A Practical Approach to Multimodality Imaging in Hypertrophic Cardiomyopathy
Abstract
1. Introduction
2. Diagnostic Challenges and Imaging Targets in HCM
2.1. Left Ventricular Hypertrophy and Morphology
2.2. Systolic Function and Myocardial Deformation
2.3. Diastolic Dysfunction
2.4. Apical Aneurysm
2.5. Left Ventricular Outflow Tract Obstruction
2.6. Abnormalities in Mitral Valve Apparatus and Mitral Regurgitation
2.7. Myocardial Ischemia and Microvascular Dysfunction
2.8. Myocardial Fibrosis
3. Multimodality Imaging in the Evaluation of HCM
3.1. Echocardiography
3.2. Stress Echocardiography
3.3. Cardiac Magnetic Resonance Imaging
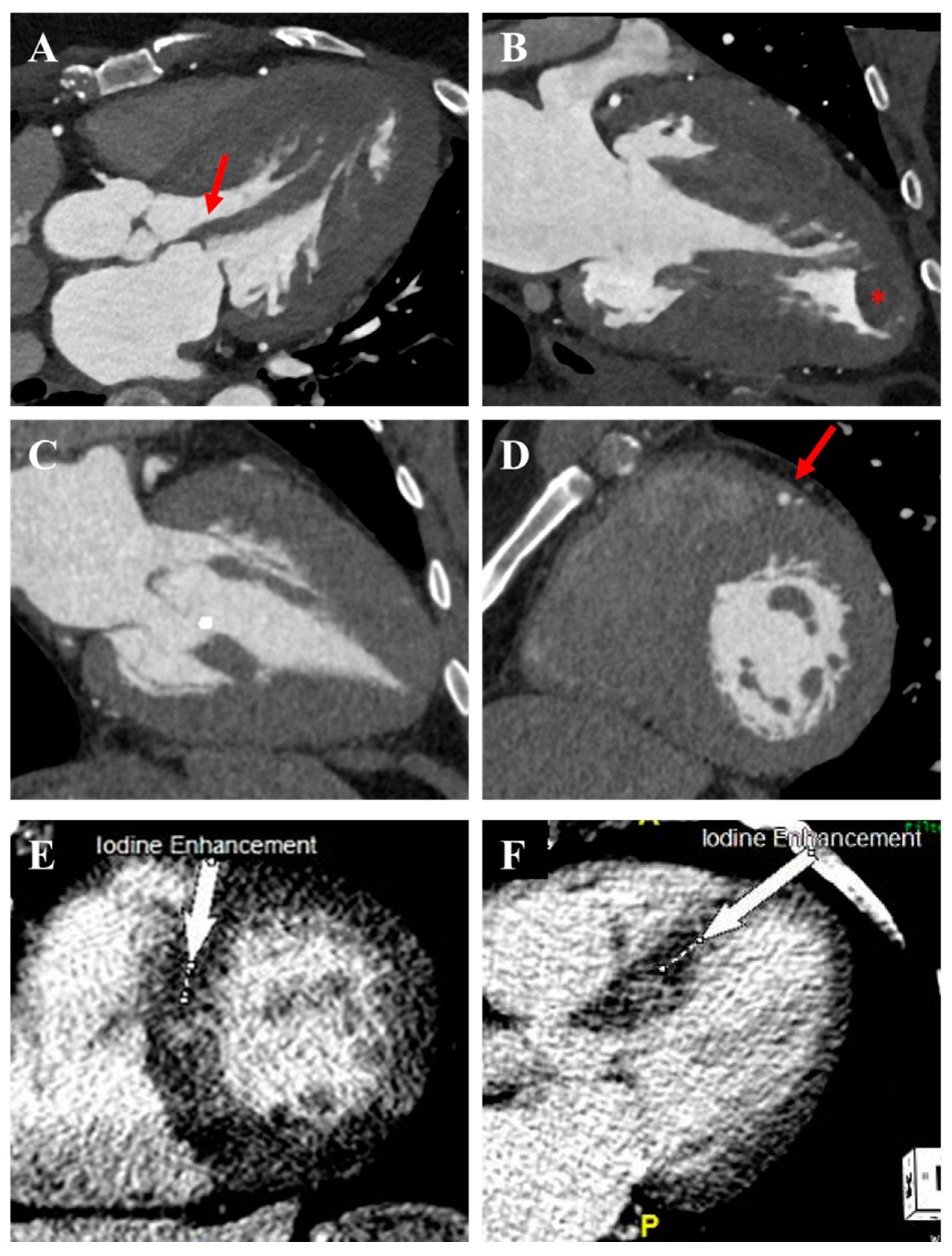
3.4. Cardiac Computed Tomography
3.5. Nuclear Myocardial Perfusion Imaging
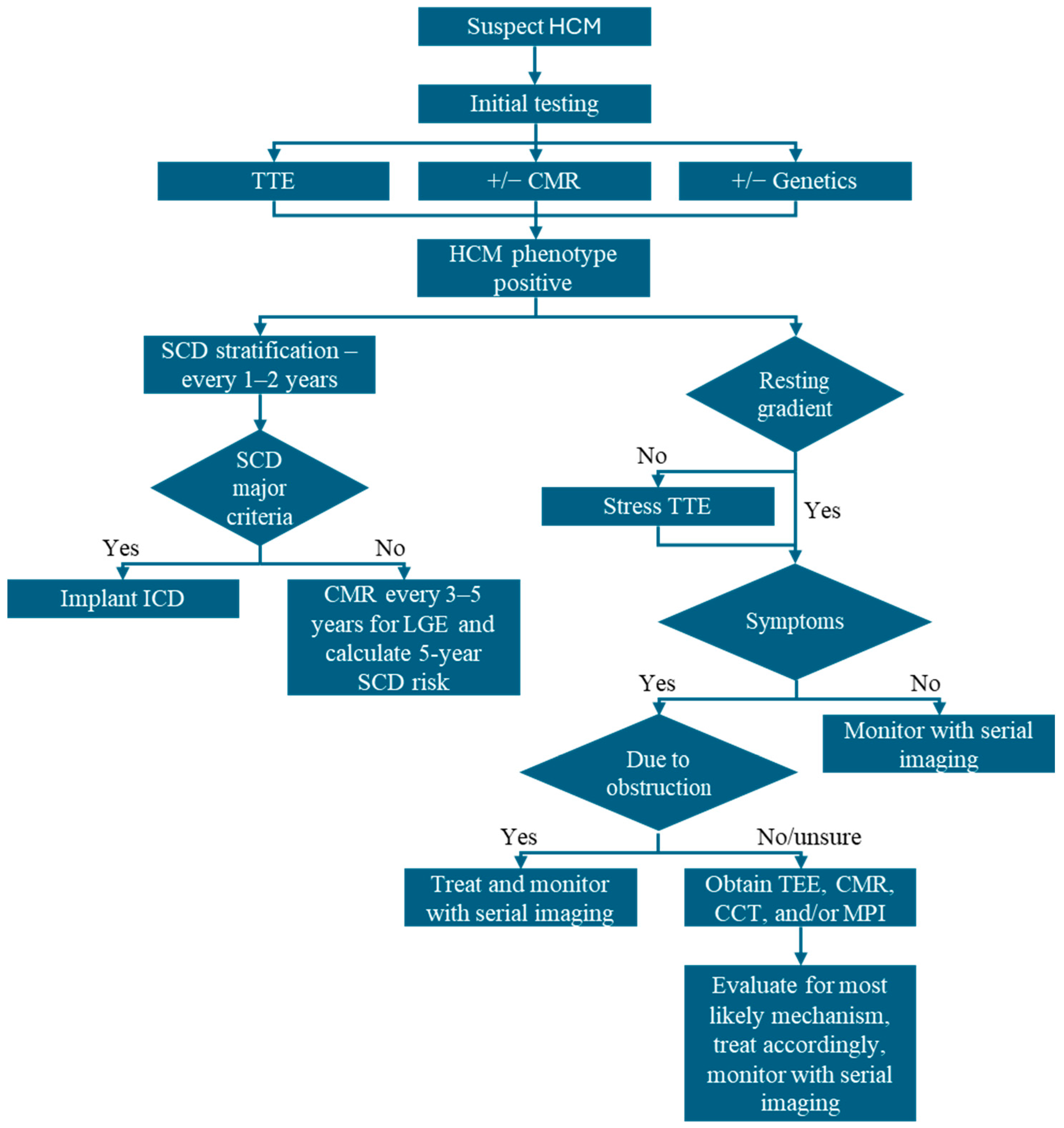
4. Imaging-Guided Management of HCM
4.1. Diagnosis and Classification
4.2. Symptom Management and Treatment of LVOTO
4.3. Risk Stratification for SCD and ICD Implantation
4.4. Assess Response to Therapy and Reverse Remodeling
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagueh, S.F.; Phelan, D.; Abraham, T.; Armour, A.; Desai, M.Y.; Dragulescu, A.; Gilliland, Y.; Lester, S.J.; Maldonado, Y.; Mohiddin, S.; et al. Recommendations for Multimodality Cardiovascular Imaging of Patients with Hypertrophic Cardiomyopathy: An Update from the American Society of Echocardiography, in Collaboration with the American Society of Nuclear Cardiology, the Society for Cardiovascular Magnetic Resonance, and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2022, 35, 533–569. [Google Scholar] [PubMed]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Hellawell, J.L.; Lucove, J.C.; Farzaneh-Far, R.; Olivotto, I. Occurrence of Clinically Diagnosed Hypertrophic Cardiomyopathy in the United States. Am. J. Cardiol. 2016, 117, 1651–1654. [Google Scholar] [CrossRef]
- Maron, B.J. Clinical Course and Management of Hypertrophic Cardiomyopathy. N. Engl. J. Med. 2018, 379, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Owens, A.; Geske, J.B.; Wolski, K.; Naidu, S.S.; Smedira, N.G.; Cremer, P.C.; Schaff, H.; McErlean, E.; Sewell, C.; et al. Myosin Inhibition in Patients with Obstructive Hypertrophic Cardiomyopathy Referred for Septal Reduction Therapy. J. Am. Coll. Cardiol. 2022, 80, 95–108. [Google Scholar] [CrossRef]
- Alashi, A.; Desai, R.M.; Khullar, T.; Hodges, K.; Rodriguez, E.R.; Tan, C.; Popovic, Z.B.; Thamilarasan, M.; Wierup, P.; Lever, H.M.; et al. Different Histopathologic Diagnoses in Patients with Clinically Diagnosed Hypertrophic Cardiomyopathy After Surgical Myectomy. Circulation 2019, 140, 344–346. [Google Scholar] [CrossRef]
- Goldie, F.C.; Lee, M.M.Y.; Coats, C.J.; Nordin, S. Advances in Multimodality Imaging in Hypertrophic Cardiomyopathy. J. Clin. Med. 2024, 13, 842. [Google Scholar] [CrossRef]
- Shiwani, H.; Davies, R.H.; Topriceanu, C.C.; Ditaranto, R.; Owens, A.; Raman, B.; Augusto, J.; Hughes, R.K.; Torlasco, C.; Dowsing, B.; et al. Demographic-Based Personalized Left Ventricular Hypertrophy Thresholds for Hypertrophic Cardiomyopathy Diagnosis. J. Am. Coll. Cardiol. 2025, 85, 685–695. [Google Scholar] [CrossRef]
- Hughes, R.K.; Shiwani, H.; Rosmini, S.; Augusto, J.B.; Burke, L.; Jiang, Y.; Pierce, I.; Joy, G.; Castelletti, S.; Orini, M.; et al. Improved Diagnostic Criteria for Apical Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2024, 17, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Klues, H.G.; Schiffers, A.; Maron, B.J. Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: Morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients. J. Am. Coll. Cardiol. 1995, 26, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Bos, J.M.; Towbin, J.A.; Ackerman, M.J. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 201–211. [Google Scholar] [CrossRef]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Hypertrophic Cardiomyopathy with Left Ventricular Systolic Dysfunction: Insights from the SHaRe Registry. Circulation 2020, 141, 1371–1383. [Google Scholar] [CrossRef]
- Marstrand, P.; Han, L.; Day, S.M.; Olivotto, I.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Wittekind, S.G.; Helms, A.; Saberi, S.; et al. Exercise response in hypertrophic cardiomyopathy: Blunted left ventricular deformational and twisting reserve with altered systolic-diastolic coupling. Circ. Cardiovasc. Imaging 2012, 5, 324–332. [Google Scholar]
- Kubo, T.; Gimeno, J.R.; Bahl, A.; Steffensen, U.; Steffensen, M.; Osman, E.; Thaman, R.; Mogensen, J.; Elliott, P.M.; Doi, Y.; et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J. Am. Coll. Cardiol. 2007, 49, 2419–2426. [Google Scholar] [CrossRef]
- Garcia, M.J. Multimodality Cardiovascular Imaging; Elsevier: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Lee, D.Z.J.; Montazeri, M.; Bataiosu, R.; Hoss, S.; Adler, A.; Nguyen, E.T.; Rakowski, H.; Chan, R.H. Clinical Characteristics and Prognostic Importance of Left Ventricular Apical Aneurysms in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2022, 15, 1696–1711. [Google Scholar] [CrossRef]
- Elliott, P.M.; Gimeno, J.R.; Tome, M.T.; Shah, J.; Ward, D.; Thaman, R.; Mogensen, J.; McKenna, W.J. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur. Heart J. 2006, 27, 1933–1941. [Google Scholar] [CrossRef]
- Allen, R.D.; Edwards, W.D.; Tazelaar, H.D.; Danielson, G.K. Surgical pathology of subaortic septal myectomy not associated with hypertrophic cardiomyopathy: A study of 98 cases (1996–2000). Cardiovasc. Pathol. 2003, 12, 207–215. [Google Scholar] [CrossRef]
- Minami, Y.; Kajimoto, K.; Terajima, Y.; Yashiro, B.; Okayama, D.; Haruki, S.; Nakajima, T.; Kawashiro, N.; Kawana, M.; Hagiwara, N. Clinical implications of midventricular obstruction in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2011, 57, 2346–2355. [Google Scholar] [CrossRef]
- Hughes, R.K.; Augusto, J.B.; Knott, K.; Davies, R.; Shiwani, H.; Seraphim, A.; Malcolmson, J.W.; Khoury, S.; Joy, G.; Mohiddin, S.; et al. Apical Ischemia Is a Universal Feature of Apical Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2023, 16, e014907. [Google Scholar] [CrossRef] [PubMed]
- Raphael, C.E.; Cooper, R.; Parker, K.H.; Collinson, J.; Vassiliou, V.; Pennell, D.J.; de Silva, R.; Hsu, L.Y.; Greve, A.M.; Nijjer, S.; et al. Mechanisms of Myocardial Ischemia in Hypertrophic Cardiomyopathy: Insights from Wave Intensity Analysis and Magnetic Resonance. J. Am. Coll. Cardiol. 2016, 68, 1651–1660. [Google Scholar] [CrossRef]
- Schlittler, M.; Pramstaller, P.P.; Rossini, A.; De Bortoli, M. Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Perspective from Fibroblasts. Int. J. Mol. Sci. 2023, 24, 14845. [Google Scholar] [CrossRef] [PubMed]
- Kiaos, A.; Daskalopoulos, G.N.; Kamperidis, V.; Ziakas, A.; Efthimiadis, G.; Karamitsos, T.D. Quantitative Late Gadolinium Enhancement Cardiac Magnetic Resonance and Sudden Death in Hypertrophic Cardiomyopathy: A Meta-Analysis. JACC Cardiovasc. Imaging 2024, 17, 489–497. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Schwammenthal, E.; Hagege, A.A.; Levine, R.A. Does the Flow Know? Mitral Regurgitant Jet Direction and Need for Valve Repair in Hypertrophic Obstructive Cardiomyopathy. J. Am. Soc. Echocardiogr. 2019, 32, 341–343. [Google Scholar] [CrossRef]
- Schwammenthal, E.; Nakatani, S.; He, S.; Hopmeyer, J.; Sagie, A.; Weyman, A.E.; Lever, H.M.; Yoganathan, A.P.; Thomas, J.D.; Levine, R.A. Mechanism of mitral regurgitation in hypertrophic cardiomyopathy: Mismatch of posterior to anterior leaflet length and mobility. Circulation 1998, 98, 856–865. [Google Scholar] [CrossRef]
- Moon, J.C.; Fisher, N.G.; McKenna, W.J.; Pennell, D.J. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart 2004, 90, 645–649. [Google Scholar] [CrossRef]
- Maron, M.S.; Rowin, E.J.; Maron, B.J. How to Image Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005372. [Google Scholar] [CrossRef]
- Rickers, C.; Wilke, N.M.; Jerosch-Herold, M.; Casey, S.A.; Panse, P.; Panse, N.; Weil, J.; Zenovich, A.G.; Maron, B.J. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005, 112, 855–861. [Google Scholar] [CrossRef]
- Hindieh, W.; Weissler-Snir, A.; Hammer, H.; Adler, A.; Rakowski, H.; Chan, R.H. Discrepant Measurements of Maximal Left Ventricular Wall Thickness Between Cardiac Magnetic Resonance Imaging and Echocardiography in Patients with Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e006309. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Maron, B.J.; Maron, M.S. Reply: Hypertrophic Cardiomyopathy with Left Ventricular Apical Aneurysm. J. Am. Coll. Cardiol. 2017, 70, 407. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Grasso, A.; Roughton, M.; Moon, J.C.; Clark, S.; Wage, R.; Webb, J.; Kulkarni, M.; Dawson, D.; Sulaibeekh, L.; et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Green, J.J.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc. Imaging 2012, 5, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Mentias, A.; Raeisi-Giglou, P.; Smedira, N.G.; Feng, K.; Sato, K.; Wazni, O.; Kanj, M.; Flamm, S.D.; Thamilarasan, M.; Popovic, Z.B.; et al. Late Gadolinium Enhancement in Patients with Hypertrophic Cardiomyopathy and Preserved Systolic Function. J. Am. Coll. Cardiol. 2018, 72, 857–870. [Google Scholar] [CrossRef]
- Rahman, S.U.; Rowin, E.J. T1 Mapping and Interstitial Fibrosis as a Marker for Heart Failure in Hypertrophic Cardiomyopathy. Circ. Cardiovasc Imaging 2025, 18, e017938. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Pu, L.; Qi, W.; Xu, Y.; Wan, K.; Zhu, Y.; Gkoutos, G.V.; Han, Y.; Chen, Y. The Prognostic Value of Left Ventricular Entropy from T1 Mapping in Patients with Hypertrophic Cardiomyopathy. JACC Asia 2024, 4, 389–399. [Google Scholar] [CrossRef]
- Ye, Y.; Ji, Z.; Zhou, W.; Pu, C.; Li, Y.; Zhou, C.; Hu, X.; Chen, C.; Sun, Y.; Huang, Q.; et al. Mean Scar Entropy by Late Gadolinium Enhancement Cardiac Magnetic Resonance Is Associated with Ventricular Arrhythmias Events in Hypertrophic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 758635. [Google Scholar] [CrossRef]
- Chiribiri, A.; Leuzzi, S.; Conte, M.R.; Bongioanni, S.; Bratis, K.; Olivotti, L.; De Rosa, C.; Lardone, E.; Di Donna, P.; Villa, A.D.; et al. Rest perfusion abnormalities in hypertrophic cardiomyopathy: Correlation with myocardial fibrosis and risk factors for sudden cardiac death. Clin. Radiol. 2015, 70, 495–501. [Google Scholar] [CrossRef]
- Camaioni, C.; Knott, K.D.; Augusto, J.B.; Seraphim, A.; Rosmini, S.; Ricci, F.; Boubertakh, R.; Xue, H.; Hughes, R.; Captur, G.; et al. Inline perfusion mapping provides insights into the disease mechanism in hypertrophic cardiomyopathy. Heart 2020, 106, 824–829. [Google Scholar] [CrossRef]
- Shariat, M.; Thavendiranathan, P.; Nguyen, E.; Wintersperger, B.; Paul, N.; Rakowski, H.; Crean, A.M. Utility of coronary CT angiography in outpatients with hypertrophic cardiomyopathy presenting with angina symptoms. J. Cardiovasc. Comput. Tomogr. 2014, 8, 429–437. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, N.; Huurman, R.; Yamasaki, Y.; Kardys, I.; Galema, T.W.; Budde, R.P.; Zijlstra, F.; Krestin, G.P.; Schinkel, A.F.; Michels, M.; et al. Frequency and Significance of Coronary Artery Disease and Myocardial Bridging in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2020, 125, 1404–1412. [Google Scholar] [CrossRef]
- Gatti, M.; De Filippo, O.; Cura Cura, G.; Dusi, V.; Di Vita, U.; Gallone, G.; Morena, A.; Palmisano, A.; Pasinato, E.; Solano, A.; et al. Diagnostic accuracy of late iodine enhancement on cardiac CT for myocardial tissue characterization: A systematic review and meta-analysis. Eur. Radiol. 2024. [Google Scholar] [CrossRef]
- Takayama, H.; Yu, S.N.; Sorabella, R.; Leb, J.; Pulerwitz, T.C.; Cooper, C.; Argenio, M.; Shimada, Y.J.; Weiner, S.; Ginns, J.N. Virtual septal myectomy for preoperative planning in hypertrophic cardiomyopathy. J. Thorac. Cardiovasc. Surg. 2019, 158, 455–463. [Google Scholar] [CrossRef]
- Cooper, R.M.; Binukrishnan, S.R.; Shahzad, A.; Hasleton, J.; Sigwart, U.; Stables, R.H. Computed tomography angiography planning identifies the target vessel for optimum infarct location and improves clinical outcome in alcohol septal ablation for hypertrophic obstructive cardiomyopathy. EuroIntervention 2017, 12, e2194–e2203. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E. Is there a role for cardiac positron emission tomography in hypertrophic cardiomyopathy? J. Nucl. Cardiol. 2019, 26, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Camici, P.; Chiriatti, G.; Lorenzoni, R.; Bellina, R.C.; Gistri, R.; Italiani, G.; Parodi, O.; Salvadori, P.A.; Nista, N.; Papi, L.; et al. Coronary vasodilation is impaired in both hypertrophied and nonhypertrophied myocardium of patients with hypertrophic cardiomyopathy: A study with nitrogen-13 ammonia and positron emission tomography. J. Am. Coll. Cardiol. 1991, 17, 879–886. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Shetty, A.; Winson, G.; Kim, B.; Musat, D.; Alviar, C.L.; Homel, P.; Balaram, S.K.; Swistel, D.G. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with beta-blockade or verapamil. Circ. Heart Fail 2013, 6, 694–702. [Google Scholar] [CrossRef]
- Mehra, N.; Veselka, J.; Smedira, N.; Desai, M.Y. Invasive therapies for symptomatic obstructive hypertrophic cardiomyopathy. Prog. Cardiovasc. Dis. 2023, 80, 46–52. [Google Scholar] [CrossRef]
- La Canna, G.; Scarfo, I.; Arendar, I.; Colombo, A.; Torracca, L.; Margonato, D.; Montorfano, M.; Alfieri, O. Targeting Alcohol Septal Ablation in Patients with Obstructive Hypertrophic Cardiomyopathy Candidates for Surgical Myectomy: Added Value of Three-Dimensional Intracoronary Myocardial Contrast Echocardiography. J. Clin. Med. 2021, 10, 2166. [Google Scholar] [CrossRef]
- Authors/Task Force members; Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Dimitrow, P.P.; Chojnowska, L.; Rudzinski, T.; Piotrowski, W.; Ziolkowska, L.; Wojtarowicz, A.; Wycisk, A.; Dabrowska-Kugacka, A.; Nowalany-Kozielska, E.; Sobkowicz, B.; et al. Sudden death in hypertrophic cardiomyopathy: Old risk factors re-assessed in a new model of maximalized follow-up. Eur. Heart J. 2010, 31, 3084–3093. [Google Scholar] [CrossRef]
- Maron, B.J. Risk stratification and role of implantable defibrillators for prevention of sudden death in patients with hypertrophic cardiomyopathy. Circ. J. 2010, 74, 2271–2282. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.H.; Maron, B.J.; Olivotto, I.; Pencina, M.J.; Assenza, G.E.; Haas, T.; Lesser, J.R.; Gruner, C.; Crean, A.M.; Rakowski, H.; et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014, 130, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Olivotto, I.; Betocchi, S.; Casey, S.A.; Lesser, J.R.; Losi, M.A.; Cecchi, F.; Maron, B.J. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 2003, 348, 295–303. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur. Heart J. 2014, 35, 2010–2020. [Google Scholar] [CrossRef]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; van Zwet, E.W.; Delgado, V.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Marsan, N.A. Global Longitudinal Strain and Left Atrial Volume Index Provide Incremental Prognostic Value in Patients with Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005706. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Mohananey, D.; To, A.; Lever, H.M.; Popovic, Z.B.; Desai, M.Y. Prognostic Value of Global Longitudinal Strain in Hypertrophic Cardiomyopathy: A Systematic Review of Existing Literature. JACC Cardiovasc. Imaging 2019, 12, 1930–1942. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.K.; Lee, S.C.; Kim, J.; Park, J.B.; Hwang, I.C.; Choi, Y.J.; Lee, S.P.; Chang, S.A.; Lee, W.; et al. Supplementary role of left ventricular global longitudinal strain for predicting sudden cardiac death in hypertrophic cardiomyopathy. Eur. Heart J. Cardiovasc Imaging 2022, 23, 1108–1116. [Google Scholar] [CrossRef]
- Lu, D.Y.; Yalcin, H.; Yalcin, F.; Zhao, M.; Sivalokanathan, S.; Valenta, I.; Tahari, A.; Pomper, M.G.; Abraham, T.P.; Schindler, T.H.; et al. Stress Myocardial Blood Flow Heterogeneity Is a Positron Emission Tomography Biomarker of Ventricular Arrhythmias in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2018, 121, 1081–1089. [Google Scholar] [CrossRef]
- Zhao, X.; Jin, F.; Wang, J.; Zhao, X.; Wang, L.; Wei, H. Entropy of left ventricular late gadolinium enhancement and its prognostic value in hypertrophic cardiomyopathy a new CMR assessment method. Int. J. Cardiol. 2023, 373, 134–141. [Google Scholar] [PubMed]
- Desai, M.Y.; Owens, A.; Wolski, K.; Geske, J.B.; Saberi, S.; Wang, A.; Sherrid, M.; Cremer, P.C.; Lakdawala, N.K.; Tower-Rader, A.; et al. Mavacamten in Patients with Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 56 Results From the VALOR-HCM Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 968–977. [Google Scholar] [PubMed]
- Olivotto, I.; Oreziak, A.; Barriales-Villa, R.; Abraham, T.P.; Masri, A.; Garcia-Pavia, P.; Saberi, S.; Lakdawala, N.K.; Wheeler, M.T.; Owens, A.; et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020, 396, 759–769. [Google Scholar] [PubMed]
- Desai, M.Y.; Okushi, Y.; Gaballa, A.; Wang, Q.; Geske, J.B.; Owens, A.T.; Saberi, S.; Wang, A.; Cremer, P.C.; Sherrid, M.; et al. Serial Changes in Ventricular Strain in Symptomatic Obstructive Hypertrophic Cardiomyopathy Treated with Mavacamten: Insights From the VALOR-HCM Trial. Circ. Cardiovasc. Imaging 2024, 17, e017185. [Google Scholar]
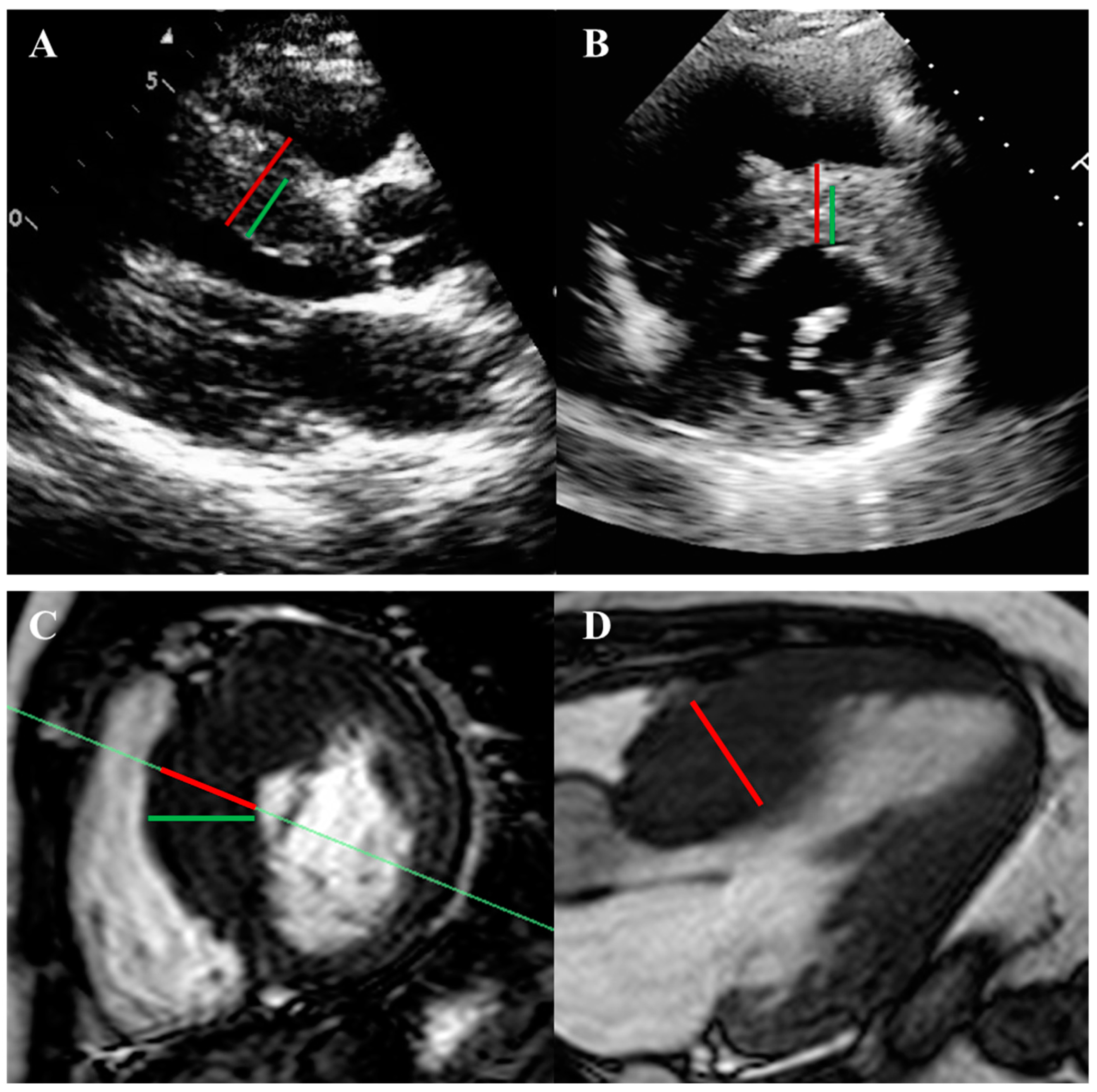

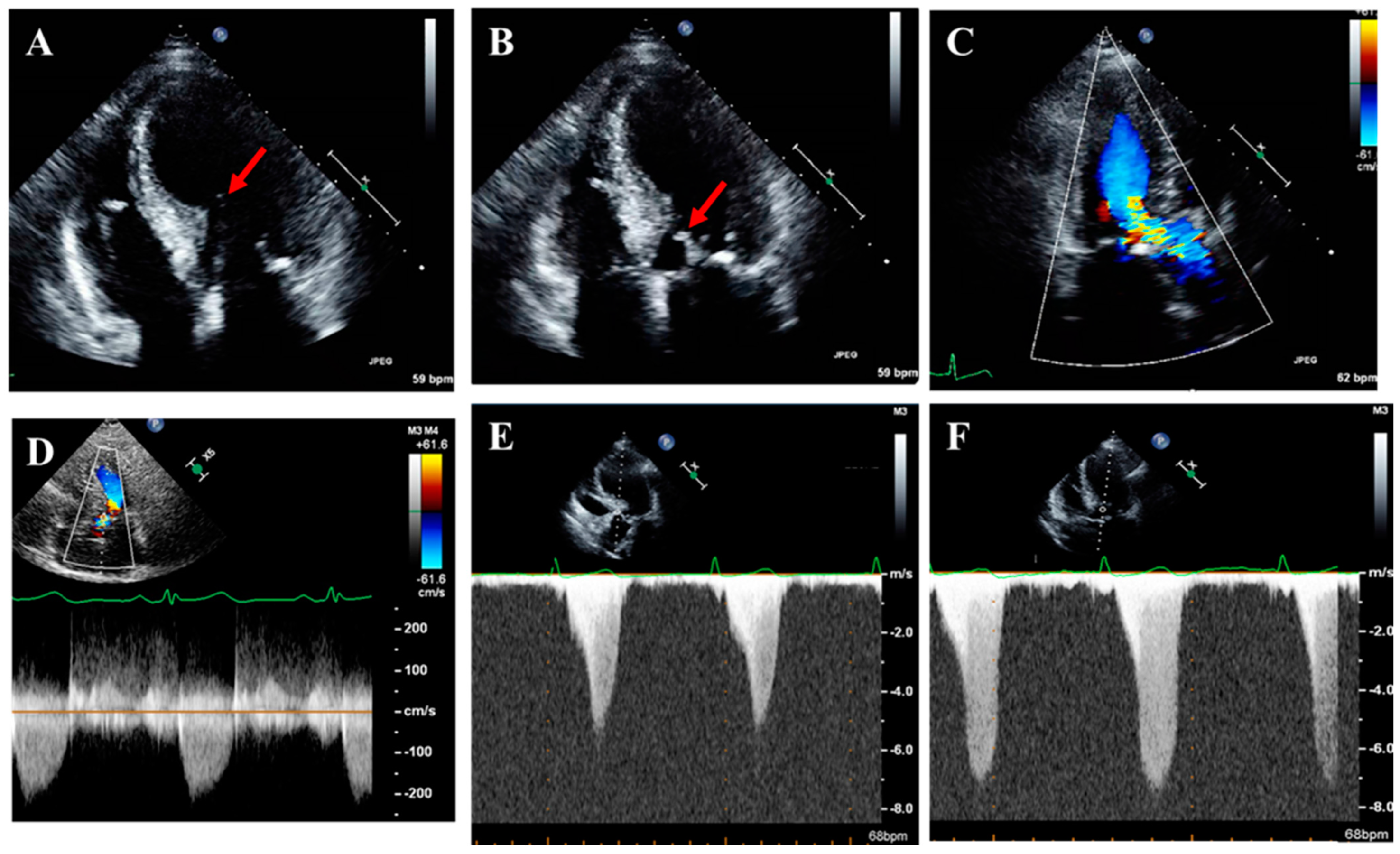


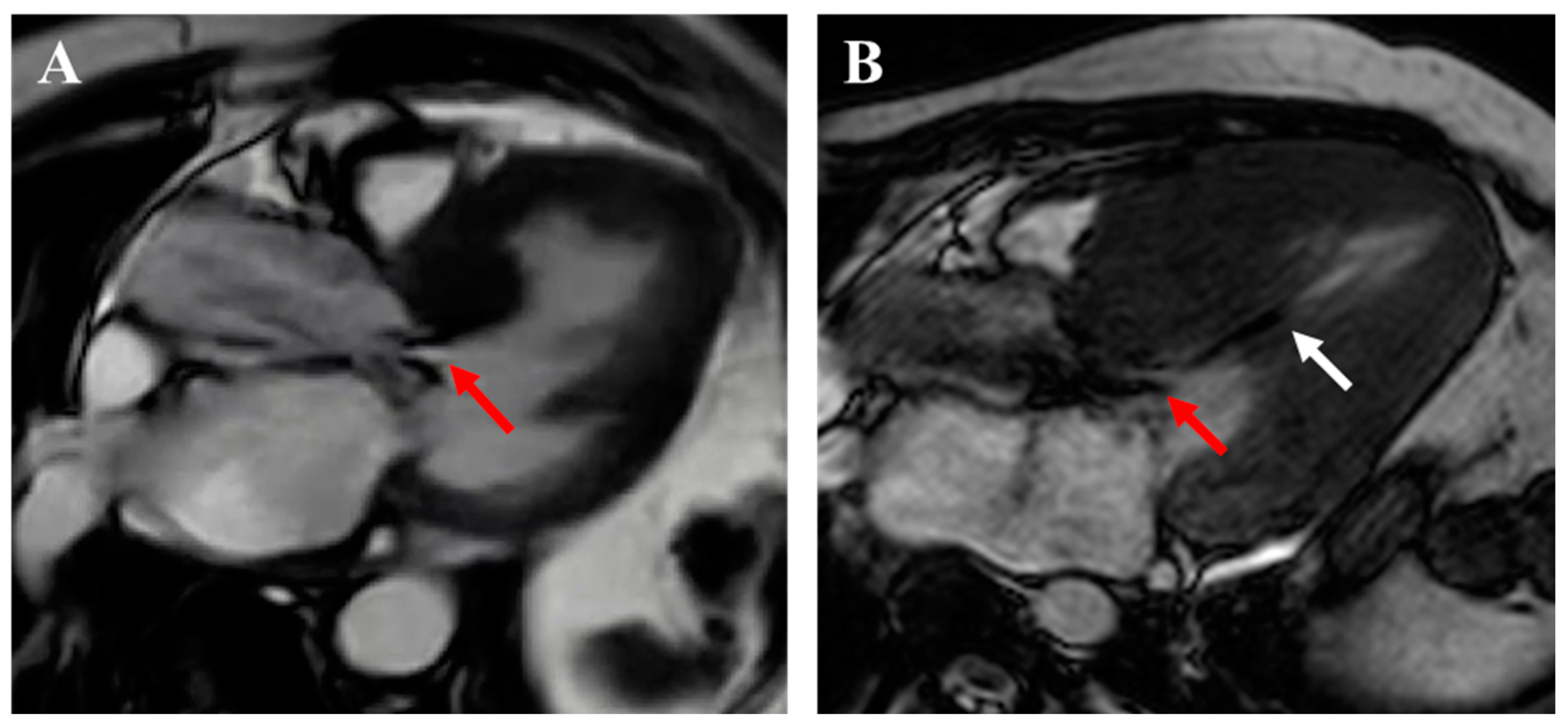
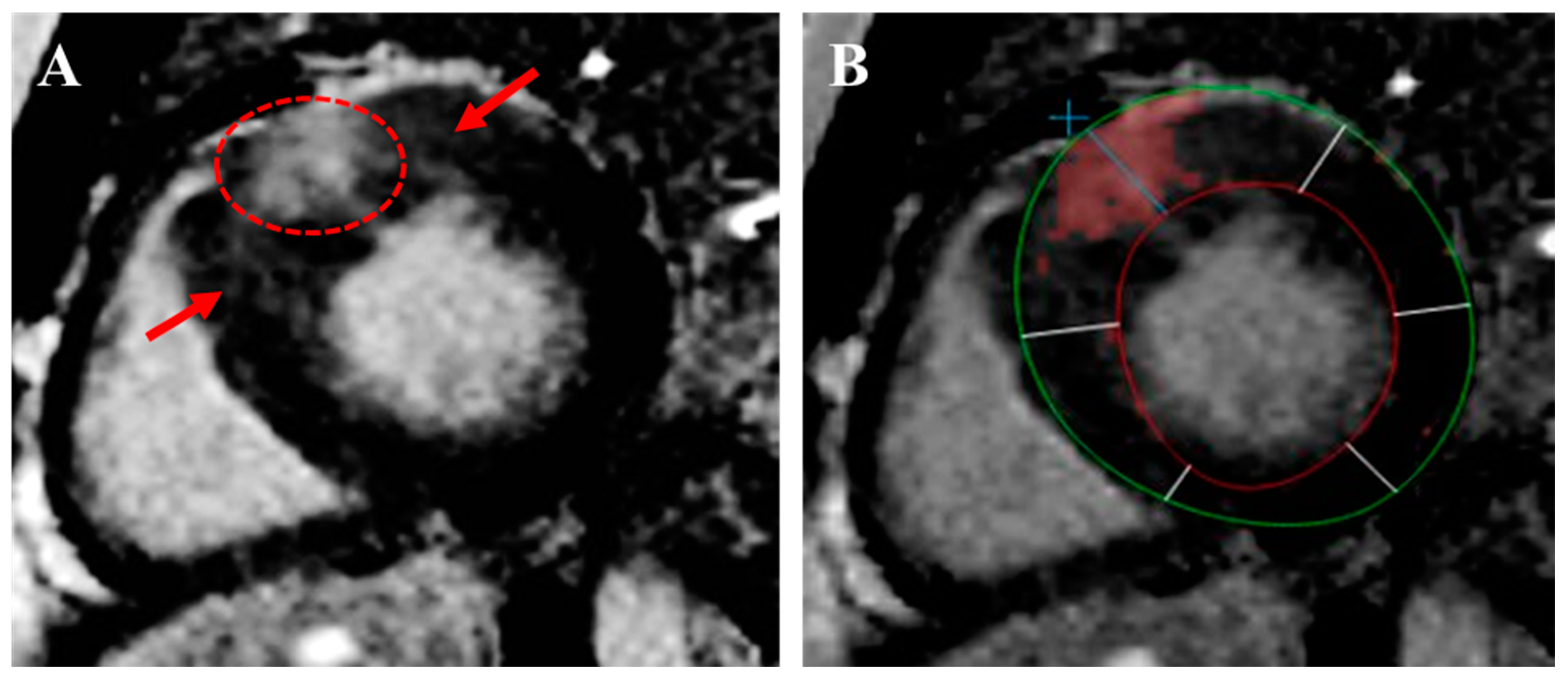

| Guideline-Supported Criteria | Supporting Findings |
|---|---|
| LV end-diastolic wall thickness ≥ 15 mm in any segment not entirely explained by another condition |
|
| LV end-diastolic wall thickness 13–14 mm with a positive pathogenic genetic variant or in primary relatives of patients with HCM | |
| In children: Indexed LV end-diastolic wall thickness z score ≥ 2 | |
| Emerging criteria | |
| Demographically adjusted LVH | |
| Indexed apical wall thickness > 5.6 mm/m2 in apical HCM |
| Modality | When to Obtain? | When to Repeat? |
|---|---|---|
| TTE |
| 1–2 years, or functional decline As per cardiac myosin inhibitor protocol |
| TEE |
| Personalized |
| Stress TTE |
| 2–3 years, or functional decline |
| CMR |
| 3–5 years for SCD risk if no ICD present |
| CCT |
| Personalized |
| Nuclear MPI |
| Personalized |
| Modality | Imaging Targets | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LVH | Systolic Function | Diastolic Function | Apical Aneurysm | LVOTO (Gradient) | MR/LVOTO Mechanisms | Epicardial CAD | MVD | Fibrosis | |
| TTE | + | + | ++ | +/++ with UEA | ++ | + | − | − | − |
| TEE | ++ | + | + | + | ++ | +++ | − | − | − |
| Stress TTE | + | + | +++ (exercise diastology) | +/++ with UEA | +++ (latent LVOTO) | ++ (dynamic MR with exercise) | + | + (LAD CFR) | − |
| CMR | +++ | +++ | + | +++ | + | +++ | ++ (Stress CMR) | ++ | ++ (LGE) |
| CCT | +++ | ++ | − | +++ | − | +/− (anatomical abnormalities) | +++ | +/− (CCT perfusion) | + (LIE) |
| Nuclear MPI | +/− (Asymmetric uptake) | + | +/− (PFR/TPFR) | − | − | − | ++ | +++ | − |
| Steps | Practical Guidance |
|---|---|
| 1. Identify MR signal with CW Doppler | Begin by placing the CW Doppler beam through the easily identifiable MR jet on color Doppler. Obtain and save the signal. |
| 2. Maneuver toward the LVOT | With the CW Doppler, slowly sweep the transducer anteriorly toward the LVOT. |
| 3. Observe for LVOT signal | Look for a signal that: ● Starts later in systole ● Has a late-peaking, dagger-shaped appearance ● Has a shorter duration than the MR signal ● Has a lower peak velocity than the MR signal |
| 4. PW Doppler mapping | Use PW Doppler to map velocities around the LVOT, starting deep in the LV cavity and moving toward the aorta. A sudden increase in the velocity helps confirm the LVOT signal’s location. |
| 5. Compare and contrast | Compare the saved MR signal with the suspected LVOT signal. Note the differences in timing, shape, velocity, and duration. |
| 6. Valsalva | If uncertain, perform a Valsalva maneuver while monitoring the LVOT signal. An increase in velocity and a more pronounced dagger shape support the presence of a dynamic LVOTO. |
| Parameter | Cut-Off |
|---|---|
| Major | |
| LV wall thickness | ≥28–30 mm |
| Apical aneurysm | Presence |
| LVEF | <50% |
| Non-major | |
| LGE | ≥15% |
| 5-year risk estimation | |
| LVOT gradient | Continuous, but higher risk at >30 mmHg |
| Left atrial diameter | Continuous |
| Investigational | |
| Left atrial volume index | >34 mL/m2 |
| GLS | >−15% |
| Flow heterogeneity | ≥1.85 |
| LGE entropy | ≥5.873 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalsania, A.K.; Park, C.M.; Nagraj, S.; Lorenzatti, D.; Filtz, A.; Weissler-Snir, A.; Garcia, M.J.; Slipczuk, L.; Schenone, A.L. A Practical Approach to Multimodality Imaging in Hypertrophic Cardiomyopathy. J. Clin. Med. 2025, 14, 2606. https://doi.org/10.3390/jcm14082606
Dalsania AK, Park CM, Nagraj S, Lorenzatti D, Filtz A, Weissler-Snir A, Garcia MJ, Slipczuk L, Schenone AL. A Practical Approach to Multimodality Imaging in Hypertrophic Cardiomyopathy. Journal of Clinical Medicine. 2025; 14(8):2606. https://doi.org/10.3390/jcm14082606
Chicago/Turabian StyleDalsania, Ankur K., Christine M. Park, Sanjana Nagraj, Daniel Lorenzatti, Annalisa Filtz, Adaya Weissler-Snir, Mario J. Garcia, Leandro Slipczuk, and Aldo L. Schenone. 2025. "A Practical Approach to Multimodality Imaging in Hypertrophic Cardiomyopathy" Journal of Clinical Medicine 14, no. 8: 2606. https://doi.org/10.3390/jcm14082606
APA StyleDalsania, A. K., Park, C. M., Nagraj, S., Lorenzatti, D., Filtz, A., Weissler-Snir, A., Garcia, M. J., Slipczuk, L., & Schenone, A. L. (2025). A Practical Approach to Multimodality Imaging in Hypertrophic Cardiomyopathy. Journal of Clinical Medicine, 14(8), 2606. https://doi.org/10.3390/jcm14082606






