Multimodal Imaging of Immune Checkpoint Inhibitor Myocarditis
Abstract
:1. Introduction
1.1. Risk Factors
1.2. Pathogenesis
1.3. Clinical Features
1.4. Biomarkers
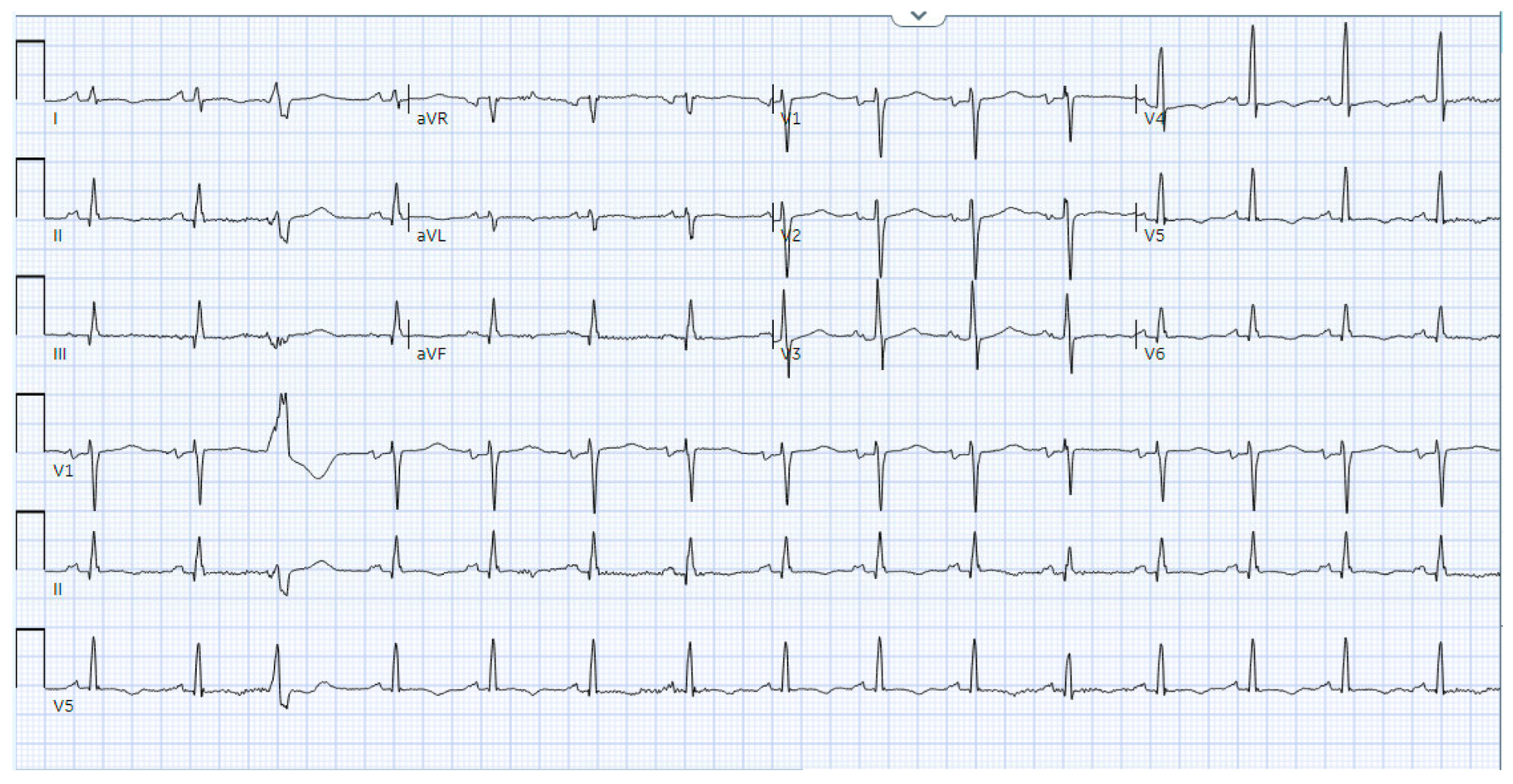
1.5. Electrocardiography
2. Multimodal Imaging
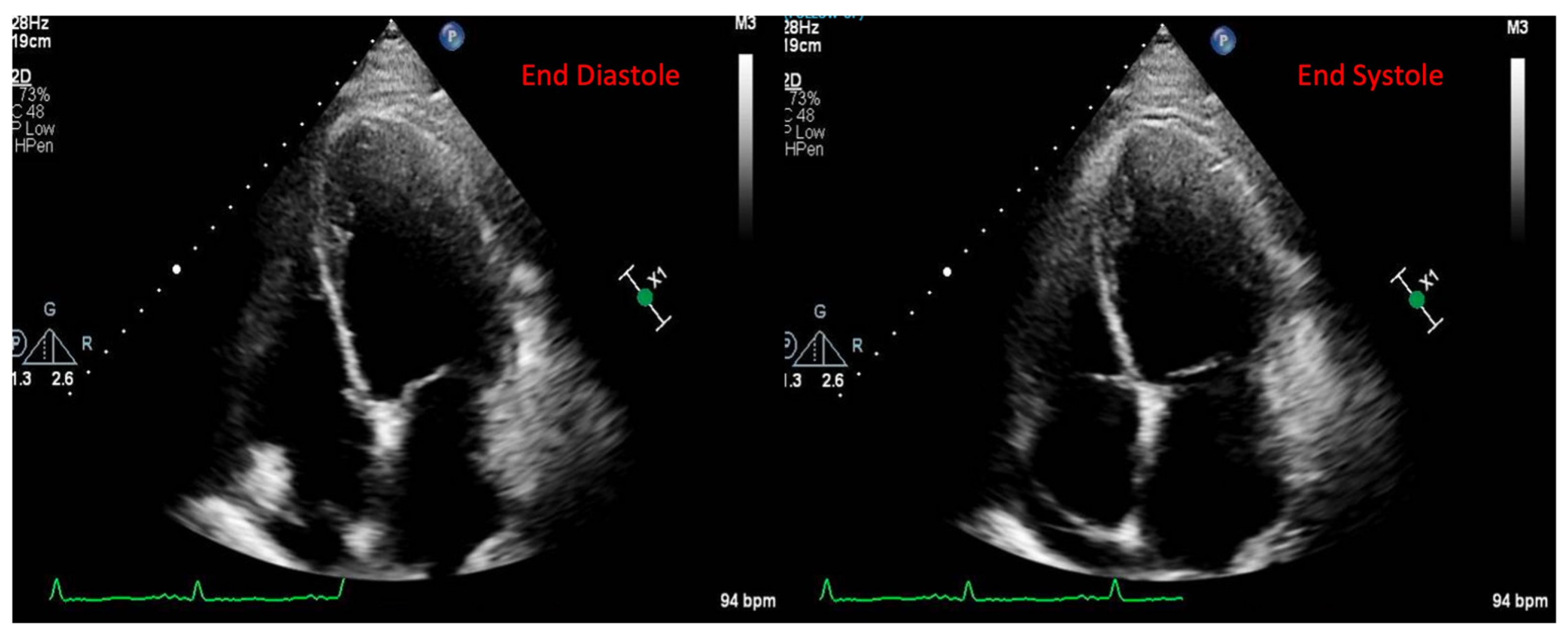
2.1. Echocardiography
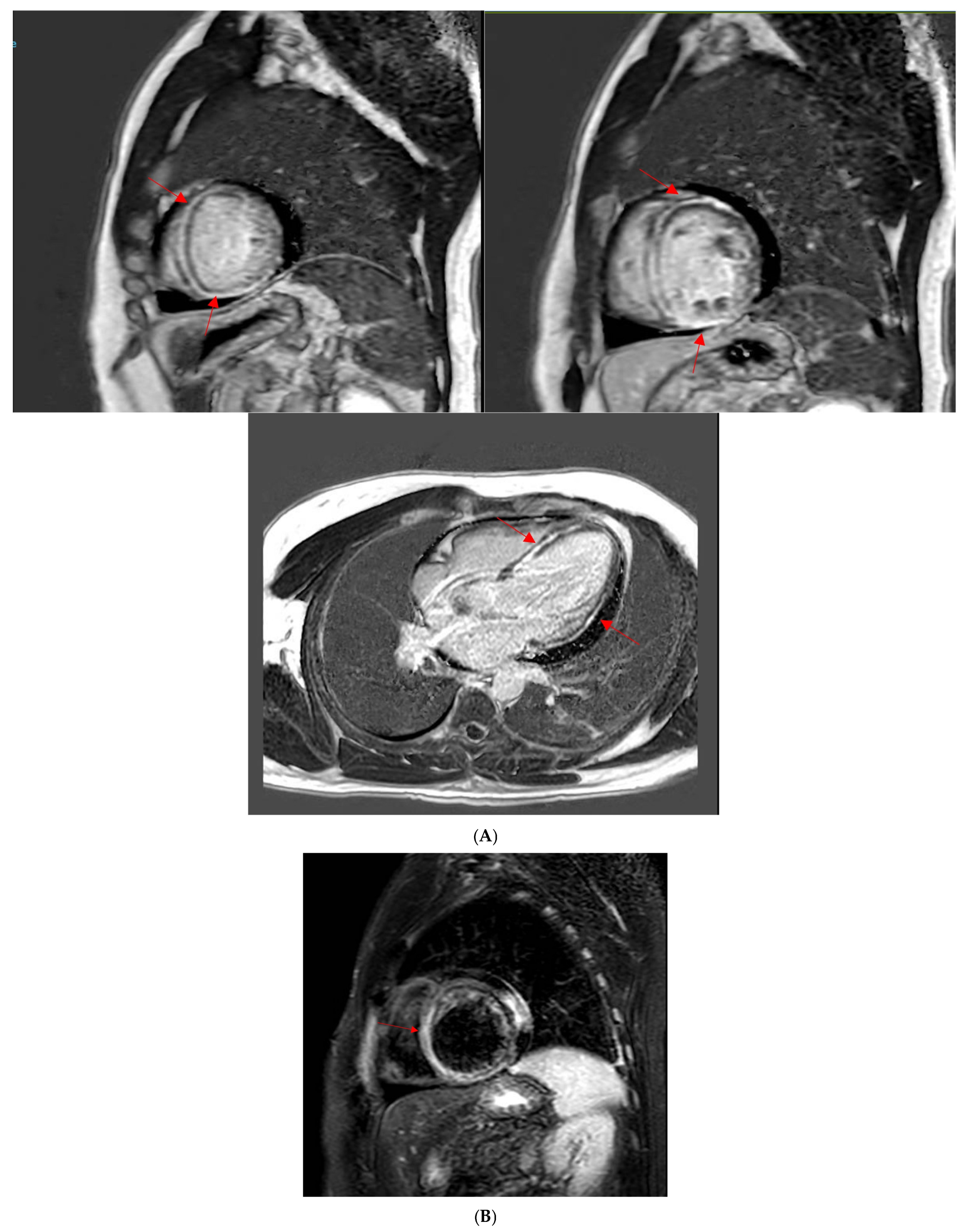
2.2. Cardiac Magnetic Resonance Imaging
2.3. Computed Tomography
2.4. Nuclear Imaging
2.5. Endomyocardial Biopsy
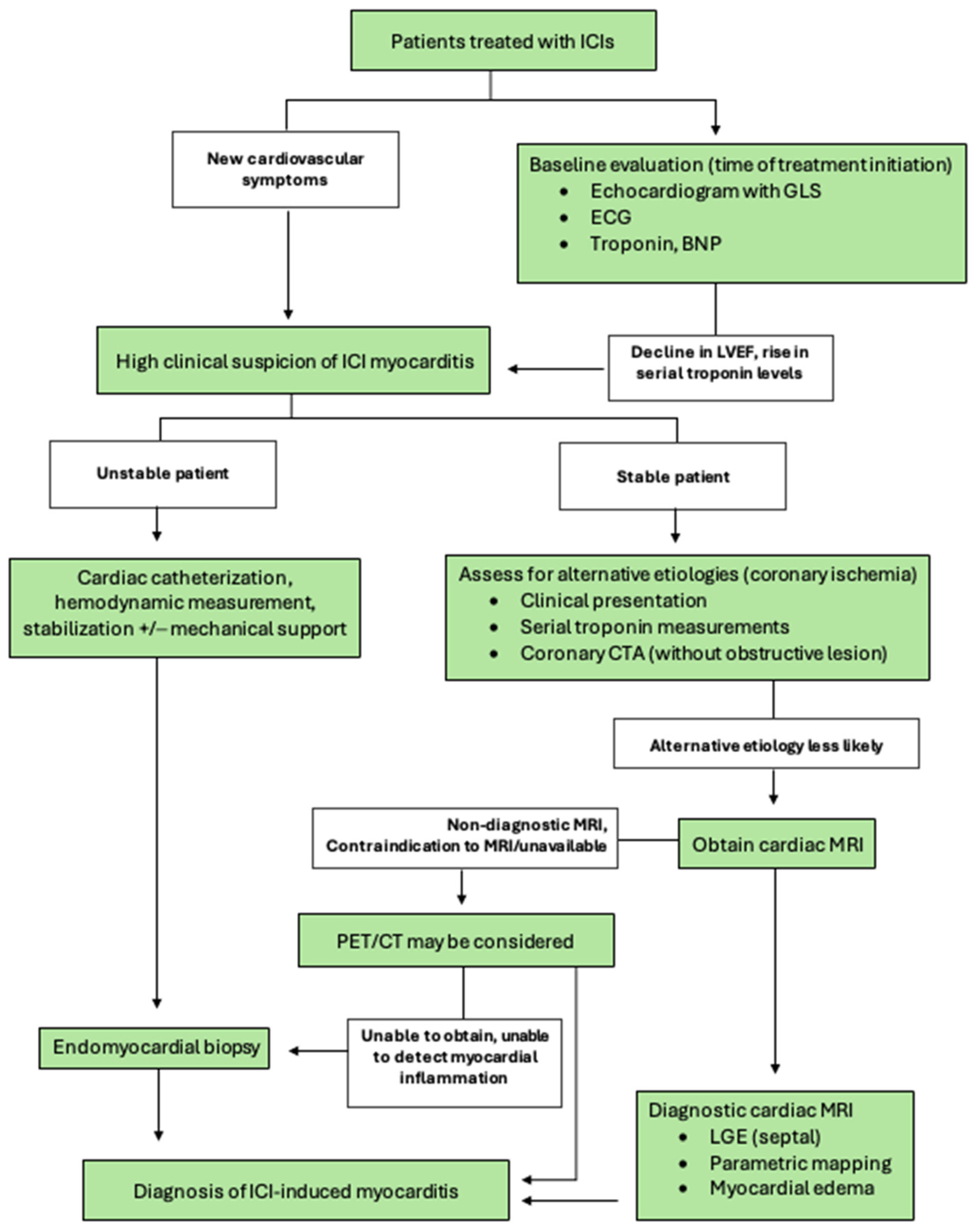
3. Diagnosis
3.1. Monitoring
3.2. Management of ICI Myocarditis
Clinical Case
4. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Tocchetti, C.G.; Farmakis, D.; Koop, Y.; Andres, M.S.; Couch, L.S.; Formisano, L.; Ciardiello, F.; Pane, F.; Au, L.; Emmerich, M.; et al. Cardiovascular toxicities of immune therapies for cancer—A scientific statement of the Heart Failure Association (HFA) of the ESC and the ESC Council of Cardio-Oncology. Eur. J. Heart Fail. 2024, 26, 2055–2076. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Haslam, A.; Olivier, T. Updated estimates of eligibility and response: Immune checkpoint inhibitors. J. Clin. Oncol. 2024, 42 (Suppl. S16), e14613. [Google Scholar] [CrossRef]
- Zhang, L.; Reynolds, K.L.; Lyon, A.R.; Palaskas, N.; Neilan, T.G. The Evolving Immunotherapy Landscape and the Epidemiology, Diagnosis, and Management of Cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncol. 2021, 3, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- Salem, J.E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Oren, O.; Yang, E.H.; Molina, J.R.; Bailey, K.R.; Blumenthal, R.S.; Kopecky, S.L. Cardiovascular Health and Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors. Am. J. Cardiol. 2020, 125, 1920–1926. [Google Scholar] [CrossRef]
- Braghieri, L.; Gharaibeh, A.; Nkashama, L.; Abushouk, A.; Abushawer, O.; Mehdizadeh-Shrifi, A.; Honnekeri, B.; Calabrese, C.; Menon, V.; Funchain, P.; et al. Long-term cardiovascular outcomes of immune checkpoint inhibitor-related myocarditis: A large single-centre analysis. ESC Heart Fail. 2024, 12, 1237–1245. [Google Scholar] [CrossRef]
- Escudier, M.; Cautela, J.; Malissen, N.; Ancedy, Y.; Orabona, M.; Pinto, J.; Monestier, S.; Grob, J.J.; Scemama, U.; Jacquier, A.; et al. Clinical Features, Management, and Outcomes of Immune Checkpoint Inhibitor-Related Cardiotoxicity. Circulation 2017, 136, 2085–2087. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Vasbinder, A.; Chen, Y.; Procureur, A.; Gradone, A.; Azam, T.U.; Perry, D.; Shadid, H.; Anderson, E.; Catalan, T.; Blakely, P.; et al. Biomarker Trends, Incidence, and Outcomes of Immune Checkpoint Inhibitor-Induced Myocarditis. JACC CardioOncol. 2022, 4, 689–700. [Google Scholar] [CrossRef]
- Itzhaki Ben Zadok, O.; Levi, A.; Divakaran, S.; Nohria, A. Severe vs Nonsevere Immune Checkpoint Inhibitor-Induced Myocarditis: Contemporary 1-Year Outcomes. JACC CardioOncol. 2023, 5, 732–744. [Google Scholar] [CrossRef]
- Vasbinder, A.; Anderson, E.; Catalan, T.; Ismail, A.; Banerjee, M.; Pizzo, I.; Machado, K.; Blakely, P.; Salem, J.E.; Hayek, S.S. Incidence of Immune Checkpoint Inhibitor-Induced Myocarditis During the COVID-19 Pandemic. J. Am. Heart Assoc. 2024, 13, e032667. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, T.; Du, Z.; Chen, S.; Li, Y.; Lv, Y.; Du, X.; Hu, Y.; Liu, Z. Prognosis of immune checkpoint inhibitor-related myocarditis: Retrospective experience of a single institution. Int. Immunopharmacol. 2024, 136, 112385. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, A.F.; Sayer, M.; Hamano, H.; Nagasaka, M.; Lee, B.J.; Doh, J.; Naqvi, A.; Nowrouzi, N.; Zamami, Y.; Patel, P.M. Incidence and survival outcomes of myocarditis and pericardial diseases associated with immune checkpoint inhibitor therapy. Cardio-Oncology 2025, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Zamami, Y.; Niimura, T.; Okada, N.; Koyama, T.; Fukushima, K.; Izawa-Ishizawa, Y.; Ishizawa, K. Factors Associated With Immune Checkpoint Inhibitor-Related Myocarditis. JAMA Oncol. 2019, 5, 1635–1637. [Google Scholar] [CrossRef]
- Otto, S.M.; Martinez, A.L.; Dains, J.E. Risk Factors for Immune Checkpoint Inhibitor-Related Myocarditis: An Integrative Review. J. Adv. Pr. Oncol. 2024, 15, 111–123. [Google Scholar] [CrossRef]
- Zlotoff, D.A.; Hassan, M.Z.O.; Zafar, A.; Alvi, R.M.; Awadalla, M.; Mahmood, S.S.; Zhang, L.; Chen, C.L.; Ederhy, S.; Barac, A.; et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J. Immunother. Cancer 2021, 9, e002007. [Google Scholar] [CrossRef]
- Awadalla, M.; Golden, D.L.A.; Mahmood, S.S.; Alvi, R.M.; Mercaldo, N.D.; Hassan, M.Z.O.; Banerji, D.; Rokicki, A.; Mulligan, C.; Murphy, S.P.T.; et al. Influenza vaccination and myocarditis among patients receiving immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 53. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Sobol, I.; Chen, C.L.; Mahmood, S.S.; Borczuk, A.C. Histopathologic Characterization of Myocarditis Associated With Immune Checkpoint Inhibitor Therapy. Arch. Pathol. Lab. Med. 2020, 144, 1392–1396. [Google Scholar] [CrossRef]
- Cihakova, D. T Cells and Macrophages Drive Pathogenesis of Immune Checkpoint Inhibitor Myocarditis. Circulation 2024, 149, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Won, T.; Kalinoski, H.M.; Wood, M.K.; Hughes, D.M.; Jaime, C.M.; Delgado, P.; Talor, M.V.; Lasrado, N.; Reddy, J.; Cihakova, D. Cardiac myosin-specific autoimmune T cells contribute to immune-checkpoint-inhibitor-associated myocarditis. Cell Rep. 2022, 41, 111611. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, M.L.; Meijers, W.C.; Screever, E.M.; Qin, J.; Carroll, M.G.; Sun, X.; Tannous, E.; Zhang, Y.; Sugiura, A.; Taylor, B.C.; et al. T cells specific for alpha-myosin drive immunotherapy-related myocarditis. Nature 2022, 611, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef]
- Moslehi, J.J.; Salem, J.E.; Sosman, J.A.; Lebrun-Vignes, B.; Johnson, D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018, 391, 933. [Google Scholar] [CrossRef]
- Norwood, T.G.; Westbrook, B.C.; Johnson, D.B.; Litovsky, S.H.; Terry, N.L.; McKee, S.B.; Gertler, A.S.; Moslehi, J.J.; Conry, R.M. Smoldering myocarditis following immune checkpoint blockade. J. Immunother. Cancer 2017, 5, 91. [Google Scholar] [CrossRef]
- Ang, E.; Mweempwa, A.; Heron, C.; Ahn, Y.; Rivalland, G.; Ha, L.Y.; Deva, S. Cardiac Troponin I and T in Checkpoint Inhibitor-associated Myositis and Myocarditis. J. Immunother. 2021, 44, 162–163. [Google Scholar] [CrossRef]
- Beaulieu, C.; Wu, K.Y.; Fuzery, A.K.; Raizman, J.E.; Tsui, A.K.Y.; Ye, C.; Basappa, N.S.; Gyenes, G.T.; Koshman, S.L. Elevated Troponin T in Immune-Checkpoint Inhibitor Myositis: A Case of Mistaken Myocarditis. JACC Case Rep. 2024, 29, 102462. [Google Scholar] [CrossRef]
- Barac, A.; Wadlow, R.C.; Deeken, J.F.; deFilippi, C. Cardiac Troponin I and T in ICI Myocarditis Screening, Diagnosis, and Prognosis. JACC CardioOncol. 2024, 6, 804–807. [Google Scholar] [CrossRef]
- Lehmann, L.H.; Heckmann, M.B.; Bailly, G.; Finke, D.; Procureur, A.; Power, J.R.; Stein, F.; Bretagne, M.; Ederhy, S.; Fenioux, C.; et al. Cardiomuscular Biomarkers in the Diagnosis and Prognostication of Immune Checkpoint Inhibitor Myocarditis. Circulation 2023, 148, 473–486. [Google Scholar] [CrossRef]
- Power, J.R.; Alexandre, J.; Choudhary, A.; Ozbay, B.; Hayek, S.; Asnani, A.; Tamura, Y.; Aras, M.; Cautela, J.; Thuny, F.; et al. Electrocardiographic Manifestations of Immune Checkpoint Inhibitor Myocarditis. Circulation 2021, 144, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Abboud, F.M. Ejection Fraction: Misunderstood and Overrated (Changing the Paradigm in Categorizing Heart Failure). Circulation 2017, 135, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Granato, A.; Bonanomi, A.; Rigamonti, E.; Lombardo, M. Influence of chest wall conformation on reproducibility of main echocardiographic indices of left ventricular systolic function. Minerva Cardiol. Angiol. 2024, 72, 111–124. [Google Scholar] [CrossRef]
- Awadalla, M.; Mahmood, S.; Groarke, J.; Liu, S.; Hassan, M.Z.O.; Murphy, S.P.T.; Jones-O’Connor, M.; Nohria, A.; Heinzerling, L.M.; Sullivan, R.J.; et al. Decreased global longitudinal strain with myocarditis from immune checkpoint inhibitors and occurrence of major adverse cardiac events. J. Am. Coll. Cardiol. 2019, 73, 1532. [Google Scholar] [CrossRef]
- Tamura, Y.; Tamura, Y.; Takemura, R.; Yamada, K.; Taniguchi, H.; Iwasawa, J.; Yada, H.; Kawamura, A. Longitudinal Strain and Troponin I Elevation in Patients Undergoing Immune Checkpoint Inhibitor Therapy. JACC CardioOncol. 2022, 4, 673–685. [Google Scholar] [CrossRef]
- Quinaglia, T.; Gongora, C.; Awadalla, M.; Hassan, M.Z.O.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Zhang, L.; Coelho-Filho, O.R.; Suero-Abreu, G.A.; et al. Global Circumferential and Radial Strain Among Patients With Immune Checkpoint Inhibitor Myocarditis. JACC. Cardiovasc. Imaging 2022, 15, 1883–1896. [Google Scholar] [CrossRef]
- Rini, B.I.; Moslehi, J.J.; Bonaca, M.; Schmidinger, M.; Albiges, L.; Choueiri, T.K.; Motzer, R.J.; Atkins, M.B.; Haanen, J.; Mariani, M.; et al. Prospective Cardiovascular Surveillance of Immune Checkpoint Inhibitor-Based Combination Therapy in Patients With Advanced Renal Cell Cancer: Data From the Phase III JAVELIN Renal 101 Trial. J. Clin. Oncol. 2022, 40, 1929–1938. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Zhang, L.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zlotoff, D.A.; Murphy, S.P.; Stone, J.R.; Golden, D.L.A.; et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur. Heart J. 2020, 41, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Aquaro, G.D.; Perfetti, M.; Camastra, G.; Monti, L.; Dellegrottaglie, S.; Moro, C.; Pepe, A.; Todiere, G.; Lanzillo, C.; Scatteia, A.; et al. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J. Am. Coll. Cardiol. 2017, 70, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Mahrholdt, H.; Wagner, A.; Deluigi, C.C.; Kispert, E.; Hager, S.; Meinhardt, G.; Vogelsberg, H.; Fritz, P.; Dippon, J.; Bock, C.T.; et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006, 114, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Cadour, F.; Cautela, J.; Rapacchi, S.; Varoquaux, A.; Habert, P.; Arnaud, F.; Jacquier, A.; Meilhac, A.; Paganelli, F.; Lalevée, N.; et al. Cardiac MRI Features and Prognostic Value in Immune Checkpoint Inhibitor-induced Myocarditis. Radiology 2022, 303, 512–521. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Zhang, L.; Zafar, A.; Drobni, Z.D.; Mahmood, S.S.; Cabral, M.; Awadalla, M.; Nohria, A.; Zlotoff, D.A.; Thuny, F.; et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients With Immune Checkpoint Inhibitor-Associated Myocarditis. J. Am. Coll. Cardiol. 2021, 77, 1503–1516. [Google Scholar] [CrossRef]
- Zhao, S.H.; Yun, H.; Chen, C.Z.; Chen, Y.Y.; Lin, J.Y.; Zeng, M.S.; Liu, T.S.; Pan, C.Z.; Jin, H. The prognostic value of global myocardium strain by CMR-feature tracking in immune checkpoint inhibitor-associated myocarditis. Eur. Radiol. 2022, 32, 7657–7667. [Google Scholar] [CrossRef]
- Wintersperger, B.J.; Calvillo-Argüelles, O.; Lheureux, S.; Houbois, C.P.; Spreafico, A.; Bedard, P.L.; Neilan, T.G.; Thavendiranathan, P. Immune checkpoint inhibitor-related myocarditis: An illustrative case series of applying the updated Cardiovascular Magnetic Resonance Lake Louise Criteria. Eur. Heart J. Case Rep. 2022, 6, ytab478. [Google Scholar] [CrossRef]
- Faron, A.; Isaak, A.; Mesropyan, N.; Reinert, M.; Schwab, K.; Sirokay, J.; Sprinkart, A.M.; Bauernfeind, F.G.; Dabir, D.; Pieper, C.C.; et al. Cardiac MRI Depicts Immune Checkpoint Inhibitor-induced Myocarditis: A Prospective Study. Radiology 2021, 301, 602–609. [Google Scholar] [CrossRef]
- Armenian, S.H.; Lacchetti, C.; Barac, A.; Carver, J.; Constine, L.S.; Denduluri, N.; Dent, S.; Douglas, P.S.; Durand, J.B.; Ewer, M.; et al. Prevention and Monitoring of Cardiac Dysfunction in Survivors of Adult Cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2017, 35, 893–911. [Google Scholar] [CrossRef]
- Lopez-Mattei, J.; Yang, E.H.; Baldassarre, L.A.; Agha, A.; Blankstein, R.; Choi, A.D.; Chen, M.Y.; Meyersohn, N.; Daly, R.; Slim, A.; et al. Cardiac computed tomographic imaging in cardio-oncology: An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). Endorsed by the International Cardio-Oncology Society (ICOS). J. Cardiovasc. Comput. Tomogr. 2023, 17, 66–83. [Google Scholar] [CrossRef]
- Suero-Abreu, G.A.; Zanni, M.V.; Neilan, T.G. Atherosclerosis With Immune Checkpoint Inhibitor Therapy: Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 598–615. [Google Scholar] [CrossRef] [PubMed]
- Addison, D.; Neilan, T.G.; Barac, A.; Scherrer-Crosbie, M.; Okwuosa, T.M.; Plana, J.C.; Reding, K.W.; Taqueti, V.R.; Yang, E.H.; Zaha, V.G. Cardiovascular Imaging in Contemporary Cardio-Oncology: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, L.A.; Ganatra, S.; Lopez-Mattei, J.; Yang, E.H.; Zaha, V.G.; Wong, T.C.; Ayoub, C.; DeCara, J.M.; Dent, S.; Deswal, A.; et al. Advances in Multimodality Imaging in Cardio-Oncology: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 1560–1578. [Google Scholar] [CrossRef] [PubMed]
- Nensa, F.; Kloth, J.; Tezgah, E.; Poeppel, T.D.; Heusch, P.; Goebel, J.; Nassenstein, K.; Schlosser, T. Feasibility of FDG-PET in myocarditis: Comparison to CMR using integrated PET/MRI. J. Nucl. Cardiol. 2018, 25, 785–794. [Google Scholar] [CrossRef]
- Ederhy, S.; Devos, P.; Pinna, B.; Funck-Brentano, E.; Abbar, B.; Fenioux, C.; Cohen, A.A.; Moslehi, J.; Bretagne, M.; Allenbach, Y.; et al. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography imaging for the diagnosis of immune checkpoint inhibitor-associated myocarditis. Arch. Cardiovasc. Dis. 2022, 115, 114–116. [Google Scholar] [CrossRef]
- Tong, J.; Vogiatzakis, N.; Andres, M.S.; Senechal, I.; Badr, A.; Ramalingam, S.; Rosen, S.D.; Lyon, A.R.; Nazir, M.S. Complementary use of cardiac magnetic resonance and 18 F-FDG positron emission tomography imaging in suspected immune checkpoint inhibitor myocarditis. Cardio-Oncology 2024, 10, 53. [Google Scholar] [CrossRef]
- Boughdad, S.; Latifyan, S.; Fenwick, C.; Bouchaab, H.; Suffiotti, M.; Moslehi, J.J.; Salem, J.E.; Schaefer, N.; Nicod-Lalonde, M.; Costes, J.; et al. (68)Ga-DOTATOC PET/CT to detect immune checkpoint inhibitor-related myocarditis. J. Immunother. Cancer 2021, 9, e003594. [Google Scholar] [CrossRef]
- Finke, D.; Heckmann, M.B.; Herpel, E.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Early Detection of Checkpoint Inhibitor-Associated Myocarditis Using (68)Ga-FAPI PET/CT. Front. Cardiovasc. Med. 2021, 8, 614997. [Google Scholar] [CrossRef]
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.T.; Fenoglio, J.J., Jr.; Olsen, E.G.; Schoen, F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987, 1, 3–14. [Google Scholar]
- Baughman, K.L. Diagnosis of myocarditis: Death of Dallas criteria. Circulation 2006, 113, 593–595. [Google Scholar] [CrossRef]
- Baandrup, U.; Florio, R.A.; Olsen, E.G.J. Do endomyocardial biopsies represent the morphology of the rest of the myocardium?: A quantitative light microscopic study of single v. multiple biopsies with the King’s bioptome. Eur. Heart J. 1982, 3, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.L.; Segura, A.; Lelenwa, L.; Siddiqui, B.A.; Subudhi, S.K.; Lopez-Mattei, J.; Durand, J.B.; Deswal, A.; Zhao, B.; Maximilian Buja, L.; et al. Immune checkpoint inhibitor myocarditis: Elucidating the spectrum of disease through endomyocardial biopsy. Eur. J. Heart Fail. 2021, 23, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef] [PubMed]
- Bonaca, M.P.; Olenchock, B.A.; Salem, J.E.; Wiviott, S.D.; Ederhy, S.; Cohen, A.; Stewart, G.C.; Choueiri, T.K.; Di Carli, M.; Allenbach, Y.; et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation 2019, 140, 80–91. [Google Scholar] [CrossRef]
- Waliany, S.; Neal, J.W.; Reddy, S.; Wakelee, H.; Shah, S.A.; Srinivas, S.; Padda, S.K.; Fan, A.C.; Colevas, A.D.; Wu, S.M.; et al. Myocarditis Surveillance with High-Sensitivity Troponin I During Cancer Treatment with Immune Checkpoint Inhibitors. JACC CardioOncol. 2021, 3, 137–139. [Google Scholar] [CrossRef]
- Berg, P.F.v.d.; Bracun, V.; Noordman, M.; Meer, P.v.d.; Shi, C.; Oosting, S.F.; Aboumsallem, J.P.; Wit, S.d.; Meijers, W.C.; Jalving, M.; et al. Elevations of Cardiac Troponin in Patients Receiving Immune Checkpoint Inhibitors. JACC Adv. 2024, 3, 101375. [Google Scholar] [CrossRef]
- Thuny, F.; Alexandre, J.; Salem, J.-E.; Mirabel, M.; Dolladille, C.; Cohen-Solal, A.; Cohen, A.; Ederhy, S.; Cautela, J. Management of Immune Checkpoint Inhibitor–Induced Myocarditis. JACC CardioOncol. 2021, 3, 157–161. [Google Scholar] [CrossRef]
- Zhang, L.; Zlotoff, D.A.; Awadalla, M.; Mahmood, S.S.; Nohria, A.; Hassan, M.Z.O.; Thuny, F.; Zubiri, L.; Chen, C.L.; Sullivan, R.J.; et al. Major Adverse Cardiovascular Events and the Timing and Dose of Corticosteroids in Immune Checkpoint Inhibitor-Associated Myocarditis. Circulation 2020, 141, 2031–2034. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Nielsen, D.L.; Juhl, C.B.; Nielsen, O.H.; Chen, I.M.; Herrmann, J. Immune Checkpoint Inhibitor-Induced Cardiotoxicity: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 1390–1399. [Google Scholar] [CrossRef]
- Katsumoto, T.R.; Wilson, K.L.; Giri, V.K.; Zhu, H.; Anand, S.; Ramchandran, K.J.; Martin, B.A.; Yunce, M.; Muppidi, S. Plasma exchange for severe immune-related adverse events from checkpoint inhibitors: An early window of opportunity? Immunother. Adv. 2022, 2, ltac012. [Google Scholar] [CrossRef] [PubMed]
- Heemelaar, J.C.; Louisa, M.; Neilan, T.G. Treatment of Immune Checkpoint Inhibitor-associated Myocarditis. J. Cardiovasc. Pharmacol. 2024, 83, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.; Salem, J.-E. Immune Checkpoint Inhibitor Myocarditis Treatment Strategies and Future Directions. JACC CardioOncol. 2022, 4, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Allenbach, Y.; Vozy, A.; Brechot, N.; Johnson, D.B.; Moslehi, J.J.; Kerneis, M. Abatacept for Severe Immune Checkpoint Inhibitor-Associated Myocarditis. N. Engl. J. Med. 2019, 380, 2377–2379. [Google Scholar] [CrossRef]
- Wang, C.; Lin, J.; Wang, Y.; Hsi, D.H.; Chen, J.; Liu, T.; Zhou, Y.; Ren, Z.; Zeng, Z.; Cheng, L.; et al. Case Series of Steroid-Resistant Immune Checkpoint Inhibitor Associated Myocarditis: A Comparative Analysis of Corticosteroid and Tofacitinib Treatment. Front. Pharmacol. 2021, 12, 770631. [Google Scholar] [CrossRef]
- Salem, J.E.; Bretagne, M.; Abbar, B.; Leonard-Louis, S.; Ederhy, S.; Redheuil, A.; Boussouar, S.; Nguyen, L.S.; Procureur, A.; Stein, F.; et al. Abatacept/Ruxolitinib and Screening for Concomitant Respiratory Muscle Failure to Mitigate Fatality of Immune-Checkpoint Inhibitor Myocarditis. Cancer Discov. 2023, 13, 1100–1115. [Google Scholar] [CrossRef]

| Study Year and Author | Cohort Year | Cohort Size (# of Patients) | Prevalence of ICI Myocarditis |
|---|---|---|---|
| 2016 Johnson et al. [9] | Up to 2016 | 20,594 | 0.09% |
| 2018 Mahmood et al. [4] | 2013–2017 | 964 | 1.14% |
| 2018 Salem et al. [5] | 2008–2018 | 31,321 | 0.39% |
| 2020 Oren et al. [6] | 2010–2019 | 3326 | 0.32% |
| 2022 Vasbinder at al. [10] | 2014–2021 | 2606 | 1.04% |
| 2023 Zadok et al. [11] | 2015–2022 | 10,046 | 1.2% |
| 2024 Bragheiri et al. [7] | 2011–2019 | 4195 | 1.8% |
| 2024 Vasbinder et al. [12] | 2014–2023 | 4056 | 0.8% |
| 2024 Qin et al. [13] | 2018–2023 | 8875 | 0.35% |
| 2025 Ozaki et al. [14] | 2011–2022 | 88,928 | 0.48% |
| IC-OS 2021 Consensus Criteria for the Diagnosis of ICI-rM | ||
|---|---|---|
| Pathological diagnosis | Multifocal inflammatory cell infiltrates with overt cardiomyocyte loss on light microscopy of cardiac tissue samples | |
| Clinical diagnosis | After excluding ACS or acute infectious myocarditis: cTn elevation + 1 major criteria or cTn elevation + 2 minor criteria | Major:
|
| Modifiers of severity | ||
| Severe | Hemodynamic instability, HF with or without mechanical ventilation, complete or high-grade heart block, ventricular arrhythmia | |
| Non-severe | Symptomatic but hemodynamically and electrically stable, may have a reduced LVEF, no features of severe disease | |
| Smoldering | Incidentally diagnosed myocarditis without any clinical signs or symptoms | |
| Steroid Refractory | Non-resolving or worsening myocarditis (clinical worsening or a persistent troponin elevation after the exclusion of other etiologies) despite high-dose methylprednisolone treatment | |
| Recovery from Myocarditis | ||
| Complete Recovery | Complete resolution of symptoms, normalization of biomarkers, and recovery of LVEF after the discontinuation of immunosuppression; CMR may show LGE or elevated T1 due to fibrosis, but no edema | |
| Recovering | Ongoing improvement without normalization in signs, symptoms, biomarkers, and imaging parameters while on tapering doses of immunosuppressants | |
| Time Period or Disease Grade | NCCN Guidelines | SITC Guidelines | ASCO Guidelines | ESMO Guidelines | 2022 ESC Guidelines |
|---|---|---|---|---|---|
| Baseline | Consider ECG; individualized assessment in consultation with cardiology, as indicated | No recommendation | No recommendation; no clear evidence regarding the efficacy of routine baseline ECG and troponin level measurement; some centers perform baseline testing | Heart rate, blood pressure, ECG, chest radiograph, TTE and levels of troponin I and/or T, CK, NT-pro-BNP; blood electrolytes | Cardiovascular assessment, ECG, TTE (high risk: dual ICI, combination of ICIs and cardiotoxic therapy); non cardiovascular events, prior cardiovascular disease, cancer therapy-related cardiac dysfunction, consider TTE in all patients, cardiac troponin, natriuretic peptides (BNP and NT-proBNP) |
| Monitoring | Consider periodic testing for patients with abnormal baseline or symptoms | No recommendation | No recommendation; no clear evidence regarding the efficacy of routine serial ECG and troponin level measurements; some centers conduct testing during the initial period of therapy | Repeat before each ICI cycle, measurement of levels of troponin T, NT-pro-BNP, CK, troponin I | ECG, cardiac troponin level measurements in cycles 1,2,and 4 and then every 3 cycles of ICIs; cardiovascular assessment every 3–6 mo for >12 mo: cardiovascular assessment; ECG; measurement of cardiac troponin and natriuretic peptides (BNP and NT-proBNP) levels |
| Assessment | Alternative reasons for elevation should be ruled out: inflammatory biomarkers, erythrocyte sedimentation rate, levels of C-reactive protein, WBC counts, echocardiography, cardiac MRI; severe symptoms: consider cardiac biopsy | Hospital attendance and consultation with a cardiologist; ECG; measurement of troponin levels, cardiac MRI (with or without right heart catheterization and biopsy) | Early cardiology involvement: ECG, continuous monitoring, measurement of troponin and CK levels; alternative reasons for elevation should be ruled out; echocardiography and cardiac MRI (preferred); guided by cardiology; stress test and catheterization and biopsy if warranted | Clinical ECG biomarker, echocardiography, cardiac MRI (if MRI is not available, cardiac CT or cardiac PET-CT recommended), preferentially with gallium-68-DOTATOC | NA |
| Grade 1 | No recommendation | Suspected ICI-induced myocarditis; methylprednisolone 1000 mg/d, for 3–5 d as soon as possible when diagnosis is likely; permanent discontinuation of ICI should be considered.; admit to coronary care unit; if no response within 24 h consider ATG, MMF, abatacept, alemtuzumab; caution is advised against infliximab; consider transient pacemaker. | Hold ICI if the troponin level is above the upper limit of normal; control troponin level 6 h later; resuming ICI if troponin level normalizes may be considered | Discontinue ICI; first line methylprednisolone, 500–1000 mg for 3 days or until clinically stable; second line MMF or tocilizumab; third line ATG, alemtuzumab, or abatacept | NA |
| Grade 2 | No recommendation | Discontinue ICI within 24 h; initiate prednisone 1–2 mg/kg/d; admit for cardiology consultation; consider immediate transfer to coronary care unit for patients with troponin or conduction abnormalities; consider pacemaker for new conduction delay; for patients with a high clinical suspicion, treatment should be offered empirically; in the absence of immediate response to high dose corticosteroids, administer methylprednisolone, 1000 mg/d, and add MMF, ATG, or infliximab (contraindicated I patients with moderate to severe HF); consider abatacept or alemtuzumab | NA | ||
| Grades 3 and 4 | Discontinue ICI; consider methylprednisolone, 1000 mg/d, for 3–5 d; without improvement within 24 h, consider transient pacemaker for arrhythmia; consider adding ATG, infliximab (contraindicated in patient with moderate to severe HF), IVIG, MMF; intensive care unit-level monitoring: may be used: alemtuzumab, abatacept | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Dave, K.; Garcia, M.J.; Gongora, C.A.; Travin, M.I.; Zhang, L. Multimodal Imaging of Immune Checkpoint Inhibitor Myocarditis. J. Clin. Med. 2025, 14, 2850. https://doi.org/10.3390/jcm14082850
Patel S, Dave K, Garcia MJ, Gongora CA, Travin MI, Zhang L. Multimodal Imaging of Immune Checkpoint Inhibitor Myocarditis. Journal of Clinical Medicine. 2025; 14(8):2850. https://doi.org/10.3390/jcm14082850
Chicago/Turabian StylePatel, Shreyans, Kartikeya Dave, Mario J. Garcia, Carlos A. Gongora, Mark I. Travin, and Lili Zhang. 2025. "Multimodal Imaging of Immune Checkpoint Inhibitor Myocarditis" Journal of Clinical Medicine 14, no. 8: 2850. https://doi.org/10.3390/jcm14082850
APA StylePatel, S., Dave, K., Garcia, M. J., Gongora, C. A., Travin, M. I., & Zhang, L. (2025). Multimodal Imaging of Immune Checkpoint Inhibitor Myocarditis. Journal of Clinical Medicine, 14(8), 2850. https://doi.org/10.3390/jcm14082850






