. CoQ10 Metabolism
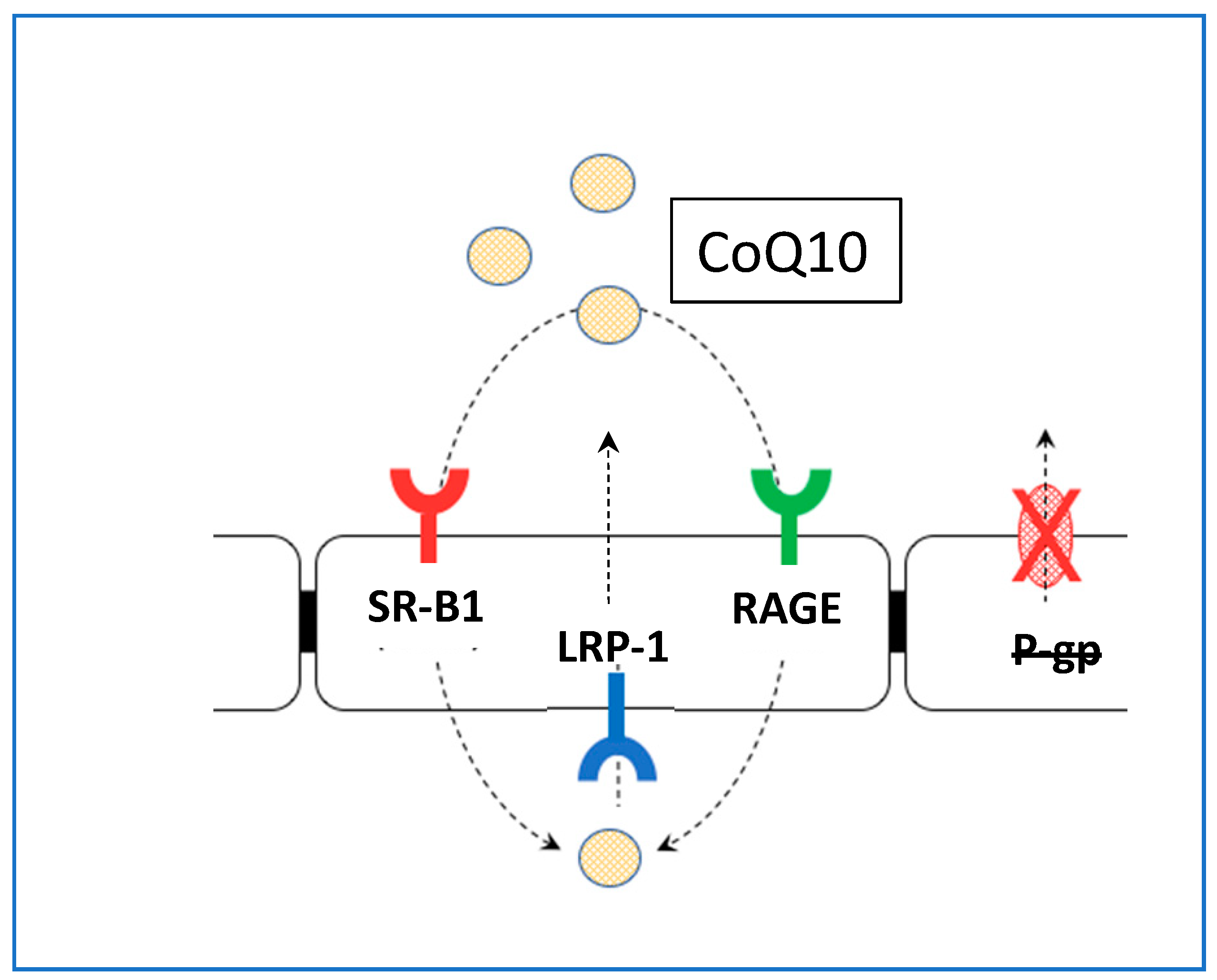
5. CoQ10 and Brain Access
6. Summary
- There are essentially two barriers preventing access of CoQ10 into the human brain, the BBB and BCSFB. The BCSFB is relatively leaky compared to the BBB, and access of CoQ10 (in either ubiquinone or ubiquinol form) into the CSF via the BCSFB does not equate to access of CoQ10 into the brain parenchyma—a common misconception.
- There are essentially two ways for substances to access the BBB-diffusion and carrier/receptor-mediated transport. For access via diffusion, the substance must be a small lipophilic molecule, with a molecular weight of <400 Da. On the basis of this molecular weight restriction, Q10 would be unlikely to cross the BBB via diffusion, in either ubiquinone or ubiquinol forms. With regard to carrier or receptor-mediated transport, to date there have been no studies that have identified carriers/receptors for Q10 (in either ubiquinone or ubiquinol form) in the human brain.
- No clinical studies were identified in which access of orally administered CoQ10 (in either form) across the BBB was directly demonstrated in humans.
- The use of CoQ10 analogues such as mitoquinone and idebenone to access the BBB/mitochondria has been proposed; however, although these analogues have antioxidant action in common with CoQ10, they differ from CoQ10 in other aspects of intracellular function.
- A possible mechanism for delivering CoQ10 into the human brain, which bypasses the BBB, is intranasal drug delivery. However, to date no clinical studies have been carried out to establish the efficacy and safety of this route for delivery of CoQ10 into the human brain. Boyuklieva et al. (2024) [36] have described the preparation of idebenone-loaded nanocomposite microspheres suitable for nasal administration. However, as noted in item 3 above, the intracellular function of idebenone differs from that of CoQ10.
Author Contributions
Funding
Conflicts of Interest
References
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Hargreaves, I.P. Mitochondrial dysfunction and neurodegenerative disorders: Role of nutritional supplementation. Int. J. Mol. Sci. 2022, 23, 12603. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Lopez-Lluch, G.; Hargreaves, I.P. Coenzyme Q10 metabolism: A review of unresolved issues. Int. J. Mol. Sci. 2023, 24, 2585. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An overview of the absorption process and subsequent metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Pardridge, W.M. Delivery of biologics across the blood-brain barrier with molecular Trojan horse technology. BioDrugs 2017, 31, 503–519. [Google Scholar] [CrossRef]
- Fromm, M.F. P-glycoprotein: A defense mechanism limiting oral bioavailability and CNS accumulation of drugs. Int. J. Clin. Pharmacol. Ther. 2000, 38, 69–74. [Google Scholar] [CrossRef]
- Mahringer, A.; Fricker, G. ABC transporters at the blood-brain barrier. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 499–508. [Google Scholar] [CrossRef]
- Pardridge, W.M. Treatment of Alzheimer’s disease and blood-brain barrier drug delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Pardridge, W.M. A historical review of brain drug delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport in brain via the cerebrospinal fluid. Fluids Barriers CNS 2011, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Yagi, Y.; Takao, M.; Tano, M.; Umetsu, M.; Hirano, S.; Usui, T.; Tachikawa, M.; Terasaki, T. Comparison of absolute protein abundances of Ttansporters and receptors among blood-brain barriers at different cerebral regions and the blood-spinal cord barrier in humans and rats. Mol. Pharm. 2020, 17, 2006–2020. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.M.; Bradley, M.C.; Fernández-Del-Río, L.; Nag, A.; Tsui, H.S.; Clarke, C.F. Coenzyme Q 10 Deficiencies: Pathways in Yeast and Humans. Essays Biochem. 2018, 62, 361–376. [Google Scholar] [CrossRef]
- Kemmerer, Z.A.; Robinson, K.P.; Schmitz, J.M.; Manicki, M.; Paulson, B.R.; Jochem, A.; Hutchins, P.D.; Coon, J.J.; Pagliarini, D.J. UbiB proteins regulate cellular CoQ distribution in Saccharomyces cerevisiae. Nat. Commun. 2021, 12, 4769. [Google Scholar] [CrossRef]
- Jin, G.; Kubo, H.; Kashiba, M.; Horinouchi, R.; Hasegawa, M.; Suzuki, M.; Sagawa, T.; Oizumi, M.; Fujisawa, A.; Tsukamoto, H.; et al. Saposin B is a human coenzyme q10-binding/transfer protein. J. Clin. Biochem. Nutr. 2008, 42, 167–174. [Google Scholar] [CrossRef]
- Mantle, D.; Millichap, L.; Castro-Marrero, J.; Hargreaves, I.P. Primary CoQ10 deficiency: An update. Antioxidants 2023, 12, 1652. [Google Scholar] [CrossRef]
- Beal, M.; Matthews, R.T.; Tieleman, A.; Shults, C.W. Coenzyme Q10 attenuates the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced loss of striatal dopamine and dopaminergic axons in aged mice. Brain Res. 1998, 783, 109–114. [Google Scholar] [CrossRef]
- Dumont, M.; Kipiani, K.; Yu, F.; Wille, E.; Katz, M.; Calingasan, N.Y.; Gouras, G.K.; Lin, M.T.; Beal, M.F. Coenzyme Q10 decreases amyloid pathology and improves behavior in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011, 27, 211–223. [Google Scholar] [CrossRef]
- Ari, C.; Poff, A.M.; Held, H.E.; Landon, C.S.; Goldhagen, C.R.; Mavromates, N.; D’Agostino, D.P. Metabolic therapy with Deanna Protocol supplementation delays disease progression and extends survival in amyotrophic lateral sclerosis (ALS) mouse model. PLoS ONE 2014, 9, e103526. [Google Scholar] [CrossRef]
- Miquel, E.; Cassina, A.; Martínez-Palma, L.; Souza, J.M.; Bolatto, C.; Rodríguez-Bottero, S.; Logan, A.; Smith, R.A.; Murphy, M.P.; Barbeito, L.; et al. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2014, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Thompson, J.L.; Levy, G.; Buchsbaum, R.; Shefner, J.; Krivickas, L.S.; Katz, J.; Rollins, Y.; Barohn, R.J.; Jackson, C.E.; et al. Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 2009, 66, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.G.; Sun, M.X.; Zhang, W.L.; Wang, W.W.; Jin, Y.M.; Xie, C.L. The efficacy and safety of coenzyme Q10 in Parkinson’s disease: A meta-analysis of randomized controlled trials. Neurol. Sci. 2017, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Matthews, R.T.; Yang, L.; Browne, S.; Baik, M.; Beal, M.F. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA 1998, 95, 8892–8897. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohsawa, I.; Shirasawa, T.; Takahashi, M. Early-onset motor impairment and increased accumulation of phosphorylated α-synuclein in the motor cortex of normal aging mice are ameliorated by coenzyme Q. Exp. Gerontol. 2016, 81, 65–75. [Google Scholar] [CrossRef]
- Mitsui, J.; Koguchi, K.; Momose, T.; Takahashi, M.; Matsukawa, T.; Yasuda, T.; Tokushige, S.I.; Ishiura, H.; Goto, J.; Nakazaki, S.; et al. Three-Year follow-up of high-dose ubiquinol supplementation in a case of familial multiple system atrophy with compound heterozygous COQ2 mutations. Cerebellum 2017, 16, 664–672. [Google Scholar] [CrossRef]
- Duberley, K.E.; Hargreaves, I.P.; Chaiwatanasirikul, K.; Heales, S.J.; Rahman, S.; Mills, K.; Eaton, S. Development of a mass spectrometry method for quantification of coenzyme Q10 in CSF. J. Inherit. Metab. Dis. 2012, 35 (Suppl. S1), S124. [Google Scholar]
- Artuch, R.; Aracil, A.; Mas, A.; Monros, E.; Vilaseca, M.A.; Pineda, M. Cerebrospinal fluid concentrations of idebenone in Friedreich ataxia patients. Neuropediatrics 2004, 35, 95–98. [Google Scholar] [CrossRef]
- Isobe, C.; Abe, T.; Terayama, Y. Levels of reduced and oxidized coenzyme Q10 and 8-hydroxy-2-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson’s disease demonstrate that mitochondrial oxidative stress damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neurosci. Lett. 2010, 469, 159–163. [Google Scholar]
- Duncan, A.J.; Heales, S.J.; Mills, K.; Eaton, S.; Land, J.M.; Hargreaves, I.P. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin. Chem. 2005, 51, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, B.; Frassineti, C.; Martinelli, P.; Iotti, S.; Lodi, R.; Cortelli, P.; Montagna, P. Coenzyme Q10 improves mitochondrial respiration in patients with mitochondrial cytopathies. An in vivo study on brain and skeletal muscle by phosphorous magnetic resonance spectroscopy. Cell. Mol. Biol. 1997, 43, 741–749. [Google Scholar]
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.R.; Preston, J.E. CoQ10 Deficient endothelial cell culture model for the investigation of CoQ10 blood-brain barrier transport. J. Clin. Med. 2020, 9, 3236. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 analogues: Benefits and challenges for therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chen, J.; Gao, J. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: Focus on recent advances. Asian J. Pharm. Sci. 2019, 14, 480–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, S.; Che, Y.; Wang, Z.; Xing, K.; Xie, X.; Chen, Y. Mitochondrion-targeted carboxymethyl chitosan hybrid nanoparticles loaded with Coenzyme Q10 protect cardiac grafts against cold ischaemia–reperfusion injury in heart transplantation. J. Transl. Med. 2023, 21, 925. [Google Scholar] [CrossRef]
- Boyuklieva, R.; Katsarov, P.; Zagorchev, P.; Abarova, S.; Hristozova, A.; Pilicheva, B. Idebenone-loaded nanocomposite microspheres for nasal administration-A perspective in the treatment of Alzheimer’s disease. Discov Med. 2024, 36, 1527–1587. [Google Scholar] [CrossRef]
| Tissues | Method to Detect CoQ10 | Reference |
|---|---|---|
| Muscle and other tissue including brain | HPLC UV detection at 275 nm HPLC linked to electrochemical detection | [30] [28] |
| Blood, plasma, and serum | HPLC UV detection at 275 nm HPLC linked to electrochemical detection | [30] [28] |
| Cerebrospinal fluid (CSF) | HPLC linked to electrochemical detection Liquid chromatography-mass spectrometry | [28] [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantle, D.; Hargreaves, I. Coenzyme Q10 and the Blood–Brain Barrier: An Overview. J. Clin. Med. 2025, 14, 2748. https://doi.org/10.3390/jcm14082748
Mantle D, Hargreaves I. Coenzyme Q10 and the Blood–Brain Barrier: An Overview. Journal of Clinical Medicine. 2025; 14(8):2748. https://doi.org/10.3390/jcm14082748
Chicago/Turabian StyleMantle, David, and Iain Hargreaves. 2025. "Coenzyme Q10 and the Blood–Brain Barrier: An Overview" Journal of Clinical Medicine 14, no. 8: 2748. https://doi.org/10.3390/jcm14082748
APA StyleMantle, D., & Hargreaves, I. (2025). Coenzyme Q10 and the Blood–Brain Barrier: An Overview. Journal of Clinical Medicine, 14(8), 2748. https://doi.org/10.3390/jcm14082748






