Chronic Obstructive Pulmonary Disease and COVID-19: The Impact of Hematological Biomarkers on Disease Severity and Outcomes
Abstract
:1. Introduction
2. Methodology
3. Shared Mechanisms Between COPD and COVID-19
3.1. Chronic Inflammation and Cytokine Storms
3.2. Hypoxia and Oxidative Stress
3.2.1. Hypoxia-Induced Hematological and Coagulation Alterations in COPD and Severe COVID-19
3.2.2. Increased Production of Reactive Oxygen Species (ROS) and Their Impact on Blood Cells
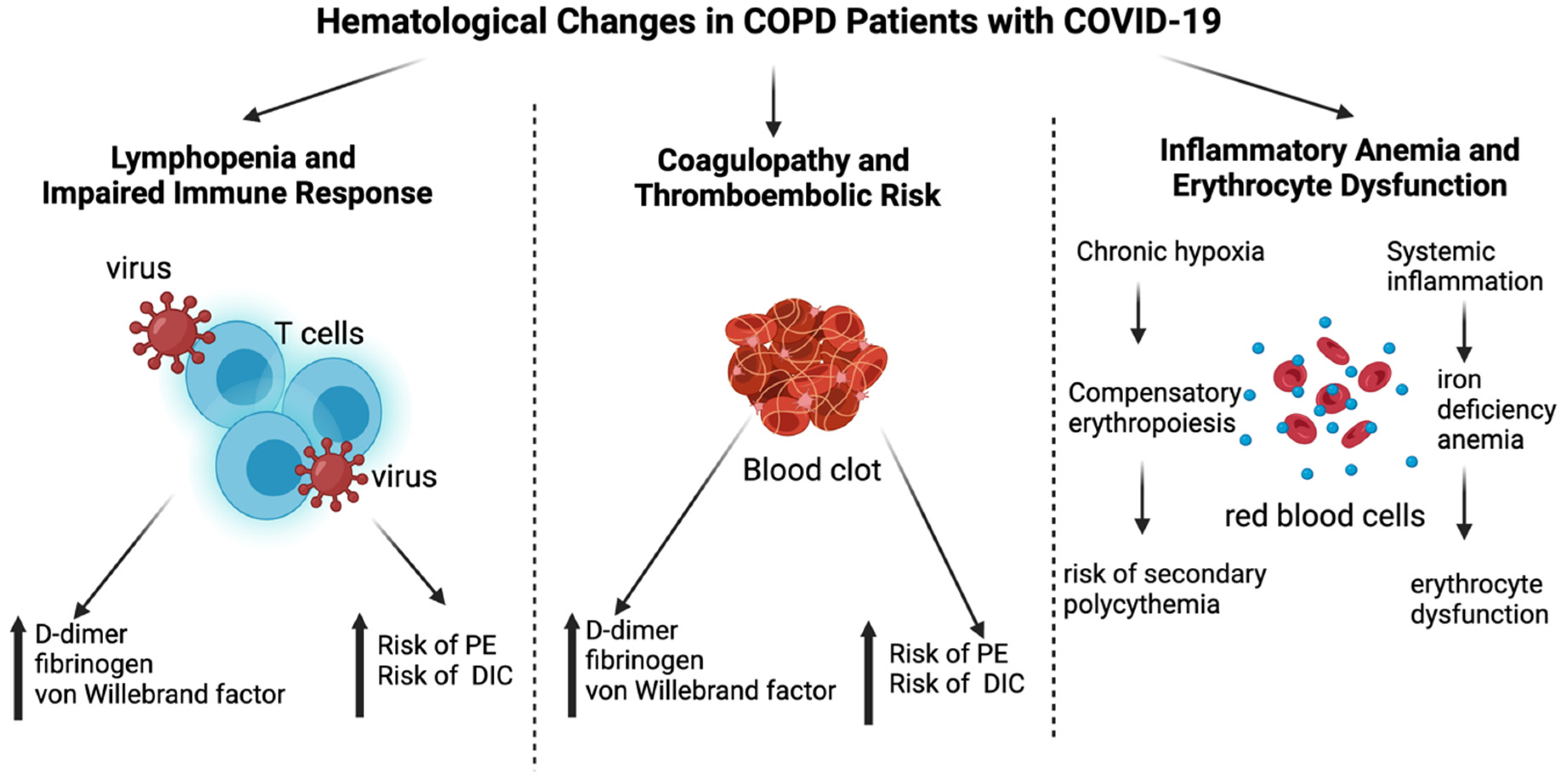
4. Hematological Changes in COPD Patients with COVID-19
4.1. Lymphopenia and Impaired Immune Responses
4.2. Coagulopathy and Thromboembolic Risk
4.3. Inflammatory Anemia and Erythrocyte Dysfunction
5. Clinical Implications and Therapeutic Strategies
5.1. Monitoring Hematological Markers
- D-dimer > 1.0 µg/mL, which is associated with increased thrombotic risk and may justify the initiation or intensification of anticoagulation;
- NLR > 7, which is predictive of severe disease progression and a poor prognosis;
- Fibrinogen > 4 g/L, which is indicative of high inflammatory burden.
5.2. Anticoagulation Therapy in COPD and COVID-19
5.3. Immunomodulators and Anti-Inflammatory Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Meng, Y.; Ji, Q.; Zhang, A.; Zhan, Y. Trends in the prevalence and incidence of chronic obstructive pulmonary disease among adults aged ≥50 years in the United States, 2000–2020. Chronic Dis. Transl. Med. 2024, 10, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Al Wachami, N.; Guennouni, M.; Iderdar, Y.; Boumendil, K.; Arraji, M.; Mourajid, Y.; Bouchachi, F.Z.; Barkaoui, M.; Louerdi, M.L.; Hilali, A.; et al. Estimating the global prevalence of chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. BMC Public Health 2024, 24, 297. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir. Med. 2020, 167, 105941. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S.; Oyelade, T.; Aldhahir, A.M.; Alghamdi, S.M.; Almehmadi, M.; Alqahtani, A.S.; Quaderi, S.; Mandal, S.; Hurst, J.R. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS ONE 2020, 15, e0233147. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.; Alghamdi, R.; Guallart, I.; Bergamini, M.; Yu, P.; Froum, S.; Cho, S.-C. Patient-Related Risk Factors for Maxillary Sinus Augmentation Procedures: A Systematic Literature Review. Int. J. Periodontics Restor. Dent. 2021, 41, e121–e128. [Google Scholar] [CrossRef]
- Ashmore, P.; Sherwood, E. An overview of COVID-19 global epidemiology and discussion of potential drivers of variable global pandemic impacts. J. Antimicrob. Chemother. 2023, 78 (Suppl. S2), ii2–ii11. [Google Scholar] [CrossRef]
- Marginean, C.M.; Popescu, M.; Vasile, C.M.; Cioboata, R.; Mitrut, P.; Popescu, I.A.S.; Biciusca, V.; Docea, A.O.; Mitrut, R.; Marginean, I.C.; et al. Challenges in the Differential Diagnosis of COVID-19 Pneumonia: A Pictorial Review. Diagnostics 2022, 12, 2823. [Google Scholar] [CrossRef]
- Zeng, Y.; Cai, S.; Chen, Y.; Duan, J.; Zhao, Y.; Li, X.; Ma, L.; Liu, Q.; Zhu, Y.; Chen, M.; et al. Current Status of the Treatment of COPD in China: A Multicenter Prospective Observational Study. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.M.; Luppi, F.; Beghé, B.; Rabe, K.F. Complex chronic comorbidities of COPD. Eur. Respir. J. 2008, 31, 204–212. [Google Scholar] [CrossRef]
- Alvarez-Martins, I.; Remédio, L.; Matias, I.; Diogo, L.N.; Monteiro, E.C.; Dias, S. The impact of chronic intermittent hypoxia on hematopoiesis and the bone marrow microenvironment. Pflüg. Arch.-Eur. J. Physiol. 2016, 468, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Anand, K.; Palanisamy, S.; Anathy, V. Editorial: Oxidative stress related to cellular metabolism in lung health and diseases. Front. Pharmacol. 2022, 13, 1015423. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of Erythropoiesis by the Hypoxia-Inducible Factor Pathway: Effects of Genetic and Pharmacological Perturbations. Annu. Rev. Med. 2023, 74, 307–319. [Google Scholar] [CrossRef]
- Zhang, J.; DeMeo, D.L.; Silverman, E.K.; Make, B.J.; Wade, R.C.; Wells, J.M.; Cho, M.H.; Hobbs, B.D. Secondary polycythemia in chronic obstructive pulmonary disease: Prevalence and risk factors. BMC Pulm. Med. 2021, 21, 235. [Google Scholar] [CrossRef]
- Patel, A.R.; Hurst, J.R. Extrapulmonary comorbidities in chronic obstructive pulmonary disease: State of the art. Expert Rev. Respir. Med. 2011, 5, 647–662. [Google Scholar] [CrossRef]
- Santos, S.; Manito, N.; Sánchez-Covisa, J.; Hernández, I.; Corregidor, C.; Escudero, L.; Rhodes, K.; Nordon, C. Risk of severe cardiovascular events following COPD exacerbations: Results from the EXACOS-CV study in Spain. Rev. Esp. Cardiol. 2025, 78, 138–150. [Google Scholar] [CrossRef]
- Morgan, A.; Herrett, E.; De Stavola, B.; Smeeth, L.; Quint, J. COPD disease severity and the risk of venous thromboembolic events: A matched case-control study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 899. [Google Scholar] [CrossRef]
- Van Der Vorm, L.N.; Li, L.; Huskens, D.; Hulstein, J.J.J.; Roest, M.; De Groot, P.G.; Ten Cate, H.; De Laat, B.; Remijn, J.A.; Simons, S.O. Acute exacerbations of COPD are associated with a prothrombotic state through platelet-monocyte complexes, endothelial activation and increased thrombin generation. Respir. Med. 2020, 171, 106094. [Google Scholar] [CrossRef]
- Hanson, A.L.; Mulè, M.P.; Ruffieux, H.; Mescia, F.; Bergamaschi, L.; Pelly, V.S.; Turner, L.; Kotagiri, P.; Cambridge Institute of Therapeutic Immunology and Infectious Disease–National Institute for Health Research (CITIID–NIHR) COVID BioResource Collaboration; Göttgens, B.; et al. Iron dysregulation and inflammatory stress erythropoiesis associates with long-term outcome of COVID-19. Nat. Immunol. 2024, 25, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, L.; Pirotte, M.; Lebeau, A.; Ernst, M.; Fillet, M.; Devey, A.; Schmitt, J.; Cobraiville, G.; Binsfeld, M.; Gofflot, S.; et al. Alterations of erythropoiesis in COVID-19 patients: Prevalence of positive Coombs tests and iron metabolism. Ther. Adv. Hematol. 2023, 14, 20406207231199836. [Google Scholar] [CrossRef]
- Saad, E.; Maamoun, B.; Nimer, A. Increased Red Blood Cell Distribution Predicts Severity of Chronic Obstructive Pulmonary Disease Exacerbation. J. Pers. Med. 2023, 13, 843. [Google Scholar] [CrossRef]
- Elemam, N.M.; Talaat, I.M.; Bayoumi, F.A.; Zein, D.; Georgy, R.; Altamimi, A.; Alkhayyal, N.; Habbal, A.; Al Ali, F.; ElKhider, A.; et al. Peripheral blood cell anomalies in COVID-19 patients in the United Arab Emirates: A single-centered study. Front. Med. 2022, 9, 1072427. [Google Scholar] [CrossRef] [PubMed]
- Chandran, N.; Sigamani, K.; Khadeja Bi, A. Hematological Profile in COVID-19 Infection Among Patients in a Tertiary Care Hospital in Tamil Nadu, South India. Cureus 2022, 14, e30731. [Google Scholar] [CrossRef]
- Hacievliyagil, S.S.; Gunen, H.; Mutlu, L.C.; Karabulut, A.B.; Temel, İ. Association between cytokines in induced sputum and severity of chronic obstructive pulmonary disease. Respir. Med. 2006, 100, 846–854. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-R.; Wang, A.-L.; Li, Y.-Q. Hypoxia-inducible factor 1-alpha is a driving mechanism linking chronic obstructive pulmonary disease to lung cancer. Front. Oncol. 2022, 12, 984525. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Yang, J.-J.; Zhang, S.-A.; Zhang, S.-M.; Wang, J.-X.; Xu, Z.-Y.; Lin, R.-Y. HIF-1α promotes inflammatory response of chronic obstructive pulmonary disease by activating EGFR/PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6077–6084. [Google Scholar]
- Walmsley, S.R.; Print, C.; Farahi, N.; Peyssonnaux, C.; Johnson, R.S.; Cramer, T.; Sobolewski, A.; Condliffe, A.M.; Cowburn, A.S.; Johnson, N.; et al. Hypoxia-induced neutrophil survival is mediated by HIF-1α–dependent NF-κB activity. J. Exp. Med. 2005, 201, 105–115. [Google Scholar] [CrossRef]
- Serebrovska, Z.O.; Chong, E.Y.; Serebrovska, T.V.; Tumanovska, L.V.; Xi, L. Hypoxia, HIF-1α, and COVID-19: From pathogenic factors to potential therapeutic targets. Acta Pharmacol. Sin. 2020, 41, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Jahani, M.; Dokaneheifard, S.; Mansouri, K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J. Inflamm. Lond. Engl. 2020, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Zhao, Y.-Y.; Evans, C.E. The stimulation of thrombosis by hypoxia. Thromb. Res. 2019, 181, 77–83. [Google Scholar] [CrossRef]
- Ninivaggi, M.; de Laat, M.; Lancé, M.M.D.; Kicken, C.H.; Pelkmans, L.; Bloemen, S.; Dirks, M.L.; van Loon, L.J.C.; Govers-Riemslag, J.W.P.; Lindhout, T.; et al. Hypoxia Induces a Prothrombotic State Independently of the Physical Activity. PLoS ONE 2015, 10, e0141797. [Google Scholar] [CrossRef]
- Malone, P.C.; Agutter, P.S. Deep venous thrombosis: The valve cusp hypoxia thesis and its incompatibility with modern orthodoxy. Med. Hypotheses 2016, 86, 60–66. [Google Scholar] [CrossRef]
- Goldhaber, S.Z.; Elliott, C.G. Acute Pulmonary Embolism: Part I: Epidemiology, Pathophysiology, and Diagnosis. Circulation 2003, 108, 2726–2729. [Google Scholar] [CrossRef]
- Subramanian, M.; Ramadurai, S.; Arthur, P.; Gopalan, S. Hypoxia as an independent predictor of adverse outcomes in pulmonary embolism. Asian Cardiovasc. Thorac. Ann. 2018, 26, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Yamashita, A.; Iwakiri, T.; Sugita, C.; Okuyama, N.; Kitamura, K.; Asada, Y. Vascular wall hypoxia promotes arterial thrombus formation via augmentation of vascular thrombogenicity. Thromb. Haemost. 2015, 114, 158–172. [Google Scholar] [CrossRef]
- Jones, D.T.; Macdonald, J.H.; Sandoo, A.; Oliver, S.J.; Rossetti, G.M. The deleterious effects of acute hypoxia and large vessel endothelial function. Exp. Physiol. 2021, 106, 1699–1709. [Google Scholar] [CrossRef]
- Delaney, C.; Davizon-Castillo, P.; Allawzi, A.; Posey, J.; Gandjeva, A.; Neeves, K.; Tuder, R.M.; Di Paola, J.; Stenmark, K.R.; Nozik, E.S. Platelet activation contributes to hypoxia-induced inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L413–L421. [Google Scholar] [CrossRef]
- Bhagat, S.; Biswas, I.; Ahmed, R.; Khan, G.A. Hypoxia induced up-regulation of tissue factor is mediated through extracellular RNA activated Toll-like receptor 3-activated protein 1 signalling. Blood Cells. Mol. Dis. 2020, 84, 102459. [Google Scholar] [CrossRef]
- Ding, C.; Wang, R.; Gong, X.; Yuan, Y. Stroke risk of COPD patients and death risk of COPD patients following a stroke: A systematic review and meta-analysis. Medicine 2023, 102, e35502. [Google Scholar] [CrossRef] [PubMed]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; Van De Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.; Patolia, S.; Looby, M.; Bade, N.; Khangoora, V.; King, C.S. Association of D-dimer and Fibrinogen With Hypercoagulability in COVID-19 Requiring Extracorporeal Membrane Oxygenation. J. Intensive Care Med. 2021, 36, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell. Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef]
- Xie, J.; Yuan, C.; Yang, S.; Ma, Z.; Li, W.; Mao, L.; Jiao, P.; Liu, W. The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-induced cell death. Cell. Mol. Biol. Lett. 2024, 29, 138. [Google Scholar] [CrossRef]
- Wang, H.; Song, T.-Y.; Reyes-García, J.; Wang, Y.-X. Hypoxia-Induced Mitochondrial ROS and Function in Pulmonary Arterial Endothelial Cells. Cells 2024, 13, 1807. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Li, C.; Liu, R.; Wang, X. Mechanism of hypoxia-induced damage to the mechanical property in human erythrocytes-band 3 phosphorylation and sulfhydryl oxidation of membrane proteins. Front. Physiol. 2024, 15, 1399154. [Google Scholar] [CrossRef]
- Tariq, S.; Ismail, D.; Thapa, M.; Goriparthi, L.; Pradeep, R.; Khalid, K.; Cooper, A.C.; Jean-Charles, G. Chronic Obstructive Pulmonary Disease and Its Effect on Red Blood Cell Indices. Cureus 2023, 15, e36100. [Google Scholar] [CrossRef] [PubMed]
- Mullen, E.; Bergin, S.; Healy, G.; Quinn, J.; Glavey, S.; Murphy, P.T. Red blood cells from COVID-19 patients suffer from increased oxidative stress and may have increased lactate influx. Blood Res. 2022, 57, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Finicelli, M.; Digilio, F.A.; Galderisi, U.; Peluso, G. The Emerging Role of Macrophages in Chronic Obstructive Pulmonary Disease: The Potential Impact of Oxidative Stress and Extracellular Vesicle on Macrophage Polarization and Function. Antioxidants 2022, 11, 464. [Google Scholar] [CrossRef]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [Google Scholar] [CrossRef] [PubMed]
- Mallah, H.; Ball, S.; Sekhon, J.; Parmar, K.; Nugent, K. Platelets in chronic obstructive pulmonary disease: An update on pathophysiology and implications for antiplatelet therapy. Respir. Med. 2020, 171, 106098. [Google Scholar] [CrossRef]
- Yang, M. Redox stress in COVID-19: Implications for hematologic disorders. Best Pract. Res. Clin. Haematol. 2022, 35, 101373. [Google Scholar] [CrossRef]
- Jo, Y.S. Long-Term Outcome of Chronic Obstructive Pulmonary Disease: A Review. Tuberc. Respir. Dis. 2022, 85, 289–301. [Google Scholar] [CrossRef]
- El-Korashy, R.I.; Amin, Y.M.; Moussa, H.A.; Badawy, I.; Bakr, S.M. Study the relationship of erythropoietin and chronic obstructive pulmonary disease. Egypt. J. Chest Dis. Tuberc. 2012, 61, 53–57. [Google Scholar] [CrossRef]
- Soliz, J.; Schneider-Gasser, E.M.; Arias-Reyes, C.; Aliaga-Raduan, F.; Poma-Machicao, L.; Zubieta-Calleja, G.; Furuya, W.I.; Trevizan-Baú, P.; Dhingra, R.R.; Dutschmann, M. Coping with hypoxemia: Could erythropoietin (EPO) be an adjuvant treatment of COVID-19? Respir. Physiol. Neurobiol. 2020, 279, 103476. [Google Scholar] [CrossRef]
- Ehrenreich, H.; Weissenborn, K.; Begemann, M.; Busch, M.; Vieta, E.; Miskowiak, K.W. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol. Med. 2020, 26, 58. [Google Scholar] [CrossRef]
- Thapa, K.B.; Paudel, A.; Dhital, S.; Shrestha, A.; Ojha, L.; Shrestha, A. Polycythemia among Patients with Chronic Obstructive Pulmonary Disease Admitted to the Department of Medicine in a Tertiary Care Center: A Descriptive Cross-sectional Study. JNMA J. Nepal Med. Assoc. 2023, 61, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Ayako, R.M.; Patel, K.; Ndede, I.; Nordgren, J.; Larrson, M.; Mining, S.K. Inflammatory, Hematological, and Biochemical Biomarkers in COVID-19 Patients. Immun. Inflamm. Dis. 2024, 12, e70078. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Ma, X.; Zhang, H.; Chen, J.; Liu, M.; Chen, L.; Xu, H. Role of HIF-1α in hypercoagulable state of COPD in rats. Arch. Biochem. Biophys. 2024, 753, 109903. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Liu, W.; Li, X.; Zhao, P.; Shereen, M.A.; Zhu, C.; Huang, S.; Liu, S.; Yu, X.; Yue, M.; et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct. Target. Ther. 2021, 6, 308. [Google Scholar] [CrossRef]
- Moulaeian, M.; Ferdousmakan, S.; Banihashemi, S.; Homayounfar, S.; Pasupulla, A.P.; Malekzadegan, Y. Reactive oxygen species induced by SARS-CoV-2 infection can induce EMT in solid tumors: Potential role of COVID-19 in chemo-resistance and metastasis. Heliyon 2024, 10, e40297. [Google Scholar] [CrossRef]
- Voskresenska, N.; Voicehovska, J.; Vojcehovska, A.; Orlikovs, G.; Skesters, A. Malondialdehyde level in chronic obstructive pulmonary disease exacerbations. In 52 Monitoring Airway Disease; European Respiratory Society: Lausanne, Switzerland, 2015; p. OA2920. [Google Scholar]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in oxidative markers in COVID-19 patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [CrossRef]
- Bowler, R.P.; Hokanson, J.; Taylor, M.; Levy, S.; Canaham, E.M.; Regan, E.; Wheeler, C.; Nicks, M.; Chan, E.; Crapo, J.D. Extracellular superoxide dismutase (EC-SOD) as a protective factor for risk of chronic obstructive pulmonary disease. Eur. Respir. Rev. 2006, 15, 200–201. [Google Scholar] [CrossRef]
- Chu, J.; Hua, L.; Liu, X.; Xiong, H.; Jiang, F.; Zhou, W.; Wang, L.; Xue, G. Superoxide dismutase alterations in COVID-19: Implications for disease severity and mortality prediction in the context of omicron variant infection. Front. Immunol. 2024, 15, 1362102. [Google Scholar] [CrossRef]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Guloyan, V.; Oganesian, B.; Baghdasaryan, N.; Yeh, C.; Singh, M.; Guilford, F.; Ting, Y.-S.; Venketaraman, V. Glutathione Supplementation as an Adjunctive Therapy in COVID-19. Antioxidants 2020, 9, 914. [Google Scholar] [CrossRef] [PubMed]
- Satała, J.; Woźniak, A.; Fabiś, M.; Gorzelak-Pabiś, P.; Pawlos, A.; Fabiś, J.; Broncel, M.; Woźniak, E. Severe COVID-19 classified by simple covid risk index is associated with higher levels of advanced oxidation protein products and 8-hydroxy 2 deoxyguanosine. Epidemiol. Infect. 2023, 151, e140. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Pérez-Cejas, A.; Cáceres, J.J.; Perez, A.; Ramos-Gómez, L.; Solé-Violán, J.; Ramos, J.A.M.Y.; Ojeda, N.; et al. DNA and RNA Oxidative Damage and Mortality of Patients With COVID-19. Am. J. Med. Sci. 2021, 361, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, J.; Janson, C.; Bröms, K.; Hårdstedt, M.; Högman, M.; Lisspers, K.; Palm, A.; Ställberg, B.; Malinovschi, A. CRP, Fibrinogen, White Blood Cells, and Blood Cell Indices as Prognostic Biomarkers of Future COPD Exacerbation Frequency: The TIE Cohort Study. J. Clin. Med. 2024, 13, 3855. [Google Scholar] [CrossRef]
- Rahaghi, F.N.; Pistenmaa, C.L. Hypercoagulation in COPD: The clot thickens. ERJ Open Res. 2021, 7, 00534–02021. [Google Scholar] [CrossRef]
- Higham, A.; Mathioudakis, A.; Vestbo, J.; Singh, D. COVID-19 and COPD: A narrative review of the basic science and clinical outcomes. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2020, 29, 200199. [Google Scholar] [CrossRef]
- Hilda, F.; Liana, P.; Nurtjahyo, A.; Hudari, H.; Sari, N.P.; Umar, T.P.; Amin, C.A.; Afifah, A.R. D-Dimer as a Sensitive Biomarker of Survival Rate in Patients with COVID-19. Eurasian J. Med. 2022, 54, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hafez, W.; Nasa, P.; Khairy, A.; Jose, M.; Abdelshakour, M.; Ahmed, S.; Abdulaal, F.; Nair, N.; Ahmad, M.; Rashid, V.J.; et al. Interleukin-6 and the determinants of severe COVID-19: A retrospective cohort study. Medicine 2023, 102, e36037. [Google Scholar] [CrossRef]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. IJCB 2021, 36, 440–450. [Google Scholar] [CrossRef]
- Lodge, K.M.; Vassallo, A.; Liu, B.; Long, M.; Tong, Z.; Newby, P.R.; Agha-Jaffar, D.; Paschalaki, K.; Green, C.E.; Belchamber, K.B.R.; et al. Hypoxia Increases the Potential for Neutrophil-mediated Endothelial Damage in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2022, 205, 903–916. [Google Scholar] [CrossRef]
- Teo, A.; Chan, L.L.Y.; Cheung, C.; Chia, P.Y.; Ong, S.W.X.; Fong, S.W.; Ng, L.F.P.; Renia, L.; Lye, D.C.; Young, B.E.; et al. Myeloperoxidase inhibition may protect against endothelial glycocalyx shedding induced by COVID-19 plasma. Commun. Med. 2023, 3, 62. [Google Scholar] [CrossRef] [PubMed]
- Awatade, N.T.; Wark, P.A.B.; Chan, A.S.L.; Mamun, S.M.A.A.; Mohd Esa, N.Y.; Matsunaga, K.; Rhee, C.K.; Hansbro, P.M.; Sohal, S.S.; On Behalf of The Asian Pacific Society of Respirology Apsr Copd Assembly. The Complex Association between COPD and COVID-19. J. Clin. Med. 2023, 12, 3791. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, M.; Kumar, R.; Wu, Y.; Huang, J.; Deng, Y.; Weng, Z.; Yang, L. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020, 96, 131–135. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Pothast, C.R.; Dijkland, R.C.; Thaler, M.; Hagedoorn, R.S.; Kester, M.G.D.; Wouters, A.K.; Hiemstra, P.S.; van Hemert, M.J.; Gras, S.; Falkenburg, J.H.F.; et al. SARS-CoV-2-specific CD4+ and CD8+ T cell responses can originate from cross-reactive CMV-specific T cells. eLife 2022, 11, e82050. [Google Scholar] [CrossRef]
- Kervevan, J.; Chakrabarti, L.A. Role of CD4+ T Cells in the Control of Viral Infections: Recent Advances and Open Questions. Int. J. Mol. Sci. 2021, 22, 523. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, B.; Liu, Y.; Wu, Y.; Chen, Y. Immune damage mechanisms of COVID-19 and novel strategies in prevention and control of epidemic. Front. Immunol. 2023, 14, 1130398. [Google Scholar] [CrossRef]
- Lian, J.; Jin, C.; Hao, S.; Zhang, X.; Yang, M.; Jin, X.; Lu, Y.; Hu, J.; Zhang, S.; Zheng, L.; et al. High neutrophil-to-lymphocyte ratio associated with progression to critical illness in older patients with COVID-19: A multicenter retrospective study. Aging 2020, 12, 13849–13859. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Negri, S.; Carru, C.; Zinellu, A. Neutrophil to lymphocyte ratio and clinical outcomes in COPD: Recent evidence and future perspectives. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2018, 27, 170113. [Google Scholar] [CrossRef]
- Grobler, C.; Maphumulo, S.C.; Grobbelaar, L.M.; Bredenkamp, J.C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. COVID-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int. J. Mol. Sci. 2020, 21, 5168. [Google Scholar] [CrossRef]
- Singh, D.; Mathioudakis, A.G.; Higham, A. Chronic obstructive pulmonary disease and COVID-19: Interrelationships. Curr. Opin. Pulm. Med. 2022, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef] [PubMed]
- Alisamir, M.; Ebrahimi, M.; Rahim, F. Anemia in chronic obstructive pulmonary disease: A systematic review. Respir. Investig. 2022, 60, 510–521. [Google Scholar] [CrossRef]
- Tao, Z.; Xu, J.; Chen, W.; Yang, Z.; Xu, X.; Liu, L.; Chen, R.; Xie, J.; Liu, M.; Wu, J.; et al. Anemia is associated with severe illness in COVID-19: A retrospective cohort study. J. Med. Virol. 2021, 93, 1478–1488. [Google Scholar] [CrossRef]
- Benoit, J.L.; Benoit, S.W.; de Oliveira, M.H.S.; Lippi, G.; Henry, B.M. Anemia and COVID-19: A prospective perspective. J. Med. Virol. 2021, 93, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Wessling-Resnick, M. Iron homeostasis and the inflammatory response. Annu. Rev. Nutr. 2010, 30, 105–122. [Google Scholar] [CrossRef]
- Mendonça, M.M.; da Cruz, K.R.; Pinheiro, D.d.S.; Moraes, G.C.A.; Ferreira, P.M.; Ferreira-Neto, M.L.; da Silva, E.S.; Gonçalves, R.V.; Pedrino, G.R.; Fajemiroye, J.O.; et al. Dysregulation in erythrocyte dynamics caused by SARS-CoV-2 infection: Possible role in shuffling the homeostatic puzzle during COVID-19. Hematol. Transfus. Cell Ther. 2022, 44, 235–245. [Google Scholar] [CrossRef]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 199–208. [Google Scholar]
- Cavezzi, A.; Troiani, E.; Corrao, S. COVID-19: Hemoglobin, Iron, and Hypoxia beyond Inflammation. A Narrative Review. Clin. Pract. 2020, 10, 1271. [Google Scholar] [CrossRef]
- Hardang, I.M.; Søyseth, V.; Kononova, N.; Hagve, T.-A.; Einvik, G. COPD: Iron Deficiency and Clinical Characteristics in Patients With and Without Chronic Respiratory Failure. Chronic Obstr. Pulm. Dis. J. COPD Found. 2024, 11, 261–269. [Google Scholar] [CrossRef]
- Şan, İ.; Gemcioğlu, E.; Davutoğlu, M.; Çatalbaş, R.; Karabuğa, B.; Kaptan, E.; Erden, A.; Küçükşahin, O.; Ateş, İ.; Karaahmetoğlu, S.; et al. Which hematological markers have predictive value as early indicators of severe COVID-19 cases in the emergency department? Turk. J. Med. Sci. 2021, 51, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Gupta, S.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Noubouossie, D.F.; Gandotra, S.; Cao, L. Elevated Plasma Fibrinogen Is Associated With Excessive Inflammation and Disease Severity in COVID-19 Patients. Front. Cell. Infect. Microbiol. 2021, 11, 734005. [Google Scholar] [CrossRef]
- Simadibrata, D.M.; Calvin, J.; Wijaya, A.D.; Ibrahim, N.A.A. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.M.; Bory, O.M.; Diehl, J.-L.; Mareau, A.; Gendron, N.; Jannot, A.-S.; Chocron, R. Daily Monitoring of D-Dimer Allows Outcomes Prediction in COVID-19. TH Open 2022, 06, e21–e25. [Google Scholar] [CrossRef]
- Patil, S.; Khule, S.; Toshniwal, S. Role of D-Dimer in assessing severity, monitoring, and predicating outcome in COVID-19 pneumonia: A single center study. Glob. J. Health Sci. Res. 2023, 1, 31–37. [Google Scholar] [CrossRef]
- Beidollahkhani, S.; Fayedeh, F.; Shoja, A.; Hassan Nejad, E.; Hoseinpour, M.; Fazlpour, F.; Payandeh, A.; Pezeshki Rad, M.; Moodi Ghalibaf, A. d-dimer as a biomarker for COVID-19-associated pulmonary thromboembolism: A narrative review from molecular pathways to the imaging findings. Egypt. J. Bronchol. 2023, 17, 44. [Google Scholar] [CrossRef]
- Kornblith, L.Z.; Sadhanandhan, B.; Arun, S.; Long, R.; Johnson, A.J.; Noll, J.; Ramchand, C.N.; Olynyk, J.K.; Farrell, D.H. γ′ fibrinogen levels as a biomarker of COVID-19 respiratory disease severity. Blood Cells. Mol. Dis. 2023, 101, 102746. [Google Scholar] [CrossRef]
- Long, W.; Yang, J.; Li, Z.; Li, J.; Chen, S.; Chen, D.; Wang, S.; Li, Q.; Hu, D.; Huang, J.; et al. Abnormal Fibrinogen Level as a Prognostic Indicator in Coronavirus Disease Patients: A Retrospective Cohort Study. Front. Med. 2021, 8, 687220. [Google Scholar] [CrossRef]
- Toori, K.U.; Qureshi, M.A.; Chaudhry, A.; Safdar, M.F. Neutrophil to lymphocyte ratio (NLR) in COVID-19: A cheap prognostic marker in a resource constraint setting. Pak. J. Med. Sci. 2021, 37, 1435–1439. [Google Scholar] [CrossRef]
- Ribeiro Carvalho, C.R.; Lamas, C.D.A.; Visani De Luna, L.A.; Chate, R.C.; Salge, J.M.; Yamada Sawamura, M.V.; Toufen, C.; Garcia, M.L.; Scudeller, P.G.; Nomura, C.H.; et al. Post-COVID-19 respiratory sequelae two years after hospitalization: An ambidirectional study. Lancet Reg. Health-Am. 2024, 33, 100733. [Google Scholar] [CrossRef] [PubMed]
- Asperges, E.; Albi, G.; Zuccaro, V.; Sambo, M.; Pieri, T.C.; Calia, M.; Colaneri, M.; Maiocchi, L.; Melazzini, F.; Lasagna, A.; et al. Dynamic NLR and PLR in Predicting COVID-19 Severity: A Retrospective Cohort Study. Infect. Dis. Ther. 2023, 12, 1625–1640. [Google Scholar] [CrossRef] [PubMed]
- Gemicioglu, B.; Uzun, H.; Borekci, S.; Karaali, R.; Kurugoglu, S.; Atukeren, P.; Sirolu, S.; Durmus, S.; Dirican, A.; Kuskucu, M.A.; et al. Focusing on Asthma and Chronic Obstructive Pulmonary Disease with COVID-19. J. Infect. Dev. Ctries. 2021, 15, 1415–1425. [Google Scholar] [CrossRef]
- Labbé, V.; Contou, D.; Heming, N.; Megarbane, B.; Razazi, K.; Boissier, F.; Ait-Oufella, H.; Turpin, M.; Carreira, S.; Robert, A.; et al. Effects of Standard-Dose Prophylactic, High-Dose Prophylactic, and Therapeutic Anticoagulation in Patients With Hypoxemic COVID-19 Pneumonia: The ANTICOVID Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 520. [Google Scholar] [CrossRef]
- Kaptein, F.H.J.; Stals, M.a.M.; Huisman, M.V.; Klok, F.A. Prophylaxis and treatment of COVID-19 related venous thromboembolism. Postgrad. Med. 2021, 133 (Suppl. S1), 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, D.; Heber, S.; Schrottmaier, W.C.; Santol, J.; Pirabe, A.; Schmuckenschlager, A.; Kammerer, K.; Ammon, D.; Sorz, T.; Fritsch, F.; et al. Low-molecular-weight heparin use in coronavirus disease 2019 is associated with curtailed viral persistence: A retrospective multicentre observational study. Cardiovasc. Res. 2021, 117, 2807–2820. [Google Scholar] [CrossRef]
- Roguljić, H.; Arambašić, J.; Ninčević, V.; Kuna, L.; Šesto, I.; Tabll, A.; Smolić, R.; Včev, A.; Primorac, D.; Wu, G.Y.; et al. The role of direct oral anticoagulants in the era of COVID-19: Are antiviral therapy and pharmacogenetics limiting factors? Croat. Med. J. 2022, 63, 287–294. [Google Scholar] [CrossRef]
- Batra, G.; Modica, A.; Renlund, H.; Larsson, A.; Christersson, C.; Held, C. Oral anticoagulants, time in therapeutic range and renal function over time in real-life patients with atrial fibrillation and chronic kidney disease. Open Heart 2022, 9, e002043. [Google Scholar] [CrossRef]
- Lai, J.; Feng, S.; Xu, S.; Liu, X. Effects of oral anticoagulant therapy in patients with pulmonary diseases. Front. Cardiovasc. Med. 2022, 9, 987652. [Google Scholar] [CrossRef]
- Aursulesei, V.; Costache, I.I. Anticoagulation in chronic kidney disease: From guidelines to clinical practice. Clin. Cardiol. 2019, 42, 774–782. [Google Scholar] [CrossRef]
- Ballestri, S.; Romagnoli, E.; Arioli, D.; Coluccio, V.; Marrazzo, A.; Athanasiou, A.; Di Girolamo, M.; Cappi, C.; Marietta, M.; Capitelli, M. Risk and Management of Bleeding Complications with Direct Oral Anticoagulants in Patients with Atrial Fibrillation and Venous Thromboembolism: A Narrative Review. Adv. Ther. 2023, 40, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.Y.; Ward, S.M. The Anti-Factor Xa Range For Low Molecular Weight Heparin Thromboprophylaxis. Hematol. Rep. 2015, 7, 5844. [Google Scholar] [CrossRef]
- Alsagaff, M.Y.; Mulia, E.P.B.; Maghfirah, I.; Azmi, Y.; Rachmi, D.A.; Yutha, A.; Andira, L.H.; Semedi, B.P. Low molecular weight heparin is associated with better outcomes than unfractionated heparin for thromboprophylaxis in hospitalized COVID-19 patients: A meta-analysis. Eur. Heart J.-Qual. Care Clin. Outcomes 2022, 8, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Wumaier, K.; Li, W.; Chen, N.; Cui, J. Direct oral anticoagulants versus low molecular weight heparins for the treatment of cancer-associated thrombosis: A cost-effectiveness analysis. Thromb. J. 2021, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Kustos, S.; Fasinu, P. Direct-Acting Oral Anticoagulants and Their Reversal Agents—An Update. Medicines 2019, 6, 103. [Google Scholar] [CrossRef]
- Lutz, J.; Jurk, K.; Schinzel, H. Direct oral anticoagulants in patients with chronic kidney disease: Patient selection and special considerations. Int. J. Nephrol. Renov. Dis. 2017, 10, 135–143. [Google Scholar] [CrossRef]
- Olie, R.H.; Winckers, K.; Rocca, B.; Ten Cate, H. Oral Anticoagulants Beyond Warfarin. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 551–575. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Tirpude, N.V.; Sharma, S.; Padwad, Y.S.; Kumar, S. Pharmaco-immunomodulatory interventions for averting cytokine storm-linked disease severity in SARS-CoV-2 infection. Inflammopharmacology 2022, 30, 23–49. [Google Scholar] [CrossRef]
- van Paassen, J.; Vos, J.S.; Hoekstra, E.M.; Neumann, K.M.I.; Boot, P.C.; Arbous, S.M. Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes. Crit. Care 2020, 24, 696. [Google Scholar] [CrossRef]
- Singanayagam, A.; Glanville, N.; Girkin, J.L.; Ching, Y.M.; Marcellini, A.; Porter, J.D.; Toussaint, M.; Walton, R.P.; Finney, L.J.; Aniscenko, J.; et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat. Commun. 2018, 9, 2229. [Google Scholar] [CrossRef]
- Ohadian Moghadam, S. A Review on Currently Available Potential Therapeutic Options for COVID-19. Int. J. Gen. Med. 2020, 13, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Balta, M.G.; Papathanasiou, E.; Christopoulos, P.F. Specialized Pro-Resolving Mediators as Potential Regulators of Inflammatory Macrophage Responses in COVID-19. Front. Immunol. 2021, 12, 632238. [Google Scholar] [CrossRef] [PubMed]
- Bahsoun, A.; Fakih, Y.; Zareef, R.; Bitar, F.; Arabi, M. Corticosteroids in COVID-19: Pros and cons. Front. Med. 2023, 10, 1202504. [Google Scholar] [CrossRef]
- Abidi, E.; El Nekidy, W.S.; Alefishat, E.; Rahman, N.; Petroianu, G.A.; El-Lababidi, R.; Mallat, J. Tocilizumab and COVID-19: Timing of Administration and Efficacy. Front. Pharmacol. 2022, 13, 825749. [Google Scholar] [CrossRef]
- Rosas, I.O.; Bräu, N.; Waters, M.; Go, R.C.; Hunter, B.D.; Bhagani, S.; Skiest, D.; Aziz, M.S.; Cooper, N.; Douglas, I.S.; et al. Tocilizumab in Hospitalized Patients with Severe COVID-19 Pneumonia. N. Engl. J. Med. 2021, 384, 1503–1516. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Scherger, S.; Franco-Paredes, C.; Gharamti, A.; Henao-Martinez, A.F. Anakinra authorized to treat severe coronavirus disease 2019; Sepsis breakthrough or time to reflect? Front. Microbiol. 2023, 14, 1250483. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Akinosoglou, K.; Florou, E.; Kouriannidi, E.; Bogosian, A.; Tsachouridou, O.; Syrigos, K.N.; Gatselis, N.; Milionis, H.; Papanikolaou, I.C.; et al. Anakinra efficacy in COVID-19 pneumonia guided by soluble urokinase plasminogen activator receptor: Association with the inflammatory burden of the host. Int. J. Antimicrob. Agents 2025, 65, 107405. [Google Scholar] [CrossRef]
- Cioboata, R.; Nicolosu, D.; Streba, C.T.; Vasile, C.M.; Olteanu, M.; Nemes, A.; Gheorghe, A.; Calarasu, C.; Turcu, A.A. Post-COVID-19 Syndrome Based on Disease Form and Associated Comorbidities. Diagnostics 2022, 12, 2502. [Google Scholar] [CrossRef]
| Mechanism | Description | Relevance to COPD | Relevance to Severe COVID-19 |
|---|---|---|---|
| Increased IL-6 levels | IL-6 is a key pro-inflammatory cytokine involved in immune activation. | Elevated in COPD, especially in frequent exacerbators; associated with reduced lung function. | Correlated with disease severity and cytokine storms; higher serum levels linked to poor outcomes. |
| Elevated TNF-α levels | Promotes macrophage activation and lung tissue damage. | Increased in COPD; contributes to alveolar destruction and exacerbations. | Associated with ARDS and lung injury; a potential target for anti-TNF therapies. |
| Increased IL-8 (CXCL8) expression | Recruits neutrophils and amplifies inflammation. | Associated with exacerbations and lung function decline. | Enhances neutrophil infiltration, worsening alveolar injury. |
| Macrophage and dendritic cell activation | Innate immune cells regulate inflammatory responses. | Overactive macrophages produce cytokines and sustain inflammation. | Excessive activation linked to cytokine storms and multiorgan failure. |
| NET formation and neutrophilia | Neutrophils release extracellular traps that damage tissue. | Higher levels correlate with worse symptoms and exacerbation frequency. | Associated with disease severity and increased cfDNA and mortality. |
| Hematological alterations and coagulopathy | Inflammation affects coagulation and increases thrombosis risk. | Advanced COPD increases VTE risk and worsens the prognosis if thrombotic events occur. | Coagulopathy may result in pulmonary embolism and stroke; associated with increased mortality. |
| Oxidative stress and lung damage | ROS generation exacerbates inflammation and tissue injury. | Drives COPD progression and systemic inflammation. | Contributes to endothelial injury, pulmonary fibrosis, and cytokine-mediated damage. |
| Biomarker | Description | Relevance in COPD | Relevance in Severe COVID-19 |
|---|---|---|---|
| Erythropoietin (EPO) | Hormone produced by the kidneys to stimulate red blood cell (RBC) production in response to hypoxia. | Increased due to chronic hypoxia, leading to secondary polycythemia [59] | Variable: May be low or normal due to inflammation-related erythropoiesis [60,61] |
| Hemoglobin (Hb) and Hematocrit (Hct) | Measures of oxygen-carrying capacity in blood. | Increased in some COPD patients due to chronic hypoxia [62] | Decreased in severe COVID-19, often due to anemia of inflammation [63] |
| Hypoxia-Inducible Factor 1-alpha (HIF-1α) | A transcription factor activated by low oxygen levels; regulates cellular responses to hypoxia. | Upregulated, promoting erythropoiesis, angiogenesis, and glycolysis [64]. | Highly upregulated, contributing to immune activation, inflammation, and vascular remodeling [65]. |
| Reactive Oxygen Species (ROS) | Free radicals produced by mitochondria and immune cells during oxidative stress. | Increased, leading to oxidative damage in lung tissue [45]. | Increased, contributing to endothelial dysfunction and tissue injury [66]. |
| Malondialdehyde (MDA) | Malondialdehyde (MDA) | Elevated, reflecting cell membrane damage due to chronic inflammation [67]. | Elevated, linked to severe oxidative injury in the lung and vascular endothelium [68]. |
| Superoxide Dismutase (SOD) | An antioxidant enzyme that neutralizes superoxide radicals. | Increased, leading to higher oxidative stress and lung injury [69]. | Decreased, reducing protection against inflammation-induced ROS [70]. |
| Glutathione (GSH) | A major intracellular antioxidant that protects against oxidative damage. | Depleted, leading to chronic lung tissue damage [71]. | Depleted, impairing the immune response and increasing lung injury risk [72]. |
| 8-Hydroxy-2′-deoxyguanosine (8-OHdG) | A DNA oxidation marker, indicating oxidative DNA damage. | Increased, reflecting oxidative stress-induced genetic damage in lung cells [73]. | Increased, linked to severe systemic inflammation and endothelial dysfunction [74]. |
| Fibrinogen | An acute-phase protein involved in blood clot formation. | Elevated, contributing to hypercoagulability and increased thrombosis risk [75]. | Highly elevated, associated with coagulation disorders and microthrombosis [76]. |
| D-dimer | A fibrin degradation product and a marker of clot formation and breakdown. | Mildly elevated, especially in exacerbations or comorbid cardiovascular disease [77]. | Significantly elevated, indicating high thrombotic activity and a poor prognosis [78]. |
| Interleukin-6 (IL-6) | A pro-inflammatory cytokine involved in immune regulation and inflammation. | Increased, associated with disease severity and systemic inflammation [79]. | Markedly increased, linked to cytokine storms and respiratory failure [80]. |
| Myeloperoxidase (MPO) | An enzyme released by neutrophils and a marker of oxidative stress and inflammation. | Elevated, contributing to neutrophil-mediated lung damage [81]. | Elevated, associated with endothelial dysfunction and multi-organ injury [82]. |
| Therapeutic Strategy | Purpose | Advantages | Risks/Limitations | References |
|---|---|---|---|---|
| D-dimer monitoring | Assess thrombotic risk and predict severity | Early detection of coagulopathy and guides anticoagulation | False positives must be interpreted with other markers | [106,107,108] |
| Fibrinogen monitoring | Evaluate inflammation and coagulation status | Helps in risk stratification | Can be elevated due to multiple factors that are not always specific to COVID-19 | [109,110] |
| NLR assessment | Gauge severity of inflammation | Quick and cost-effective | Non-specific, influenced by other infections or stress responses | [111,112,113] |
| Anticoagulant Type | Mechanism | Benefits | Risks/Limitations | References |
|---|---|---|---|---|
| LMWH | Inhibits factor Xa and prevents clot formation | Reliable dosing; fewer drug interactions | Requires injection and costlier than DOACs | [123,124,125] |
| DOACs | Directly inhibit factor Xa or thrombin | Oral administration; no need for monitoring | Bleeding risk; contraindications in renal impairment | [125,126,127] |
| VKAs (e.g., warfarin) | Inhibits vitamin K-dependent clotting factors (II, VII, IX, X) | Effective for patients with mechanical valves or antiphospholipid syndrome; cost-effective | Requires regular INR monitoring; dietary restrictions; drug interactions | [122,128] |
| Immunomodulator | Mechanism | Benefits | Risks/Limitations | References |
|---|---|---|---|---|
| Corticosteroids | Reduce inflammatory cytokine release | Lower mortality and improved oxygenation | Increased infection risk and hyperglycemia | [134] |
| Tocilizumab | An IL-6 receptor blocker; suppresses inflammation | Reduces cytokine storm severity | May cause hepatotoxicity and secondary infections | [135,136] |
| Anakinra | Blocks IL-1 receptor and mitigates hyperinflammation | Beneficial in severe cases | Limited evidence and high cost | [137,138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mara, G.; Nini, G.; Cotoraci, C. Chronic Obstructive Pulmonary Disease and COVID-19: The Impact of Hematological Biomarkers on Disease Severity and Outcomes. J. Clin. Med. 2025, 14, 2765. https://doi.org/10.3390/jcm14082765
Mara G, Nini G, Cotoraci C. Chronic Obstructive Pulmonary Disease and COVID-19: The Impact of Hematological Biomarkers on Disease Severity and Outcomes. Journal of Clinical Medicine. 2025; 14(8):2765. https://doi.org/10.3390/jcm14082765
Chicago/Turabian StyleMara, Gabriela, Gheorghe Nini, and Coralia Cotoraci. 2025. "Chronic Obstructive Pulmonary Disease and COVID-19: The Impact of Hematological Biomarkers on Disease Severity and Outcomes" Journal of Clinical Medicine 14, no. 8: 2765. https://doi.org/10.3390/jcm14082765
APA StyleMara, G., Nini, G., & Cotoraci, C. (2025). Chronic Obstructive Pulmonary Disease and COVID-19: The Impact of Hematological Biomarkers on Disease Severity and Outcomes. Journal of Clinical Medicine, 14(8), 2765. https://doi.org/10.3390/jcm14082765






