Prediction of Pituitary Adenoma’s Volumetric Response to Gamma Knife Radiosurgery Using Machine Learning-Supported MRI Radiomics
Abstract
1. Introduction
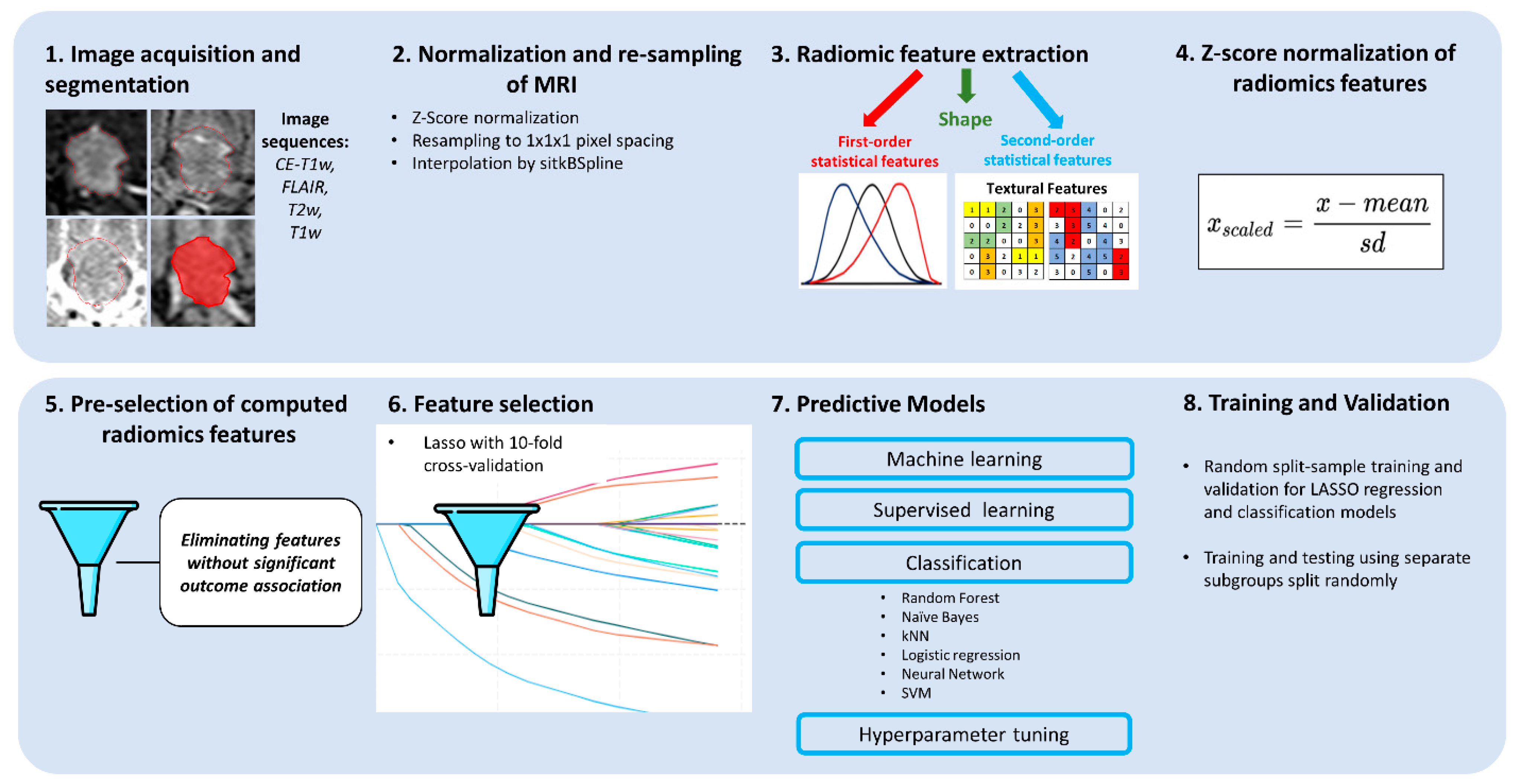
2. Methods
2.1. Ethics Approval Statement
2.2. Patients
2.3. Sample Size Calculation
2.4. Gamma Knife Treatment
2.5. MRI
- Three-dimensional T1w magnetization-prepared rapid acquisition (MPRAGE) sequence: gradient echo; TR/TE/TI, 6.8/3.2/900 ms; flip angle, 8°; measured voxel size, 0.6 × 0.6 × 1.0 mm, before and after intravenous injection of contrast medium.
- T2w sequence: TR/TE 3693.8/80 ms; 150 transversal slices; thickness, 1 mm; matrix, 512 × 512.
- Fluid-attenuated inversion recovery (FLAIR) sequence: TR/TE/TI, 11,000/120/2800 ms; 90 transversal slices; thickness, 2 mm; matrix, 512 × 512.
2.6. Postprocessing
2.7. Follow-Up
2.8. Feature Extraction
2.9. Model Selection
2.10. Evaluation of Predictive Performance
2.11. Validation
3. Results
3.1. Patients’ Characteristics
3.2. Experimental Design
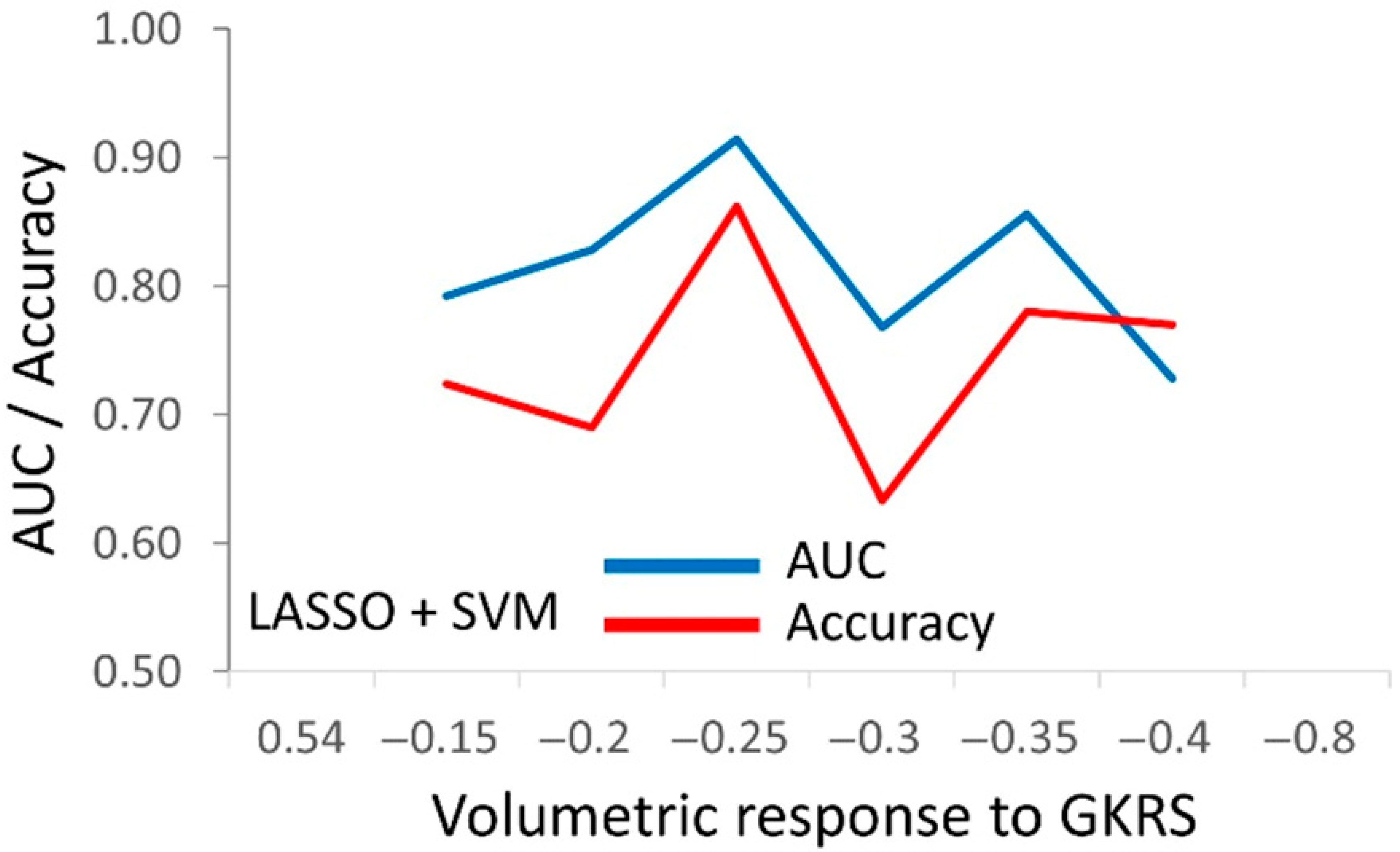
3.3. The Predictive Models for PA’s Response to Radiosurgery
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trifiletti, D.M.; Dutta, S.W.; Lee, C.C.; Sheehan, J.P. Pituitary Tumor Radiosurgery. Prog. Neurol. Surg. 2019, 34, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Sahgal, A.; Rubens, M.; De Salles, A.; Fariselli, L.; Pollock, B.E.; Levivier, M.; Ma, L.; Paddick, I.; Regis, J.; et al. Stereotactic radiosurgery for non-functioning pituitary adenomas: Meta-analysis and International Stereotactic Radiosurgery Society practice opinion. Neuro Oncol. 2020, 22, 318–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lehrer, E.J.; Kowalchuk, R.O.; Trifiletti, D.M.; Sheehan, J.P. The Role of Stereotactic Radiosurgery for Functioning and Nonfunctioning Pituitary Adenomas. Neurol. India. 2023, 71 (Suppl. S1), S133–S139. [Google Scholar] [CrossRef] [PubMed]
- Dayawansa, S.; Abbas, S.O.; Mantziaris, G.; Dumot, C.; Donahue, J.H.; Sheehan, J.P. Volumetric Assessment of Nonfunctional Pituitary Adenoma Treated With Stereotactic Radiosurgery: An Assessment of Long-Term Response. Neurosurgery 2023, 93, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Pomeraniec, I.J.; Xu, Z.; Lee, C.C.; Yang, H.C.; Chytka, T.; Liscak, R.; Martinez-Alvarez, R.; Martinez-Moreno, N.; Attuati, L.; Picozzi, P.; et al. Dose to neuroanatomical structures surrounding pituitary adenomas and the effect of stereotactic radiosurgery on neuroendocrine function: An international multicenter study. J. Neurosurg. 2021, 136, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mantziaris, G.; Pikis, S.; Chytka, T.; Liščák, R.; Sheehan, K.; Sheehan, D.; Peker, S.; Samanci, Y.; Bindal, S.K.; Niranjan, A.; et al. Adjuvant versus on-progression Gamma Knife radiosurgery for residual nonfunctioning pituitary adenomas: A matched-cohort analysis. J. Neurosurg. 2022, 138, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Speckter, H.; Radulovic, M.; Trivodaliev, K.; Vranes, V.; Joaquin, J.; Hernandez, W.; Mota, A.; Bido, J.; Hernandez, G.; Rivera, D.; et al. MRI radiomics in the prediction of the volumetric response in meningiomas after gamma knife radiosurgery. J. Neurooncol. 2022, 159, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Djuričić, G.J.; Ahammer, H.; Rajković, S.; Kovač, J.D.; Milošević, Z.; Sopta, J.P.; Radulovic, M. Directionally Sensitive Fractal Radiomics Compatible with Irregularly Shaped Magnetic Resonance Tumor Regions of Interest: Association with Osteosarcoma Chemoresistance. J. Magn. Reason. Imaging 2023, 57, 248–258. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J. The linear quadratic model: Usage, interpretation and challenges. Phys. Med. Biol. 2018, 64, 01TR01. [Google Scholar] [CrossRef]
- Speckter, H.; Santana, J.; Miches, I.; Hernandez, G.; Bido, J.; Rivera, D.; Suazo, L.; Valenzuela, S.; Garcia, J.; Stoeter, P. Assessment of the alpha/beta ratio of the optic pathway to adjust hypofractionated stereotactic radiosurgery regimens for perioptic lesions. J. Radiat. Oncol. 2019, 8, 279–289. [Google Scholar] [CrossRef]
- Speckter, H.; Hernandez, G.; Bido, J.; Rivera, D.; Suazo, L.; Valenzuela, S.; Santana, J.; Hernandez, W.; Moreno, L.; Peralta, I.; et al. Assessment of the alpha/beta Ratios of Pituitary Adenomas and Craniopharyngiomas. In Proceedings of the International Stereotactic Radiosurgery Society (ISRS) Meeting, Milan, Italy, 19–23 June 2022. [Google Scholar]
- Speckter, H.; Santana, J.; Lara, G.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Stoeter, P. Assessment of The Alpha/Beta Ratios of Pituitary Adenomas and Craniopharyngiomas for The Quantification of Single Fraction Equivalent Dose Benefits from Hypofractionated Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, E714. [Google Scholar] [CrossRef]
- van Griethuysen, J.J.M.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.H.H.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J.W.L. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 7, e104–e107. [Google Scholar] [CrossRef] [PubMed]
- Speckter, H.; Palque-Santos, S.; Mota-Gonzalez, R.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Gonzalez-Curi, M.; Stoeter, P. Can Apparent Diffusion Coefficient (ADC) maps replace Diffusion Tensor Imaging (DTI) maps to predict the volumetric response of meningiomas to Gamma Knife Radiosurgery? J. Neurooncol. 2023, 161, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Bi, W.L.; Weller, M.; Kaley, T.; Blakeley, J.; Dunn, I.; Galanis, E.; Preusser, M.; McDermott, M.; Rogers, L.; et al. Proposed response assessment and endpoints for meningioma clinical trials: Report from the response assessment in neuro-oncology working group. Neuro-Oncol. 2019, 21, 26–36. [Google Scholar] [CrossRef]
- Imber, B.S.; Lin, A.L.; Zhang, Z.; Keshavamurthy, K.N.; Deipolyi, A.R.; Beal, K.; Cohen, M.A.; Tabar, V.; DeAngelis, L.M.; Geer, E.B.; et al. Comparison of Radiographic Approaches to Assess Treatment Response in Pituitary Adenomas: Is RECIST or RANO Good Enough? J. Endocr. Soc. 2019, 3, 1693–1706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Speckter, H.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Miches, I.; Oviedo, J.; Gonzalez, C.; Stoeter, P. Pretreatment texture analysis of routine MR images and shape analysis of the diffusion tensor for prediction of volumetric response after radiosurgery for meningioma. J. Neurosurg. 2018, 129, 31–37. [Google Scholar] [CrossRef]
- Speckter, H.; Santana, J.; Bido, J.; Hernandez, G.; Rivera, D.; Suazo, L.; Valenzuela, S.; Oviedo, J.; Gonzalez, C.F.; Stoeter, P. Texture Analysis of Standard Magnetic Resonance Images to Predict Response to Gamma Knife Radiosurgery in Vestibular Schwannomas. World Neurosurg. 2019, 132, e228–e234. [Google Scholar] [CrossRef] [PubMed]
- Langenhuizen, P.; Sebregts, S.H.P.; Zinger, S.; Leenstra, S.; de Verheul, J.B. With PHN Prediction of transient tumor enlargement using MRI tumor texture after radiosurgery on vestibular schwannoma. Med. Phys. 2020, 47, 1692–1701. [Google Scholar] [CrossRef]
- George-Jones, N.A.; Wang, K.; Wang, J.; Hunter, J.B. Prediction of Vestibular Schwannoma Enlargement After Radiosurgery Using Tumor Shape and MRI Texture Features. Otol. Neurotol. 2021, 42, e348–e354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Geng, D.; Yu, T.; Xia, W.; She, D.; Liu, L.; Yin, B. Prognostic value of pretreatment MRI texture features in breast cancer brain metastasis treated with Gamma Knife radiosurgery. Acta Radiol. 2021, 62, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, B.S.; Han, J.H.; Kim, C.Y.; Cho, J.; Bae, Y.J.; Sunwoo, L.; Kim, J.H. MRI Texture Analysis for the Prediction of Stereotactic Radiosurgery Outcomes in Brain Metastases from Lung Cancer. J. Clin. Med. 2021, 10, 237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langenhuizen, P.; Zinger, S.; Leenstra, S.; Kunst, H.P.M.; Mulder, J.J.S.; Hanssens, P.E.J.; de With, P.H.N.; Verheul, J.B. Radiomics based prediction of long-term treatment response of vestibular schwannomas following stereotactic radiosurgery. Otol. Neurotol. 2020, 41, e1321–e1327. [Google Scholar] [CrossRef]
- Yang, H.C.; Wu, C.C.; Lee, C.C.; Huang, H.E.; Lee, W.K.; Chung, W.Y.; Wu, H.M.; Guo, W.Y.; Wu, Y.T.; Lu, C.F. Prediction of pseudoprogression and long-term outcome of vestibular schwannoma after Gamma Knife radiosurgery based on preradiosurgical MR radiomics. Radiother. Oncol. 2021, 155, 123–130. [Google Scholar] [CrossRef]
- Mouraviev, A.; Detsky, J.; Sahgal, A.; Ruschin, M.; Lee, Y.K.; Karam, I.; Heyn, C.; Stanisz, G.J.; Martel, A.L. Use of radiomics for the prediction of local control of brain metastases after stereotactic radiosurgery. Neuro. Oncol. 2020, 22, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.Y.; Lee, C.C.; Yang, H.C.; Chen, C.J.; Chung, W.Y.; Wu, H.M.; Guo, W.Y.; Liu, R.S.; Lu, C.F. Enhancement of radiosurgical treatment outcome prediction using MRI radiomics in patients with non-small cell lung cancer brain metastases. Cancers 2021, 13, 4030. [Google Scholar] [CrossRef]
- Wang, H.; Xue, J.; Qu, T.; Bernstein, K.; Chen, T.; Barbee, D.; Silverman, J.S.; Kondziolka, D. Predicting local failure of brain metastases after stereotactic radiosurgery with radiomics on planning MR images and dose maps. Med. Phys. 2021, 48, 5522–5530. [Google Scholar] [CrossRef]
- Mulford, K.; Chen, C.; Dusenbery, K.; Yuan, J.; Hunt, M.A.; Chen, C.C.; Sperduto, P.; Watanabe, Y.; Wilke, C. A radiomics-based model for predicting local control of resected brain metastases receiving adjuvant SRS. Clin. Transl. Radiat. Oncol. 2021, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Meng, X.; Jin, H.; Liu, A.; Sun, S. Assessment of gamma knife radiosurgery for unruptured cerebral arteriovenous malformations based on multi-parameter radiomics of MRI. Magn. Reason Imaging 2022, 92, 251–259. [Google Scholar] [CrossRef]
- Meng, X.; Gao, D.; Jin, H.; Wang, K.; Bao, E.; Liu, A.; Li, Y.; Sun, S. Factors affecting volume reduction velocity for arteriovenous malformations after treatment with dose-stage stereotactic radiosurgery. Front. Oncol. 2021, 11, 769533. [Google Scholar] [CrossRef]
- Meng, X.; Gao, D.; He, H.; Sun, S.; Liu, A.; Jin, H.; Li, Y. A machine learning model predicts the outcome of SRS for residual arteriovenous malformations after partial embolization: A realworld clinical obstacle. World Neurosurg. 2022, 163, e73–e82. [Google Scholar] [CrossRef]
- Chen, T.C.; Zee, C.S.; Miller, C.A.; Weiss, M.H.; Tang, G.; Chin, L.; Levy, M.L.; Apuzzo, M.L. Magnetic resonance imaging and pathological correlates of meningiomas. Neurosurgery 1992, 31, 1015–1021. [Google Scholar] [PubMed]
- Suzuki, Y.; Sugimoto, T.; Shibuya, M.; Sugita, K.; Patel, S.J. Meningiomas: Correlation between MRI characteristics and operative findings including consistency. Acta Neurochir. 1994, 129, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Iaconetta, G.; de Divitiis, O.; Cirillo, S.; Di Salle, F.; De Caro, M.L. Intracranial meningiomas: Correlations between MR imaging and histology. Eur. J. Radiol 1999, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Rutland, J.W.; Verma, G.; Banihashemi, A.; Padormo, F.; Tsankova, N.M.; Delman, B.N.; Shrivastava, R.K.; Balchandani, P. Pituitary adenoma consistency: Direct correlation of ultrahigh field 7T MRI with histopathological analysis. Eur. J. Radiol. 2020, 126, 108931. [Google Scholar] [CrossRef] [PubMed]
- Sitthinamsuwan, B.; Khampalikit, I.; Nunta-aree, S.; Srirabheebhat, P.; Witthiwej, T.; Nitising, A. Predictors of meningioma consistency: A study in 243 consecutive cases. Acta Neurochir. 2012, 154, 1383–1389. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Kawase, T.; Sagoh, M.; Ohira, T.; Shiga, H.; Toya, S. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surg. Neurol. 1997, 48, 579–583. [Google Scholar] [CrossRef]
- Smith, K.A.; Leever, J.D.; Chamoun, R.B. Predicting consistency of meningioma by magnetic resonance imaging. J. Neurol. Surg. B Skull Base 2015, 76, 225–229. [Google Scholar] [CrossRef]
- Černý, M.; Sedlák, V.; Lesáková, V.; Francůz, P.; Netuka, D. Methods of preoperative prediction of pituitary adenoma consistency: A systematic review. Neurosurg. Rev. 2022, 46, 11. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wu, C.; Meng, M.; Liu, T.; Li, C.; Ma, J.; Qin, Z. Radiomic Features on Multiparametric MRI for Preoperative Evaluation of Pituitary Macroadenomas Consistency: Preliminary Findings. J. Magn. Reason. Imaging 2022, 55, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
| All PAs | Functional PAs | Nonfunctional PAs | ||||
|---|---|---|---|---|---|---|
| Patient and treatment characteristics | Value | Range | Value | Range | Value | Range |
| Number of patients | 81 | - | 29 [36%] | - | 52 [64%] | - |
| Age in years (mean, range) | 45.6 | (11.4/83.2) | 38.3 | (11.4/65.5) | 49.7 | (21.2/83.2) |
| Pre-SRS tumor volume in cm3 (mean, range) | 6.30 | (0.16/40.33) | 6.00 | (0.18/33.54) | 6.39 | (0.16/40.33) |
| Previous RT, SRS | 0 | - | 0 | - | 0 | - |
| Previous surgery | 69 [85%] | - | 25 [86%] | - | 44 [85%] | - |
| KPS before SRS (mean, range) | 83.3 | (60/100) | 82.7 | (60/100) | 83.8 | (60/100) |
| Single fraction SRS treatments | 49 [60%] | - | 13 [45%] | - | 36 [69%] | - |
| Hypofractionated SRS treatments | 32 [40%] | - | 16 [55%] | - | 16 [31%] | - |
| Number of fractions (mean, range) | 2.21 | (1/5) | 2.83 | (1/5) | 1.86 | (1/4) |
| Gradient index (mean, range) | 2.83 | (2.42/3.60) | 2.82 | (2.48/3.48) | 2.83 | (2.42/3.60) |
| Coverage index (mean, range) | 96.7% | (91.0%/100%) | 96.8% | (91.0%/100%) | 96.6% | (91.0%/100%) |
| Selectivity index (mean, range) | 67.8% | (18.0%/89.0%) | 63.6% | (18.0%/85.0%) | 70.3% | (39.0%/89.0%) |
| Paddick conformity index (mean, range) | 65.5% | (17.6%/83.7%) | 61.5% | (17.6%/80.8%) | 67.8% | (39.0%/83.7%) |
| Margin physical dose in Gy (mean, range) | 20.5 | (12/40) | 26.7 | (15/40) | 17.0 | (12/24) |
| Margin BED in Gy (mean, range) | 100.9 | (40.4/284.5) | 106.2 | (40.4/284.5) | 97.8 | (60.5/181.9) |
| Margin SFED in Gy (mean, range) | 16.2 | (11.1/35.0) | 19.6 | (11.8/35.0) | 14.2 | (11.1/20.0) |
| Treatment results | Value | Range | Value | Range | Value | Range |
| Follow-up period in months (mean, range) | 40.4 | (6.7/105.5) | 38.6 | (6.7/85.1) | 41.4 | (7.2/105.5) |
| Complete response | 0 [0%] | - | 0 [0%] | - | 0 [0%] | - |
| Partial response (PR, decrease by 30%) | 60 [74.1%] | - | 27 [93%] | - | 33 [63.5%] | - |
| Stable disease (SD, neither PR, no PD) | 20 [24.7%] | - | 2 [7%] | - | 18 [34.6%] | - |
| Progressive disease (PD, increase by ≥20%) | 1 [1.2%] | - | 0 [0%] | - | 1 [1.9%] | - |
| Absolute volume change in cm3 (mean, range) | −2.80 | (−15.83/1.80) | −3.35 | (−15.00/−0.06) | −2.49 | (−15.83/1.80) |
| Relative volume change (mean, range) | −45.7% | (−90.2%/92.7%) | −55.4% | (−81.0%/−27.6%) | −40.2% | (−90.2%/92.7%) |
| Volume change per month (mean, range) | −1.58% | (−9.84%/1.75%) | −2.21% | (−9.84%/−0.57%) | −1.22% | (−4.83%/1.75%) |
| Test Folds (10) | ||
|---|---|---|
| Model | R2 | Selected l |
| CP | 0.272 | 0.0092 |
| T1w | 0.464 | 0.0086 |
| CE-T1w | 0.281 | 0.0130 |
| T1w + CE-T1w | 0.502 | 0.0144 |
| CP + T1w + CE-T1w | 0.584 | 0.0138 |
| T2w | 0.665 | 0.0115 |
| FLAIR | 0.312 | 0.0149 |
| Clinical Parameters | ||||
|---|---|---|---|---|
| T-Test in the Entire Cohort | ||||
| Feature a | T-Statistic | p-Value | Mean ± SD | Mean Difference ± SD |
| Age | −3.38 | 0.001 | 45.5 ± 14.2 | 10.6 ± 3.0 |
| Fraction number | 2.62 | 0.011 | 2.2 ± 1.6 | −0.80 ± 0.32 |
| Dose per fraction | −0.06 | 0.953 | 13.1 ± 7.2 | −0.26 ± 1.4 |
| Accumulated dose | 4.99 | 0.000 | 20.6 ± 6.7 | −5.7 ± 1.2 |
| BED | 0.368 | 0.714 | 100.6 ± 44.6 | −4.7 ± 9.1 |
| SFED | 2.588 | 0.012 | 16.2 ± 5.0 | −2.5 ± 0.93 |
| Coverage | 0.711 | 0.479 | 96.7 ± 2.0 | −0.32 ± 0.47 |
| Selectivity | 0.008 | 0.994 | 67.8 ± 11.6 | 0.37 ± 2.7 |
| PCI | 0.096 | 0.924 | 0.66 ± 0.11 | 0.001 ± 0.027 |
| BOT | 0.641 | 0.523 | 40.9 ± 24.6 | −4.9 ± 5.2 |
| Chi-square test in the entire cohort | ||||
| Pearson’s Chi-square | p-value | Gamma | p-value | |
| Functionality b | 0.42 | 0.001 | 0.81 | <0.001 |
| Models c | ||||
| AUC and accuracy in the test folds (8) d | ||||
| Model | AUC | Accuracy | True positives | True negatives |
| CP | 0.846 ± 0.046 | 0.800 ± 0.049 | 0.542 ± 0.123 | 0.770 ± 0.049 |
| T1w * | 0.924 ± 0.022 | 0.859 ± 0.054 | 0.823 ± 0.049 | 0.850 ± 0.035 |
| CE-T1w | 0.759 ± 0.076 | 0.724 ± 0.091 | 0.614 ± 0.049 | 0.830 ± 0.113 |
| T1w + CE-T1w * | 0.899 ± 0.054 | 0.859 ± 0.062 | 0.810 ± 0.131 | 0.873 ± 0.089 |
| CP + T1w + CE-T1w * | 0.909 ± 0.016 | 0.854 ± 0.024 | 0.845 ± 0.051 | 0.866 ± 0.101 |
| Averages of Prognostic Evaluators ± SD | ||||
|---|---|---|---|---|
| Training Folds (8) | Testing Folds (8) | |||
| Classifier | AUC | Accuracy | AUC | Accuracy |
| Random forest | 0.941 ± 0.033 | 0.867 ± 0.060 | 0.846 ± 0.048 | 0.773 ± 0.066 |
| Naive Bayes | 0.896 ± 0.052 | 0.804 ± 0.086 | 0.795 ± 0.073 | 0.704 ± 0.088 |
| kNN | 0.962 ± 0.028 | 0.892 ± 0.049 | 0.845 ± 0.056 | 0.790 ± 0.028 |
| Logistic regression | 0.991 ± 0.015 | 0.950 ± 0.042 | 0.877 ± 0.031 | 0.815 ± 0.064 |
| Neural network | 0.990 ± 0.020 | 0.957 ± 0.042 | 0.878 ± 0.033 | 0.824 ± 0.065 |
| SVM | 0.977 ± 0.017 | 0.927 ± 0.045 | 0.889 ± 0.043 | 0.820 ± 0.045 |
| Feature | B | 95% CI | P | |
|---|---|---|---|---|
| Age | 1.192 | −18.8 | 122.6 | 0.015 |
| Accumulated dose | −1.020 | −204.3 | 13.4 | 0.084 |
| Orig_firstorder_Entropy_T1w | −5.144 | −989.7 | −3.30 | 0.002 |
| Log_gldm_Smalldepemph_T1w | 1.775 | −84.4 | 417.5 | 0.026 |
| Log_glcm_Id_T1w | −2.936 | −601.5 | 8.9 | 0.010 |
| Lbp2D_gldm_lgdepLowGraylevemph_T1w | −0.784 | −147.4 | 400.8 | 0.296 |
| Logarithm_glcm_JointEnergy_CE-T1w | 0.681 | −33.3 | 111.3 | 0.040 |
| Lbp2D_glrlm_LongRunLowGraylevemph_T1w | 0.955 | −218.6 | 228.8 | 0.257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speckter, H.; Radulovic, M.; Lazo, E.; Hernandez, G.; Bido, J.; Rivera, D.; Suazo, L.; Valenzuela, S.; Stoeter, P.; Vranes, V. Prediction of Pituitary Adenoma’s Volumetric Response to Gamma Knife Radiosurgery Using Machine Learning-Supported MRI Radiomics. J. Clin. Med. 2025, 14, 2896. https://doi.org/10.3390/jcm14092896
Speckter H, Radulovic M, Lazo E, Hernandez G, Bido J, Rivera D, Suazo L, Valenzuela S, Stoeter P, Vranes V. Prediction of Pituitary Adenoma’s Volumetric Response to Gamma Knife Radiosurgery Using Machine Learning-Supported MRI Radiomics. Journal of Clinical Medicine. 2025; 14(9):2896. https://doi.org/10.3390/jcm14092896
Chicago/Turabian StyleSpeckter, Herwin, Marko Radulovic, Erwin Lazo, Giancarlo Hernandez, Jose Bido, Diones Rivera, Luis Suazo, Santiago Valenzuela, Peter Stoeter, and Velicko Vranes. 2025. "Prediction of Pituitary Adenoma’s Volumetric Response to Gamma Knife Radiosurgery Using Machine Learning-Supported MRI Radiomics" Journal of Clinical Medicine 14, no. 9: 2896. https://doi.org/10.3390/jcm14092896
APA StyleSpeckter, H., Radulovic, M., Lazo, E., Hernandez, G., Bido, J., Rivera, D., Suazo, L., Valenzuela, S., Stoeter, P., & Vranes, V. (2025). Prediction of Pituitary Adenoma’s Volumetric Response to Gamma Knife Radiosurgery Using Machine Learning-Supported MRI Radiomics. Journal of Clinical Medicine, 14(9), 2896. https://doi.org/10.3390/jcm14092896







